Abstract
Unexpected and attractive properties can be observed when decreasing the size of a material down to the nanoscale. Cellulose is no exception to the rule. In addition, the highly reactive surface of cellulose resulting from the high density of hydroxyl groups is exacerbated at this scale. Different forms of cellulose nanomaterials, resulting from a top-down deconstruction strategy (cellulose nanocrystals, cellulose nanofibrils) or bottom-up strategy (bacterial cellulose), are potentially useful for a large number of industrial applications. These include the paper and cardboard industry, use as reinforcing filler in polymer nanocomposites, the basis for low-density foams, additives in adhesives and paints, as well as a wide variety of filtration, electronic, food, hygiene, cosmetic and medical products. This paper focuses on the use of cellulose nanomaterials as a filler for the preparation of polymer nanocomposites. Impressive mechanical properties can be obtained for these materials. They obviously depend on the type of nanomaterial used, but the crucial point is the processing technique. The emphasis is on the melt processing of such nanocomposite materials, which has not yet been properly resolved and remains a challenge.
This article is part of a discussion meeting issue ‘New horizons for cellulose nanotechnology’.
Keywords: cellulose nanocrystal, cellulose nanofibril, nanocomposite, processing, mechanical properties
1. Introduction
Cellulose is a linear macromolecule composed of β-l,4-linked d-glucopyranose rings and the most abundant polymer on Earth. It is certainly one of the most important structural elements in plants and other living species and serves to maintain their structure. Each of these living species, from tree to bacteria, produces cellulose day by day, e.g. a tree produces about 10 g of cellulose per day and the global annual production of cellulose is estimated at 1.5 × 1012 t [1]. It has been used for centuries in highly diverse applications. More recently, the recognition that, by suitable chemical and mechanical treatments, it is possible to produce fibrous materials with one or two dimensions in the nanometre range from any naturally occurring sources of cellulose has been emphasized and opens the door to new applications. The initial concept of the chemical extraction of cellulose nanomaterials through an acid hydrolysis process was pioneered in 1947 [2]. The first report on the mechanical destructuration of cellulose fibres was published in 1983 in two companion papers [3,4].
The potential of these nanomaterials, generally grouped under the name nanocellulose, has been proved for special functional nanomaterials [5], but it is as a biobased reinforcing nanofiller that it has attracted significant interest during the last 20 years [6–9]. Cellulosic nanomaterials have the intrinsic benefits of cellulose, i.e. low cost, low density, high specific strength and modulus, renewability, biodegradability, non-toxicity, availability in a variety of forms throughout the world, a high ability for surface modification and the possibility to generate energy, without residue after burning at the end of their life cycle. They also have its drawbacks, i.e. a hydrophilic character, which limits their adhesion and dispersion in a non-polar matrix, and limited thermal stability, resulting in low permissible temperatures for processing and use. The size reduction in cellulosic particles retains most of these properties. However, some properties are amplified, e.g. mechanical stiffness and specific surface area, exacerbating the available surface hydroxyl groups. Moreover, the disintegration of naturally grown fibres allows macroscopic flaws to be eliminated by separating the almost defect-free highly crystalline nanofibres. This, therefore, avoids any large variations in structure and the huge scatter of mechanical plant fibre properties inherent to any natural products that are related to disturbances during plant growth, climatic conditions, maturity of the plant and type of soil. In this context, nanometre-scale cellulose fibres, or nanocellulose, are emerging green nanoreinforcements for polymers. The major driver for using nanocellulose as a reinforcement for polymers is the possibility of exploiting the high tensile stiffness and strength of the cellulose crystals.
2. Cellulose nanomaterials
Cellulose occurs in almost pure form in cotton fibre. However, in wood, plant leaves and stalks, it is found in combination with other materials, such as lignin and hemicelluloses. Most natural fibres are composed of cellulose fibres, which consist of helically wound cellulose microfibrils bound together by an amorphous lignin matrix. Lignin keeps water in fibres, acts as protection against biological attack and acts as a stiffener to give the stem its resistance against gravity forces and wind. Hemicellulose found in natural fibres is believed to be a compatibilizer between cellulose and lignin. Cellulose microfibrils typically have a diameter of about 10–30 nm, are made up of 30–100 cellulose molecules in extended chain conformation and provide mechanical strength to the fibre.
The first step for the preparation of cellulose nanomaterials generally consists in a purification step using chemical treatments to remove most of the non-cellulosic components. Cotton and bleached pulps are often used in order to skip the matrix removal process.
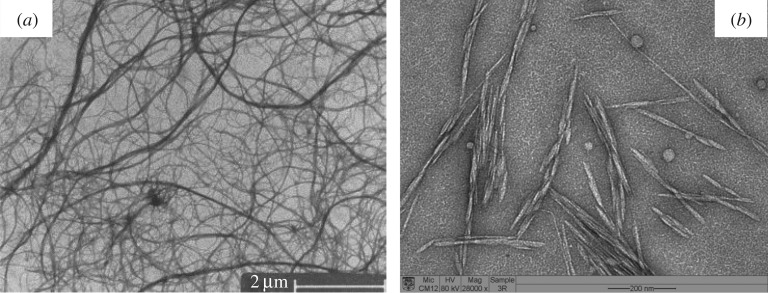
A mechanically induced destructuring strategy can be applied to a diluted cellulosic fibre suspension to release more or less individually the constitutive microfibrils. This consists in applying severe multiple mechanical shearing actions to the cellulosic fibre slurry. Different shearing equipment such as a homogenizer, a microfluidizer or an ultra-fine friction grinder are generally used. The ensuing nanomaterial is usually called microfibrillated cellulose, nanofibrillated cellulose or cellulose nanofibrils (CNFs) and it is obtained as a very dilute aqueous suspension (typically 2 wt%) because of viscosity issues. This mechanical treatment is normally associated with high energy consumptions for fibre delamination, which has limited for a long time its industrial exploitation. Different pretreatments of the cellulosic fibre raw material have, therefore, been proposed to facilitate this production route, mainly enzymatic pretreatment [10] or the introduction of charged groups through carboxymethylation [11] or 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO)-mediated oxidation [12]. CNFs occur as long, high-aspect-ratio, flexible entangled filaments consisting of both individual and aggregated nanofibrils made of alternating crystalline and amorphous cellulose domains (figure 1a). The width is generally in the range 3–100 nm depending on the source of the cellulose, the defibrillation process and the pretreatment, and the length is considered to be greater than 1 µm. It is worth noting that hydrophobic compounds are still present at the surface of CNFs and that the surface charge is fixed by the pretreatment step.
Figure 1.
Transmission electron micrographs from a dilute suspension of (a) CNFs prepared from potato pulp [13] and (b) cellulose nanocrystals extracted from mengkuang leaf (Pandanus tectorius) fibres [14].
The second strategy is chemically assisted and consists generally in applying a controlled strong acid hydrolysis treatment to cellulosic fibres. This treatment induces the hydrolysis of amorphous domains and therefore longitudinal cutting of the microfibrils. Sulfuric acid is classically used because it promotes the formation of negatively charged sulfate groups at the surface of the released nanoparticles, resulting in very stable aqueous dispersions. The ensuing nanoparticles are generally called cellulose nanocrystals (CNCs). They occur as rod-like acicular nanoparticles (figure 1b). Their geometrical dimensions depend on the origin of the cellulose substrate and hydrolysis conditions. The average length is generally of the order of a few hundred nanometres and the width is of the order of a few nanometres. An important parameter for CNCs is their aspect ratio, which is defined as the ratio of the length to the width.
The tensile modulus of a single cellulose I (native cellulose) crystal was estimated both experimentally and theoretically in the literature. Most of the reported values were determined by considering intramolecular hydrogen bonding, and much lower values were obtained when intramolecular hydrogen bonding was not taken into account; this is evidence of the important role of intramolecular hydrogen bonding in the determination of the crystallite modulus and chain deformation mechanism. A broad range of values, between 56 and 220 GPa, was reported with an average value of 130 GPa [15]. As expected, the lower crystallinity of CNFs results in a lower modulus, the average value of which is around 100 GPa [9]. The specific tensile modulus, which is the ratio between the tensile modulus and the density (1.5–1.6 g cm−3 for crystalline cellulose), was estimated to be between 85 and 65 J g−1 for CNCs and CNFs, respectively, whereas it is around 25 J g−1 for steel [16]. These data justify the numerous research efforts that have been poured into the use of cellulosic nanomaterials as reinforcement for polymers, the modulus of which is around a few GPa under the best conditions, i.e. when in the glassy state.
3. Wet processing of polymer nanocomposites
Cellulose nanomaterials are obtained as dilute suspensions, usually in water because it is the most convenient and cheapest polar liquid medium. The stability of these colloidal suspensions results from the presence of residual hemicelluloses for CNFs and charged sulfate groups in the case of sulfuric acid-hydrolysed CNCs. If the concentration increases, the viscosity of the dispersion increases sharply and it becomes a gel with only a few per cent solid content. Upon complete drying, the nanoparticles aggregate through hydrogen bonding, as occurs for paper, and the nanoscale is lost. Therefore, it is important to avoid this irreversible aggregation phenomenon and most studies used never-dried cellulose nanomaterials to prepare polymer nanocomposites.
Therefore, the simplest processing method consists in mixing the cellulose nanomaterial dispersion with a polymer/prepolymer/monomer dispersed in the same liquid medium or in a liquid that is miscible with the liquid in which the nanoparticles are suspended. This mixture can be cast and the liquid evaporated or freeze-dried, avoiding the aggregation of the nanomaterial because of intercalated matrix material and preserving its individualized state. As CNFs/CNCs are usually dispersed in water, it means that water-soluble polymers or polymer aqueous dispersions (i.e. latex) are well adapted. When using a monomer, a further in situ polymerization step is necessary. This simple strategy was obviously used in the pioneering investigations on the preparation of nanocellulose-reinforced polymer nanocomposites. It consisted in reinforcing a copolymer of styrene and butyl acrylate (poly(S-co-BuA)) in latex form with CNCs [17] or glycerol-plasticized water-soluble starch with CNFs [18].
This wet casting/evaporation processing strategy can be extended to other liquids to cover a broader range of polymer matrices. The aqueous dispersion of cellulose nanomaterial can be mixed with a polymer solution based on a solvent that is miscible with water. Homogeneous nanocomposite films based on LiClO4-doped ethylene oxide–epichlorohydrin (EO-EPI) copolymers and CNCs were prepared by solution casting of tetrahydrofuran/water mixtures and subsequent compression moulding [19]. All-cellulose films have also been prepared from CNCs and a cellulose matrix regenerated from an aqueous NaOH–urea solvent system (cellulose II) on the basis of their temperature-dependent solubility [20]. If the solvent is not miscible with water, it is necessary to suspend the cellulose nanoparticles in a suitable liquid, i.e. the solvent in which the polymer matrix is solubilized. Solvent exchange can be conducted in this way. The process generally starts with acetone, and continues with liquids of decreasing polarity. Even if the suspension is less stable than in water, it can be sufficiently stable for processing. This technique of solvent exchange can also be used for further chemical modification of the nanoparticles in a suitable solvent.
