Abstract
AIM
To evaluate the importance of the CD34+CD38- cell population when compared to the CD34+CD38+/low and CD34+CD38+/high leukemic cell sub-populations and to determine its correlations with leukemia characteristics and known prognostic factors, as well as with response to therapy and survival.
METHODS
Two hundred bone marrow samples were obtained at diagnosis from 200 consecutive patients with newly diagnosed acute myeloid leukemia (AML) were studied between September 2008 and December 2010 at our Institution (Hematology Department, Lyon, France). The CD34/CD38 cell profile was analyzed by multiparameter flowcytometry approach using 8C panels and FACS CANTO and Diva software (BD Bioscience).
RESULTS
We analyzed CD34 and CD38 expression in bone marrow samples of 200 AML patients at diagnosis, and investigated the prognostic value of the most immature CD34+CD38- population. Using a cut-off value of 1% of CD34+CD38- from total “bulk leukemic cells” we found that a high (> 1%) level of CD34+CD38- blasts at diagnosis was correlated with advanced age, adverse cytogenetics as well as with a lower rate of complete response after induction and shorter disease-free survival. In a multivariate analysis considering age, leukocytosis, the % of CD34+ blasts cells and the standardized cytogenetic and molecular risk subgroups, a percentage of CD34+CD38- leukemic cells > 1% was an independent predictor of DFS [HR = 2.8 (1.02-7.73), P = 0.04] and OS [HR = 2.65 (1.09-6.43), P = 0.03].
CONCLUSION
Taken together, these results show that a CD34/CD38 “backbone” for leukemic cell analysis by multicolour flowcytometry at diagnosis provides useful prognostic information.
Keywords: CD34+CD38-/low, Immunophenotyping, Leukemic stem cells, Acute myeloid leukemia, Prognosis
Core tip: We analyzed the bone marrow samples of 200 acute myeloid leukemia (AML) patients at diagnosis by multicolour flow cytometry and investigated the prognostic value of the most immature CD34+CD38- population. We showed that a higher then > % level of CD34+CD38- blasts at diagnosis was an independent predictor of disease free survival (DFS) and overall survival in a multivariate analysis considering age, leukocytosis, the % of CD34+ blasts cells, cytogenetic and molecular risk subgroups. Despite heterogeneity and complexity of AML leukemia stem cells, we could still use CD34+CD38- quantification at diagnosis as useful complementary prognostic parameter for risk-stratification AML patients in future clinical trials.
INTRODUCTION
It has been reported that leukemic cells able to reproduce human acute myeloid leukemia (AML) in NOD/SCID mice are found exclusively in the CD34+CD38- cell compartment[1,2]. Leukemia initiating cells (LICs) or Leukemia stem cells (LSC) in mice have shown a primitive immunophenotype (CD34+CD38-) with similarities to normal hematopoietic stem cells (HSCs) regardless of the subtype of AML or the immunophenotype of the majority of the leukemic blasts present in the bone marrow[3-5]. However, other studies showed that LSC were exclusively found in the CD34- compartment. Other investigators described LSC in both the CD34- and CD34+ cell compartments[6-8].
The importance of the CD34/CD38 subpopulations in the outcome of patients with AML remains controversial. A high frequency of CD34+CD38- immature cell population at the time of diagnosis has been correlated with a higher percentage of chemotherapy-resistant cells and minimal residual disease (MRD)[9,10].
Previously, it was showed that cancer initiation is fundamentally a dynamic, Darwinian process of mutational diversification and clonal selection[11]. Most recently, Greaves propose a “back to Darwin” model for leukaemia initiation and development where cells with variable self-renewal potential or “stem cells” are considered as the units of evolutionary diversification and selection[11]. Independent of frequency of cancer stem cells are in any cancer, if they are the critical cells for therapeutic targeting or control, the dilemma rising and question if their inherent genetic variability makes them a “moving” and therefore “elusive” target considered as the major impediment to successful therapy for advanced or relapsed leukaemia. Therefore, testing of multiple new agents on the backbones of conventional therapies will present serious challenges in the design of futures clinical trials. There is emerging evidence that personalized therapy will ultimately result, adapted for each patient having a unique combination of molecular features characterizing his leukaemia.
Most recently, Goardon et al[12] investigated 74 primary human AML patient samples. They showed that the CD34+ cells in about 80% of these cases contained two predominant populations: One CD38-CD90-CD45RA+ (lymphoid-primed multipotential progenitor LMPP-like cells) and the other CD38+CD110+CD45RA+ (representing granulocyte-monocyte progenitor GMP-like cells). Moreover, these populations showed a hierarchical organization: the CD38-CD45RA+ cells gave raise the GMP-like cells but not vice versa. These results enlarged on the previous view of AML LSC and establish a hierarchy of leukemia populations with decreasing frequency of LSC, implicate normal hematopoietic progenitors, as LMPP and/or GMP as the cell of origin for AML/LSC in the most of cases. One of the most relevant implications of a progenitor phenotype for AML LSC state to the critical stem cell property of self-renewal, the acquisition of self-renewal ability in AML LSC being an aberrant event resulting important genetic and/or epigenetic changes. Taken together, the results of our study emphasis that using CD34/CD38 “backbone” in leukaemia cells analysis by multicolour flow cytometry at AML samples at diagnosis is relatively facile method, rapidly translate in clinical practice as complementary prognostic factor in AML. Multicolour/multidimensional flow cytometry represent very useful tools to identify and characterise immunologic profile of different leukaemia compartments using CD34/CD38/CD45 as “backbone” to design more complex panels (8-10-14 colours) adapted to AML diagnosis and MRD flow evaluation.
