Abstract
Immune specificity is the degree to which a host's immune system discriminates among various pathogens or antigenic variants. Vertebrate immune memory is highly specific due to antibody responses. On the other hand, some invertebrates show immune priming, i.e. improved survival after secondary exposure to a previously encountered pathogen. Until now, specificity of priming has only been demonstrated via the septic infection route or when live pathogens were used for priming. Therefore, we tested for specificity in the oral priming route in the red flour beetle, Tribolium castaneum. For priming, we used pathogen-free supernatants derived from three different strains of the entomopathogen, Bacillus thuringiensis, which express different Cry toxin variants known for their toxicity against this beetle. Subsequent exposure to the infective spores showed that oral priming was specific for two naturally occurring strains, while a third engineered strain did not induce any priming effect. Our data demonstrate that oral immune priming with a non-infectious bacterial agent can be specific, but the priming effect is not universal across all bacterial strains.
Keywords: innate immunity, specific immune memory, invertebrate immune priming, Bacillus thuringiensis, Tribolium castaneum
1. Background
Specificity and memory are the hallmarks of the vertebrate adaptive immune response [1]. However, accumulating evidence suggests that memory is not only restricted to the adaptive immune system but also occurs within the vertebrate innate immune system, and is referred to as ‘trained immunity' [2]. In invertebrates, this phenomenon is called ‘immune priming' [3]. A number of recent studies found strong evidence for the prophylactic effects of immune priming across a wide-range of insect taxa [3,4].
While there is mounting evidence for such alternative forms of memory, the extent of specificity in these reactions often remains unstudied. Specificity measures the degree to which the immune system can discriminate between different pathogenic species and/or strains [5,6]. Specific immune priming may depend on differential recruitment of immune system components after priming and secondary exposure to the same pathogen strain. Ultimately, specificity can result in strain-specific survival differences [4,7].
A handful of studies have focused on specific memory in invertebrate host–pathogen interactions and found evidence for high degrees of immune priming specificity after infection with various bacterial species in Tribolium castaneum, Bombus terrestris, Bombyx mori and Drosophila melanogaster [4,7–9]. Additionally, a high degree of specificity on the level of parasite genotypes (sibships) was found in a copepod host after repeated exposure to live Schistocephalus solidus tapeworm larvae [10]. While homologous priming and challenge combinations generally result in increased protection, indicating that priming is specific, specific responses are not universal [11]. Moreover, many studies used live pathogens or parasites for priming, so direct interference among parasites derived from first and second exposures cannot always be fully excluded. Generally, a highly specific invertebrate immune response comparable to that of vertebrates would indicate that invertebrates have adapted to similar selection pressures over evolutionary time [3].
Immune priming in T. castaneum can be triggered through the septic and oral infection routes [4,12–14]. Once primed with Bacillus thuringiensis by septic wounding, priming in T. castaneum shows high specificity at the level of bacterial strain [4]. The Gram-positive bacterium B. thuringiensis is a natural pathogen of T. castaneum known to negatively affect the beetle's fitness by entering the host via the oral ingestion route [15]. Thus, we asked whether oral immune priming shows specificity. Using a full factorial design, we orally primed larvae with sterilized growth media previously conditioned with three different bacterial strains. Subsequently, the larvae were challenged with a potentially lethal dose of bacterial spores. This experimental set-up allowed us to test for specific priming in both naturally and non-naturally occurring host–pathogen interactions. We show that oral immune priming in T. castaneum can be specifically induced by related B. thuringiensis strains. To the best of our knowledge, this is the first study to directly address specificity in an oral priming context in an insect, and thus further advances our understanding of specificity and memory in invertebrate immune systems.
2. Material and methods
(a). Model organisms
In this study, we used larvae from a wild-caught Croatian T. castaneum population (Cro1) [12], kept under standard breeding conditions [14]. We used three B. thuringiensis strains: B. thuringiensis subsp. morrisoni bv. tenebrionis (Btt) (BGSC, Ohio State University, Columbus, OH, USA), BTS00125 L (BtS) (kindly provided by Dr Carolina Rausell, University of Valencia, Spain) and the genetically engineered strain, BtEG10327 (BtEG) (ARS Culture Collection, NRRL, USA; ref. no. B-21365). These strains harbour, among other toxins, the beetle-specific Cry toxins Cry3Aa, Cry3Ba and Cry23Aa/Cry37Aa, respectively. Bacterial cultures were incubated in sporulation medium pH 7.2, at 30°C in darkness until autolysis. Centrifugation steps were carried out for 10 min at 3700g at RT.