A pretreatment of CNCs or CNFs consisting in surface functionalization prior to composite processing can be carried out. Coating the nanoparticles with a surfactant or covalent coupling of hydrophobic moieties directly on their surface have been extensively used to tune their surface chemistry and enhance the dispersion of the filler in a broad variety of non-polar liquid media or polymers. The negatively charged surface groups setting at the surface of H2SO4-prepared CNCs can be advantageously used to promote strong interactions with cationic surfactants, and the omnipresent surface hydroxyl groups of cellulose nanomaterials can be the site of various chemical reactions because of their high reactivity. The surface chemical modification of cellulosic nanoparticles by acetylation [21], esterification [22] and silanization/silylation [23,24] has been reported, among many other modifications. Experimental conditions should avoid swelling media and the peeling effect of surface-grafted chains inducing their dissolution in the reaction medium. The chemical grafting process therefore has to be mild in order to preserve the integrity of the nanoparticle. Substitution of surface hydroxyl groups with small molecules is not the only strategy that has been investigated. Polymer surface modifications based on the ‘grafting onto’ strategy with different coupling agents [25,26] or ‘grafting from’ strategies with radical polymerization of ring-opening polymerization [27], atom transfer radical polymerization [28] and single-electron transfer living radical polymerization [29] have also been reported. The modified cellulose nanomaterials can be effectively dispersed in non-polar liquid media and mixed with the corresponding polymer solution prior to being cast and evaporated. Even if improved dispersion is usually observed for modified nanomaterials, it is not necessarily associated with improved mechanical properties of the ensuing nanocomposites.
This wet mode of processing generally leads to the highest mechanical properties. From the earliest studies, it was shown that this outstanding reinforcing effect cannot be attributed only to the high stiffness of cellulose nanoparticles [17]. Indeed, when applying the theoretical model of Halpin–Kardos (a mean field approach), it was shown that the predicted modulus failed to describe the experimental data. In a second approach, a model involving the percolation of CNCs was applied and good agreement with the experimental data was observed. It was concluded that above the percolation threshold, the value of which depends on the aspect ratio of the nanoparticles, the cellulosic nanorods can connect and form a three-dimensional (3D) continuous pathway through the nanocomposite film. The formation of this cellulose network was supposed to result from strong interactions between nanocrystals, like hydrogen bonds. This mechanical percolation effect allowed both the high reinforcing effect and the thermal stabilization of the composite modulus up to 500 K for CNC-reinforced poly(S-co-BuA) films produced from a water medium to be explained. Therefore, any factor that affects the formation of the percolating nanocrystal network or interferes with it changes the mechanical performance of the composite [30]. In particular, functionalization of CNCs or CNFs can restrict the filler–filler interactions and hinder the formation of the percolating network. This was observed, for example, for nanocomposites based on cellulose acetate butyrate and CNCs prepared from bacterial cellulose obtained by casting/evaporation in acetone [23]. A higher reinforcing effect was reported for unmodified cellulose nanoparticles than for trimethylsilylated nanocrystals.
4. Melt processing of polymer nanocomposites
However, when high-volume products are targeted, these wet processing strategies appear difficult to scale up. More conventional melt processing techniques are therefore expected to be the key processing methods. Extrusion and injection-moulding processes are cheap, fast, industrially and economically viable, and solvent-free techniques. Table 1 summarizes the different nanocomposite systems prepared from cellulose nanomaterials by melt compounding. This list is probably not exhaustive. However, this update leads to the following comments.
Table 1.
Polymer nanocomposites obtained from nanocellulose by melt compounding. ABS, acrylonitrile–butadiene–styrene; ASA, alkenyl succinic anhydride; BC, bacterial cellulose; CA, cellulose acetate; CNC, cellulose nanocrystal; CNF, cellulose nanofibril; DMF, dimethylformamide; EA, ethylene acrylic; EAA, ethylene acrylic acid; EVOH, ethylene vinyl alcohol; GMA, glycidyl methacrylate; GTA, glycerol triacetate; HDPE, high-density polyethylene; LDPE, low-density polyethylene; MAPE, maleated PE; MAPLA, maleated PLA; MAPP, maleated PP; NR, natural rubber; PA4.10, polyamide 4.10; PA6, polyamide 6; PA11, polyamide 11; PA12, polyamide 12; PBAT, polybutylene adipate-co-terephthalate; PBG, poly(butyl glutarate); PBS, polybutylene succinate; PBSA, poly(butylene succinate-co-butylene adipate); PC, polycarbonate; PCL, polycaprolactone; PE, polyethyne; PEG, polyethylene glycol; PEO, poly(ethylene oxide); PGA, polypropylene glycol alginate; PHB, polyhydroxybutyrate; PHBV, poly(3-hydroxybutyrate-co-3-hydroxyvalerate); PLA, polylactic acid; PLMA-b-PHEMA, poly(lauryl methacrylate)-block-poly(2-hydroxyethyl methacrylate); PMMA, poly(methyl methacrylate); PP, polypropylene; PPC, poly(propylene carbonate); PPO, poly(propylene oxide); PS, polystyrene; poly(S-co-BuA) = poly(styrene-co-butyl acrylate); PU, polyurethane; PVA, polyvinyl alcohol; PVAc, polyvinyl acetate; ROP, ring-opening polymerization; SMA, styrene maleic anhydride; VTS, vinyl triethoxy silane.
| polymer | nanoparticle | processing aid/surface functionalization | processing technique | references |
|---|---|---|---|---|
| ABS | CNC | masterbatch/MAPE | extrusion/hot-pressing | [31] |
| Bioplast | CNF/CNC | masterbatch with alginate | extrusion | [32] |
| CA | CNC | masterbatch | extrusion/injection moulding | [33] |
| EVOH | CNC | electrospinning/masterbatch with EVOH | mixing/hot-pressing | [34,35] |
| HDPE | CNF | MAPE/PEO | extrusion | [36] |
| MAPP/ASA | extrusion/injection moulding | [37] | ||
| PLMA-b-PHEMA | extrusion/injection moulding | [38] | ||
| masterbatch with PVA | injection moulding | [39] | ||
| CNC | VTS grafting | mixing/hot-pressing | [40] | |
| vegetable oils | extrusion/hot-pressing | [41] | ||
| MAPE | extrusion | [42] | ||
| LDPE | CNF | EA | mixing/hot-pressing | [43] |
| — | extrusion/hot-pressing | [44,45] | ||
| PEG-b-PE | extrusion/hot-pressing | [46] | ||
| CNC | organic acid chloride grafting | extrusion | [22] | |
| masterbatch with PEO | extrusion | [47,48] | ||
| — | extrusion | [49] | ||
| PEO–PPO–PEO | extrusion | [50] | ||
| NR | CNF/CNC | masterbatch with NR | milling/hot-pressing | [51] |
| CNC | masterbatch with NR | milling/hot-pressing | [52] | |
| silanization | milling/hot-pressing | [53] | ||
| PA4.10 | CNF | acetylation | extrusion | [54] |
| PA6 | CNC | masterbatch | extrusion/injection moulding | [55] |
| silanization/in situ polymerization in caprolactam | extrusion | [56] | ||
| water-assisted compounding | extrusion | [57] | ||
| masterbatch with PA6 | extrusion/injection moulding | [58] | ||
| PA11 | CNC | — | mixing/hot-pressing | [59] |
| PA12 | CNF | cationic surfactant | extrusion/injection moulding | [60] |
| CNC | — | mixing/hot-pressing | [61] | |
| PBAT | CNF | — | extrusion/injection moulding | [62] |
| CNC | — | extrusion/injection moulding | [63,64] | |
| phenylbutyl isocyanate grafting | extrusion | [65] | ||
| PBG grafting | extrusion/hot-pressing | [66] | ||
| acetylation | mixing/hot-pressing | [67] | ||
| PBS | CNC | — | mixing/hot-pressing/foaming | [68] |
| acetylation | mixing/hot-pressing/foaming | [69] | ||
| masterbatch with PEG | extrusion/injection moulding | [70] | ||
| PBSA | CNC | phthalic anhydride | mixing/hot-pressing | [71] |
| PC | CNC | masterbatch | extrusion | [72] |
| PCL | CNC | ROP PCL grafting | extrusion/injection moulding | [73] |
| — | mixing/hot-pressing | [74] | ||
| — | extrusion/injection moulding | [75] | ||
| PEO | CNF | — | mixing/hot-pressing | [76] |
| CNC | — | extrusion | [77] | |
| PHB | CNF | SMA/PGA/EAA | mixing/injection moulding | [78] |
| CNC | — | mixing | [79] | |
| PHBV | CNF | masterbatch in PHBV | mixing/injection moulding | [80] |
| CNC | PEG | extrusion/injection moulding | [81] | |
| ball milling | hot-pressing | [82] | ||
| PLA | CNF | SMA/PGA/EAA | mixing/injection moulding | [78] |
| — | mixing/hot-pressing | [83,84] | ||
| masterbatch with PLA in acetone/chloroform | extrusion/injection | [85] | ||
| — | injection | [86] | ||
| masterbatch/carboxymethylation/esterification | extrusion/injection | [87] | ||
| liquid feeding/GTA | extrusion/hot-pressing | [88] | ||
| CNC | PEG/MAPLA | extrusion | [89] | |
| PVA | extrusion | [90] | ||
| ROP PLA grafting | extrusion/injection | [91] | ||
| silanization | extrusion/injection | [92] | ||
| surfactant | extrusion | [93] | ||
| masterbatch/acetylation | extrusion | [94] | ||
| GMA grafting/masterbatch | mixing | [95] | ||
| — | mixing/hot-pressing | [96] | ||
| masterbatch with PEO | mixing/hot-pressing | [97] | ||
| lactate and acetate modification | extrusion/injection moulding | [98] | ||
| PLA grafting reactive extrusion | extrusion | [99] | ||
| — | extrusion | [100] | ||
| liquid feeding | extrusion | [101] | ||
| ROP PLA grafting | extrusion | [102] | ||
| ROP PLA grafting | extrusion/hot-pressing | [103] | ||
| PMMA grafting | extrusion/injection moulding | [104] | ||
| masterbatch with GMA-g-PLA | extrusion | [105,106] | ||
| ball milling | hot-pressing | [107] | ||
| organic solvent-assisted centrifugation/acetylation | injection moulding | [108] | ||
| maleic anhydride | mixing/injection moulding | [109] | ||
| masterbatch with PVAc or PVAc/GMA | extrusion | [110] | ||
| masterbatch with PEG | mixing/hot-pressing | [111] | ||
| BC | acetylation | mixing/hot-pressing | [112] | |
| PLA/limonene | CNC | — | extrusion | [113] |
| PLA/NR | CNC | n-octadecyl isocyanate/ROP PLA grafting | extrusion | [114] |
| PLA/PHB | CNC | masterbatch/surfactant | extrusion/hot-pressing | [115,116] |
| PP | CNF | EA | mixing/hot-pressing | [117] |
| masterbatch with PCL | mixing/hot-pressing | [118] | ||
| in situ nanofibrillation/MAPP | extrusion/hot-pressing/injection moulding | [119] | ||
| surfactant/MAPP | extrusion/hot-pressing/injection moulding | [120] | ||
| MAPP/cationic polymer | extrusion/injection moulding | [121] | ||
| solid-state pulverization/MAPP | hot-pressing/injection moulding | [122] | ||
| masterbatch/MAPP | extrusion | [123] | ||
| CNC | n-octadecyl/MAPP | extrusion/hot-pressing | [124] | |
| spray freeze drying | mixing | [125,126] | ||
| masterbatch | extrusion/injection moulding | [127] | ||
| — | extrusion | [49] | ||
| surfactant | extrusion | [128] | ||
| PPC | CNC | masterbatch with PPC | mixing/injection moulding | [129] |
| PS | CNC | PEO/PEG grafting | extrusion | [130] |
| ionic liquids | extrusion | [131] | ||
| poly(S-co-BuA) | CNF | — | extrusion | [132] |
| CNC | — | extrusion/hot-pressing | [133] | |
| PU | CNC | reactive extrusion | extrusion | [134] |
| PVA | CNF | water-assisted extrusion | extrusion/foaming | [135] |
| CNC | water-assisted mixing | mixing/injection moulding | [136] | |
| PVAc | CNC | masterbatch | extrusion/hot-pressing | [137–139] |
| mixing in DMF | mixing/extrusion | [140] | ||
| starch | CNF | liquid feeding | extrusion/hot-pressing | [141] |
| — | extrusion/hot-pressing | [142] | ||
| CNC | — | extrusion | [143–145] | |
| — | mixing/hot-pressing | [146] | ||
| starch/PCL | CNC | — | extrusion | [144] |
(i) Melt processing has been mostly applied to CNC-reinforced polymer nanocomposites. This is not really surprising. Intuitively, melt processing methods seem to be trickier to apply to CNFs than to CNCs, because of the possibility of entanglement of the former. Indeed, a reduced percolation threshold and higher reinforcing effect are expected for higher-aspect-ratio CNFs provided that homogeneous dispersion is achieved. However, the issue of homogeneous dispersion of CNFs within the polymer melt is more difficult to obtain than for CNCs because of entanglements between CNFs. Therefore, they have been mainly developed with CNCs that are considered as more adapted.