The goal of our study was to evaluate the importance of the CD34+CD38- cell population when compared to the CD34+CD38+/low and CD34+CD38+/high leukemic cell sub-populations and to determine its correlations with leukemia characteristics and known prognostic factors, as well as with response to therapy and survival.
MATERIALS AND METHODS
Patients
Two hundred bone marrow samples were obtained at diagnosis from 200 consecutive patients with newly diagnosed AML were studied between September 2008 and December 2010 at our Institution (Hematology Department, Lyon, France). All clinical trials have been considered reviewed and approved by a suitable ethic committee and were completed in accordance with the Helsinki declaration of 1975. All patients signed informed consent according to French legislation.
Multicolor flow cytometry
Briefly, EDTA-anticoagulated fresh bone marrow samples were processed using the whole-blood lysis technique for immunophenotypic analysis. The CD34/CD38 cell profile was analyzed in one single tube containing the following MoAbs: CD7 FITC (clone 8H8.1, Becton Dickinson), CD13 PE (clone L138, Becton Dickinson), CD33 PerCPcy5.5 (clone P67.6, Becton Dickinson), and CD34 APC (clone 8G12, Becton Dickinson), CD38 PEcy7 (cloneHB7, Becton Dickinson), CD45 APCH7 (clone2D1 Becton Dickinson), CD19 Pacific Blue (clone SJ25-C1, Invitrogen). Data analyses were made using FACS Diva software (BD Bioscience). Instrument setup was regularly optimized by analysing Calibrite beads-Rainbows 8 picks beads and CST beads system for checking cytometer stability. The required minimal events of CD34+ was set at 20 and the total number events range between 100000-500000. Isotype IgG staining was used as a negative control for ratio rMFI evaluation. Strategy of gating was based on two distinct analyses: CD45low/SSC total blasts and CD34+/CD45low gated cells from total FSC/SSC viable cells. Within CD34+ compartment we divided three subpopulations: CD34+CD38-, CD34+CD38lo, and CD34+CD38hi, based on intensity of CD38 expression; FMO (Fluorescence Minus One) was used for CD38- level, and hematogones populations for CD38hi level. The stem cell compartment CD34+CD38- contain very few events in some patients but these events should tightly cluster in a FSC/SSC plot and CD45/SSC plot. We also measured the intensity of fluorescence signal for CD38 quantified as rMFICD38 from CD34+ gated cells and rMFICD38 from CD45lo/SSC total blasts cells (Supplemental Figure 1C). We evaluated a comparative analysis between %CD34+CD38- cells and rMFI CD38 intensity in 30 normal bone marrow samples (NBM) from 10 volunteers donors (nBM) and in 20 regenerative bone marrow samples (rBM) from different hematologic diseases obtained after chemotherapeutic treatment and considered in molecular remission status. We observed a strong linear correlation between % CD34+CD38- and rMFI CD38 in all samples, with comparable median in nBM and Rbm (Figure 1B) (P-value < 0.0001).
Figure 1.
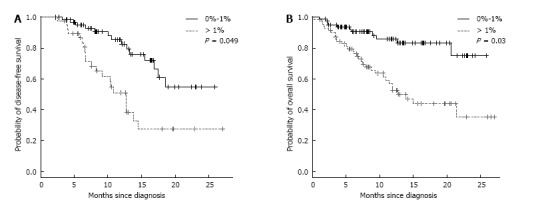
Survival of acute myeloid leukemia patients (without palliative) according to 1% CD34+CD38- cut-off. A: Disease free survival; B: Overall survival.
Cytogenetics risk classification and molecular characteristics
Karyotypes were classified into three categories (favorable, intermediate, and unfavorable) according to the Medical Research Council (MRC) classification[13]. NPM1, CEBPA, FLT3 mutations and ITD, MLL partial tandem duplications and Evi1 and WT1 expression were analyzed as previously described[14-19].
Statistical analysis
R program (version 2.13) was used for statistical analyses. Non-parametric tests were performed to test the impact of prognostic factors. Correlation tests were computed to compare continuous variables. Survival curves were obtained with the Kaplan-Meier method. Log-Rank test and Cox models were performed on overall survival (OS) and event-free survival for univariate and multivariate analyses, respectively. A cut-off value of 1% was used, corresponding to the median percentage of CD34+CD38 population contained in the total blast cell population gated in the biparametric CD45/SSC plots in all 200 AML patients.
