Abstract
Hypoperfusion injury related to blood pressure decrease in acute hypertensive intracerebral hemorrhage continues to be a controversial topic. Aggressive treatment is provided with the intent to stop the ongoing bleeding. However, there may be additional factors, including autoregulation and increased intracranial pressure, that may limit this approach. We present here a case of acute hypertensive intracerebral hemorrhage, in which aggressive blood pressure management to levels within the normal range led to global cerebral ischemia within multiple border zones. Global cerebral ischemia may be of concern in the management of hypertensive hemorrhage in the presence of premorbid poorly controlled blood pressure and increased intracranial pressure.
Keywords: Intracranial hemorrhage, Neurocritial care, Stroke management, Perihematoma ischemia
Core tip: The current case report highlights the risk of aggressive management of acute hypertension in the setting of intracerebral hemorrhage causing global cerebral hypoperfusion, despite maintenance of cerebral perfusion pressure above the lower threshold of autoregulation. The authors suggest the use of accurate method to measure cerebral oxygenation, such as brain-tissue oxygen monitoring, which could help individualize aggressive blood pressure control in patients with acute hypertensive intracerebral hemorrhage.
INTRODUCTION
Up to one-third of spontaneous intracerebral hemorrhages (ICHs) expand, typically, within the first 6 h after the ictus. This expansion contributes to clinical deterioration and worse outcome[1-3]. Persistent high blood pressure may promote recurrent early bleeding[4-6]. Despite several reports that supported aggressive blood pressure control, reducing the risk of bleeding and improving the outcome, recent randomized larger trials have failed to prove it[7-9]. The safety of this approach has been questioned, mainly based on the concern that the perihematoma region may already be ischemic, due to local tissue pressure and have impaired autoregulation. But it may also be because autoregulation may be globally impaired after the ictus, or autoregulation may be retained but shifted substantially toward higher perfusion pressures. Based on these concerns, aggressive blood pressure reduction might lead to local or global ischemia.
There is evidence suggesting that autoregulation is retained locally in the perihematoma region. Blood flow decreases in areas adjacent to the hematoma; although, there is an accompanying decrease in metabolism without evidence of ischemia[10]. Cerebral autoregulation has been shown to be preserved in small- and medium-sized hematomas; however, it is variably shifted to higher levels in patients with chronic hypertension[11,12]. Determining whether blood pressure management prevents hematoma growth after ICH and how blood pressure reduction can be safely performed are key research priorities[13].
We present herein a case of spontaneous ICH, for which aggressive treatment of hypertension did not lead to infarction around the hematoma but globally in multiple border zone areas.
CASE REPORT
A 52-year-old black woman suddenly developed slurred speech and mild right hemiparesis. Medical history included poorly controlled chronic hypertension, and no known history of atrial fibrillation. In the emergency department, the initial blood pressure reading was 264/218 mmHg. The patient had right hemiparesis and dysarthria, and it was also noted that she was confused. After an episode of emesis she became lethargic, requiring emergent endotracheal intubation. A computerized tomography (CT) scan of the head showed a left thalamic hemorrhage (volume 36 mL, A × B × C/2) with intraventricular extension and developing hydrocephalus. Treatment to control blood pressure included intravenous labetalol boluses and nitroprusside infusion. External ventricular drainage was placed to manage hydrocephalus, and intracranial pressure and cerebral perfusion pressure were then monitored (Figure 1).
Figure 1.
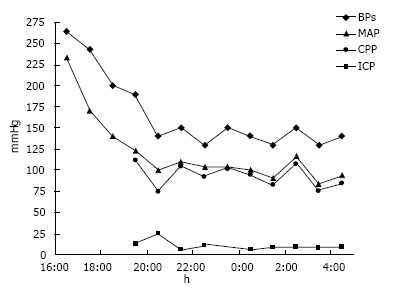
Diagram showing the evolution of systolic blood pressure, mean arterial blood pressure, cerebral perfusion pressure and intracranial pressure within the first 12 h. BP: Blood pressure; MAP: Mean arterial blood pressure; CPP: Cerebral perfusion pressure; ICP: Intracranial pressure.
