Abstract
Invariant natural killer T (iNKT) cells are innate T cells restricted by CD1d molecules. They are positively selected in the thymic cortex and migrate to the medullary area, in which they differentiate into 3 different lineages. Promyelocytic leukemia zinc finger (PLZF) modulates this process, and PLZFhigh, PLZFintermediate, and PLZFlow iNKT cells are designated as NKT2, NKT17, and NKT1 cells, respectively. Analogous to conventional helper CD4 T cells, each subset expresses distinct combinations of transcription factors and produces different cytokines. In lymphoid organs, iNKT subsets have unique localizations, which determine their cytokine responses upon antigenic challenge. The lineage differentiation programs of iNKT cells are differentially regulated in various mice strains in a cell-intrinsic manner, and BALB/c mice contain a high frequency of NKT2 cells. In the thymic medulla, steady state IL-4 from NKT2 cells directly conditions CD8 T cells to become memory-like cells expressing Eomesodermin, which function as premade memory effectors. The genetic signature of iNKT cells is more similar to that of γδ T cells and innate lymphoid cells (ILCs) than of conventional helper T cells, suggesting that ILCs and innate T cells share common developmental programs.
Keywords: Natural killer T cells, Thymus gland, Growth and development
INTRODUCTION
Innate or unconventional T cells develop in the thymus but are phenotypically distinct from conventional T cells. Invariant natural killer T (iNKT), γδ T, mucosal-associated invariant T (MAIT), and major histocompatibility complex (MHC) class Ib-reactive T cells belong to this category (1,2). Among them, iNKT cells are γδ T cells derived from double positive cortical thymocytes (3) and are selected by thymocytes presenting lipid antigens in the context of CD1d molecules (4), whereas conventional CD4 and CD8 T lymphocytes are selected by peptide antigens presented by MHC class Ia and class II molecules. NKT cells develop as memory cells in the thymus, and promyelocytic leukemia zinc finger (PLZF) regulates their development and effector differentiation (5,6).
There are 3 functional subsets of iNKT cells in the thymus, which produce distinct combination of cytokines and lineage-specific transcription factors. These subsets are designated as NKT1, NKT2, and NKT17, and they respectively express T-box 21 (T-bet), GATA binding protein-3 (GATA3), and retinoic acid receptor-related orphan nuclear receptor gamma (RORγt) to produce interferon-γ (IFN-γ), IL-4, and IL-17. NKT2 cells produce copious amounts of IL-4 at steady state, which elicits various systemic effects through the phosphorylation of signal transducer and activator of transcription 6 (STAT6) (7). IL-4 directly conditions CD8 T cells to become memory-like cells in the thymus, stimulates B cells to make IgE, and induces dendritic cells to secrete T helper (Th) 2-type chemokines like chemokine (C-C motif) ligand (CCL) 17 and CCL22. The development of iNKT cells is differentially regulated amongst various mouse strains, and BALB/c mice contain a high frequency of the IL-4-producing iNKT subset. In lymphoid organs, each iNKT subset displayed unique anatomic localization, which determined their responsiveness to intravenous or oral antigenic challenges (8). RNAseq analysis showed each iNKT subset has distinctive genetic signatures, and these features are more similar to γδ T cells and innate lymphoid cells (ILCs) than conventional T cells (9).
When iNKT cells are stimulated with α-galactosylceramide (αGalcer), they produce copious amounts of both Th1 and Th2 type cytokines and modulate immunity (10). In B16 melanoma tumor models, they activate natural killer (NK) cells which eradicate tumor cells. In autoimmune disease models, activated NKT cells could suppress type I diabetes and experimental allergic encephalitis (11,12,13). In pneumococcal infection model, NKT cells recognized bacterial glycolipids and it was essential to control lung infection (14). Defective development of NKT cells were suggested to be a cause of type I diabetes in NOD mice and systemic lupus erythematosus in NZB×NZW F1 mice (15). As a prominent source of Th2 type cytokines, however, NKT cells aggravated allergic lug inflammation and induced oxazolone induced colitis (16,17). By secreting IFN-γ and IL-4, NKT cells suppressed transforming growth factor β1 (TGF-β1) production and worsened antibody induced joint inflammation (18). These extreme variety of immunological function of NKT cells at the steady state and upon activation, however, were not fully explained by previous model of NKT cell development and this review will describe recent advances of our knowledge about their ontogeny and effector differentiation.