RESULTS
CD34+CD38- cell population in AML at diagnosis and correlation with other biological parameters
The proportion of CD34+CD38- immature leukemia cells was highly variable among the 200 adult and pediatric AML samples included in this series with a median value of 0.95% (range 0.01%-85.5%), with similar values in the adult (0.99%) and pediatric (0.5%) samples. In the adult patients, the proportion of CD34+CD38- immature leukemia cells was significantly correlated with age, FAB classification, cytogenetics and the expression of main molecular markers. The intensity of CD38 expression was significantly lower in patients older than 60 years than in younger patients (≤ 60 years) (P < 0.001, data not shown). When FAB classification was considered, we found a higher proportion of immature CD34+CD38- leukemia cells in M0 and M7 AML as compared to the other subtypes (Supplemental Figure 1A). The CD34+CD38- immature leukemic cell frequency was significantly higher in patients with unfavorable karyotypes than in those with favorable cytogenetics (excluding APL) or those with intermediate1-risk cytogenetics (P < 0.001). The proportion of CD34+CD38- cells was significantly higher in the NPM1-FLT3ITD+ patient group than in the other groups but was not correlated with EVI1 status (Supplemental Tables 1 and 2).
In the favourable risk group, we observed a close correlation of % LSC (from total CD45/SSC blasts cells) with OS and quite significant for DFS, with longer survival for patients with lower level of CD34+CD38- LSC < 1% (median of OS and DFS not attempt > 50%) compared with patients where %LSC was > 1% (median of OS 21.5 mo and for DFS 10.4 mo respectively), P-value = 0.0005 and 0.06. Interestingly, in the intermediate risk group, we observed the same significant correlation between the frequency of most immature CD34+CD38- blasts cells and survival: Median of OS and DFS 12.8 mo for patients with %LSC > 1% and not attempt for those with %LSC < 1%); when only CD34+ compartment was analysed, we obtained similar results, a shorter OS and DFS (median > 50% and 12.7 mo respectively) for %LSC > 20% compared with not attempt (> 50%) for patients with %LSC < 20% (P-value = 0.004 and 0.009 respectively) (Data not showed).
CD34+CD38- LSC evolution between diagnosis and relapse
Among 109 patients in CR after induction chemotherapy, 33 relapsed (30%), with median time between diagnosis of 10 mo[2-24], the majority from the group with higher frequency of LSC CD34+CD38- > 1% in blasts CD45/SSC (47%) compared with patients with LSC < 1% (20%) (P-value = 0.006). (Data not showed). Interestingly, there are no difference in terms of incidence of relapse between two groups when consider LSC% from CD34+ cells, suggested that the proportion of most immature stem cells from whole CD45 pool leukaemia cells is most predicted for aggressive clone than we regarded specifically CD34+ compartment, probably affected by residual normal HSC CD34+. There were significantly differences between relapsed and no-relapsed patients in term of %CD34+CD38- as % from total blasts cells (median 1.53% vs 0.45% respectively) (P-value = 0.0098) but no difference when regarding %CD34+CD38- in CD34+ compartment (data not showed). The majority of patients that relapsed had unfavourable (13/33) or intermediate 2 (12/33) karyotype and 6/33 were in favourable group (3 patients with t(8;21) and 2 patients with inv16, and one had normal karyotype but AML1/ETO positivity) (Supplemental Table 3). Regarding molecular features, 4/33 relapsed patients had Evi1 overexpression, 4 patients FLT3ITD and 3 patients NPM1 mutation. Interestingly, most patients which relapsed had NPM1-/FLT3ITD- profile (26/33) with median of % LSC CD34+CD38- from CD45 total blasts cells > 1% [1.62% (0.04-56.8)]. Among 33 relapsed patients, 13 received allogeneic stem cell transplant, and 10 died for refractory disease.
Concerning favourable group, the frequency of CD34+CD38- cells was observed slightly higher at relapse compared at diagnosis for 4/6 patients suggested a negative influence of most immature CD34+CD38- leukaemia cells level in these patients considered initially as favourable group.
We also evaluated the %CD34+CD38- frequency at relapse time for 26 patients with available immunophenotype data for both diagnosis and relapse time, and we compared with diagnosis level. We observed globally an increase of the most immature stem cell compartment at relapse in almost half of relapsed patients (12/26 patients; 46.15%) with accumulation of leukaemia blasts in CD34+CD38- compartment or eventually clonal selection of most immature LSC (median %LSC at relapse 2.16% vs 1.53% in diagnosis) but statistically not significantly (P-value = 0.2). Moreover, when compared intensity of CD38 level (express as ratio MFI of CD38/Isotype control) between diagnosis and relapsed paired samples we observed a significant higher expression at diagnosis total CD45/SSC blasts cells but also in CD34+ leukaemia subpopulation compared with relapse (median of rMFI CD38 in CD45/SSC blasts cells 25 vs 54 and 25.7 vs 66.7 in CD34+ compartment). The level of CD34+ expression in total blasts cells was significantly higher at relapse compared at diagnosis with median of 94% and 77% respectively (P-value = 0.001). These results suggest a continuous dynamic of LSC with clonal evolution and continuous selection in phenotype level of CD34+ leukaemia cells and most immature self-renewal LSC.