The patient underwent CT angiography of the head on arrival, which revealed no vessel occlusion. In addition, further work-up including continuous cardiac monitoring in telemetry unit in the intensive care unit (ICU) for several days, electrocardiogram and echocardiogram did not reveal any cardioembolic etiology, except for left ventricular hypertrophy. For the following days, the patient remained comatose, although examination was limited due to sedation. A follow-up CT of the head at 96 h post-admission showed a cerebellar hypodensity. Magnetic resonance imaging (MRI) of the brain showed areas of restricted diffusion consistent with acute ischemia in multiple internal border zone areas of bilateral cerebral and cerebellar hemispheres (Figure 2). No significant stenosis was found in the magnetic angiography of the neck or brain. Cerebral perfusion pressure was above 70 mmHg, except for two measurements (68 mmHg on day 2 and 62 mmHg on day 4). On day 2, intraventricular thrombolytics were administered and the ventriculostomy was clamped. On day 4, blood pressure was 110/55 (the lowest recorded) and the nitroprusside drip was reduced with rapid improvement. The patient survived, but was aphasic and right hemiparetic at the time of discharge to a nursing home.
Figure 2.
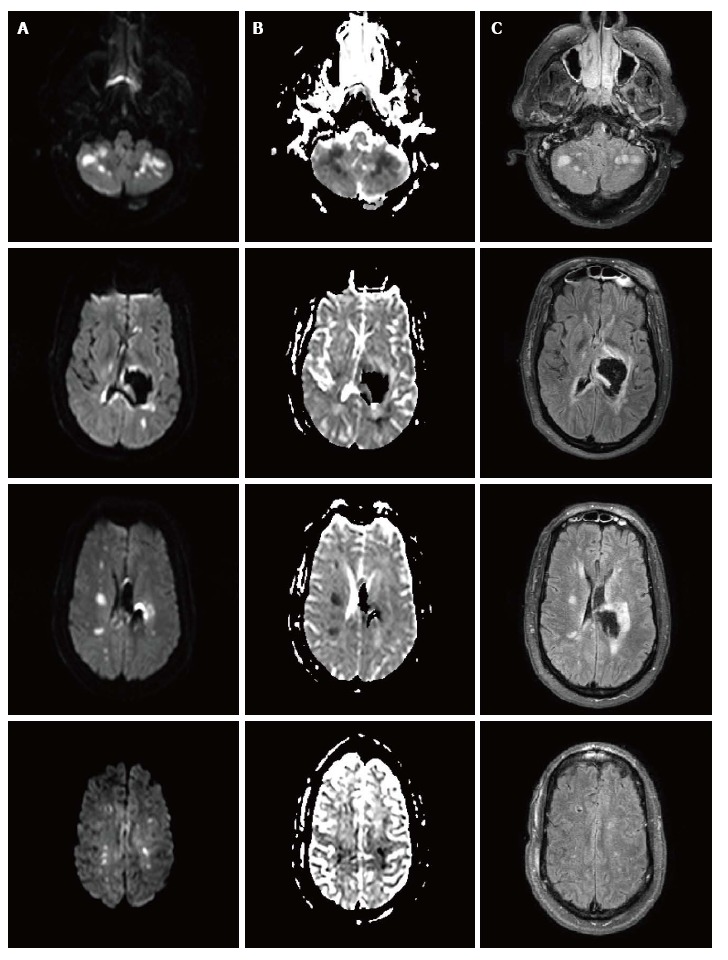
Brain magnetic resonance imaging with diffusion weighted imaging (A), apparent diffusion coefficient (B) and FLAIR sequences (C) showing multiple areas of infarction in the internal border zone areas of bilateral cerebral and cerebellar hemispheres. There is no evidence of perihematoma infarction. MRI: Magnetic resonance imaging.