(ii) When using a polar matrix, such as poly(ethylene oxide) (PEO) or starch, extrusion can be performed directly with raw cellulose nanoparticles because strong filler–matrix interactions are expected. An exception to this rule is for polyamides because of their high melting points; this requires protection of the nanoparticles to prevent thermal degradation. Nevertheless, functionalization of the nanofiller can be implemented (e.g. with poly(ethylene glycol) (PEG)) to prevent strong self-aggregation, while preserving its polar nature.
(iii) Polylactic acid (PLA) has been extensively used. Apart from being a biodegradable polymer derived from renewable resources, thus resulting in fully biodegradable nanocomposites, its extensive use can be explained by its slow crystallization compared with conventional semicrystalline polymers such as polypropylene (PP). It, therefore, requires longer moulding cycles, but usually a mostly amorphous polymer with low modulus at high temperature is obtained, thus limiting its applications. Hence, accelerating the crystallization kinetics of PLA to obtain high crystallinity in a short time and/or enhancing its high-temperature modulus have been extensively studied. Polyethylene (PE) and PP have also been extensively used to prepare cellulose nanomaterial-reinforced nanocomposites. These commodity polyolefins appear to be quite challenging because of their highly hydrophobic character.
(iv) As shown in figure 2, more than 90% and 64% of the studies reported in table 1 have been published since 2011 and since 2014, respectively, showing that the melt processing strategy of cellulose-based nanocomposites is mostly a newly developed topic.
Figure 2.
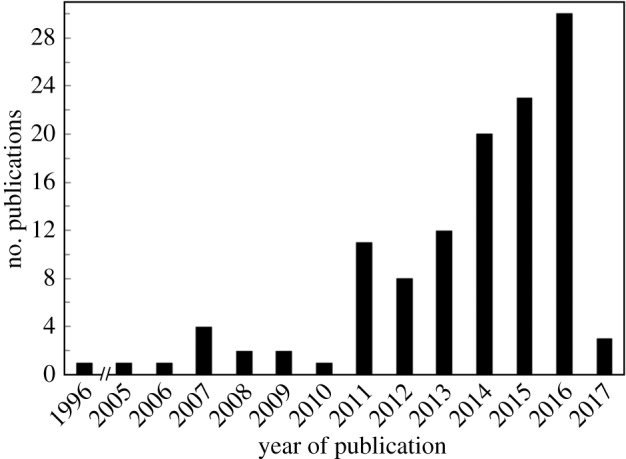
Historical distribution of the publications reported in table 1.
(v) Not surprisingly, physical methods, such as the preparation of a masterbatch or physical coating of the nanoparticles with a polymer or a surfactant, are generally preferred over chemical modification of the cellulose nanomaterial. Indeed, covalent functionalization of the nanofiller normally involves complicated and expensive steps which are quite difficult to scale up and can be prohibitive for most industrial uses. It frequently requires tedious time-consuming solvent exchange procedures and this strategy is often inconsistent with fast and solvent-free melt processing techniques. Among the studies reported in table 1, 21% involved a chemical modification of the cellulose nanomaterial, whereas 56% involved a physical method to improve processing, and 23% used unmodified nanoparticles.
The main issues to overcome for an efficient melt processing of CNF/CNC-reinforced polymer nanocomposites are (i) the irreversible aggregation of the nanofiller upon drying prior to melt processing, (ii) its non-uniform dispersion within the polymer melt, (iii) its thermal stability, (iv) its structural integrity, and (v) its orientation.
(i) When water is removed from the CNC/CNF suspension, irreversible aggregation occurs because of the highly reactive surface of the nanoparticles and strong hydrogen bonding forces. It is therefore necessary to use drying techniques that limit this effect and produce a porous weakly bonded solid material, retaining as far as possible the nanosize structure. Different drying methods, namely oven drying, freeze drying, supercritical drying and spray drying of CNC/CNF suspensions, have been applied and compared [147]. Spray drying was proposed as the technically suitable process to dry nanocellulose suspensions and composite processing because particle sizes ranging from nano- to micrometre were obtained. However, another study showed that conventional spray drying produces a compact solid structure with very low porosity, and spray freeze drying was suggested as a more suitable technique [96]. Conflicting results were reported for high-density polyethylene (HDPE)-based nanocomposites and it was shown that freeze-dried CNCs were less aggregated than spray-dried CNCs [42]. A possible way to overcome self-aggregation of the cellulose nanomaterial during drying prior to melt extrusion consists in using never-dried nanoparticles. Liquid feeding of cellulose nanomaterial dispersed in water or a mixture of water and other liquids and subsequent extrusion with PLA [88,101] or thermoplastic starch [141] was reported. However, effective venting and vacuum ports are needed, and the possible hydrolytic degradation of the polymer (PLA) can be questionable. Moreover, only low filler content composites can be prepared because of the high viscosity of the nanoparticle dispersion. Coating or functionalization of the nanoparticles can avoid their irreversible aggregation through hydrogen bonding upon drying.
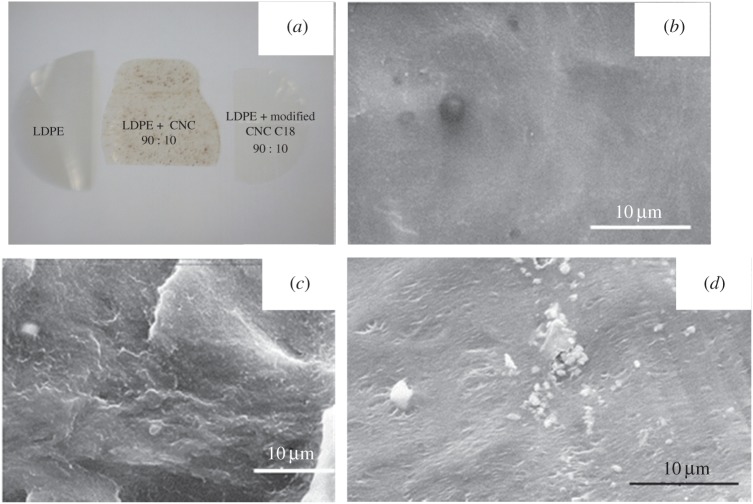
(ii) The issue of uniform dispersion of CNCs in a polymer melt, which is generally hydrophobic, results from the intrinsic hydrophilicity of cellulose. The situation is similar to mixing oil with water. It is, therefore, necessary to match the surface properties of the filler and the matrix. The strategies applied to suspend the cellulose nanomaterials in a non-polar liquid medium can be applied, i.e. surface functionalization using a surfactant or covalently grafted hydrophobic moieties. The quality of the dispersion of the nanofiller within the polymeric matrix can be difficult to evaluate. If strong aggregation occurs, a simple visual inspection with the naked eye is enough. An example is provided in figure 3a, which shows an extruded, neat, low-density polyethylene (LDPE) film (left) that is obviously translucent, as expected for any low-thickness polymeric film with a relatively low degree of crystallinity [22]. When extruding the polymer with 10 wt% CNCs (middle), the film becomes dotted with black spots. These heterogeneities reveal the poor and inhomogeneous dispersion of the filler within the polymeric matrix as well as the thermal degradation of the cellulosic nanomaterial. When using chemically modified CNCs (right), the occurrence of these aggregates progressively vanishes and the appearance of the composite film becomes similar to the one of the unfilled film as a result of improved dispersion. Scanning electron microscopy (SEM) can be used for closer inspection. Nevertheless, the resolution is generally insufficient to identify individual nanoparticles, but it can be used to detect the presence of aggregates as shown in figure 3b–d. Figure 3b shows the cryofractured surface of a PEO film obtained by casting/evaporation from water. The cryofractured surface of a PEO film reinforced with 6 wt% CNCs obtained by casting/evaporation is shown in figure 3c. The surface of the nanocomposite film is more chaotic than for the matrix but displays a homogeneous dispersion of white dots. The cross-section of these dots does not correspond to the one for isolated CNCs, because their dimensions are far higher than those of CNCs. They result from electrical charge effects that increase the apparent cross-section of the nanorods or possible CNC aggregates. The morphology of the extruded nanocomposite film reinforced with 6 wt% CNCs (figure 3d) is similar but less chaotic than its cast/evaporated counterpart. However, large domains of white dots are observed, indicating that the nanoparticles were not well dispersed in the PEO matrix. The dots observed are much larger than those obtained for the cast/evaporated film. The extrusion process induced aggregation of the CNCs. A combination of Raman imaging with image analysis has been suggested as a powerful and useful tool to quantify the degree of mixing of CNCs with melt compounded HDPE [42].
Figure 3.
(a) Extruded neat LDPE film (left), and extruded nanocomposite films reinforced with 10 wt% neat CNCs (middle), and C18 acid chloride-grafted CNCs (right) [22]. (b–d) Scanning electron micrographs of the cryofractured surface of (b) cast/evaporated PEO film, (c) cast/evaporated PEO film reinforced with 6 wt% CNCs and (d) extruded PEO film reinforced with 6 wt% CNCs [77].
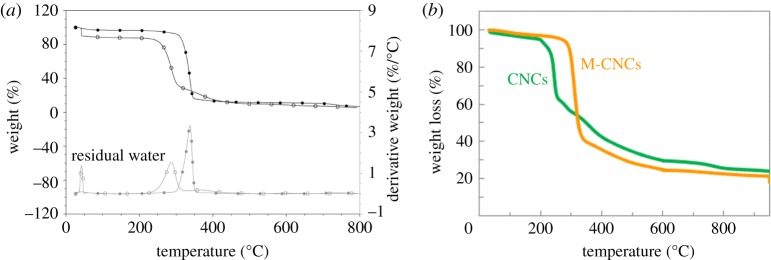
(iii) The low thermal stability of cellulose is an issue that can restrict melt processing to low-melting-point polymer matrices. It is particularly sensitive for H2SO4-hydrolysed CNCs because the acid hydrolysis process introduces less thermally stable sulfate groups on the surface [148,149]. The degradation was described as a two-step process, namely a low-temperature process and a high-temperature process. The low-temperature process involves the degradation of most accessible amorphous regions, which are also highly sulfated. The high-temperature process involves the degradation of less accessible interior crystalline regions that are comparatively less sulfated. The thermal stability of CNCs is then related to the conditions of the acid hydrolysis step, but also to the specific surface area of the nanoparticles [150]. Improved thermal stability of CNCs can be achieved by neutralizing the CNC suspension with a 1 wt% NaOH solution, as shown in figure 4a [27]. Another strategy consists in using another acid for hydrolysing cellulose. Hydrochloric acid can be used but, because of hydrogen bonding between the surface hydroxyl groups, HCl-prepared CNCs tend to aggregate easily and are often difficult to redisperse. It was shown that, on account of ionic repulsion between charged surface groups, slightly phosphorylated CNCs, prepared by controlled hydrolysis with phosphoric acid, are readily dispersible and form stable dispersions in polar solvents. The H3PO4-prepared CNCs were found to exhibit a much higher thermal stability than H2SO4-prepared CNCs [151]. Coating the cellulose nanomaterial through either chemical grafting or physical wrapping with a surfactant or long chains (masterbatch approach) also imparts improved thermal stability to the nanoparticle. This is exemplified in figure 4b, which shows thermogravimetric analysis (TGA) curves for neat CNCs and CNCs modified with quaternary ammonium salt bearing long alkyl chains (M-CNCs) [128].