Concerning refractory or non-responders AML patients (55/163; 33.7%); we observed a significantly increase of median LSC CD34+CD38- levels in total CD45/SSC blasts cells > cut-off de 1% (median of 2.1%). The majority of these refractory/non-responder patients (34/55; 61.81%) presented at diagnosis with higher frequency of most immature CD34+/CD38-leukemia cells (> 1%) suggested again the major impact of these LSC population CD34+CD38- in mechanism of chemoresistance.
Correlation of CD34+CD38- population with patient outcome
Induction therapy achieved CR in 67% of cases, with a median follow-up of 7.6 mo (1-27.1) and a median time to relapse of 10.4 mo (1.9-27.1 mo). The CR rate was significantly higher in patients with a lower proportion of CD34+CD38- (< 1%) than in those with higher CD34+CD38- (> 1%) (79% vs 52%, P = 0.0005). The proportion of CD34+CD38- cells was significantly lower in patients achieving CR when compared to that of those who failed (0.53% vs 2% respectively, P = 0.0005). Similarly, a significant difference was observed among relapsing and non-relapsing patients regarding the percentage of immature CD34+CD38- leukemia cell observed at the time of diagnosis (Supplemental Figure 1B). A high percentage of CD34+CD38- was significantly associated with a shorter DFS (median DFS: 12.7 mo in patients with CD34+CD38- > 1% vs not reached in patients with CD34+CD38- < 1%) (Figure 1A) and a shorter OS (median OS: 14 mo in patients with CD34+CD38- > 1% vs not reached in patients with CD34+CD38- < 1%) (P = 0.03) (Figure 1B). In univariate analysis, a high percentage of CD34+CD38- (> 1%) was correlated with a significantly shorter DFS (P < 0.0001) and OS (P = 0.0004) (Supplemental Table 4). In a multivariate analysis considering age, white blood cell count, percentage of CD34+ blasts and molecular characteristics, this factor appeared as an independent prognostic parameter for both DFS and OS (Table 1).
Table 1.
Impact of prognostic factors on disease free survival and overall survival
| Prognostic factors |
Impact of prognostic factors on overall survival OS (wo palliatives, wo M3), n = 153 |
Impact of prognostic factors on disease free survival DFS (wo palliatives, wo M3), n = 101 |
||||||
| Univariate analysis P value | Multivariate analysis P value | 95%CI | HR | Univariate analysis P value | Multivariate analysis P value | 95%CI | HR | |
| Age > 60 yr | P < 0.0001 | P = 0.02268 | 1.15-6.13 | 2.65 | P = 0.21 | P = 0.76834 | 0.33-2.25 | 0.87 |
| WBC | P = 0.74 | P = 0.10429 | 1-1.02 | 1.01 | P = 0.13 | P = 0.00184 | 1.01-1.02 | 1.01 |
| Cytogenetic risk subgroup | P < 0.0001 | |||||||
| Favorable | P = 0.31675 | 0.43-13.33 | 2.4 | |||||
| Intermediate | P = 0.05414 | 0.97-33.01 | 5.66 | |||||
| Unfavorable | ||||||||
| Molecular anomalies | ||||||||
| FLT3ITD- | P = 0.82 | P = 0.70619 | 0.32-5.27 | 1.31 | ||||
| NMP1- | P = 0.04 | P = 0.01139 | 1.79-98.56 | 13.29 | ||||
| EVI1- | P = 0.89 | P = 0.47958 | 0.38-8 | 1.74 | ||||
| %CD34 of Blasts | P = 0.47 | P = 0.08765 | 1-1.03 | 1.01 | P = 0.15 | P = 0.95058 | 0.99-1.02 | 1 |
| %CD34+CD38- of Blasts > 1% | P = 0.0001 | P = 0.03091 | 1.09-6.43 | 2.65 | P = 0.0005 | P = 0.04663 | 1.02-7.73 | 2.8 |
This analysis excluded palliative cases and M3 subtypes. OS: Overall survival; DFS: Disease free survival; WBC: White blood cell.
DISCUSSION
In this study, we confirmed that high stem cell frequency based on CD34/CD38 profile at diagnosis is a prognostic significance regarding to OS and disease-free survival (DFS). Our data are in agreement with data previously published by van Rhenen et al[9,10] showing a prognostic impact of the proportion of the CD34+CD38- stem cell population in 92 AML patients. Relapse of AML is thought to originate from resistant leukemic cells, residual cells at very few level as minimal residual disease (MRD), a higher CD34+CD38- population has no major impact on the CR rate and MRD after induction, but the most resistant fractions of the CD34+CD38- compartment seems to be selected with additional courses of chemotherapy. Keyhani et al[20] demonstrated that patients with AML showed a high CD38 intensity had significantly longer CR duration and survival compared with those with lower expression, suggesting that CD38 expression is a potentially useful independent marker of disease outcome. Several studies have demonstrated the prognostic value of CD34 expression in AML leukemic cells at diagnosis[21], suggesting that CD34 expression is associated with lower CR rates and a shorter OS, but this has been not confirmed by all groups[22]. In our series, we observed any significant correlations between CD34 expression and both OS and DFS in a multivariate analysis. Conversely, we observed a correlation between the percentage of CD34+CD38- leukemia cells and the total level of CD34+ blasts cells, more immature CD34+CD38- leukaemia cells correlating well with total level of CD34+ blasts cells, suggested anyway more aggressive potential of CD34 compartment when associated with CD38 analyse. These results emphasize the strong heterogeneity in CD34+ leukemia cells and the need for more detailed simultaneous analyses of CD38 combined with CD34 and quantification of the most immature CD34+CD38- stem cell compartment.