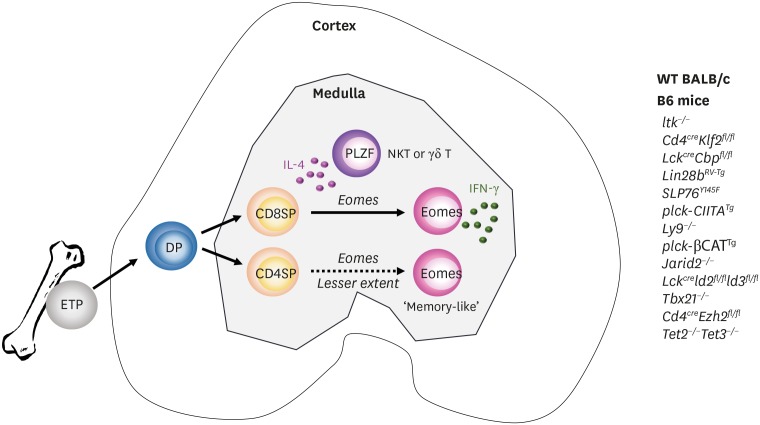
IL-4 AND MEMORY-LIKE CD8 T CELLS
Bone marrow transplantation from wild type (WT) mice to β2m−/− mice resulted in the development of CD8 T cells with unique features (19). They were positively selected by donor hematopoietic cells, and restricted by unconventional MHC Ib molecules. In the thymus, these cells displayed the CD44high CD24low memory phenotype without cognate antigenic challenge and, as a preformed memory population, rapidly responded to Listeria monocytogenes in the periphery in H2-M3 dependent manner. Due to these properties, they were called innate (born as memory cells) or unconventional (restricted by MHC Ib) T cells. Similar phenotypes were observed in IL-2 inducible T cell kinase (ITK)−/−, and cAMP responsive element binding protein binding protein (CBP)−/− mice, in which the majority of CD8 single positive thymocytes displayed the CD44high CD24low memory phenotype in the thymus (20,21,22). However, unlike innate CD8 T cells they were restricted by conventional MHC Ia molecules expressed on cortical epithelial cells and recognized peptide antigens. As ITK and CBP are involved in T cell receptor (TCR) signaling, it was initially thought that altered TCR signaling strength redirected the fate of conventional T cells to innate lineages (23). However, later this was found not to be CD8 T cell-intrinsic, but was solely mediated by IL-4 cytokine secreted from PLZFhigh CD4 T cells, which expanded in the absence of ITK, CBP or Krüppel-like factor 2 (KLF2), as illustrated in Fig. 1 (24,25,26,27). Unlike conventional memory CD8 T cells, IL-4 induced memory CD8 T cells expressed only Eomes, but not T-bet, and Weinreich et al. (24) first used the term ‘memory-like CD8 T cells’ to describe this novel population. Eomes alone were sufficient to make rapid IFN-γ production from memory-like CD8 T cells upon stimulation. They were also positive for other memory surface markers including CD44, CD122, and CXCR3 and later NKG2d and CCL5 were identified to be unique markers of these cells (28). In various genetic alterations, PLZFhigh CD4 T cells were mainly αβ iNKT or γδ T cells and, so far, 13 different genotypes have been identified in B6 mice with Eomes-expressing CD8 T cells expanded by this mechanism (Fig. 1) (27,29,30,31). Interestingly, compared to B6 mice, WT BALB/c mice had an approximately 10-fold higher number of PLZFhigh iNKT cells, and Eomes-expressing memory-like CD8 T cells (7). This was also true in fetal humans, in which PLZF-expressing innate CD4 T cells develop via thymocyte-thymocyte interactions generate Eomes-expressing memory CD8 T cells (32,33). These findings indicate it is an evolutionarily conserved mechanism in mice and humans that IL-4 secreted from expanded PLZFhigh CD4 T cells drives the development of Eomes-expressing memory-like CD8 T cells.
Figure 1.
CD8 SP thymocytes become memory-like CD8+ T cells by IL-4 produced from PLZFhigh T cells. ETPs from bone marrow migrate to the thymus and differentiate into CD8 or CD4 SP thymocytes in the medulla. In WT BALB/c mice, IL-4 from iNKT cells conditions SP thymocytes to become memory-like cells expressing Eomes. CD4 SP thymocytes (dotted line) are much less efficient to become Eomes-positive memory-like cells compared to CD8 SP thymocytes (solid line). These features are also seen in genetically altered B6 mice (listed on the right side), in which PLZF-positive iNKT or γδ T cells expand.
SP, single positive; ETP, early T cell progenitor; DP, double positive.
Subsequently, additional pathways generating Eomes-expressing CD8 T cells were revealed. In NFκB−/− and B-cell lymphoma/leukemia (Bcl11b) hypomorphic mice, they develop in a cell-intrinsic manner, and in NEDD4 family-interacting protein 1 deficient (Ndfip1−/−) mice, these cells were only present in the periphery, but not in the thymus (27). In BALB/c Klf13−/− mice, the development of IL-4-producing iNKT cells was suppressed and Eomes+ CD8 T cells were not observed (34). In B6 mice, genetic deletion of various genes, including ubiquitously transcribed tetratricopeptide repeat, X chromosome (Utx), Jumonji Domain Containing 3, Histone Lysine Demethylase (Jmjd3), Histone Deacetylase 3 (Hdac3), and Transcription Factor E2α (E2a)/Helix-Loop-Helix Transcription Factor 4 (Heb) resulted in complete loss or decreased development of iNKT cells (35,36,37), but their phenotype was not tested in a BALB/c background.
Physiologically, IL-4 receptor expression on CD8 T cells was critical for the anti-malaria resistance of BALB/c mice (38,39) and developmental exposure of CD8 T cells to IL-4 promoted Th1 responses during acute and chronic lymphocytic choriomeningitis virus (LCMV) infection (40,41). Therefore, memory-like CD8 T cells may be important for host defense against pathogenic invasions during the fetal and neonatal periods, when antigen-specific memory T cells have not yet developed (42).