(b). Oral immune priming
The priming diet for Experiment 1 was prepared using all three strains. For Experiment 2, only the BtEG strain was used. The general protocol used for preparation of priming diets for both experiments is described elsewhere [13,14] with the following modifications. After an initial overnight incubation on lysogeny broth (LB) plates, 5 ml of DSG sporulation medium [16] were inoculated with five colonies from the same strain each and incubated overnight. Then, 100 ml of modified sporulation medium (MSM) [17] were inoculated with 1 ml of overnight culture and incubated for 7 days at 200 r.p.m. On day 7, the priming diets and plates were prepared using bacteria-conditioned media that were derived from sterile-filtered, i.e. bacteria and spore-free, spore-culture supernatants (filter size 0.2 μm) [13,14]. For controls, we used unconditioned MSM (Medium) or phosphate-buffered saline (PBS). The PBS was omitted in Experiment 2. Fourteen days post oviposition (PO), larvae were standardized for size and orally primed [14]. We used in total 1200 larvae for Experiment 1 and 1152 larvae for Experiment 2 (electronic supplementary material, table S1). Larvae were left on priming/control diet for 24 h, after which we transferred them and left them to feed on a naive diet for 4 days.
(c). Challenge
The challenge diet was prepared following the protocol described above with a few modifications. For overnight cultures, two replicates of 5 ml DSG medium were inoculated with five colonies of each strain. For sporulation, two replicates of 600 ml of MSM were inoculated with 5 ml of DSG overnight culture for each strain. After 7 days of incubation, spores were harvested by centrifugation, pooled for each strain, and the supernatant was discarded. For Experiment 1, the spore pellet of each strain was resuspended in PBS and the spore concentration of the diets was adjusted to 1 × 1010 ml−1 by adding PBS. For Experiment 2, five separate challenge diets were prepared containing 1 × 108, 5 × 108, 1 × 109, 5 × 109 and 1 × 1010 BtEG spores per millilitre of diet. In both experiments, 0.15 g of flour was added per ml of spore suspension. As a control, a 0.15 g ml−1 flour–PBS mixture was used in both experiments. On day 19 PO, larvae were individually transferred from naive to challenge diet. All plates were sealed, and larvae were kept on the challenge diet for eight days. Motility and death-induced larval discoloration were used as discrimination criteria for daily survival checks.
(d). Statistical analyses
Statistical analyses were performed in R v. 3.3.1 [18] using generalized linear models with binomial errors with cumulative survival as a response variable or Cox proportional hazards models with day of death as the response variable [19]. For Experiment 1, we ran a single overarching model (generalized linear model, GLM) to ask if there was an effect of priming and challenge on the proportion of animals that survived to day 8 in all priming and challenge combinations. We then broke the model up by challenge treatment (BtEG, BtS, Btt, PBS) to examine survival over the entire experimental period. For Experiment 2, we asked if there was a priming effect on survival over the 8-day experimental period. Where applicable, the assumptions of Cox proportional hazards were met in all cases.