DISCUSSION
Two features in this case are to be discussed. First, global cerebral ischemia after aggressive blood pressure reduction seemed more a concern than the presence of ischemia around the hematoma; and, second, global ischemia developed despite maintenance of cerebral perfusion pressure above the lower threshold of autoregulation in normals.
The fact that the region around the hematoma was spared, with a mean arterial pressure reduction of about 50%, goes along with recent work by Powers et al[12] and Zazulia et al[14]. Although ischemia around the hematoma was initially thought to be present and to contribute to secondary brain injury based on experimental animal models[15-17], nowadays it is more controversial and it is becoming more evident about its absence, based on human studies. Using MRI, no markers of ischemia were associated with the perihematomal region in acute ICH[18,19]. Positron emission tomography (PET) studies also reported perihematomal cerebral blood flow reductions, without evidence of ischemia[20]. Reduced perihematomal cerebral blood flow was associated with a decreased metabolic rate of oxygen and oxygen extraction fraction, suggesting that flow changes represent hypoactive rather than ischemic tissue[14].
On the basis of evidence derived from laboratory and clinical studies, three phases have been identified[10]. The hibernation phase, an acute period of concomitant hypoperfusion and hypometabolism, predominantly involves the perihematoma region and occurs during the first 2 d. The reperfusion phase is observed between days 2 and 14, with a heterogeneous pattern of cerebral blood flow, consisting of areas of relatively normal flow, persistent hypoperfusion and hyperperfusion. And, the normalization phase is observed thereafter, with normal cerebral blood flow reestablished in all viable regions. In this case, hypometabolism in the area around the hematoma might have prevented the tissue from infarction.
The reduction of about 50% in the mean arterial pressure may seem unsafe; although, once the intracranial pressure was monitored, the cerebral perfusion pressure (CPP) could be calculated, and in fact it was kept above the lower range for normals. Nevertheless, strong evidence-based guidelines for the management of blood pressure in patients with spontaneous ICH with systolic blood pressure more than 220 mm Hg are not clearly established. The writing group of the stroke council for the American Heart Association encourages in their guidelines, aggressive treatment of high blood pressure to prevent ongoing bleeding with the caveat that aggressive treatment may decrease cerebral perfusion and worsen brain damage, especially[21-23]. Based on these two rationales, the recommendation is for patients presenting with a systolic blood pressure between 150 and 220 mmHg, acute lowering of systolic blood pressure to 140 mmHg is safe (ClassI; Level of Evidence A); however, if the systolic blood pressure at presentation is above 220 mmHg, the recommendation of aggressive reduction is less clear (Class IIb; Level of Evidence C). Intracranial pressure monitoring is also considered in patients with significant intraventricular hemorrhage or hydrocephalus, with a reasonable goal CPP between 50-70 mmHg (Class IIb; Level of Evidence C).
Cerebral autoregulation maintains cerebral blood flow by modifying the cerebrovascular resistance when cerebral perfusion pressure fluctuates, keeping cerebral blood flow constant in normal subjects at a CPP between 50-70 mmHg[24]. In chronic hypertension, cerebral autoregulation is shifted to higher levels and the degree correlates with the severity of hypertension. Thickening in the vascular wall increases the resistance, providing tissue protection if CPP is high; however, the ability to dilate when CPP lowers is lessened[25,26]. While perfusion pressure is calculable at bedside, quantitative tissue flow cannot be measured without employing a method such PET or a more invasive one using brain-tissue oxygen monitors[27]. Recently, it has been shown in traumatic brain injury that up to one-third of patients may demonstrate low brain tissue oxygen despite adequate CPP[28,29].
The patient presented herein developed multiple cerebral border zone infarcts after aggressive but carefully monitored treatment of blood pressure with labetalol and nitroprusside. Several modern imaging studies suggest that an internal watershed infarction is primarily caused by hypoperfusion as seen in our patient; this should not be confused with cortical watershed infarct, which is primarily caused by microembolism[30-33].