DEVELOPMENT OF iNKT CELLS
The frequency of total iNKT cells was 3–5 times higher in BALB/c mice compared to B6 mice, and BALB/c iNKT cells produced more IL-4 per cell (7). In the conventional model of iNKT cell development, or linear maturation model, CD24highCD44lowNK1.1− (stage 0) cells differentiate into CD24lowCD44lowNK1.1− cells (stage 1), which then upregulate CD44 (stage 2), and finally acquire NK1.1 (stage 3; Fig. 2A) (10). IL-4 is produced in stage 1 and 2 immature cells, and mature stage 3 cells produce IFN-γ. According to this model, it is likely that BALB/c iNKT cells remain immature and produce more IL-4. However, it was not possible to directly compare the development of iNKT cells between B6 and BALB/c mice, due to the absence of NK1.1 expression in the BALB/c strain. Instead, a transcription factor-based approach could distinguish between the 3 distinctive subsets of iNKT cells, which use a combination of transcription factors, including PLZF, T-bet, GATA3, and RORγt (Fig. 2B) (7,43). These cells are designated as NKT1, NKT2, and NKT17 cells analogous to conventional CD4 T cells. IL17RB is an IL25 receptor that defines the IL-4 producing capacity of iNKT cells (44) and PLZFhigh iNKT cells could be divided into IL17RB-positive NKT2 cells and IL17RB-negative natural killer T progenitor (NKTp) cells. NKT2 cells were GATA3- and IL17RB-positive and produced IL-4 at steady state, PLZFintermediate NKT17 cells were RORγt-positive and secreted IL-17 upon stimulation, and PLZFlow NKT1 cells were T-bet positive and secreted IFN-γ. In KN2 mice, which express human CD2 (huCD2) on the surface of IL-4 secreting cells (45), GATA-3 expression was highest in huCD2 positive NKT2 cells (unpublished data), NKTp and NKT17 cells expressed lower levels of GATA-3, and NKT1 cells, while also GATA-3 positive, had the lowest levels (Fig. 2B) (7). At steady state, only NKT2 cells produced IL-4. However, when activated with αGalcer, GATA3 and PLZF expression was upregulated in NKT1 cells, which could produce IFN-γ and IL-4 simultaneously (7). The molecular mechanism of these complex transcription factor expression patterns is not clear, but it appears that PLZF modulates these processes, as iNKT cells fail to differentiate in its absence (5,6). Compared to B6 mice, BALB/c mice had higher numbers of NKT2 and NKT17 cells, but the total numbers of NKT1 cells were not different, indicating that the development of NKT2 and NKT17 cells, but not NKT1 cells, are differentially regulated in BALB/c background (7).
Figure 2.
Comparison between the linear maturation and lineage differentiation models of iNKT cell development. Invariant TCR-expressing thymocytes are positively selected by CD1d+ cortical thymocytes. In the linear maturation model, NKT cells that produce IL-4 or IL-17 are considered immature, while mature cells produce IFN-γ. In the lineage differentiation model, undifferentiated PLZFhigh NKTp give rise to terminally differentiated NKT1, NKT2, and NKT17 cells.
According to the linear maturation model, IL-4 is produced from immature stage 1 or 2 cells, which are dependent on GATA3. However, this does not explain the normal development of stage 3 or NKT1 cells in GATA3-deficient mice (46). Alternatively, defective development of NKT1 or stage 3 cells in Tbx21−/− mice could facilitate the expansion of NKT2 and NKT17, corresponding to stage 1 or 2 cells (7,47). This is also unlikely to be explained by the lineage maturation model, as we generally do not expect progenitors to proliferate when their differentiation is blocked. Therefore, the conventional model of iNKT cell development fails to explain the phenotypes of iNKT cells in Tbx21 and Gata3 knockout mice. In the lineage differentiation model, terminally differentiated NKT1, NKT2, and NKT17 cells are derived from NKTp cells, and GATA3 is not required for their development (Fig. 2) (7). Therefore, GATA3-deficient iNKT cells preferentially differentiate into NKT1 cells, whereas T-bet deficient iNKT cells become NKT2 or NKT17 cells. This new model predicts that IL-4 producing NKT2 cells are terminally differentiated and are not the precursors of NKT1 (stage 3) cells. This was proven in cell transfer assays using KN2 mice, in which huCD2−IL17RB−NKTp cells, but not huCD2+IL17RB+ NKT2 cells, gave rise to NKT1 cells (7) and NKT1 and NKT17 cells did not change their phenotypes (unpublished data). The reciprocal expansion of NKT2 and NKT1 cells in Tbx21−/− and Gata3−/− mice, respectively (7,46) raises the question of whether the fates of individual cells are determined by TCR specificity at the progenitor level, or determined stochastically. A recent publication showed that a monoclonal iNKT TCR transgenic mouse had all 3 effector subsets of iNKT cells, indicating that the tissue microenvironment, rather than TCR specificity, plays a major role in lineage differentiation (48).
Amongst 6 different mice strains, 3 displayed Eomes-expressing memory CD8 T cells in the thymus, and their frequencies were proportional to the level of IL-4 produced from PLZFhigh NKT2 cells (7). Therefore, it appears that NKT2 cells are the primary determinants of IL-4 levels in the mouse thymus.
LOCALIZATION OF iNKT CELLS
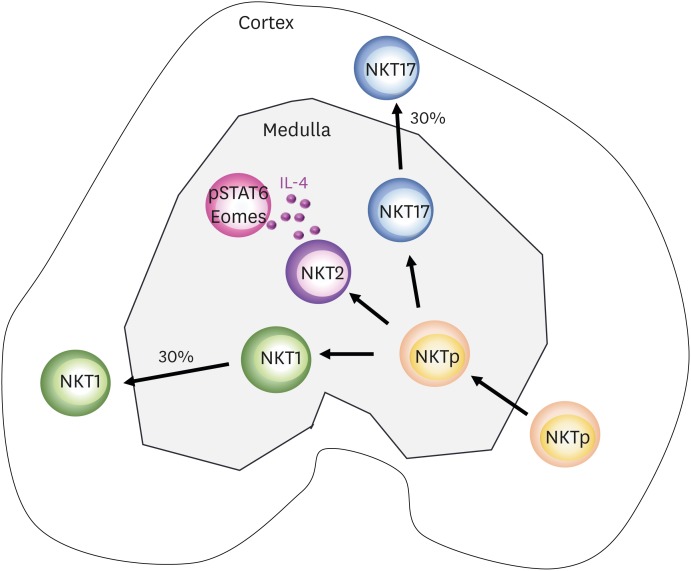
The CD1d tetramer is the gold standard biomarker for the detection of iNKT cells. However, the direct detection of iNKT cells using CD1d tetramers in fresh frozen or paraformaldehyde-fixed tissue sections is not technically possible, as their binding affinity is much lower than antibodies and they require an intact TCR tertiary structure. Although previous studies have used the CD1d tetramer for immunohistochemistry, they did not validate the sensitivity of detection, leading to the preferential analysis of cells expressing high levels of CD1d tetramer (49,50) as NKT1 cells display lower TCR expression than NKT2 and NKT17 cells. A new method was employed to detect iNKT cells using CD1d tetramer staining, in which fresh thymi were incubated in PE-conjugated CD1d tetramer solution, then the CD1d tetramer signal was amplified using anti-PE antibody in fixed tissue sections (8). NKT1 and NKT17 cells were further defined by