Figure 4.
TGA curves for (a) untreated H2SO4-hydrolysed CNCs (open circles) and 1 wt% NaOH-neutralized CNCs (filled circles) (under helium flow) [27]; and (b) untreated H2SO4-hydrolysed CNCs (CNCs) and CNCs modified with quaternary ammonium salt bearing long alkyl chains (M-CNCs) (under air flow) [128]. (Online version in colour.)
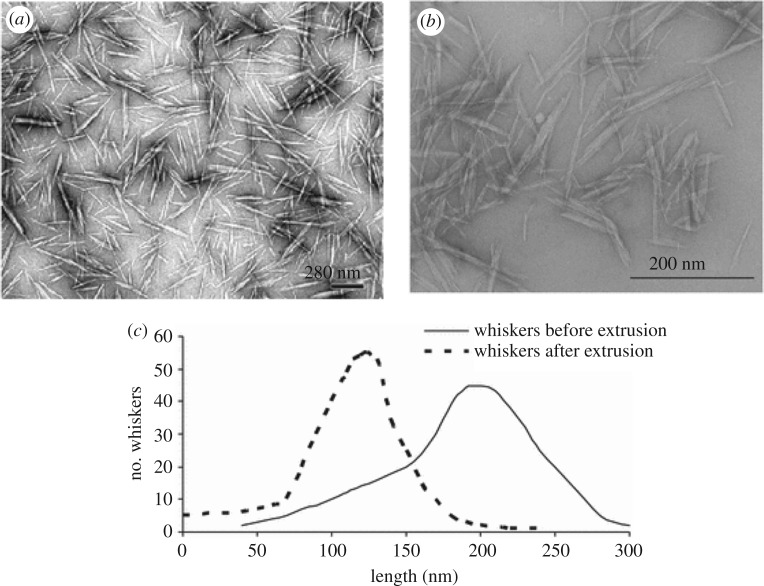
(iv) Extrusion and injection-moulding processes involve high shear rates that can impact the structural integrity of CNCs. This effect is seldom reported in the literature [77,140], and strongly depends on the extrusion conditions and the viscosity of the polymer melt. It has been reported for CNC-reinforced PEO nanocomposites prepared by extrusion [77]. To evaluate the influence of the extrusion process, the length and diameter of CNCs before and after extrusion were determined through microscopic observations and compared (figure 5a,b). The nanoparticles were extracted from the extruded nanocomposite by dissolving the PEO matrix in water. The length and cross-section of the nanocrystals were found to decrease from about 200 to 120 nm, and from 7 to 5 nm, respectively (figure 5c). No significant change in the aspect ratio, from 28 to 24, was observed after extrusion. Moreover, a significant narrowing of the length distribution was reported, showing that the degradation was more efficient for longer CNCs.
Figure 5.
Transmission electron micrographs (TEM) of CNCs (whiskers) extracted from ramie fibres (a) before extrusion; (b) after extrusion with PEO; (c) their length distributions [77].
The impact of processing methods on the morphology and mechanical properties of nanocomposites made from poly(vinyl acetate) (PVAc) and CNCs was investigated [140]. Homogeneously mixed reference PVAc/CNC nanocomposites of various compositions were first prepared by solution casting. These materials were post-processed by mixing in a roller blade mixer or a twin-screw extruder with subsequent compression moulding. When solution-cast materials were re-processed using low-shear-mixing conditions in a roller blade mixer, similar reinforcement was observed, suggesting that, if CNCs are pre-dispersed within the matrix, the morphology can be maintained during re-processing. The high-shear-melt mixing environment in a twin-screw extruder proved to be responsible for mechanical degradation and in particular for a reduction in the CNC length, affecting the formation of the percolation network.
(iv) Another phenomenon which is inherent to the melt processing technique is the possible orientation of the elongated nanoparticles in the extrusion direction, limiting the formation of a percolating network, which is the basis of the reinforcing effect of cellulose nanomaterials. It obviously depends on the processing conditions and viscosity of the polymer. This preferential orientation was evidenced with small-angle X-ray scattering (SAXS) experiments for CNC-reinforced LDPE prepared by extrusion, as shown in figure 6 [50]. A triblock copolymer PEO–PPO–PEO was used as the processing aid. The SAXS patterns exhibit an anisotropic shape with a higher intensity in the horizontal direction, which corresponds to a preferential orientation of CNCs in the flow direction during the extrusion process. For increasing CNC contents, the anisotropic level is amplified, which could be attributed to a higher orientation of the nanorods or to an increasing quantity of CNC nanoparticles orientated in the flow direction during the extrusion process.
Figure 6.
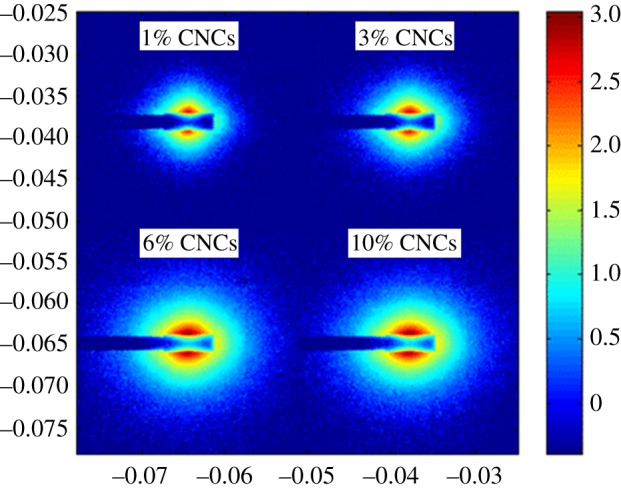
Two-dimensional SAXS patterns for LDPE nanocomposites with different CNC contents [50].
The orientation of CNCs upon melt processing was also observed using two-dimensional small-amplitude oscillatory shear experiments for injection-moulded CNC-reinforced polybutyrate adipate terephthalate nanocomposites [64]. The effect of a thermal annealing treatment of the nanocomposites on the possible auto-reorganization of the nanofiller as a first step to create an isotropic material, the initial condition to induce 3D network formation under mild conditions, was investigated [64]. Even if the high viscosity of the polymer melt limited the movement of the nanoparticles and hindered the formation of the desired percolating network, a spatial reorganization of the nanorods was observed after a short conditioning time. This suggests that it should be possible to partially alter the organization of the particle within the polymeric matrix imposed by the injection-moulding process.
Even if different strategies can be used to limit the effect of the previously listed issues related to melt processing of CNF/CNC-reinforced polymer nanocomposites, disappointing mechanical properties are often reported. If improvement is generally observed compared with direct extrusion of unmodified nanoparticles, the mechanical performance is far from what is observed for wet-processed nanocomposites. Moreover, it is worth noting that, in most cases, a semicrystalline polymeric matrix is used and it was clearly shown that cellulose nanomaterials generally act as a nucleating agent inducing an increase in the degree of crystallinity of the matrix. This enhanced crystallinity of the polymeric matrix obviously contributes to the improvement in the stiffness of the material, which is difficult to dissociate from the real direct reinforcing effect of the nanoparticle. The main difficulty is attributed to difficulties in forming a percolating nanoparticle network during melt processing.
5. Conclusion
Cellulose nanomaterials in the form of CNFs or CNCs exhibit properties such as high stiffness and specific surface area and low density, making them good candidates for the preparation of advanced polymer nanocomposites. The processing step is obviously crucial as it conditions the morphology and the properties of the final material. Two main processing methods can be considered, i.e. wet and melt processing. Wet processing methods such as casting/evaporation of the mixture of a cellulose nanoparticle dispersion and a polymer matrix solution/dispersion procure the most efficient and well-performing nanocomposites. This results from the colloidal stability of cellulose nanomaterials in water that can be trapped within the polymeric matrix upon drying of the mixture, preserving their nanoscale dimensions. This technique can be extended to other liquid media by tailoring the surface properties of the nanoparticles. However, it is important to preserve the surface hydroxyl groups because, during liquid evaporation, strong interactions between nanoparticles can settle and promote the formation of a strong percolating network through H-bonding. This network ensures the mechanical stiffness of the material, with the intrinsic stiffness of the nanoparticles surprisingly playing only a minor role. In addition, one of the shortcomings of wet processing is that the drying of complex shapes and thick mouldings presents difficulties in part drying and solvent removal. Melt processing methods, such as extrusion and injection moulding, are usually more challenging to implement, but this is a step towards a larger scale use of cellulose nanomaterials. The main issues to overcome for an efficient melt processing of CNF/CNC-reinforced polymer nanocomposites are the irreversible aggregation of the nanofiller upon drying prior to melt processing, its non-uniform dispersion within the polymer melt, its thermal stability, its structural integrity and its orientation. Different strategies have been proposed in the literature to overcome these issues, but the fact remains that the final mechanical properties cannot compete with those obtained for nanocomposites prepared by casting/evaporation. The solution might be to adapt the coating of cellulose nanomaterials in order to retain some free OH groups for further nanoparticle interaction. Even more ideally, it might be to hide surface OH groups during processing with a compound that could be eliminated by a specific treatment (thermal, radiative, …) after shaping the product.
Data accessibility
This article has no additional data.
Competing interests
I declare I have no competing interests.
Funding
LGP2 is part of the LabEx Tec 21 (Investissements d'Avenir—grant agreement no. ANR-11-LABX-0030) and of the PolyNat Carnot Institut (Investissements d'Avenir—grant agreement no. ANR-11- CARN-030–01).