Interestingly, we observed a very high level of CD34+ CD38- quantified in CD34+ compartment among the AML patients with leukaemia cells negative for CD34 (< 1%), previously considered as normal residual HSC in NPM1 mutated AML subtype[5]. Anyway, reduced expression of CD38 in these CD34+ cells suggested a block in differentiation of the residual HSC. It was demonstrated recently[23] that normal HSC in bone marrow from AML patients were more quiescent compared to HSC in normal healthy group, normal CD34+CD38- cells divided less when cultured with AML than alone, even when they are not in direct contact with HSC suggesting a soluble factor responsible for increased quiescence in normal residual HSC, the Taussig group trying to identify this factor. Whether this quiescent of normal residual HSC has possible impact in prognostic of AML CD34- patients should be confirmed in the large prospective studies.
Considering our understanding of leukemogenesis one of the most important questions arise from the cellular origin of the leukaemia stem cells. It was showed previously that AML-LSC were identified and purified in CD34+CD38- fraction cells between all bulk blast population in AML patients, and represented the only AML cells capable of self/renewal[3,4,6]. Nonetheless, importantly heterogeneity has been revealed. Recent studies suggest that in some patients, AML-LSC could have a progenitor phenotype CD34+CD38+ and in patients with NPM mutation, LSC could be identified in the CD34- fraction[5,7,8]. Recently more evidence on the heterogeneity of AML was described in terms of karyotype, differentiation stage of the blasts and clinical outcome, consequently it is remarkable that AML-LSC is more complex than previously thought, and can be different from patient to patient but also in the same patient regarding on the stage of disease with other mutations acquired in the original LSC that might occur during the development of the leukaemia, suggesting that LSC might represent a “moving target” even in the same patient.
Most recently, Goardon et al[12] investigated 74 primary human AML patient samples. It was showed that the CD34+ cells in about 80% of these cases contained two most frequent populations: One CD38-CD90-CD45RA+ (lymphoid-primed multipotential progenitor LMPP-like cells) and the other CD38+CD110+CD45RA+ (representing granulocyte-monocyte progenitor GMP-like cells). Both corresponded to normal hematopoietic progenitor populations rather than HSC and possess LSC activity based on their capacity of serially transplant the AML in immunodeficient NOD/SCID/IL2Rgamma null mice. Moreover, these populations showed a hierarchically organisation; whereby the CD38-CD45RA+ cells gave rise the GMP-like cells but not vice versa. Summary, these results enlarge on the previous view of AML LSC and settled a hierarchy of populations with decreasing frequency of LSC, implicate normal hematopoietic progenitors, LMPP and/or GMP as the cell of origin for AML/LSC in much of cases. One of the crucial implication of a progenitor phenotype profile for AML LSC connect to the critical stem cell property of self-renewal, the acquisition of self-renewal ability in AML LSC being consider as an aberrant event resulting drum genetic and/or epigenetic features. Thereafter, the most significant incrimination of the leukaemia stem cell model is that to eradicate the leukaemia and cure the patient. The final goal could be eradication of all LSC, the analyses of gene expression data reported by Goardon in this study would be one of the most important step toward the identification of these LSC specific genes or pathways.
Taken together, the results of our study emphasis that using CD34/CD38 “backbone” in leukaemia cells analysis by multicolour flow cytometry at AML samples at diagnosis is relatively facile method, rapidly translate in clinical practice as complementary prognostic factor in AML. Multicolour/multidimensional flow cytometry represent very useful tools to identify and characterise immunologic profile of different leukaemia compartments using CD34/CD38/CD45 as “backbone” to design more complex panels (8-10-14 colours) adapted to AML diagnosis and MRD flow evaluation, including most specific LSC markers described previously as CLL-1, TIM3, CD123, CD45RA, CD97, CD47, CD44, CD49f, to better discriminate between nHSC and LSC as described recently[24,25]. The origin of AML LSC is still controversy, however all fundamental experience to elucidate the function of stem cells are based to first in CD34/CD38 selected populations subsequent transplanted to immunodeficient mice, suggested the importance of CD34/CD38 analyse of all AML leukaemia cell at diagnosis and relapse and quantification of most immature “profile” CD34+CD38- in all “bulk” leukaemia blasts population as basically immunophenotype that should be used in all leukaemia immunological evaluation, associated with others lineage or stem cells markers.
In summary, we showed that the presence of a CD34+CD38- subpopulation representing more than 1% of total “bulk leukemic cells” at diagnosis could help to identify patients at risk of induction failure and poor outcome. This method is simple, rapid and accurate and can easily be applied to the clinical practice. Despite heterogeneity and complexity of AML LSC we could still use CD34+CD38- to predict patient outcome and it is still a potential tool.