T-bet and RORγt expression among CD1d tetramer-positive cells. The localization of all 3 subsets of iNKT cells was analyzed in lymphoid organs, and the results demonstrated that each subset has distinct anatomic localization. In the thymus, 70% of NKT1 and NKT17 cells resided in the medulla, and 30% in the cortex. Ninety percent of IL-4 producing NKT2 cells were in the medullary area, and half of NKTp cells were estimated to be in the cortex (Fig. 3). Younger mice did not have a higher frequency of cortical iNKT cells, suggesting that cortical iNKT cells migrated from the medulla rather than being developed in the cortex. Overall, these findings suggest that NKTp cells migrate from the cortex to the medulla and differentiate into NKT1, NKT2, and NKT17 cells, and 30% of NKT1 and NKT17 cells migrate back to the cortex (Fig. 3). It is not clear why and how NKT1 and NKT17 cells, but not NKT2 cells, migrate back to the cortex. It is unlikely that they move by passive overflow, as 99% of FOXP3-expressing regulatory T cells are found in the medullary area (8). Instead, NKT2 cells seem to be short lived, because NKT1 and NKT17 cells are long-term components of the thymic populations of aged mice, while NKT2 cells gradually decrease, along with Eomes-expressing CD8 T cells (7). In the spleen, NKT1 cells are located in the red pulp and NKT2 cells are located in the T cell zone of the white pulp. In mesenteric lymph nodes, NKT2 cells are dominant and located in the T cell zone, while NKT1 cells are evenly distributed throughout the T and B cell areas. In other peripheral lymph nodes, NKT17 cells are enriched in the subcapsular area; consistent with a previous report that IL17-producing T cells reside there (51). The liver contains mostly NKT1 cells, with few NKT2 and NKT17 cells. These patterns determine the cytokine responses of iNKT cells upon administration of αGalcer, a potent stimulating ligand of iNKT cells. When αGalcer was intravenously injected, intravascular NKT1 cells in the splenic red pulp and liver were activated and produced IFN-γ and IL-4 simultaneously, which diffused systemically and affected other immune cells (8). Upon oral administration, αGalcer was delivered to the mesenteric lymph nodes, where it activated local resident NKT2 cells to produce IL-4 but not IFN-γ. In this case, IL-4 only activated neighboring lymphocytes, without systemic effects (8). Therefore, the tissue-specific distribution of iNKT subsets determines their cytokine responses, which might have potential applications in immune modification by localized conditioning of the cytokine milieu (8).
Figure 3.
Schematic representation of iNKT cell development and localization in the thymus. Upon positive selection, NKTp cells move to the medullary area and undergo differentiation into various subsets. NKT2 cells produce IL-4 at steady state, which can condition medullary thymocytes to phosphorylate STAT6 and express Eomes. A portion of NKT1 and NKT17 cells migrate back to the cortical area.
STAT6, signal transducer and activator of transcription 6.
GENETIC SIGNATURE OF iNKT CELLS
Affymetrix gene chip analysis of iNKT cells revealed that the transcriptional profile of iNKT cells is shared with NK and activated memory CD8 T cells, and partially overlaps with that of γδ T cells (52). However, this analysis used total splenic iNKT cells from B6 mice, in which NKT1 cells predominate. To analyze the genetic signatures of NKT2 and NKT17 cells, iNKT subsets were isolated from BALB/c Tbx21GFP/KN2 dual reporter mice (9). Among CD1d tetramer-positive cells, NKT1 cells were sorted as Tbx21bright cells, and NKT17 cells were T-bet, CD4, and CD27 triple-negative cells. NKT2 cells were T-bet−huCD2+IL17RB+CD4+CD27+ cells, and NKTp cells were defined as T-bet−huCD2−IL17RB−CD4+CD27+. As a result, NKTp, NKT1, NKT2, and NKT17 cells all have distinct transcriptional natures, and clustering analysis revealed that NKT2 cells were transcriptionally more similar to NKT1 and NKT17 cells than NKTp cells, indicating that NKT2 and NKTp cells are distinct populations among PLZFhigh NKT cells. Pathway analysis showed that NKTp cells were enriched in genes involved in Myc signaling, and indeed, previous analysis of the iNKT phenotype in Myc-deficient mice showed that the development of iNKT cells was immediately arrested after stage 0 (53,54). Single cell-based RNA sequencing analysis further revealed that the functional iNKT cell subsets contain highly divergent subpopulations at the transcriptional and epigenomic levels (55).
Upon analysis of iNKT cells, the signature genes of each iNKT subset were compared with those of ILCs, γδ T cells, and conventional CD4 Th cells using public datasets (56,57,58). ILCs are composed of ILC1, ILC2, and ILC3 cells, which produce IFN-γ, IL-4, and IL-17, respectively, and previous analysis showed that ILC1 cells are indistinguishable from conventional NK cells at the transcriptional level (56). Interestingly, ILCs develop from cells that have expressed PLZF during their development (59), suggesting their potential transcriptional similarity with other PLZF-expressing T cells, including iNKT cells. Three molecularly distinct thymic γδ T cells were identified based on their TCR usage: V1 (Vγ1.1+Vδ6.3−), V6 (Vγ1.1+Vδ6.3+), and V2 (Vγ2+) cells, which produce IFN-γ, IL-4, and IL-17 respectively (58). V5 (Vγ5+) γδ T cells are rare in the thymus but enriched in the intestinal mucosa, and produce IFN-γ. A total of 14 different subsets, encompassing iNKT cells, ILCs, γδ T cells, and Th CD4 T cells, were analyzed at the transcriptional level. However, the 4 datasets used different platforms to generate their raw data, and it was not possible to directly compare the genetic similarities among them. Instead, genes that were uniquely enriched in one of subsets in each cell type were compared by gene set enrichment analysis to obtain enrichment scores. It was found that the genetic signatures of iNKT subsets were most similar to those of γδ T cells, followed by ILCs. Th subsets had the least similarity, indicating that ILCs and innate T cells share similar genetics, which are distinguishable from conventional Th cells (9).