References
- 1.Klemm D, Heublein B, Fink H-P, Bohn A. 2005. Cellulose: fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 36, 3358–3393. ( 10.1002/anie.200460587) [DOI] [PubMed] [Google Scholar]
- 2.Nickerson RF, Habrle JA. 1947. Cellulose intercrystalline structure. Ind. Eng. Chem. 39, 1507–1512. ( 10.1021/ie50455a024) [DOI] [Google Scholar]
- 3.Herrick FW, Casebier RL, Hamilton JK, Sandberg KR. 1983. Microfibrillated cellulose: morphology and accessibility. J. Appl. Polym. Sci. Polym. Symp. 37, 797–813. [Google Scholar]
- 4.Turbak AF, Snyder FW, Sandberg KR. 1983. Microfibrillated cellulose: a new cellulose product—properties, uses, and commercial potential. J. Appl. Polym. Sci. Polym. Symp. 37, 815–827. [Google Scholar]
- 5.Lin N, Huang J, Dufresne A. 2012. Preparations, properties and applications of polysaccharide nanocrystals in advanced functional nanomaterials: a review. Nanoscale 4, 3274–3294. ( 10.1039/C2NR30260H) [DOI] [PubMed] [Google Scholar]
- 6.Azizi SMAS, Alloin F, Dufresne A. 2005. A review of recent research into cellulosic whiskers, their properties and their application in nanocomposite field. Biomacromolecules 6, 612–626. ( 10.1021/bm0493685) [DOI] [PubMed] [Google Scholar]
- 7.Eichhorn SJ, et al. 2010. Review: current international research into cellulose nanofibres and nanocomposites. J. Mater. Sci. 45, 1–33. ( 10.1007/s10853-009-3874-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moon RJ, Martini A, Nairn J, Simonsen J, Youngblood J. 2011. Cellulose nanomaterials review: structure, properties and nanocomposites. Chem. Soc. Rev. 40, 3941–3994. ( 10.1039/C0CS00108B) [DOI] [PubMed] [Google Scholar]
- 9.Dufresne A. 2012. Nanocellulose: from nature to high performance tailored materials. Berlin, Germany: Walter de Gruyter GmbH & Co. KG. [Google Scholar]
- 10.Henriksson M, Henriksson G, Berglund LA, Lindström T. 2007. An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose (MFC) nanofibers. Eur. Polym. J. 43, 3434–3441. ( 10.1016/j.eurpolymj.2007.05.038) [DOI] [Google Scholar]
- 11.Wågberg L, Decher G, Norgren M, Lindström T, Ankerfors M, Axnäs K. 2008. The build-up of polyelectrolyte multilayers of microfibrillated cellulose and cationic polyelectrolytes. Langmuir 24, 784–795. ( 10.1021/la702481v) [DOI] [PubMed] [Google Scholar]
- 12.Saito T, Kimura S, Nishiyama Y, Isogai A. 2007. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 8, 2485–2491. ( 10.1021/bm0703970) [DOI] [PubMed] [Google Scholar]
- 13.Dufresne A, Dupeyre D, Vignon MR. 2000. Cellulose microfibrils from potato tuber cells: processing and characterization of starch-cellulose microfibril composites. J. Appl. Polym. Sci. 76, 2080–2092. ( 10.1002/(SICI)1097-4628(20000628)76:14%3C2080) [DOI] [Google Scholar]
- 14.Sheltami RM, Ahmad I, Abdullah I, Dufresne A. 2012. Extraction of cellulose nanocrystals from mengkuang leaves. Carbohydr. Polym. 88, 772–779. ( 10.1016/j.carbpol.2012.01.062) [DOI] [Google Scholar]
- 15.Dufresne A. 2017. Cellulose nanomaterial reinforced polymer nanocomposites. Curr. Opin. Colloid Interface Sci. 29, 1–8. ( 10.1016/j.cocis.2017.01.004) [DOI] [Google Scholar]
- 16.Dufresne A. 2013. Nanocellulose: a new ageless bionanomaterial. Mater. Today 16, 220–227. ( 10.1016/j.mattod.2013.06.004) [DOI] [Google Scholar]
- 17.Favier V, Canova GR, Cavaillé JY, Chanzy H, Dufresne A, Gauthier C. 1995. Nanocomposites materials from latex and cellulose whiskers. Polym. Adv. Technol. 6, 351–355. ( 10.1002/pat.1995.220060514) [DOI] [Google Scholar]
- 18.Dufresne A, Vignon MR. 1998. Improvement of starch films performances using cellulose microfibrils. Macromolecules 31, 2693–2696. ( 10.1021/ma971532b) [DOI] [Google Scholar]
- 19.Schroers M, Kokil A, Weder C. 2004. Solid polymer electrolytes based on nanocomposites of ethylene oxide-epichlorohydrin copolymers and cellulose whiskers. J. Appl. Polym. Sci. 93, 2883–2888. ( 10.1002/app.20870) [DOI] [Google Scholar]
- 20.Qi H, Cai J, Zhang L, Kuga S. 2009. Properties of films composed of cellulose nanowhiskers and a cellulose matrix regenerated from alkali/urea solution. Biomacromolecules 10, 1597–1602. ( 10.1021/bm9001975) [DOI] [PubMed] [Google Scholar]
- 21.Sassi JF, Chanzy H. 1995. Ultrastructural aspects of the acetylation of cellulose. Cellulose 2, 111–127. ( 10.1007/BF00816384) [DOI] [Google Scholar]
- 22.de Menezes AJ, Siqueira G, Curvelo AAS, Dufresne A. 2009. Extrusion and characterization of functionalized cellulose whisker reinforced polyethylene nanocomposites. Polymer 50, 4552–4563. (doi:0.1016/j.polymer.2009.07.038) [Google Scholar]
- 23.Grunert M, Winter WT. 2002. Nanocomposites of cellulose acetate butyrate reinforced with cellulose nanocrystals. J. Polym. Environ. 10, 27–30. ( 10.1023/A:1021065905986) [DOI] [Google Scholar]
- 24.Goussé C, Chanzy H, Excoffier G, Soubeyrand L, Fleury E. 2002. Stable suspensions of partially silylated cellulose whiskers dispersed in organic solvents. Polymer 43, 2645–2651. ( 10.1016/S0032-3861(02)00051-4) [DOI] [Google Scholar]
- 25.Kloser E, Gray DG. 2010. Surface grafting of cellulose nanocrystals with poly(ethylene oxide) in aqueous media. Langmuir 26, 13 450–13 456. ( 10.1021/la101795s) [DOI] [PubMed] [Google Scholar]
- 26.Habibi Y, Dufresne A. 2008. Highly filled bionanocomposites from functionalized polysaccharide nanocrystals. Biomacromolecules 9, 1974–1980. ( 10.1021/bm8001717) [DOI] [PubMed] [Google Scholar]
- 27.Habibi Y, Goffin AL, Schiltz N, Duquesne E, Dubois P, Dufresne A. 2008. Bionanocomposites based on poly(ε-caprolactone)-grafted cellulose nanocrystals by ring opening polymerization. J. Mater. Chem. 18, 5002–5010. ( 10.1039/B809212E) [DOI] [Google Scholar]
- 28.Yi J, Xu Q, Zhang X, Zhang H. 2008. Chiral-nematic self-ordering of rodlike cellulose nanocrystals grafted with poly(styrene) in both thermotropic and lyotropic states. Polymer 49, 4406–4412. ( 10.1016/j.polymer.2008.08.008) [DOI] [Google Scholar]
- 29.Zoppe JO, Habibi Y, Rojas OJ, Venditti RA, Johansson LS, Efimenko K, Österberg M, Laine J. 2010. Poly(N-isopropylacrylamide) brushes grafted from cellulose nanocrystals via surface-initiated single-electron transfer living radical polymerization. Biomacromolecules 11, 2683–2691. ( 10.1021/bm100719d) [DOI] [PubMed] [Google Scholar]
- 30.Dufresne A. 2006. Comparing the mechanical properties of high performances polymer nanocomposites from biological sources. J. Nanosci. Nanotechnol. 6, 322–330. ( 10.1166/jnn.2006.906) [DOI] [PubMed] [Google Scholar]
- 31.Ma L, Zhang Y, Meng Y, Anusonti-Inthra P, Wang S. 2015. Preparing cellulose nanocrystal/acrylonitrile-butadiene-styrene nanocomposites using the master-batch method. Carbohydr. Polym. 125, 352–359. ( 10.1016/j.carbpol.2015.02.062) [DOI] [PubMed] [Google Scholar]
- 32.Lemahieu L, Bras J, Tiquet P, Augier S, Dufresne A. 2011. Extrusion of nanocellulose reinforced nanocomposites using the dispersed nano-objects protective encapsulation (DOPE) process. Macromol. Mater. Eng. 296, 984–991. ( 10.1002/mame.201100015) [DOI] [Google Scholar]
- 33.Leite LSF, Battirola LC, da Silva LCE, Gonçalves MC. 2016. Morphological investigation of cellulose acetate/cellulose nanocrystal composites obtained by melt extrusion. J. Appl. Polym. Sci. 133, 44201 ( 10.1002/app.44201) [DOI] [Google Scholar]
- 34.Martínez-Sanz M, Lopez-Rubio A, Lagaron JM. 2013. Nanocomposites of ethylene vinyl alcohol copolymer with thermally resistant cellulose nanowhiskers by melt compounding (I): morphology and thermal properties. J. Appl. Polym. Sci. 128, 2666–2678. ( 10.1002/app.38433) [DOI] [Google Scholar]
- 35.Martínez-Sanz M, Lopez-Rubio A, Lagaron JM. 2013. Nanocomposites of ethylene vinyl alcohol copolymer with thermally resistant cellulose nanowhiskers by melt compounding (II): water barrier and mechanical properties. J. Appl. Polym. Sci. 128, 2197–2207. ( 10.1002/app.38432) [DOI] [Google Scholar]
- 36.Li J, Song Z, Li D, Shang S, Guo Y. 2014. Cotton cellulose nanofiber-reinforced high density polyethylene composites prepared with two different pretreatment methods. Ind. Crops Prod. 59, 318–328. ( 10.1016/j.indcrop.2014.05.033) [DOI] [Google Scholar]
- 37.Sato A, Kabusaki D, Okumura H, Nakatani T, Nakatsubo F, Yano H. 2016. Surface modification of cellulose nanofibers with alkenyl succinic anhydride for high-density polyethylene reinforcement. Compos. Part A 83, 72–79. ( 10.1016/j.compositesa.2015.11.009) [DOI] [Google Scholar]
- 38.Sakakibara K, Yano H, Tsujii Y. 2016. Surface engineering of cellulose nanofiber by adsorption of diblock copolymer dispersant for green nanocomposite materials. ACS Appl. Mater. Interfaces 8, 24 893–24 900. ( 10.1021/acsami.6b07769) [DOI] [PubMed] [Google Scholar]
- 39.Kiziltas A, Nazari B, Kiziltas EE, Gardner DJS, Han Y, Rushing TS. 2016. Cellulose nanofiber-polyethylene nanocomposites modified with polyvinyl alcohol. J. Appl. Polym. Sci. 133, 42933 ( 10.1002/app.42933) [DOI] [Google Scholar]
- 40.Mokhena AS, Luyt AS. 2014. Investigation of polyethylene/sisal whiskers nanocomposites prepared under different conditions. Polym. Compos. 35, 2221–2233. ( 10.1002/pc.22887) [DOI] [Google Scholar]
- 41.Castro DO, Frollini E, Ruvolo-Filho A, Dufresne A. 2015. ‘Green polyethylene’ and curauá cellulose nanocrystal based nanocomposites: effect of vegetable oils as coupling agent and processing technique. J. Polym. Sci. Polym. Phys. 53, 1010–1019. ( 10.1002/polb.23729) [DOI] [Google Scholar]
- 42.Lewandowska AE, Eichhorn SJ. 2016. Quantification of the degree of mixing of cellulose nanocrystals in thermoplastics using Raman spectroscopy. J. Raman Spectrosc. 47, 1337–1342. ( 10.1002/jrs.4966) [DOI] [Google Scholar]