3. Results and discussion
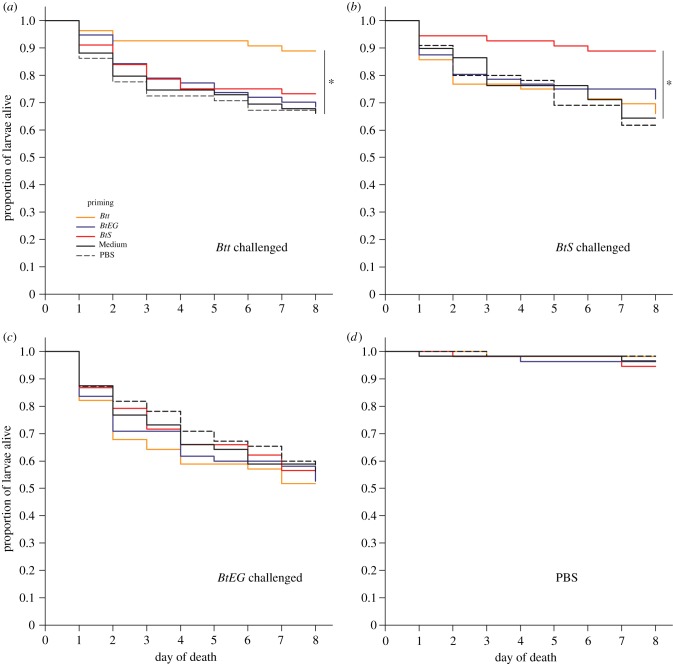
In this study, larvae homologously primed and challenged with two strains (Btt, BtS) showed significantly improved survival rates compared with the heterologous treatment combinations and corresponding controls (figures 1a and 2a,b; electronic supplementary material, table S2–4). Our data show that the beetle's immune system can discriminate between related bacterial strains. This indicates a surprisingly high degree of specificity in oral priming. By contrast, in all treatment groups challenged with BtEG no significant differences in the survival probability were observed (figure 1a; figure 2c; electronic supplementary material, table S2–4). Most notably, individuals receiving a homologous combination (BtEG/BtEG) did not show a priming effect (figures 1a and 2c). Therefore, in Experiment 2, we tested if a potential priming effect of BtEG might have been masked by the challenge dose (figure 1b; electronic supplementary material, S1, table S5). However, the survival rates did not differ significantly between primed and unprimed larvae within the respective challenge-dose spectrum (electronic supplementary material, table S5).
Figure 1.
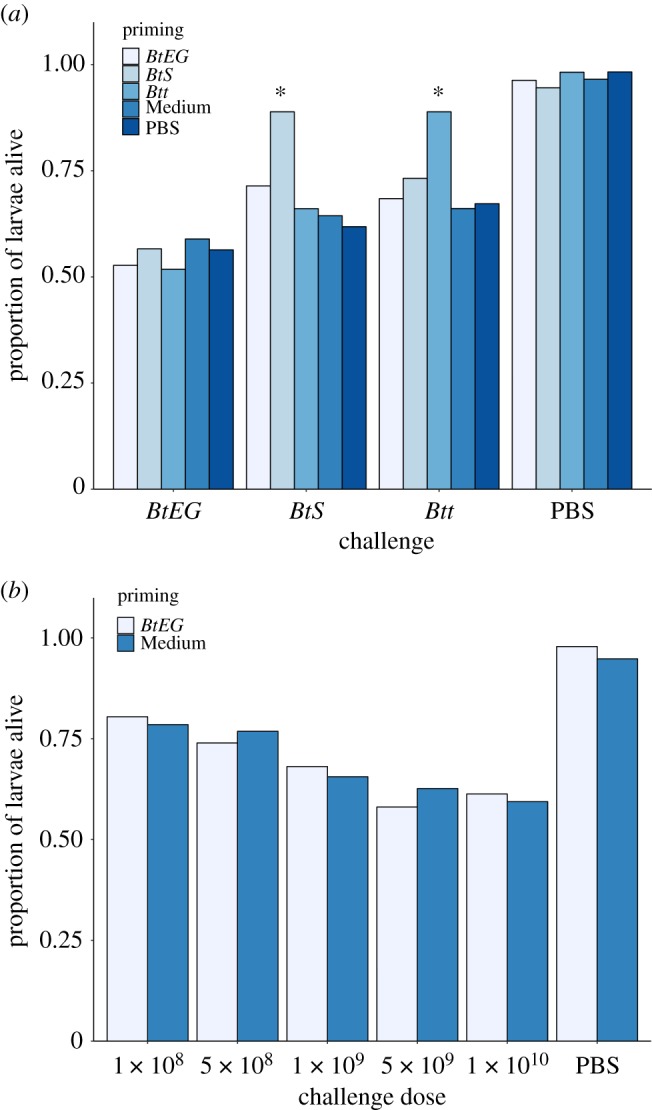
Proportion of T. castaneum larvae which survived to day 8 post-challenge with: (a) spores of either one of three B. thuringiensis strains (BtEG, BtS, Btt) and (b) with various concentrations of BtEG spores. Priming was specifically triggered by two strains (Btt, BtS), significant difference indicated by asterisks (a), while no priming was observed with any of the BtEG spore concentrations used (b). (Online version in colour.)