We hypothesize that the lower limit of autoregulation was shifted to higher levels secondary to chronic untreated hypertension. What is an adequate cerebral perfusion pressure in a normal subject was not so in her case, resulting in insufficient cerebral blood flow and ischemia. Alternatively, decreased cerebral perfusion pressure could have occurred during treatment of hypertension prior to intracranial pressure monitoring (Figure 2).
In conclusion, aggressive management of acute hypertension in ICH is controversial. Global but not perihematoma hypoperfusion may be of more concern in this approach, since cerebral autoregulation in chronic hypertensive patients is variably shifted to higher levels. An accurate method to measure cerebral oxygenation, such as brain-tissue oxygen monitoring, could help individualize aggressive blood pressure control in patients with acute hypertensive ICH.
ARTICLE HIGHLIGHTS
Case characteristics
This case illustrates diffuse border zone infarcts caused by rapid and dramatic reduction in blood pressure in a patient presenting with intracerebral hemorrhage and chronic uncontrolled hypertension.
Clinical diagnosis
Stroke, sudden onset of slurred speech, right-sided hemiparesis, dysarthria, lethargy, blood pressure of 264/218.
Differential diagnosis
Ischemic stroke, hemorrhagic stroke, transient ischemic attack, hypoglycemia, Todd’s paralysis, intracerebral aneurysm rupture.
Laboratory diagnosis
No laboratory test was diagnostic; the patient, however, presented with very elevated blood pressure levels, and normal blood sugar levels.
Imaging diagnosis
Non-contrast computed tomography (CT) scan of the head showed left thalamic hemorrhage with intraventricular extension and developing hydrocephalus. Follow-up non-contrast CT scan of the head at 96 h showed a cerebellar hypodensity. Electrocardiogram revealed normal sinus rhythm. Echocardiogram did not reveal any cardioembolic etiology, except for left ventricular hypertrophy. Magnetic resonance imaging of the brain showed areas of restricted diffusion consistent with acute ischemia in multiple internal border zone areas of bilateral cerebral and cerebellar hemispheres; the location in the border zone areas makes embolic etiology of the ischemia unlikely. Magnetic resonance angiography of the brain and neck revealed no significant stenosis that could have contributed to the cerebral ischemia.
Treatment
Blood pressure control with intravenous labetalol boluses and nitroprusside infusion. For the management of hydrocephalus and monitoring of intracranial and cerebral perfusion pressures, external ventricular drain was placed. Intraventricular thrombolytics were given to prevent clot formation in the ventricles and facilitate the cerebrospinal fluid drainage.
Related reports
There are several randomized controlled trials that have focused on blood pressure control in the setting of intracerebral hemorrhage; however, strong evidence-based guidelines for the management of blood pressure in patients with spontaneous intracerebral hemorrhage and systolic blood pressure more than 220 mmHg are not clearly established.
Term explanation
Hematoma expansion: An increase in size of the initial intracerebral hemorrhage that occurs in up to one-third of the patients, usually within the first 24 h. Borderline or watershed infarctions: Those that occur in areas shared by two vascular territories; those areas are more susceptible to perfusion reduction as it happens with blood pressure reduction and/or shifted cerebral autoregulation. Cerebral autoregulation: A physiological mechanism that maintains cerebral blood flow at different blood pressure levels. In patients with chronic hypertension, the curve shifts to the right (“right shifted”); autoregulation is used for higher blood pressure levels, and it is more protective to elevated blood pressure but fails to react in case of lower blood pressure levels.
Experiences and lessons
Caution should be advised when blood pressure reduction is considered in patients with intracerebral hemorrhage, especially if arriving with very elevated blood pressure and known to have untreated chronic hypertension. In addition, the presence of concomitant increased intracranial pressure due to the mass effect and/or hydrocephalus after intraventricular extension increases the intracranial pressure and thus decreases the cerebral perfusion pressure. Cerebral autoregulation shifts to the right in patients with chronic untreated hypertension and a reduction in blood pressure may not be tolerated leading to ischemia; therefore, such intervention in certain cases may be unsafe, as described in the article.