- 43.Wang B, Sain M. 2007. Dispersion of soybean stock-based nanofiber in a plastic matrix . Polym. Int. 56, 538–546. ( 10.1002/pi.2167) [DOI] [Google Scholar]
- 44.Farahbakhsh N, Venditti RA, Jur JS. 2014. Mechanical and thermal investigation of thermoplastic nanocomposite films fabricated using micro- and nano-sized fillers from recycled cotton T-shirts. Cellulose 21, 2743–2755. ( 10.1007/s10570-014-0285-4) [DOI] [Google Scholar]
- 45.Farahbakhsh N, Roodposhti PS, Ayoub A, Venditti RA, Jur JS. 2015. Melt extrusion of polyethylene nanocomposites reinforced with nanofibrillated cellulose from cotton and wood sources. J. Appl. Polym. Sci. 132, 41857 ( 10.1002/app.41857) [DOI] [Google Scholar]
- 46.Volk N, He R, Magniez K. 2015. Enhanced homogeneity and interfacial compatibility in melt-extruded cellulose nano-fibers reinforced polyethylene via surface adsorption of poly(ethylene glycol)-block-poly(ethylene) amphiphiles. Eur. Polym. J. 72, 270–281. ( 10.1016/j.eurpolymj.2015.09.025) [DOI] [Google Scholar]
- 47.Ben Azouz K, Ramires EC, Van den Fonteyne W, El Kissi N, Dufresne A. 2012. Simple method for the melt extrusion of a cellulose nanocrystal reinforced hydrophobic polymer. ACS Macro Lett. 1, 236–240. ( 10.1021/mz2001737) [DOI] [PubMed] [Google Scholar]
- 48.Pereda M, El Kissi N, Dufresne A. 2014. Extrusion of polysaccharide nanocrystal reinforced polymer nanocomposites through compatibilization with poly(ethylene oxide). ACS Appl. Mater. Interfaces 6, 9365–9375. ( 10.1021/am501755p) [DOI] [PubMed] [Google Scholar]
- 49.Iyer KA, Schueneman GT, Torkelson JM. 2015. Cellulose nanocrystal/polyolefin biocomposites prepared by solid-state shear pulverization: superior dispersion leading to synergistic property enhancements. Polymer 56, 464–475. ( 10.1016/j.polymer.2014.11.017) [DOI] [Google Scholar]
- 50.Nagalakshmaiah M, Pignon F, El Kissi N, Dufresne A. 2016. Surface adsorption of triblock copolymer (PEO-PPO-PEO) on cellulose nanocrystals and their melt extrusion with polyethylene. RSC Adv. 6, 66 224–66 232. ( 10.1039/c6ra11139d) [DOI] [Google Scholar]
- 51.Visakh PM, Thomas S, Oksman K, Mathew AP. 2012. Cellulose nanofibres and cellulose nanowhiskers based natural rubber composites: diffusion, sorption, and permeation of aromatic organic solvents. J. Appl. Polym. Sci. 124, 1614–1623. ( 10.1002/app.35176) [DOI] [Google Scholar]
- 52.Visakh PM, Thomas S, Oksman K, Mathew AP. 2012. Crosslinked natural rubber nanocomposites reinforced with cellulose whiskers isolated from bamboo waste: processing and mechanical/thermal properties. Compos. Part A 43, 735–741. ( 10.1016/j.compositesa.2011.12.015) [DOI] [Google Scholar]
- 53.Xu SH, Gu J, Luo YF, Jia DM. 2012. Effects of partial replacement of silica with surface modified nanocrystalline cellulose on properties of natural rubber nanocomposites. Exp. Polym. Lett. 6, 14–25. ( 10.3144/expresspolymlett.2012.3) [DOI] [Google Scholar]
- 54.Leszczyńska A, Kiciliński P, Pielichowski K. 2015. Biocomposites of polyamide 4.10 and surface modified microfibrillated cellulose (MFC): influence of processing parameters on structure and thermomechanical properties. Cellulose 22, 2551–2569. ( 10.1007/s10570-015-0657-4) [DOI] [Google Scholar]
- 55.Corrêa AC, Teixeira EM, Carmona VB, Teodoro KBR, Ribeiro C, Mattoso LHC, Marconcini JM. 2014. Obtaining nanocomposites of polyamide 6 and cellulose whiskers via extrusion and injection molding. Cellulose 21, 311–322. ( 10.1007/s10570-013-0132-z) [DOI] [Google Scholar]
- 56.Rahimi SK, Otaigbe JU. 2016. The role of particle surface functionality and microstructure development in isothermal and non-isothermal crystallization behavior of polyamide 6/cellulose nanocrystals nanocomposites. Polymer 107, 316–331. ( 10.1016/j.polymer.2016.11.023) [DOI] [Google Scholar]
- 57.Peng J, Walsh PJ, Sabo RC, Turng L-S, Clemons CM. 2016. Water-assisted compounding of cellulose nanocrystals into polyamide 6 for use as a nucleating agent for microcellular foaming. Polymer 84, 158–166. ( 10.1016/j.polymer.2015.12.050) [DOI] [Google Scholar]
- 58.Yousefian H, Rodrigue D. 2016. Effect of nanocrystalline cellulose on morphological, thermal, and mechanical properties of nylon 6 composites. Polym. Compos. 37, 1473–1479. ( 10.1002/pc.23316) [DOI] [Google Scholar]
- 59.Panaitescu DM, Frone AN, Nicolae C. 2013. Micro- and nano-mechanical characterization of polyamide 11 and its composites containing cellulose nanofibers. Eur. Polym. J. 49, 3857–3866. ( 10.1016/j.eurpolymj.2013.09.031) [DOI] [Google Scholar]
- 60.Semba T, Ito A, Kitagawa K, Nakatani T, Yano H, Sato A. 2014. Thermoplastic composites of polyamide-12 reinforced by cellulose nanofibers with cationic surface modification. J. Appl. Polym. Sci. 131, 40920 ( 10.1002/app.40920) [DOI] [Google Scholar]
- 61.Nicharat A, Sapkota J, Weder C, Foster EJ. 2015. Melt processing of polyamide 12 and cellulose nanocrystals nanocomposites. J. Appl. Polym. Sci. 132, 42752 ( 10.1002/app.42752) [DOI] [Google Scholar]
- 62.Mukherjee T, Czaka M, Kao N, Gupta RK, Choi HJ, Bhattacharya S. 2014. Dispersion study of nanofibrillated cellulose based poly(butyleneadipate-co-terephthalate) composites. Carbohydr. Polym. 102, 537–542. ( 10.1016/j.carbpol.2013.11.047) [DOI] [PubMed] [Google Scholar]
- 63.Mariano M, Chirat C, El Kissi N, Dufresne A. 2016. Impact of cellulose nanocrystal aspect ratio on crystallization and reinforcement of poly(butylene adipate-co-terephthalate). J. Polym. Sci. Polym. Phys. 54, 2284–2297. ( 10.1002/polb.24139) [DOI] [Google Scholar]
- 64.Mariano M, El Kissi N, Dufresne A. 2016. Structural reorganization of CNC in injection-molded CNC/PBAT materials under thermal annealing. Langmuir 32, 10 093–10 103. ( 10.1021/acs.langmuir.6b03220) [DOI] [PubMed] [Google Scholar]
- 65.Morelli CL, Belgacem N, Bretas RES, Bras J. 2016. Melt extruded nanocomposites of polybutylene adipate-co-terephthalate (PBAT) with phenylbutyl isocyanate modified cellulose nanocrystals. J. Appl. Polym. Sci. 133, 43678 ( 10.1002/app.43678) [DOI] [Google Scholar]
- 66.Morelli CL, Belgacem MN, Branciforti MC, Salon MCB, Bras J, Bretas RES. 2016. Nanocomposites of PBAT and cellulose nanocrystals modified by in situ polymerization and melt extrusion. Polym. Eng. Sci. 56, 1339–1348. ( 10.1002/pen.24367) [DOI] [Google Scholar]
- 67.Zhang X, Ma P, Zhang Y. 2016. Structure and properties of surface-acetylated cellulose nanocrystal/poly(butylene adipate-co-terephthalate) composites. Polym. Bull. 73, 2073–2085. ( 10.1007/s00289-015-1594-y) [DOI] [Google Scholar]
- 68.Lin N, Chen Y, Hu F, Huang J. 2015. Mechanical reinforcement of cellulose nanocrystals on biodegradable microcellular foams with melt-compounding process. Cellulose 22, 2629–2639. ( 10.1007/s10570-015-0684-1) [DOI] [Google Scholar]
- 69.Hu F, Lin N, Chang PR, Huang J. 2015. Reinforcement and nucleation of acetylated cellulose nanocrystals in foamed polyester composites. Carbohydr. Polym. 129, 208–215. ( 10.1016/j.carbpol.2015.04.061) [DOI] [PubMed] [Google Scholar]
- 70.Ludueña LN, Fortunati E, Morán JI, Alvarez VA, Cyras VP, Puglia D, Manfredi LB, Pracella M. 2016. Preparation and characterization of polybutylene-succinate/poly(ethylene-glycol)/cellulose nanocrystals ternary composites. J. Appl. Polym. Sci. 133, 43302 ( 10.1002/app.43302) [DOI] [Google Scholar]
- 71.Zhang X, Zhang Y. 2015. Poly(butylene succinate-co-butylene adipate)/cellulose nanocrystal composites modified with phthalic anhydride. Carbohydr. Polym. 134, 52–59. ( 10.1016/j.carbpol.2015.07.078) [DOI] [PubMed] [Google Scholar]
- 72.Mariano M, El Kissi N, Dufresne A. 2015. Melt processing of cellulose nanocrystal reinforced polycarbonate from a masterbatch process. Eur. Polym. J. 69, 208–223. ( 10.1016/j.eurpolymj.2015.06.007) [DOI] [Google Scholar]
- 73.Goffin A, Raquez J-M, Duquesne E, Siqueira G, Habibi Y, Dufresne A, Dubois P. 2011. Poly(ε-caprolactone) based nanocomposites reinforced by surface-grafted cellulose nanowhiskers via extrusion processing: morphology, rheology, and thermo-mechanical properties. Polymer 52, 1532–1538. ( 10.1016/j.polymer.2011.02.004) [DOI] [Google Scholar]
- 74.Khan A, Beck S, Dussault D, Salmieri S, Bouchard J, Lacroix M. 2013. Mechanical and barrier properties of nanocrystalline cellulose reinforced poly(caprolactone) composites: effect of gamma radiation. J. Appl. Polym. Sci. 129, 3038–3046. ( 10.1002/app.38896) [DOI] [Google Scholar]
- 75.Mi H-Y, Jing X, Peng J, Salick MR, Peng X-F, Turng L-S. 2014. Poly(e-caprolactone) (PCL)/cellulose nano-crystal (CNC) nanocomposites and foams. Cellulose 21, 2727–2741. ( 10.1007/s10570-014-0327-y) [DOI] [Google Scholar]
- 76.Safdari F, Carreau PJ, Heuzey M-C, Kamal MR, Sain MM. 2017. Enhanced properties of poly(ethylene oxide)/cellulose nanofiber biocomposites. Cellulose 24, 755–767. ( 10.1007/s10570-016-1137-1) [DOI] [Google Scholar]
- 77.Alloin F, D'Aprea A, Dufresne A, El Kissi N, Bossard F. 2011. Poly(oxyethylene) and ramie whiskers based nanocomposites: influence of processing—extrusion and casting/evaporation. Cellulose 18, 957–973. ( 10.1007/s10570-011-9543-x) [DOI] [Google Scholar]
- 78.Wang B, Sain M. 2007. The effect of chemically coated nanofiber reinforcement on biopolymer based nanocomposites. BioResources 2, 371–388. [Google Scholar]
- 79.Chen J, Xu C, Wu D, Pan K, Qian A, Sha Y, Wang L, Tong W. 2015. Insights into the nucleation role of cellulose crystals during crystallization of poly(β-hydroxybutyrate). Carbohydr. Polym. 134, 508–515. ( 10.1016/j.carbpol.2015.08.023) [DOI] [PubMed] [Google Scholar]