Figure 2.
Survival of primed T. castaneum larvae during 8 days post-challenge with spores of Btt (a), BtS (b), BtEG (c) and PBS (d) as a control treatment. The priming effect was specifically triggered only by two homologously primed/challenged strain combinations (a,b), while the engineered strain failed to provoke any priming response (c). Significant differences are indicated by asterisks.
Few studies have demonstrated oral immune priming in insects and those used live pathogens and/or showed a low degree of specificity. For example, in Anopheles gambiae mosquitoes, pre-exposure to Plasmodium parasites led to enhanced resistance after a challenge. The effect was mediated by haemocyte differentiation, based on the lipoxin/lipocalin complex haemocyte differentiation factor [20]. This process was induced by the natural gut microbiota that entered the haemocoel when the Plasmodium ookinetes broke the barrier between the gut and haemocoel. Given that microbiota is responsible for priming against Plasmodium parasites, this is an unspecific form of priming. Interestingly, gut microbiota is also necessary for priming against Btt in T. castaneum [14]. However, the degree of priming specificity shown in our study suggests a different mechanism, because microbiota-mediated priming alone would be unlikely to show any specificity. A number of invertebrate immune mechanisms have the potential to discriminate between different antigens, and some of these candidate mechanisms are even based on somatically diversifying immune receptors that might enable very high degrees of specificity (see [3] for review). Alternatively, the observed specificity in the primed response could also be mediated by differences among the bacterial strains, such as their toxins, leading to the induction of specific protective processes in the host upon priming.
Our findings confirm that specific priming in insects may not occur universally across all bacterial species, which is in line with other studies [4,9]. Within invertebrates, specificity in the immune system may have evolved multiple times independently as a result of similar selection pressures from a large variety of entomopathogenic species and parasites [3]. The detailed molecular mechanisms of oral priming in T. castaneum are not yet fully understood. A recent transcriptome study indicates that priming with Btt induces a shift in immunity towards responses mediated by reactive oxygen species [21]. Additional studies are required to further investigate the spectrum of immune specificity and the molecular bases of memory-like responses of invertebrate immune systems.
Supplementary Material
Acknowledgements
We thank Barbara Hasert for help in the laboratory and four anonymous referees for helping us to improve the manuscript.
Data accessibility
All raw data supporting this article are deposited on Dryad: https://dx.doi.org/10.5061/dryad.5vr86 [22].
Authors' contributions
M.F. and J.K. designed the experiments, M.F. and M.P.S. carried out the experiments, M.A.M.K. carried out the statistical analyses, all authors wrote the manuscript, approved its final version for publication and agree to be held accountable for its content.
Competing interests
We have no competing interests.
Funding
We are grateful for the financial support provided to M.F. by the DAAD grant no. A/11/91411, and to M.P.S. and J.K. by the DFG grant no. KU 1929/8-1 within the Priority Programme SPP 1819.