STRAIN SPECIFICITY
In the thymus, iNKT cells develop and differentiate into 3 distinct lineages from common progenitors. In BALB/c mice, NKT2 and NKT17 cells predominate, whereas NKT1 cells predominate in B6 mice. To address whether these differences are cell-intrinsic or -extrinsic, B6 or BALB/c fetal liver stem cells were transplanted into the fetal liver of the other strain (60). Donor cells established a stable hematopoietic mosaicism without inducing rejection or graft versus host disease. In this experiment, NKT1 cells predominated among B6-derived donor cells in the majority of BALB/c thymocytes, and NKT2 and NKT17 cells predominated among BALB/c derived donor cells in the majority of B6 thymocytes. These results demonstrate that the developmental differences in iNKT cells between B6 and BALB/c mice are cell-intrinsic rather than induced by external signals.
The frequencies of iNKT cells, especially NKT2 and NKT17 cells, were lower in adult bone marrow chimeras compared to WT mice (unpublished data), suggesting that, like γδ T cells, iNKT cells require the fetal thymic microenvironment for their development (61). Lin28b suppresses Let7 family microRNAs, and is highly expressed in fetal hematopoietic stem cells in mice and humans (62). Plzf contains regulatory motifs to bind Let7 (63) and Lin28b overexpression in retrogenic bone marrow chimeras promoted the development of NKT2 and V6 (Vγ1.1+Vδ6.3+) γδ T cells, which expressed a high level of PLZF and produced IL-4 (62). As Lin28b expression rapidly decreases in postnatal hematopoietic stem cells, it is likely that upregulation of Let7 family microRNAs, which are unrepressed by Lin28b after birth, favors the development of conventional lineage T cells. Consistent with this, we have observed that Lin28b expression is higher in the neonatal thymi of BALB/c mice compared to B6 mice (unpublished data), suggesting that Lin28b is a regulatory element driving NKT2 cell development in mice.
FUTURE PERSPECTIVES
In the periphery, iNKT cells differentiate into follicular helper NKT cells (NKTFH) and NKT10 cells, which secrete IL-21 and IL-10 respectively after αGalcer injection (64,65,66). At steady state, NKTFH cells were not present, and upon immunization with 4-hydroxy-3-nitrophenylacetyl (NP)/αGalcer, NKTFH cells provided cognate help to B cells in a BCL6-dependent manner. However, the effect was limited to IgM, and NKTFH cells did not enhance affinity maturation and long-lived plasma cell differentiation (64). NKT10 cells do not express PLZF and normally reside in fat tissue. Upon activation, NKT10 cells secrete IL-10, which can modulate regulatory T cells and M2 type macrophages, suggesting a potential role in controlling fat tissue metabolism and obesity (65). In disease models, NKT10 cells promote B16 melanoma growth and suppressed allergic encephalitis, indicating they are an immunosuppressive subset (66). In summary, there are 5 different subtypes of iNKT cells including NKT1, NKT2, NKT17, NKTFH, and NKT10 cells in the thymus and periphery, analogous to conventional CD4 helper T cells. As iNKT cells are tissue resident and do not circulate (49), tissue-specific modulation of iNKT activation by localized delivery of αGalcer could be a potential modality to use iNKT cells for immunotherapeutic purposes.
ACKNOWLEDGEMENTS
This work was supported by grants from the Research Center Program of the Academy of Immunology and Microbiology, Institute for Basic Science (IBS-R005-D1).
Abbreviations
- CBP
cAMP responsive element binding protein binding protein
- CCL
chemokine (C-C motif) ligand
- GATA3
GATA binding protein-3
- IFN-γ
interferon-γ
- ILC
innate lymphoid cell
- iNKT
invariant natural killer T
- ITK
IL-2 inducible T cell kinase
- LCMV
lymphocytic choriomeningitis virus
- MHC
major histocompatibility complex
- NK
natural killer
- NKTFH
follicular helper NKT cells
- NKTp
natural killer T progenitor
- PLZF
promyelocytic leukemia zinc finger
- RORγt
retinoic acid receptor-related orphan nuclear receptor gamma
- T-bet
T-box 21
- TCR
T cell receptor
- Th
T helper
- WT
wild type
- αGalcer
α-galactosylceramide
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
Author Contributions: Writing - original draft: Kwon DI; Writing - review & editing: Lee YJ.