- 80.Srithep Y, Ellingham T, Peng J, Sabo R, Clemons C, Turng L-S, Pilla S. 2013. Melt compounding of poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/nanofibrillated cellulose nanocomposites. Polym. Degrad. Stab. 98, 1439–1449. ( 10.1016/j.polymdegradstab.2013.05.006) [DOI] [Google Scholar]
- 81.Jiang L, Morelius E, Zhang J, Wolcott M, Holbery J. 2008. Study of the poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/cellulose nanowhiskers composites prepared by solution casting and melt processing. J. Compos. Mater. 42, 2629–2645. ( 10.1177/0021998308096327) [DOI] [Google Scholar]
- 82.Ambrosio-Martín J, Fabra MJ, López-Rubio A, Gorrasi G, Sorrentino A, Lagaron JM. 2016. Assessment of ball milling as a compounding technique to develop nanocomposites of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and bacterial cellulose nanowhiskers. J. Polym. Environ. 24, 241–254. ( 10.1007/s10924-016-0767-6) [DOI] [Google Scholar]
- 83.Iwatake A, Nogi M, Yano H. 2008. Cellulose nanofiber-reinforced polylactic acid. Compos. Sci. Technol. 68, 2103–2106. ( 10.1016/j.compscitech.2008.03.006) [DOI] [Google Scholar]
- 84.Suryanegara L, Nakagaito AN, Yano H. 2009. The effect of crystallization of PLA on the thermal and mechanical properties of microfibrillated cellulose-reinforced PLA composites. Compos. Sci. Technol. 69, 1187–1192. ( 10.1016/j.compscitech.2009.02.022) [DOI] [Google Scholar]
- 85.Jonoobi M, Harun J, Mathew AP, Oksman K. 2010. Mechanical properties of cellulose nanofiber (CNF) reinforced polylactic acid (PLA) prepared by twin screw extrusion. Compos. Sci. Technol. 70, 1742–1747. ( 10.1016/j.compscitech.2010.07.005) [DOI] [Google Scholar]
- 86.Suryanegara L, Okumura H, Nakagaito AN, Yano H. 2011. The synergetic effect of phenylphosphonic acid zinc and microfibrillated cellulose on the injection molding cycle time of PLA composites. Cellulose 18, 689–698. ( 10.1007/s10570-011-9515-1) [DOI] [Google Scholar]
- 87.Eyholzer C, Tingaut P, Zimmermann T, Oksman K. 2012. Dispersion and reinforcing potential of carboxymethylated nanofibrillated cellulose powders modified with 1-hexanol in extruded poly(lactic acid) (PLA) composites. J. Polym. Environ. 20, 1052–1062. ( 10.1007/s10924-012-0508-4) [DOI] [Google Scholar]
- 88.Herrera N, Mathew AP, Oksman K. 2015. Plasticized polylactic acid/cellulose nanocomposites prepared using melt-extrusion and liquid feeding: mechanical, thermal and optical properties. Compos. Sci. Technol. 106, 149–155. ( 10.1016/j.compscitech.2014.11.012) [DOI] [Google Scholar]
- 89.Oksman K, Mathew AP, Bondeson D, Kvien I. 2006. Manufacturing process of cellulose whiskers/polylactic acid nanocomposites. Compos. Sci. Technol. 66, 2776–2784. ( 10.1016/j.compscitech.2006.03.002) [DOI] [Google Scholar]
- 90.Bondeson D, Oksman K. 2007. Polylactic acid/cellulose whisker nanocomposites modified by polyvinyl alcohol. Compos. Part A 38, 2486–2492. ( 10.1016/j.compositesa.2007.08.001) [DOI] [Google Scholar]
- 91.Goffin A-L, Raquez J-M, Duquesne E, Siqueira G, Habibi Y, Dufresne A, Dubois P. 2011. From interfacial ring-opening polymerization to melt processing of cellulose nanowhisker-filled polylactide-based nanocomposites. Biomacromolecules 12, 2456–2465. ( 10.1021/bm200581h) [DOI] [PubMed] [Google Scholar]
- 92.Raquez JM, Murena Y, Goffin AL, Habibi Y, Ruelle B, DeBuyl F, Dubois P. 2012. Surface-modification of cellulose nanowhiskers and their use as nanoreinforcers into polylactide: a sustainably-integrated approach. Compos. Sci. Technol. 72, 544–549. ( 10.1016/j.compscitech.2011.11.017) [DOI] [Google Scholar]
- 93.Fortunati E, Armentano I, Zhou Q, Iannoni A, Saino E, Visai L, Berglund LA, Kenny JM. 2012. Multifunctional bionanocomposite films of poly(lactic acid), cellulose nanocrystals and silver nanoparticles. Carbohydr. Polym. 87, 1596–1605. ( 10.1016/j.carbpol.2011.09.066) [DOI] [Google Scholar]
- 94.Jonoobi M, Mathew AP, Mahnaz M, Abdi MM, Makinejad MD, Oksman K. 2012. A comparison of modified and unmodified cellulose nanofiber reinforced polylactic acid (PLA) prepared by twin screw extrusion. J. Polym. Environ. 20, 991–997. ( 10.1007/s10924-012-0503-9) [DOI] [Google Scholar]
- 95.Pracella M, Haque MM-U, Puglia D. 2014. Morphology and properties tuning of PLA/cellulose nanocrystals bionanocomposites by means of reactive functionalization and blending with PVAc. Polymer 55, 3720–3728. ( 10.1016/j.polymer.2014.06.071) [DOI] [Google Scholar]
- 96.Kamal MR, Khoshkava V. 2015. Effect of cellulose nanocrystals (CNC) on rheological and mechanical properties and crystallization behavior of PLA/CNC nanocomposites. Carbohydr. Polym. 123, 105–114. ( 10.1016/j.carbpol.2015.01.012) [DOI] [PubMed] [Google Scholar]
- 97.Spinella S, Lo Re G, Liu B, Habibi Y, Leclère P, Raquez J-M, Dubois P, Gross R. 2015. Polylactide/cellulose nanocrystal nanocomposites: efficient routes for nanofiber modification and effects of nanofiber chemistry on PLA reinforcement. Polymer 65, 9–17. ( 10.1016/j.polymer.2015.02.048) [DOI] [Google Scholar]
- 98.Arias A, Heuzey M-C, Huneault MA, Ausias G, Bendahou A. 2015. Enhanced dispersion of cellulose nanocrystals in melt-processed polylactide-based nanocomposites. Cellulose 22, 483–498. ( 10.1007/s10570-014-0476-z) [DOI] [Google Scholar]
- 99.Dhar P, Tarafder D, Kumar A, Katiyar V. 2016. Thermally recyclable polylactic acid/cellulose nanocrystal films through reactive extrusion process. Polymer 87, 268–282. ( 10.1016/j.polymer.2016.02.004) [DOI] [Google Scholar]
- 100.Dhar P, Bhasney SM, Kumar A, Katiyar V. 2016. Acid functionalized cellulose nanocrystals and its effect on mechanical, thermal, crystallization and surfaces properties of poly(lactic acid) bionanocomposites films: a comprehensive study. Polymer 101, 75–92. ( 10.1016/j.polymer.2016.08.028) [DOI] [Google Scholar]
- 101.Herrera N, Salaberria AM, Mathew AP, Oksman K. 2016. Plasticized polylactic acid nanocomposite films with cellulose and chitin nanocrystals prepared using extrusion and compression molding with two cooling rates: effects on mechanical, thermal and optical properties. Compos. Part A 83, 89–97. ( 10.1016/j.compositesa.2015.05.024) [DOI] [Google Scholar]
- 102.Lizundia E, Fortunati E, Dominici F, Vilas JL, León LM, Armentano I, Torre L, Kenny JM. 2016. PLLA-grafted cellulose nanocrystals: role of the CNC content and grafting on the PLA bionanocomposite film properties. Carbohydr. Polym. 142, 105–113. ( 10.1016/j.carbpol.2016.01.041) [DOI] [PubMed] [Google Scholar]
- 103.Miao C, Hamad WY. 2016. In-situ polymerized cellulose nanocrystals (CNC)-poly(l-lactide)(PLLA) nanomaterials and applications in nanocomposite processing. Carbohydr. Polym. 153, 549–558. ( 10.1016/j.carbpol.2016.08.012) [DOI] [PubMed] [Google Scholar]
- 104.Spinella S, Samuel C, Raquez J-M, McCallum SA, Gross R, Dubois P. 2016. Green and efficient synthesis of dispersible cellulose nanocrystals in biobased polyesters for engineering applications. ACS Sustain. Chem. Eng. 4, 2517–2527. ( 10.1021/acssuschemeng.5b01611) [DOI] [Google Scholar]
- 105.Yang W, Dominici F, Fortunati E, Kenny JM, Puglia D. 2015. Melt free radical grafting of glycidyl methacrylate (GMA) onto fully biodegradable poly(lactic) acid films: effect of cellulose nanocrystals and a masterbatch process. RSC Adv. 5, 32 350–32 357. ( 10.1039/c5ra00894h) [DOI] [Google Scholar]
- 106.Yang W, Fortunati E, Dominici F, Giovanale G, Mazzaglia A, Balestra GM, Kenny JM, Puglia D. 2016. Synergic effect of cellulose and lignin nanostructures in PLA based systems for food antibacterial packaging. Eur. Polym. J. 79, 1–12. ( 10.1016/j.eurpolymj.2016.04.003) [DOI] [Google Scholar]
- 107.Ambrosio-Martín J, López-Rubio A, Fabra MJ, Gorrasi G, Pantani R, Lagaron JM. 2015. Assessment of ball milling methodology to develop polylactide-bacterial cellulose nanocrystals nanocomposites. J. Appl. Polym. Sci. 132, 41605 ( 10.1002/app.41605) [DOI] [Google Scholar]
- 108.Xu C, Chen J, Wu D, Chen Y, Lv Q, Wang M. 2016. Polylactide/acetylated nanocrystalline cellulose composites prepared by a continuous route: a phase interface-property relation study. Carbohydr. Polym. 146, 58–66. ( 10.1016/j.carbpol.2016.03.058) [DOI] [PubMed] [Google Scholar]
- 109.Johari AP, Kurmvanshi SK, Mohanty S, Nayak SK. 2016. Influence of surface modified cellulose microfibrils on the improved mechanical properties of poly(lactic acid). Int. J. Biol. Macromolec. 84, 329–339. ( 10.1016/j.ijbiomac.2015.12.038) [DOI] [PubMed] [Google Scholar]
- 110.Haque MM-U, Puglia D, Fortunati E, Pracella M. 2017. Effect of reactive functionalization on properties and degradability of poly(lactic acid)/poly(vinyl acetate) nanocomposites with cellulose nanocrystals. React. Funct. Polym. 110, 1–9. ( 10.1016/j.reactfunctpolym.2016.11.003) [DOI] [Google Scholar]
- 111.Zhang P, Gao D, Zou P, Wang B. 2017. Preparation and thermomechanical properties of nanocrystalline cellulose reinforced poly(lactic acid) nanocomposites. J. Appl. Polym. Sci. 134, 44683 ( 10.1002/app.44683) [DOI] [Google Scholar]
- 112.Tomé LC, Pinto RJB, Trovatti E, Freire CSR, Silvestre AJD, Neto CP, Gandini A. 2011. Transparent bionanocomposites with improved properties prepared from acetylated bacterial cellulose and poly(lactic acid) through a simple approach. Green Chem. 13, 419–427. ( 10.1039/C0GC00545B) [DOI] [Google Scholar]
- 113.Fortunati E, Luzi F, Puglia D, Dominici F, Santulli C, Kenny JM, Torre L. 2014. Investigation of thermo-mechanical, chemical and degradative properties of PLA-limonene films reinforced with cellulose nanocrystals extracted from Phormium tenax leaves. Eur. Polym. J. 56, 77–91. ( 10.1016/j.eurpolymj.2014.03.030) [DOI] [Google Scholar]