Footnotes
Informed consent statement: Informed consent was obtained from the family of the patient for the purpose of publication.
Conflict-of-interest statement: None.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
Peer-review started: February 12, 2017
First decision: May 17, 2017
Article in press: November 29, 2017
P- Reviewer: Kim MS, Llompart-Pou JA, Shen J, Zavras N S- Editor: Kong JX L- Editor: Filipodia E- Editor: Lu YJ
Contributor Information
Jose Gavito-Higuera, Department of Neurology, Paul L. Foster School of Medicine, Texas Tech University of Health Sciences Center, El Paso, TX 79905, United States.
Rakesh Khatri, Department of Neurology, Paul L. Foster School of Medicine, Texas Tech University of Health Sciences Center, El Paso, TX 79905, United States. rakesh.khatri@ttuhsc.edu.
Ihtesham A Qureshi, Department of Neurology, Paul L. Foster School of Medicine, Texas Tech University of Health Sciences Center, El Paso, TX 79905, United States.
Alberto Maud, Department of Neurology, Paul L. Foster School of Medicine, Texas Tech University of Health Sciences Center, El Paso, TX 79905, United States.
Gustavo J Rodriguez, Department of Neurology, Paul L. Foster School of Medicine, Texas Tech University of Health Sciences Center, El Paso, TX 79905, United States.
References
- 1.Brott T, Broderick J, Kothari R, Barsan W, Tomsick T, Sauerbeck L, Spilker J, Duldner J, Khoury J. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28:1–5. doi: 10.1161/01.str.28.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Kazui S, Naritomi H, Yamamoto H, Sawada T, Yamaguchi T. Enlargement of spontaneous intracerebral hemorrhage. Incidence and time course. Stroke. 1996;27:1783–1787. doi: 10.1161/01.str.27.10.1783. [DOI] [PubMed] [Google Scholar]
- 3.Broderick JP, Brott TG, Tomsick T, Barsan W, Spilker J. Ultra-early evaluation of intracerebral hemorrhage. J Neurosurg. 1990;72:195–199. doi: 10.3171/jns.1990.72.2.0195. [DOI] [PubMed] [Google Scholar]
- 4.Kazui S, Minematsu K, Yamamoto H, Sawada T, Yamaguchi T. Predisposing factors to enlargement of spontaneous intracerebral hematoma. Stroke. 1997;28:2370–2375. doi: 10.1161/01.str.28.12.2370. [DOI] [PubMed] [Google Scholar]
- 5.Becker KJ, Baxter AB, Bybee HM, Tirschwell DL, Abouelsaad T, Cohen WA. Extravasation of radiographic contrast is an independent predictor of death in primary intracerebral hemorrhage. Stroke. 1999;30:2025–2032. doi: 10.1161/01.str.30.10.2025. [DOI] [PubMed] [Google Scholar]
- 6.Ohwaki K, Yano E, Nagashima H, Hirata M, Nakagomi T, Tamura A. Blood pressure management in acute intracerebral hemorrhage: relationship between elevated blood pressure and hematoma enlargement. Stroke. 2004;35:1364–1367. doi: 10.1161/01.STR.0000128795.38283.4b. [DOI] [PubMed] [Google Scholar]
- 7.Anderson CS, Heeley E, Huang Y, Wang J, Stapf C, Delcourt C, Lindley R, Robinson T, Lavados P, Neal B, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368:2355–2365. doi: 10.1056/NEJMoa1214609. [DOI] [PubMed] [Google Scholar]
- 8.Qureshi AI. Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH): rationale and design. Neurocrit Care. 2007;6:56–66. doi: 10.1385/ncc:6:1:56. [DOI] [PubMed] [Google Scholar]