References
- 1.Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB. The burgeoning family of unconventional T cells. Nat Immunol. 2015;16:1114–1123. doi: 10.1038/ni.3298. [DOI] [PubMed] [Google Scholar]
- 2.Bedoui S, Gebhardt T, Gasteiger G, Kastenmüller W. Parallels and differences between innate and adaptive lymphocytes. Nat Immunol. 2016;17:490–494. doi: 10.1038/ni.3432. [DOI] [PubMed] [Google Scholar]
- 3.Egawa T, Eberl G, Taniuchi I, Benlagha K, Geissmann F, Hennighausen L, Bendelac A, Littman DR. Genetic evidence supporting selection of the Valpha14i NKT cell lineage from double-positive thymocyte precursors. Immunity. 2005;22:705–716. doi: 10.1016/j.immuni.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Wei DG, Lee H, Park SH, Beaudoin L, Teyton L, Lehuen A, Bendelac A. Expansion and long-range differentiation of the NKT cell lineage in mice expressing CD1d exclusively on cortical thymocytes. J Exp Med. 2005;202:239–248. doi: 10.1084/jem.20050413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, Lantz O, Bendelac A. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kovalovsky D, Uche OU, Eladad S, Hobbs RM, Yi W, Alonzo E, Chua K, Eidson M, Kim HJ, Im JS, et al. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat Immunol. 2008;9:1055–1064. doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee YJ, Holzapfel KL, Zhu J, Jameson SC, Hogquist KA. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat Immunol. 2013;14:1146–1154. doi: 10.1038/ni.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee YJ, Wang H, Starrett GJ, Phuong V, Jameson SC, Hogquist KA. Tissue-specific distribution of iNKT cells impacts their cytokine response. Immunity. 2015;43:566–578. doi: 10.1016/j.immuni.2015.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee YJ, Starrett GJ, Lee ST, Yang R, Henzler CM, Jameson SC, Hogquist KA. Lineage-specific effector signatures of invariant NKT cells are shared amongst γδ T, innate lymphoid, and th cells. J Immunol. 2016;197:1460–1470. doi: 10.4049/jimmunol.1600643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 11.Sharif S, Arreaza GA, Zucker P, Mi QS, Sondhi J, Naidenko OV, Kronenberg M, Koezuka Y, Delovitch TL, Gombert JM, et al. Activation of natural killer T cells by alpha-galactosylceramide treatment prevents the onset and recurrence of autoimmune Type 1 diabetes. Nat Med. 2001;7:1057–1062. doi: 10.1038/nm0901-1057. [DOI] [PubMed] [Google Scholar]
- 12.Singh AK, Wilson MT, Hong S, Olivares-Villagómez D, Du C, Stanic AK, Joyce S, Sriram S, Koezuka Y, Van Kaer L. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1801–1811. doi: 10.1084/jem.194.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jahng AW, Maricic I, Pedersen B, Burdin N, Naidenko O, Kronenberg M, Koezuka Y, Kumar V. Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1789–1799. doi: 10.1084/jem.194.12.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nieuwenhuis EE, Matsumoto T, Exley M, Schleipman RA, Glickman J, Bailey DT, Corazza N, Colgan SP, Onderdonk AB, Blumberg RS. CD1d-dependent macrophage-mediated clearance of Pseudomonas aeruginosa from lung. Nat Med. 2002;8:588–593. doi: 10.1038/nm0602-588. [DOI] [PubMed] [Google Scholar]
- 15.Morshed SR, Mannoor K, Halder RC, Kawamura H, Bannai M, Sekikawa H, Watanabe H, Abo T. Tissue-specific expansion of NKT and CD5+B cells at the onset of autoimmune disease in (NZBxNZW)F1 mice. Eur J Immunol. 2002;32:2551–2561. doi: 10.1002/1521-4141(200209)32:9<2551::AID-IMMU2551>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 16.Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9:582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 17.Heller F, Fuss IJ, Nieuwenhuis EE, Blumberg RS, Strober W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity. 2002;17:629–638. doi: 10.1016/s1074-7613(02)00453-3. [DOI] [PubMed] [Google Scholar]
- 18.Kim HY, Kim HJ, Min HS, Kim S, Park WS, Park SH, Chung DH. NKT cells promote antibody-induced joint inflammation by suppressing transforming growth factor beta1 production. J Exp Med. 2005;201:41–47. doi: 10.1084/jem.20041400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urdahl KB, Sun JC, Bevan MJ. Positive selection of MHC class Ib-restricted CD8(+) T cells on hematopoietic cells. Nat Immunol. 2002;3:772–779. doi: 10.1038/ni814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broussard C, Fleischacker C, Horai R, Chetana M, Venegas AM, Sharp LL, Hedrick SM, Fowlkes BJ, Schwartzberg PL. Altered development of CD8+ T cell lineages in mice deficient for the Tec kinases Itk and Rlk. Immunity. 2006;25:93–104. doi: 10.1016/j.immuni.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Atherly LO, Lucas JA, Felices M, Yin CC, Reiner SL, Berg LJ. The Tec family tyrosine kinases Itk and Rlk regulate the development of conventional CD8+ T cells. Immunity. 2006;25:79–91. doi: 10.1016/j.immuni.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Fukuyama T, Kasper LH, Boussouar F, Jeevan T, van Deursen J, Brindle PK. Histone acetyltransferase CBP is vital to demarcate conventional and innate CD8+ T-cell development. Mol Cell Biol. 2009;29:3894–3904. doi: 10.1128/MCB.01598-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berg LJ. Signalling through TEC kinases regulates conventional versus innate CD8(+) T-cell development. Nat Rev Immunol. 2007;7:479–485. doi: 10.1038/nri2091. [DOI] [PubMed] [Google Scholar]