- 114.Bitinis N, Verdejo R, Bras J, Fortunati E, Kenny JM, Torre L, Lopez-Manchado MA. 2013. Poly(lactic acid)/natural rubber/cellulose nanocrystal bionanocomposites Part I. Processing and morphology. Carbohydr. Polym. 96, 611–620. ( 10.1016/j.carbpol.2013.02.068) [DOI] [PubMed] [Google Scholar]
- 115.Arrieta MP, Fortunati E, Dominici F, Rayón E, López J, Kenny JM. 2014. PLA-PHB/cellulose based films: mechanical, barrier and disintegration properties. Polym. Degrad. Stab. 107, 139–149. ( 10.1016/j.polymdegradstab.2014.05.010) [DOI] [Google Scholar]
- 116.Arrieta MP, Fortunati E, Dominici F, López J, Kenny JM. 2015. Bionanocomposite films based on plasticized PLA-PHB/cellulose nanocrystal blends. Carbohydr. Polym. 121, 265–275. ( 10.1016/j.carbpol.2014.12.056) [DOI] [PubMed] [Google Scholar]
- 117.Wang B, Sain M. 2007. Isolation of nanofibers from soybean source and their reinforcing capability on synthetic polymers. Compos. Sci. Technol. 67, 2521–2527. ( 10.1016/j.compscitech.2006.12.015) [DOI] [Google Scholar]
- 118.Lee S-H, Teramoto Y, Endo T. 2011. Cellulose nanofiber-reinforced polycaprolactone/polypropylene hybrid nanocomposite. Compos. Part A 42, 151–156. ( 10.1016/j.compositesa.2010.10.014) [DOI] [Google Scholar]
- 119.Suzuki K, Okumura H, Kitagawa K, Sato S, Nakagaito AN, Yano H. 2013. Development of continuous process enabling nanofibrillation of pulp and melt compounding. Cellulose 20, 201–210. ( 10.1007/s10570-012-9843-9) [DOI] [Google Scholar]
- 120.Iwamoto S, Yamamoto S, Lee S-H, Endo T. 2014. Mechanical properties of polypropylene composites reinforced by surface-coated microfibrillated cellulose. Compos. Part A 59, 26–29. ( 10.1016/j.compositesa.2013.12.011) [DOI] [Google Scholar]
- 121.Suzuki K, Sato A, Okumura H, Hashimoto T, Nakagaito AN, Yano H. 2014. Novel high-strength, micro fibrillated cellulose-reinforced polypropylene composites using a cationic polymer as compatibilizer. Cellulose 21, 507–518. ( 10.1007/s10570-013-0143-9) [DOI] [Google Scholar]
- 122.Iwamoto S, Yamamoto S, Lee S-H, Endo T. 2014. Solid-state shear pulverization as effective treatment for dispersing lignocellulose nanofibers in polypropylene composites. Cellulose 21, 1573–1580. ( 10.1007/s10570-014-0195-5) [DOI] [Google Scholar]
- 123.Peng Y, Gallegos SA, Gardner DJ, Han Y, Cai Z. 2016. Maleic anhydride polypropylene modified cellulose nanofibril polypropylene nanocomposites with enhanced impact strength. Polym. Compos. 37, 782–793. ( 10.1002/pc.23235) [DOI] [Google Scholar]
- 124.Hassan ML, Mathew AP, Hassan EA, Fadel SM, Oksman K. 2014. Improving cellulose/polypropylene nanocomposites properties with chemical modified bagasse nanofibers and maleated polypropylene. J. Reinf. Plast. Compos. 33, 26–36. ( 10.1177/0731684413509292) [DOI] [Google Scholar]
- 125.Khoshkava V, Kamal MR. 2014. Effect of cellulose nanocrystals (CNC) particle morphology on dispersion and rheological and mechanical properties of polypropylene/CNC nanocomposites. ACS Appl. Mater. Interfaces 6, 8146–8157. ( 10.1021/am500577e) [DOI] [PubMed] [Google Scholar]
- 126.Khoshkava V, Ghasemi H, Kamal MR. 2015. Effect of cellulose nanocrystals (CNC) on isothermal crystallization kinetics of polypropylene. Thermochim. Acta 608, 30–39. ( 10.1016/j.tca.2015.04.007) [DOI] [Google Scholar]
- 127.Yousefian H, Rodrigue D. 2015. Nano-crystalline cellulose, chemical blowing agent, and mold temperature effect on morphological, physical/mechanical properties of polypropylene. J. Appl. Polym. Sci. 132, 42845 ( 10.1002/APP.42845) [DOI] [Google Scholar]
- 128.Nagalakshmaiah M, El Kissi N, Dufresne A. 2016. Ionic compatibilization of cellulose nanocrystals with quaternary ammonium salt and their melt extrusion with polypropylene. ACS Appl. Mater. Interfaces 8, 8755–8764. ( 10.1021/acsami.6b01650) [DOI] [PubMed] [Google Scholar]
- 129.Hu X, Xu C, Gao J, Yang G, Geng C, Chen F, Fu Q. 2013. Toward environment-friendly composites of poly(propylene carbonate) reinforced with cellulose nanocrystals. Compos. Sci. Technol. 78, 63–68. ( 10.1016/j.compscitech.2013.02.002) [DOI] [Google Scholar]
- 130.Lin N, Dufresne A. 2013. Physical and/or chemical compatibilization of extruded cellulose nanocrystal reinforced polystyrene nanocomposites. Macromolecules 46, 5570–5583. ( 10.1021/ma4010154) [DOI] [Google Scholar]
- 131.Fox DM, Rodriguez RS, Devilbiss MN, Woodcock J, Davis CS, Sinko R, Keten S, Gilman JW. 2016. Simultaneously tailoring surface energies and thermal stabilities of cellulose nanocrystals using ion exchange: effects on polymer composite properties for transportation, infrastructure, and renewable energy applications. ACS Appl. Mater. Interfaces 8, 27 270–27 281. ( 10.1021/acsami.6b06083) [DOI] [PubMed] [Google Scholar]
- 132.Besbes I, Magnin A, Boufi S. 2011. Rheological behavior of nanofibrillated cellulose/acrylic polymer nanocomposites: effect of melt extrusion. Polym. Compos. 32, 2070–2075. ( 10.1002/pc.21232) [DOI] [Google Scholar]
- 133.Hajji P, Cavaillé J-Y, Favier V, Gauthier C, Vigier G. 1996. Tensile behavior of nanocomposites from latex and cellulose whiskers. Polym. Compos. 17, 612–619. ( 10.1002/pc.10651) [DOI] [Google Scholar]
- 134.Amin KNM, Amiralian N, Annamalai PK, Edwards G, Chaleat C, Martin DJ. 2016. Scalable processing of thermoplastic polyurethane nanocomposites toughened with nanocellulose. Chem. Eng. J. 302, 406–416. ( 10.1016/j.cej.2016.05.067) [DOI] [Google Scholar]
- 135.Zhao N, Mark LH, Zhu C, Park CB, Li Q, Glenn R, Thompson TR. 2014. Foaming poly(vinyl alcohol)/microfibrillated cellulose composites with CO2 and water as co-blowing agents. Ind. Eng. Chem. Res. 53, 11 962–11 972. ( 10.1002/app.42551) [DOI] [Google Scholar]
- 136.Zhang W, He X, Li C, Zhang X, Lu C, Zhang X, Deng Y. 2014. High performance poly(vinyl alcohol)/cellulose nanocrystals nanocomposites manufactured by injection molding. Cellulose 21, 485–494. ( 10.1007/s10570-013-0141-y) [DOI] [Google Scholar]
- 137.Mathew AP, Gong G, Bjorngrim N, Wixe D, Oksman K. 2011. Moisture absorption behavior and its impact on the mechanical properties of cellulose whiskers-based polyvinylacetate nanocomposites. Polym. Eng. Sci. 51, 2136–2142. ( 10.1002/pen.22063) [DOI] [Google Scholar]
- 138.Gong G, Mathew AP, Oksman K. 2011. Toughening effect of cellulose nanowhiskers on polyvinyl acetate: fracture toughness and viscoelastic analysis. Polym. Compos. 32, 1492–1498. ( 10.1002/pc.21170) [DOI] [Google Scholar]
- 139.Gong G, Pyo J, Mathew AP, Oksman K. 2011. Tensile behavior, morphology and viscoelastic analysis of cellulose nanofiber-reinforced (CNF) polyvinyl acetate (PVAc). Compos. Part A 42, 1275–1282. ( 10.1016/j.compositesa.2011.05.009) [DOI] [Google Scholar]
- 140.Sapkota J, Kumar S, Weder C, Foster EJ. 2015. Influence of processing conditions on properties of poly (vinyl acetate)/cellulose nanocrystal nanocomposites. Macromol. Mater. Eng. 300, 562–571. ( 10.1002/mame.201400313) [DOI] [Google Scholar]
- 141.Hietala M, Mathew AP, Oksman K. 2013. Bionanocomposites of thermoplastic starch and cellulose nanofibers manufactured using twin-screw extrusion. Eur. Polym. J. 49, 950–956. ( 10.1016/j.eurpolymj.2012.10.016) [DOI] [Google Scholar]
- 142.Ferreira AM, Carvalho AJF. 2014. TPS nanocomposite reinforced with MFC by melt process. Mater. Res. 17, 807–810. ( 10.1590/S1516-14392014005000090) [DOI] [Google Scholar]
- 143.Orts WJ, Shey J, Imam SH, Glenn GM, Guttman ME, Revol JF. 2005. Application of cellulose microfibrils in polymer nanocomposites. J. Polym. Environ. 13, 301–306. ( 10.1007/s10924-005-5514-3) [DOI] [Google Scholar]
- 144.Campos A, Teodoro KBR, Teixeira EM, Corrêa AC, Marconcini JM, Wood DF, Williams TG, Mattoso LHC. 2013. Properties of thermoplastic starch and TPS/polycaprolactone blend reinforced with sisal whiskers using extrusion processing. Polym. Eng. Sci. 53, 800–808. ( 10.1002/pen.23324) [DOI] [Google Scholar]
- 145.Cordeiro EMS, Nunes YL, Mattos ALA, Rosa MF, Sousa Filho MSM, Ito EN. 2014. Polymer biocomposites and nanobiocomposites obtained from mango seeds. Macromol. Symp. 344, 39–54. ( 10.1002/masy.201300217) [DOI] [Google Scholar]
- 146.Fabra MJ, López-Rubio A, Ambrosio-Martín J, Lagaron JM. 2016. Improving the barrier properties of thermoplastic corn starch-based films containing bacterial cellulose nanowhiskers by means of PHA electrospun coatings of interest in food packaging. Food Hydrocoll. 61, 261–268. ( 10.1016/j.foodhyd.2016.05.025) [DOI] [Google Scholar]
- 147.Peng Y, Gardner DJ, Han Y. 2012. Drying cellulose nanofibrils: in search of a suitable method. Cellulose 19, 91–102. ( 10.1007/s10570-011-9630-z) [DOI] [Google Scholar]
- 148.Roman M, Winter WT. 2004. Effect of sulfate groups from sulfuric acid hydrolysis on the thermal degradation behavior of bacterial cellulose. Biomacromolecules 5, 1671–1677. ( 10.1021/bm034519+) [DOI] [PubMed] [Google Scholar]
- 149.Lin N, Dufresne A. 2014. Surface chemistry, morphological analysis and properties of cellulose nanocrystals with gradient sulfation degrees. Nanoscale 6, 5384–5393. ( 10.1039/c3nr06761k) [DOI] [PubMed] [Google Scholar]
- 150.Bettaieb F, Khiari R, Dufresne A, Mhenni MF, Belgacem MN. 2015. Mechanical and thermal properties of Posidonia oceanica cellulose nanocrystal reinforced polymer. Carbohydr. Polym. 123, 99–104. ( 10.1016/j.carbpol.2015.01.026) [DOI] [PubMed] [Google Scholar]
- 151.Camarero ES, Kuhnt T, Foster EJ, Weder C. 2013. Isolation of thermally stable cellulose nanocrystals by phosphoric acid hydrolysis. Biomacromolecules 14, 1223–1230. ( 10.1021/bm400219u) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.