- 9.Qureshi AI, Palesch YY, Barsan WG, Hanley DF, Hsu CY, Martin RL, Moy CS, Silbergleit R, Steiner T, Suarez JI, et al. Intensive Blood-Pressure Lowering in Patients with Acute Cerebral Hemorrhage. N Engl J Med. 2016;375:1033–1043. doi: 10.1056/NEJMoa1603460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qureshi AI, Hanel RA, Kirmani JF, Yahia AM, Hopkins LN. Cerebral blood flow changes associated with intracerebral hemorrhage. Neurosurg Clin N Am. 2002;13:355–370. doi: 10.1016/s1042-3680(02)00012-8. [DOI] [PubMed] [Google Scholar]
- 11.Strandgaard S. Autoregulation of cerebral blood flow in hypertensive patients. The modifying influence of prolonged antihypertensive treatment on the tolerance to acute, drug-induced hypotension. Circulation. 1976;53:720–727. doi: 10.1161/01.cir.53.4.720. [DOI] [PubMed] [Google Scholar]
- 12.Powers WJ, Zazulia AR, Videen TO, Adams RE, Yundt KD, Aiyagari V, Grubb RL Jr, Diringer MN. Autoregulation of cerebral blood flow surrounding acute (6 to 22 hours) intracerebral hemorrhage. Neurology. 2001;57:18–24. doi: 10.1212/wnl.57.1.18. [DOI] [PubMed] [Google Scholar]
- 13.NINDS ICH Workshop Participants. Priorities for clinical research in intracerebral hemorrhage: report from a National Institute of Neurological Disorders and Stroke workshop. Stroke. 2005;36:e23–e41. doi: 10.1161/01.STR.0000155685.77775.4c. [DOI] [PubMed] [Google Scholar]
- 14.Zazulia AR, Diringer MN, Videen TO, Adams RE, Yundt K, Aiyagari V, Grubb RL Jr, Powers WJ. Hypoperfusion without ischemia surrounding acute intracerebral hemorrhage. J Cereb Blood Flow Metab. 2001;21:804–810. doi: 10.1097/00004647-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Kobari M, Gotoh F, Tomita M, Tanahashi N, Shinohara T, Terayama Y, Mihara B. Bilateral hemispheric reduction of cerebral blood volume and blood flow immediately after experimental cerebral hemorrhage in cats. Stroke. 1988;19:991–996. doi: 10.1161/01.str.19.8.991. [DOI] [PubMed] [Google Scholar]
- 16.Bullock R, Brock-Utne J, van Dellen J, Blake G. Intracerebral hemorrhage in a primate model: effect on regional cerebral blood flow. Surg Neurol. 1988;29:101–107. doi: 10.1016/0090-3019(88)90065-1. [DOI] [PubMed] [Google Scholar]
- 17.Nehls DG, Mendelow AD, Graham DI, Sinar EJ, Teasdale GM. Experimental intracerebral hemorrhage: progression of hemodynamic changes after production of a spontaneous mass lesion. Neurosurgery. 1988;23:439–444. doi: 10.1227/00006123-198810000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Butcher KS, Baird T, MacGregor L, Desmond P, Tress B, Davis S. Perihematomal edema in primary intracerebral hemorrhage is plasma derived. Stroke. 2004;35:1879–1885. doi: 10.1161/01.STR.0000131807.54742.1a. [DOI] [PubMed] [Google Scholar]
- 19.Schellinger PD, Fiebach JB, Hoffmann K, Becker K, Orakcioglu B, Kollmar R, Jüttler E, Schramm P, Schwab S, Sartor K, et al. Stroke MRI in intracerebral hemorrhage: is there a perihemorrhagic penumbra? Stroke. 2003;34:1674–1679. doi: 10.1161/01.STR.0000076010.10696.55. [DOI] [PubMed] [Google Scholar]
- 20.Hirano T, Read SJ, Abbott DF, Sachinidis JI, Tochon-Danguy HJ, Egan GF, Bladin CF, Scott AM, McKay WJ, Donnan GA. No evidence of hypoxic tissue on 18F-fluoromisonidazole PET after intracerebral hemorrhage. Neurology. 1999;53:2179–2182. doi: 10.1212/wnl.53.9.2179. [DOI] [PubMed] [Google Scholar]