References
- 1.Janeway C, Travers P, Walport M, Shlomchik M. 2004. Immunobiology: the immune system in health and disease. 6th edn New York, NY: Garland Science. [Google Scholar]
- 2.Netea MG, Quintin J, Van Der Meer JWM. 2011. Trained immunity: a memory for innate host defense. Cell Host Microbe 9, 355–361. ( 10.1016/j.chom.2011.04.006) [DOI] [PubMed] [Google Scholar]
- 3.Milutinović B, Kurtz J. 2016. Immune memory in invertebrates. Semin. Immunol. 28, 328–342. ( 10.1016/j.smim.2016.05.004) [DOI] [PubMed] [Google Scholar]
- 4.Roth O, Sadd BM, Schmid-Hempel P, Kurtz J. 2009. Strain-specific priming of resistance in the red flour beetle, Tribolium castaneum. Proc. R. Soc. B 276, 145–151. ( 10.1098/rspb.2008.1157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frank SA. 2002. Immunology and evolution of infectious disease. Princeton, NJ: Princeton University Press. [PubMed] [Google Scholar]
- 6.Kurtz J. 2005. Specific memory within innate immune systems. Trends Immunol. 26, 186–192. ( 10.1016/j.it.2005.02.001) [DOI] [PubMed] [Google Scholar]
- 7.Sadd BM, Schmid-Hempel P. 2006. Insect immunity shows specificity in protection upon secondary pathogen exposure. Curr. Biol. 16, 1206–1210. ( 10.1016/j.cub.2006.04.047) [DOI] [PubMed] [Google Scholar]
- 8.Wu G, Li M, Liu Y, Ding Y, Yi Y. 2015. The specificity of immune priming in silkworm, Bombyx mori, is mediated by the phagocytic ability of granular cells. J. Insect Physiol. 81, 60–68. ( 10.1016/j.jinsphys.2015.07.004) [DOI] [PubMed] [Google Scholar]
- 9.Pham LN, Dionne MS, Shirasu-Hiza M, Schneider DS. 2007. A specific primed immune response in Drosophila is dependent on phagocytes. PLoS Pathog. 3, e26 ( 10.1371/journal.ppat.0030026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurtz J, Franz K. 2003. Evidence for memory in invertebrate immunity. Nature 425, 37–38. ( 10.1038/425037a) [DOI] [PubMed] [Google Scholar]
- 11.McTaggart SJ, Wilson PJ, Little TJ. 2012. Daphnia magna shows reduced infection upon secondary exposure to a pathogen. Biol. Lett. 8, 972–975. ( 10.1098/rsbl.2012.0581) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milutinović B, Stolpe C, Peuß R, Armitage SAO, Kurtz J. 2013. The red flour beetle as a model for bacterial oral infections. PLoS ONE 8, e64638 ( 10.1371/journal.pone.0064638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milutinović B, Fritzlar S, Kurtz J. 2014. Increased survival in the red flour beetle after oral priming with bacteria-conditioned media. J. Innate Immun. 6, 306–314. ( 10.1159/000355211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Futo M, Armitage SAO, Kurtz J. 2016. Microbiota plays a role in oral immune priming in Tribolium castaneum. Front. Microbiol. 6, 1383 ( 10.3389/fmicb.2015.01383) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler DR, Dean DH. 1998. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62, 775–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donovan WP, Donovan JC, Slaney AC.2009. Methods for detecting Bacillus thuringiensis cryET33 and cryET34 polypeptides. Patent no: US 7,504,229 B2.
- 17.Stewart GS, Johnstone K, Hagelberg E, Ellar DJ. 1981. Commitment of bacterial spores to germinate. A measure of the trigger reaction. Biochem. J. 198, 101–106. ( 10.1042/bj1980101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.R Core Team. 2016. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 19.Pinheiro J, Bates D, DebRoy S, Sarkat D, R Development Core Team. 2013. nlme: linear and nonlinear mixed effects models. (R package version 3.1-111.)
- 20.Ramirez JL, De Almeida Oliveira G, Calvo E, Dalli J, Colas RA, Serhan CN, Ribeiro JM, Barillas-Mury C. 2015. A mosquito lipoxin/lipocalin complex mediates innate immune priming in Anopheles gambiae. Nat. Commun. 6, 7403 ( 10.1038/ncomms8403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenwood JM, Milutinović B, Peuß R, Behrens S, Esser D, Rosenstiel P, Schulenburg H, Kurtz J. 2017. Oral immune priming with Bacillus thuringiensis induces a shift in the gene expression of Tribolium castaneum larvae. BMC Genomics 18, 329 ( 10.1186/s12864-017-3705-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Futo M, Sell MP, Kutzer MAM, Kurtz J. 2017. Data from: Specificity of oral immune priming in the red flour beetle Tribolium castaneum Dryad Digital Repository. ( 10.5061/dryad.5vr86) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Futo M, Sell MP, Kutzer MAM, Kurtz J. 2017. Data from: Specificity of oral immune priming in the red flour beetle Tribolium castaneum Dryad Digital Repository. ( 10.5061/dryad.5vr86) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
All raw data supporting this article are deposited on Dryad: https://dx.doi.org/10.5061/dryad.5vr86 [22].