These results should be confirmed in a prospectively larger cohort of patients and could be considered as useful complementary prognostic parameter for risk-stratification AML patients in future clinical trials.
COMMENTS
Background
Multicolor flow cytometry is largely used for acute myeloid leukemia (AML) diagnosis in most of Hematology departments for lineage assessment based on ELN and WHO 2016 guidelines. Quantification of most immature CD34+CD38- leukemia blast cells could be easily included at diagnosis panel, this method being simple, rapid and accurate and be applied to the clinical practice.
Research frontiers
CD34+ compartment the authors divided three subpopulations: CD34+CD38-, CD34+CD38lo, and CD34+CD38hi, based on intensity of CD38 expression; FMO (Fluorescence Minus One) was used for CD38- level, and hematogones populations for CD38hi level. The stem cell compartment CD34+CD38- contain very few events in some patients but these events should tightly cluster in a FSC/SSC plot and CD45/SSC plot. The authors evaluated also the intensity of fluorescence signal for CD38 quantified as rMFICD38 from CD34+ gated cells and rMFICD38 from CD45lo/SSC total blasts cells.
Innovations and breakthroughs
In this study, the authors confirmed that high stem cell frequency based on CD34/CD38 profile at diagnosis is a prognostic significance regarding to overall survival (OS) and disease-free survival (DFS). Relapse of AML is thought to originate from resistant leukemic cells, residual cells at very few level as minimal residual disease (MRD), a higher CD34+CD38- population has no major impact on the CR rate and MRD after induction, but the most resistant fractions of the CD34+CD38- compartment seems to be selected with additional courses of chemotherapy. The results emphasized the strong heterogeneity in CD34+ leukemia cells and the need for more detailed simultaneous analyses of CD38 combined with CD34 and quantification of the most immature CD34+CD38- stem cell compartment.
Applications
Multicolour/multidimensional flow cytometry represent very useful tools to identify and characterise immunologic profile of different leukaemia compartments using CD34/CD38/CD45 as “backbone” to design more complex panels (8-10-14 colours) adapted to AML diagnosis and MRD flow evaluation, including most specific LSC markers described previously as CLL-1, TIM3, CD123, CD45RA, CD97, CD47, CD44, CD49f, to better discriminate between nHSC and LSC.
Terminology
Leukemia initiating cells (LICs) or Leukemia stem cells (LSC) in mice have shown a primitive immunophenotype (CD34+CD38-) with similarities to normal hematopoietic stem cells (HSCs) regardless of the subtype of AML or the immunophenotype of the majority of the leukemic blasts present in the bone marrow. However, other studies showed that LSC were exclusively found in the CD34- compartment. Other investigators described LSC in both the CD34- and CD34+ cell compartments.
Peer-review
The study is well-designed and its topic is interesting.
ACKNOWLEDGMENTS
We acknowledge technicians from Laboratory of Haematology: Eve Mattei, Anne Monin, Laurence Volkmann, Brigitte Petit, Claudine Maghba, Josiane Carret, Isabelle Bazin, Francoise Morand, Lucie Belmont, Emily Loison, Morgane Denis, for rigorous technical assistance. We also gratefully acknowledge all patients for their contribution to this study.
Footnotes
Institutional review board statement: Lyon University Hospital-Hematology Review Board.
Informed consent statement: All clinical trials have been reviewed and approved by a relevant ethic committee and were performed in accordance with the Helsinki declaration of 1975. All patients gave informed consent according to French legislation.
Conflict-of-interest statement: None of the authors have anything to disclose regarding this study.
Data sharing statement: Technical appendix, statistical code, and dataset available from the corresponding author.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: France
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: February 13, 2017
First decision: May 10, 2017
Article in press: November 8, 2017
P- Reviewer: Cao T, Kiselev SL, Ramírez M, Yao CL, Zaminy A S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
Contributor Information
Adriana Plesa, Laboratory of Hematology, Lyon-Sud Hospital, Hospices Civils de Lyon, Pierre - Bénite Cedex 69495, France; CRCL, INSERM 1052/CNRS 5286, Lyon FR-69008, France. adriana.plesa@chu-lyon.com.
Charles Dumontet, Laboratory of Hematology, Lyon-Sud Hospital, Hospices Civils de Lyon, Pierre - Bénite Cedex 69495, France; CRCL, INSERM 1052/CNRS 5286, Lyon FR-69008, France.
Eve Mattei, Laboratory of Hematology, Lyon-Sud Hospital, Hospices Civils de Lyon, Pierre - Bénite Cedex 69495, France.
Ines Tagoug, CRCL, INSERM 1052/CNRS 5286, Lyon FR-69008, France.
Sandrine Hayette, Laboratory of Hematology, Lyon-Sud Hospital, Hospices Civils de Lyon, Pierre - Bénite Cedex 69495, France.
Pierre Sujobert, Laboratory of Hematology, Lyon-Sud Hospital, Hospices Civils de Lyon, Pierre - Bénite Cedex 69495, France.
Isabelle Tigaud, Laboratory of Hematology, Lyon-Sud Hospital, Hospices Civils de Lyon, Pierre - Bénite Cedex 69495, France.