- 24.Weinreich MA, Odumade OA, Jameson SC, Hogquist KA. T cells expressing the transcription factor PLZF regulate the development of memory-like CD8+ T cells. Nat Immunol. 2010;11:709–716. doi: 10.1038/ni.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinreich MA, Takada K, Skon C, Reiner SL, Jameson SC, Hogquist KA. KLF2 transcription-factor deficiency in T cells results in unrestrained cytokine production and upregulation of bystander chemokine receptors. Immunity. 2009;31:122–130. doi: 10.1016/j.immuni.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsagaratou A, González-Avalos E, Rautio S, Scott-Browne JP, Togher S, Pastor WA, Rothenberg EV, Chavez L, Lähdesmäki H, Rao A. TET proteins regulate the lineage specification and TCR-mediated expansion of iNKT cells. Nat Immunol. 2017;18:45–53. doi: 10.1038/ni.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jameson SC, Lee YJ, Hogquist KA. Innate memory T cells. Adv Immunol. 2015;126:173–213. doi: 10.1016/bs.ai.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ventre E, Brinza L, Schicklin S, Mafille J, Coupet CA, Marçais A, Djebali S, Jubin V, Walzer T, Marvel J. Negative regulation of NKG2D expression by IL-4 in memory CD8 T cells. J Immunol. 2012;189:3480–3489. doi: 10.4049/jimmunol.1102954. [DOI] [PubMed] [Google Scholar]
- 29.Lee YJ, Jameson SC, Hogquist KA. Alternative memory in the CD8 T cell lineage. Trends Immunol. 2011;32:50–56. doi: 10.1016/j.it.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vasanthakumar A, Xu D, Lun AT, Kueh AJ, van Gisbergen KP, Iannarella N, Li X, Yu L, Wang D, Williams BR, et al. A non-canonical function of Ezh2 preserves immune homeostasis. EMBO Rep. 2017;18:619–631. doi: 10.15252/embr.201643237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dobenecker MW, Kim JK, Marcello J, Fang TC, Prinjha R, Bosselut R, Tarakhovsky A. Coupling of T cell receptor specificity to natural killer T cell development by bivalent histone H3 methylation. J Exp Med. 2015;212:297–306. doi: 10.1084/jem.20141499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee YJ, Jeon YK, Kang BH, Chung DH, Park CG, Shin HY, Jung KC, Park SH. Generation of PLZF+ CD4+ T cells via MHC class II-dependent thymocyte-thymocyte interaction is a physiological process in humans. J Exp Med. 2010;207:237–246. doi: 10.1084/jem.20091519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Min HS, Lee YJ, Jeon YK, Kim EJ, Kang BH, Jung KC, Chang CH, Park SH. MHC class II-restricted interaction between thymocytes plays an essential role in the production of innate CD8+ T cells. J Immunol. 2011;186:5749–5757. doi: 10.4049/jimmunol.1002825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lai D, Zhu J, Wang T, Hu-Li J, Terabe M, Berzofsky JA, Clayberger C, Krensky AM. KLF13 sustains thymic memory-like CD8(+) T cells in BALB/c mice by regulating IL-4-generating invariant natural killer T cells. J Exp Med. 2011;208:1093–1103. doi: 10.1084/jem.20101527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D'Cruz LM, Knell J, Fujimoto JK, Goldrath AW. An essential role for the transcription factor HEB in thymocyte survival, Tcra rearrangement and the development of natural killer T cells. Nat Immunol. 2010;11:240–249. doi: 10.1038/ni.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thapa P, Romero Arocha S, Chung JY, Sant'Angelo DB, Shapiro VS. Histone deacetylase 3 is required for iNKT cell development. Sci Rep. 2017;7:5784. doi: 10.1038/s41598-017-06102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beyaz S, Kim JH, Pinello L, Xifaras ME, Hu Y, Huang J, Kerenyi MA, Das PP, Barnitz RA, Herault A, et al. The histone demethylase UTX regulates the lineage-specific epigenetic program of invariant natural killer T cells. Nat Immunol. 2017;18:184–195. doi: 10.1038/ni.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morrot A, Hafalla JC, Cockburn IA, Carvalho LH, Zavala F. IL-4 receptor expression on CD8+ T cells is required for the development of protective memory responses against liver stages of malaria parasites. J Exp Med. 2005;202:551–560. doi: 10.1084/jem.20042463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carvalho LH, Sano G, Hafalla JC, Morrot A, Curotto de Lafaille MA, Zavala F. IL-4-secreting CD4+ T cells are crucial to the development of CD8+ T-cell responses against malaria liver stages. Nat Med. 2002;8:166–170. doi: 10.1038/nm0202-166. [DOI] [PubMed] [Google Scholar]
- 40.Renkema KR, Lee JY, Lee YJ, Hamilton SE, Hogquist KA, Jameson SC. IL-4 sensitivity shapes the peripheral CD8+ T cell pool and response to infection. J Exp Med. 2016;213:1319–1329. doi: 10.1084/jem.20151359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee A, Park SP, Park CH, Kang BH, Park SH, Ha SJ, Jung KC. IL-4 induced innate CD8+ T cells control persistent viral infection. PLoS Pathog. 2015;11:e1005193. doi: 10.1371/journal.ppat.1005193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White JT, Cross EW, Kedl RM. Antigen-inexperienced memory CD8(+) T cells: where they come from and why we need them. Nat Rev Immunol. 2017;17:391–400. doi: 10.1038/nri.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Constantinides MG, Bendelac A. Transcriptional regulation of the NKT cell lineage. Curr Opin Immunol. 2013;25:161–167. doi: 10.1016/j.coi.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watarai H, Sekine-Kondo E, Shigeura T, Motomura Y, Yasuda T, Satoh R, Yoshida H, Kubo M, Kawamoto H, Koseki H, et al. Development and function of invariant natural killer T cells producing T(h)2- and T(h)17-cytokines. PLoS Biol. 2012;10:e1001255. doi: 10.1371/journal.pbio.1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohrs K, Wakil AE, Killeen N, Locksley RM, Mohrs M. A two-step process for cytokine production revealed by IL-4 dual-reporter mice. Immunity. 2005;23:419–429. doi: 10.1016/j.immuni.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim PJ, Pai SY, Brigl M, Besra GS, Gumperz J, Ho IC. GATA-3 regulates the development and function of invariant NKT cells. J Immunol. 2006;177:6650–6659. doi: 10.4049/jimmunol.177.10.6650. [DOI] [PubMed] [Google Scholar]