- 21.Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, Fung GL, Goldstein JN, Macdonald RL, Mitchell PH, Scott PA, Selim MH, Woo D; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2015;46:2032–2060. doi: 10.1161/STR.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 22.D’Amore C, Paciaroni M. Border-zone and watershed infarctions. Front Neurol Neurosci. 2012;30:181–184. doi: 10.1159/000333638. [DOI] [PubMed] [Google Scholar]
- 23.Gerraty RP, Gilford EJ, Gates PC. Watershed cerebral infarction associated with perioperative hypotension. Clin Exp Neurol. 1993;30:82–89. [PubMed] [Google Scholar]
- 24.Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke. 1989;20:45–52. doi: 10.1161/01.str.20.1.45. [DOI] [PubMed] [Google Scholar]
- 25.Baumbach GL, Heistad DD. Cerebral circulation in chronic arterial hypertension. Hypertension. 1988;12:89–95. doi: 10.1161/01.hyp.12.2.89. [DOI] [PubMed] [Google Scholar]
- 26.Agnoli A, Fieschi C, Bozzao L, Battistini N, Prencipe M. Autoregulation of cerebral blood flow. Studies during drug-induced hypertension in normal subjects and in patients with cerebral vascular diseases. Circulation. 1968;38:800–812. doi: 10.1161/01.cir.38.4.800. [DOI] [PubMed] [Google Scholar]
- 27.Powers WJ, Zazulia AR. The use of positron emission tomography in cerebrovascular disease. Neuroimaging Clin N Am. 2003;13:741–758. doi: 10.1016/s1052-5149(03)00091-1. [DOI] [PubMed] [Google Scholar]
- 28.Stiefel MF, Udoetuk JD, Spiotta AM, Gracias VH, Goldberg A, Maloney-Wilensky E, Bloom S, Le Roux PD. Conventional neurocritical care and cerebral oxygenation after traumatic brain injury. J Neurosurg. 2006;105:568–575. doi: 10.3171/jns.2006.105.4.568. [DOI] [PubMed] [Google Scholar]
- 29.Stocchetti N, Chieregato A, De Marchi M, Croci M, Benti R, Grimoldi N. High cerebral perfusion pressure improves low values of local brain tissue O2 tension (PtiO2) in focal lesions. Acta Neurochir Suppl. 1998;71:162–165. doi: 10.1007/978-3-7091-6475-4_47. [DOI] [PubMed] [Google Scholar]
- 30.Zheng M, Sun A, Sun Q, Zhang H, Fan D. Clinical and Imaging Analysis of a Cerebellar Watershed Infarction. Chinese Medicine. 2015;6:54–60. [Google Scholar]
- 31.Moriwaki H, Matsumoto M, Hashikawa K, Oku N, Ishida M, Seike Y, Watanabe Y, Hougaku H, Handa N, Nishimura T. Hemodynamic aspect of cerebral watershed infarction: assessment of perfusion reserve using iodine-123-iodoamphetamine SPECT. J Nucl Med. 1997;38:1556–1562. [PubMed] [Google Scholar]
- 32.Lee PH, Bang OY, Oh SH, Joo IS, Huh K. Subcortical white matter infarcts: comparison of superficial perforating artery and internal border-zone infarcts using diffusion-weighted magnetic resonance imaging. Stroke. 2003;34:2630–2635. doi: 10.1161/01.STR.0000097609.66185.05. [DOI] [PubMed] [Google Scholar]
- 33.Gould B, McCourt R, Gioia LC, Kate M, Hill MD, Asdaghi N, Dowlatshahi D, Jeerakathil T, Coutts SB, Demchuk AM, et al. Acute blood pressure reduction in patients with intracerebral hemorrhage does not result in borderzone region hypoperfusion. Stroke. 2014;45:2894–2899. doi: 10.1161/STROKEAHA.114.005614. [DOI] [PubMed] [Google Scholar]