Marie Pierre Pages, Laboratory of Hematology, Lyon-Sud Hospital, Hospices Civils de Lyon, Pierre - Bénite Cedex 69495, France.
Youcef Chelghoum, Department of Hematology, Hospices Civils de Lyon, Pierre - Bénite Cedex 69495, France.
Fiorenza Baracco, Department of Hematology, Hospices Civils de Lyon, Pierre - Bénite Cedex 69495, France.
Helene Labussierre, Department of Hematology, Hospices Civils de Lyon, Pierre - Bénite Cedex 69495, France.
Sophie Ducastelle, Department of Hematology, Hospices Civils de Lyon, Pierre - Bénite Cedex 69495, France.
Etienne Paubelle, Department of Hematology, Hospices Civils de Lyon, Pierre - Bénite Cedex 69495, France.
Franck Emmanuel Nicolini, Department of Hematology, Hospices Civils de Lyon, Pierre - Bénite Cedex 69495, France.
Mohamed Elhamri, Department of Hematology, Hospices Civils de Lyon, Pierre - Bénite Cedex 69495, France.
Lydia Campos, Laboratory of Hematology, Nord Hospital, Saint Etienne 42055, France.
Claudiu Plesa, Department of Hematology, Hospices Civils de Lyon, Pierre - Bénite Cedex 69495, France.
Stéphane Morisset, Statistical and Clinical Research, Lyon-Sud Hospital, Hospices Civils de Lyon, Pierre - Bénite Cedex 69495, France.
Gilles Salles, Department of Hematology, Hospices Civils de Lyon, Pierre - Bénite Cedex 69495, France.
Yves Bertrand, Department of Pediatric Hematology and BMT, IHOP Lyon, Lyon 69001, France.
Mauricette Michallet, Department of Hematology, Hospices Civils de Lyon, Pierre - Bénite Cedex 69495, France.
Xavier Thomas, Department of Hematology, Hospices Civils de Lyon, Pierre - Bénite Cedex 69495, France.
References
- 1.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 2.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 3.Passegué E, Jamieson CH, Ailles LE, Weissman IL. Normal and leukemic hematopoiesis: are leukemias a stem cell disorder or a reacquisition of stem cell characteristics? Proc Natl Acad Sci USA. 2003;100 Suppl 1:11842–11849. doi: 10.1073/pnas.2034201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas X. Targeting leukemia stem cells: The new goal of therapy in adult acute myeloid leukemia. World J Stem Cells. 2009;1:49–54. doi: 10.4252/wjsc.v1.i1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taussig DC, Vargaftig J, Miraki-Moud F, Griessinger E, Sharrock K, Luke T, Lillington D, Oakervee H, Cavenagh J, Agrawal SG, et al. Leukemia-initiating cells from some acute myeloid leukemia patients with mutated nucleophosmin reside in the CD34(-) fraction. Blood. 2010;115:1976–1984. doi: 10.1182/blood-2009-02-206565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barabé F, Kennedy JA, Hope KJ, Dick JE. Modeling the initiation and progression of human acute leukemia in mice. Science. 2007;316:600–604. doi: 10.1126/science.1139851. [DOI] [PubMed] [Google Scholar]
- 7.Zanjani ED, Almeida-Porada G, Livingston AG, Flake AW, Ogawa M. Human bone marrow CD34- cells engraft in vivo and undergo multilineage expression that includes giving rise to CD34+ cells. Exp Hematol. 1998;26:353–360. [PubMed] [Google Scholar]
- 8.Taussig DC, Miraki-Moud F, Anjos-Afonso F, Pearce DJ, Allen K, Ridler C, Lillington D, Oakervee H, Cavenagh J, Agrawal SG, et al. Anti-CD38 antibody-mediated clearance of human repopulating cells masks the heterogeneity of leukemia-initiating cells. Blood. 2008;112:568–575. doi: 10.1182/blood-2007-10-118331. [DOI] [PubMed] [Google Scholar]
- 9.van Rhenen A, Feller N, Kelder A, Westra AH, Rombouts E, Zweegman S, van der Pol MA, Waisfisz Q, Ossenkoppele GJ, Schuurhuis GJ. High stem cell frequency in acute myeloid leukemia at diagnosis predicts high minimal residual disease and poor survival. Clin Cancer Res. 2005;11:6520–6527. doi: 10.1158/1078-0432.CCR-05-0468. [DOI] [PubMed] [Google Scholar]
- 10.van Rhenen A, Moshaver B, Kelder A, Feller N, Nieuwint AW, Zweegman S, Ossenkoppele GJ, Schuurhuis GJ. Aberrant marker expression patterns on the CD34+CD38- stem cell compartment in acute myeloid leukemia allows to distinguish the malignant from the normal stem cell compartment both at diagnosis and in remission. Leukemia. 2007;21:1700–1707. doi: 10.1038/sj.leu.2404754. [DOI] [PubMed] [Google Scholar]