- 47.Townsend MJ, Weinmann AS, Matsuda JL, Salomon R, Farnham PJ, Biron CA, Gapin L, Glimcher LH. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 2004;20:477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 48.Clancy-Thompson E, Chen GZ, Tyler PM, Servos MM, Barisa M, Brennan PJ, Ploegh HL, Dougan SK. Monoclonal invariant NKT (iNKT) cell mice reveal a role for both tissue of origin and the TCR in development of iNKT functional subsets. J Immunol. 2017;199:159–171. doi: 10.4049/jimmunol.1700214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas SY, Scanlon ST, Griewank KG, Constantinides MG, Savage AK, Barr KA, Meng F, Luster AD, Bendelac A. PLZF induces an intravascular surveillance program mediated by long-lived LFA-1-ICAM-1 interactions. J Exp Med. 2011;208:1179–1188. doi: 10.1084/jem.20102630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.King IL, Amiel E, Tighe M, Mohrs K, Veerapen N, Besra G, Mohrs M, Leadbetter EA. The mechanism of splenic invariant NKT cell activation dictates localization in vivo. J Immunol. 2013;191:572–582. doi: 10.4049/jimmunol.1300299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gray EE, Friend S, Suzuki K, Phan TG, Cyster JG. Subcapsular sinus macrophage fragmentation and CD169+ bleb acquisition by closely associated IL-17-committed innate-like lymphocytes. PLoS One. 2012;7:e38258. doi: 10.1371/journal.pone.0038258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohen NR, Brennan PJ, Shay T, Watts GF, Brigl M, Kang J, Brenner MB. ImmGen Project Consortium. Shared and distinct transcriptional programs underlie the hybrid nature of iNKT cells. Nat Immunol. 2013;14:90–99. doi: 10.1038/ni.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mycko MP, Ferrero I, Wilson A, Jiang W, Bianchi T, Trumpp A, MacDonald HR. Selective requirement for c-Myc at an early stage of V(alpha)14i NKT cell development. J Immunol. 2009;182:4641–4648. doi: 10.4049/jimmunol.0803394. [DOI] [PubMed] [Google Scholar]
- 54.Dose M, Sleckman BP, Han J, Bredemeyer AL, Bendelac A, Gounari F. Intrathymic proliferation wave essential for Valpha14+ natural killer T cell development depends on c-Myc. Proc Natl Acad Sci USA. 2009;106:8641–8646. doi: 10.1073/pnas.0812255106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Engel I, Seumois G, Chavez L, Samaniego-Castruita D, White B, Chawla A, Mock D, Vijayanand P, Kronenberg M. Innate-like functions of natural killer T cell subsets result from highly divergent gene programs. Nat Immunol. 2016;17:728–739. doi: 10.1038/ni.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robinette ML, Fuchs A, Cortez VS, Lee JS, Wang Y, Durum SK, Gilfillan S, Colonna M Immunological Genome Consortium. Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat Immunol. 2015;16:306–317. doi: 10.1038/ni.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stubbington MJ, Mahata B, Svensson V, Deonarine A, Nissen JK, Betz AG, Teichmann SA. An atlas of mouse CD4(+) T cell transcriptomes. Biol Direct. 2015;10:14. doi: 10.1186/s13062-015-0045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Narayan K, Sylvia KE, Malhotra N, Yin CC, Martens G, Vallerskog T, Kornfeld H, Xiong N, Cohen NR, Brenner MB, et al. Intrathymic programming of effector fates in three molecularly distinct γδ T cell subtypes. Nat Immunol. 2012;13:511–518. doi: 10.1038/ni.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Constantinides MG, McDonald BD, Verhoef PA, Bendelac A. A committed precursor to innate lymphoid cells. Nature. 2014;508:397–401. doi: 10.1038/nature13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strong BS, Newkold TJ, Lee AE, Turner LE, Alhajjat AM, Heusel JW, Shaaban AF. Extrinsic allospecific signals of hematopoietic origin dictate iNKT cell lineage-fate decisions during development. Sci Rep. 2016;6:28837. doi: 10.1038/srep28837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haas JD, Ravens S, Düber S, Sandrock I, Oberdörfer L, Kashani E, Chennupati V, Föhse L, Naumann R, Weiss S, et al. Development of interleukin-17-producing γδ T cells is restricted to a functional embryonic wave. Immunity. 2012;37:48–59. doi: 10.1016/j.immuni.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 62.Yuan J, Nguyen CK, Liu X, Kanellopoulou C, Muljo SA. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science. 2012;335:1195–1200. doi: 10.1126/science.1216557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pobezinsky LA, Etzensperger R, Jeurling S, Alag A, Kadakia T, McCaughtry TM, Kimura MY, Sharrow SO, Guinter TI, Feigenbaum L, et al. Let-7 microRNAs target the lineage-specific transcription factor PLZF to regulate terminal NKT cell differentiation and effector function. Nat Immunol. 2015;16:517–524. doi: 10.1038/ni.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chang PP, Barral P, Fitch J, Pratama A, Ma CS, Kallies A, Hogan JJ, Cerundolo V, Tangye SG, Bittman R, et al. Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat Immunol. 2011;13:35–43. doi: 10.1038/ni.2166. [DOI] [PubMed] [Google Scholar]
- 65.Lynch L, Michelet X, Zhang S, Brennan PJ, Moseman A, Lester C, Besra G, Vomhof-Dekrey EE, Tighe M, Koay HF, et al. Regulatory iNKT cells lack expression of the transcription factor PLZF and control the homeostasis of T(reg) cells and macrophages in adipose tissue. Nat Immunol. 2015;16:85–95. doi: 10.1038/ni.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sag D, Krause P, Hedrick CC, Kronenberg M, Wingender G. IL-10-producing NKT10 cells are a distinct regulatory invariant NKT cell subset. J Clin Invest. 2014;124:3725–3740. doi: 10.1172/JCI72308. [DOI] [PMC free article] [PubMed] [Google Scholar]