- 11.Greaves M. Cancer stem cells: back to Darwin? Semin Cancer Biol. 2010;20:65–70. doi: 10.1016/j.semcancer.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Amielańczyk W. [Psychpathological aspects of the status of patients with late diagnosis of congenital myotonia] Wiad Lek. 1976;29:55–57. [PubMed] [Google Scholar]
- 13.Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, Rees J, Hann I, Stevens R, Burnett A, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood. 1998;92:2322–2333. [PubMed] [Google Scholar]
- 14.Preudhomme C, Sagot C, Boissel N, Cayuela JM, Tigaud I, de Botton S, Thomas X, Raffoux E, Lamandin C, Castaigne S, et al. Favorable prognostic significance of CEBPA mutations in patients with de novo acute myeloid leukemia: a study from the Acute Leukemia French Association (ALFA) Blood. 2002;100:2717–2723. doi: 10.1182/blood-2002-03-0990. [DOI] [PubMed] [Google Scholar]
- 15.Boissel N, Cayuela JM, Preudhomme C, Thomas X, Grardel N, Fund X, Tigaud I, Raffoux E, Rousselot P, Sigaux F, et al. Prognostic significance of FLT3 internal tandem repeat in patients with de novo acute myeloid leukemia treated with reinforced courses of chemotherapy. Leukemia. 2002;16:1699–1704. doi: 10.1038/sj.leu.2402622. [DOI] [PubMed] [Google Scholar]
- 16.Boissel N, Renneville A, Biggio V, Philippe N, Thomas X, Cayuela JM, Terre C, Tigaud I, Castaigne S, Raffoux E, et al. Prevalence, clinical profile, and prognosis of NPM mutations in AML with normal karyotype. Blood. 2005;106:3618–3620. doi: 10.1182/blood-2005-05-2174. [DOI] [PubMed] [Google Scholar]
- 17.Poirel H, Rack K, Delabesse E, Radford-Weiss I, Troussard X, Debert C, Leboeuf D, Bastard C, Picard F, Veil-Buzyn A, et al. Incidence and characterization of MLL gene (11q23) rearrangements in acute myeloid leukemia M1 and M5. Blood. 1996;87:2496–2505. [PubMed] [Google Scholar]
- 18.Cilloni D, Renneville A, Hermitte F, Hills RK, Daly S, Jovanovic JV, Gottardi E, Fava M, Schnittger S, Weiss T, et al. Real-time quantitative polymerase chain reaction detection of minimal residual disease by standardized WT1 assay to enhance risk stratification in acute myeloid leukemia: a European LeukemiaNet study. J Clin Oncol. 2009;27:5195–5201. doi: 10.1200/JCO.2009.22.4865. [DOI] [PubMed] [Google Scholar]
- 19.Barjesteh van Waalwijk van Doorn-Khosrovani S, Erpelinck C, van Putten WL, Valk PJ, van der Poel-van de Luytgaarde S, Hack R, Slater R, Smit EM, Beverloo HB, Verhoef G, et al. High EVI1 expression predicts poor survival in acute myeloid leukemia: a study of 319 de novo AML patients. Blood. 2003;101:837–845. doi: 10.1182/blood-2002-05-1459. [DOI] [PubMed] [Google Scholar]
- 20.Keyhani A, Huh YO, Jendiroba D, Pagliaro L, Cortez J, Pierce S, Pearlman M, Estey E, Kantarjian H, Freireich EJ. Increased CD38 expression is associated with favorable prognosis in adult acute leukemia. Leuk Res. 2000;24:153–159. doi: 10.1016/s0145-2126(99)00147-2. [DOI] [PubMed] [Google Scholar]
- 21.Guinot M, Sanz GF, Sempere A, Arilla MJ, Jarque I, Gomis F, Sanz MA. Prognostic value of CD34 expression in de novo acute myeloblastic leukaemia. Br J Haematol. 1991;79:533–534. doi: 10.1111/j.1365-2141.1991.tb08075.x. [DOI] [PubMed] [Google Scholar]
- 22.Selleri C, Notaro R, Catalano L, Fontana R, Del Vecchio L, Rotoli B. Prognostic irrelevance of CD34 in acute myeloid leukaemia. Br J Haematol. 1992;82:479–481. doi: 10.1111/j.1365-2141.1992.tb06452.x. [DOI] [PubMed] [Google Scholar]
- 23.Miraki-Moud F, Anjos-Afonso F, Hodby KA, Griessinger E, Rosignoli G, Lillington D, Jia L, Davies JK, Cavenagh J, Smith M, et al. Acute myeloid leukemia does not deplete normal hematopoietic stem cells but induces cytopenias by impeding their differentiation. Proc Natl Acad Sci USA. 2013;110:13576–13581. doi: 10.1073/pnas.1301891110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terwijn M, Zeijlemaker W, Kelder A, Rutten AP, Snel AN, Scholten WJ, Pabst T, Verhoef G, Löwenberg B, Zweegman S, et al. Leukemic stem cell frequency: a strong biomarker for clinical outcome in acute myeloid leukemia. PLoS One. 2014;9:e107587. doi: 10.1371/journal.pone.0107587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanekamp D, Cloos J, Schuurhuis GJ. Leukemic stem cells: identification and clinical application. Int J Hematol. 2017;105:549–557. doi: 10.1007/s12185-017-2221-5. [DOI] [PubMed] [Google Scholar]


