Abstract
Introduction
The extent of lymphadenectomy in the surgical treatment of gastric cancer is a topic of controversy among surgeons. This study was conducted to analyze the American National Cancer Database (NCDB) and conclude the optimal extent of lymphadenectomy for gastric adenocarcinoma.
Methods
The NCDB for gastric cancer was utilized. Patients who received at least a partial gastrectomy were included. Patients with metastatic disease, unknown TNM stages, R1/R2 resection, or treated with a palliative intent were excluded. Joinpoint regression was used to identify the extent of lymphadenectomy that reflects the optimal survival. Cox regression analysis and Bayesian information criterion were used to identify significant survival predictors. Kaplan-Meier was applied to study overall survival and stage migration.
Results
40,281 patients of 168,377 met the inclusion criteria. Joinpoint analysis showed that dissection of 29 nodes provides the optimal median survival for the overall population. Regression analysis reported the cutoff ≥29 to have a better fit in the prognostic model than that of ≥15. Dissection of ≥29 nodes in the higher stages provides a comparable overall survival to the immediately lower stage. Nonetheless, the retrieval of ≥15 nodes proved to be adequate for staging without a significant stage migration compared to ≥29 nodes.
Conclusion
The extent of lymphadenectomy in gastric adenocarcinoma is a marker of improved resection which reflects in a longer overall survival. Our analysis concludes that the dissection of ≥15 nodes is adequate for staging. However, the dissection of 29 nodes might be needed to provide a significantly improved survival.
Keywords: Gastric cancer, Lymphadenectomy, Survival, Cancer staging
INTRODUCTION
In the past decade, gastric cancer (GC) was the fifth most common cancer and the third leading cause of cancer-related death worldwide, with an estimated incidence of one million new cases yearly, and a case fatality rate of 60%–70% with a 2-fold male preponderance [1]. More than 40% of GC cases occur in East Asia [2]. In the United States, approximately 26,370 new cases and 10,730 deaths from GC occurred in 2016 [3]. Meanwhile, Korea has the highest incidence rate in the world relative to the population, with 33,000 new cases of GC annually [4] and an annual mortality rate of up to 8,000 [5]. The incidence and mortality rates increased to 74,000 and 34,000 in Japan and to 283,500 and 221,500 in China, respectively [4]. This discrepancy in epidemiology may have contributed to the significant differences in the surgical expertise concerning this cancer between the eastern and the western hemispheres as well as a subsequent controversy about the adequate extent of lymphadenectomy.
D2 lymphadenectomy is considered the standard treatment for GCs beyond the T1 depth in East Asia, with numerous reports demonstrating a survival improvement in the D2 vs. D1 group dissections [6,7,8]. The Western stance against extensive nodal dissection stems from the results of large prospective, randomized trials in the United Kingdom [9] and the Netherlands [10] in the 1990s. Despite the efforts taken to standardize the procedures in these trials, a noteworthy high rate of morbidity and mortality was documented compared to the Eastern trials, without a significant survival benefit in the D2 arm. These results guided the Western surgeons to refrain from aggressive nodal dissections in GC surgeries.
The National Comprehensive Cancer Network (NCCN) currently recommends the retrieval of at least 15 nodes in GC to achieve proper staging and avoid stage migration [11]. However, the Surveillance, Epidemiology, and End Results (SEER) analysis demonstrated that the median number of examined nodes in the United States is 11 [12]. Importantly, evidence shows that even compliance with the current NCCN guidelines may lead to understaging, with an evident stage migration in approximately one-third of the patients whose nodal stages were upstaged as the number of retrieved nodes increased [13,14].
In this study, we used the National Cancer Database (NCDB), which is a large population-based cancer registry collected and maintained by the American College of Surgeons Committee on Cancer and records approximately 70% of the cancer cases nationwide. We aimed to analyze the NCDB for GC, study the impact of the extent of lymphadenectomy on staging and overall survival (OS) in an exclusively western patient population, and determine the level of nodal dissection that provides the optimal outcome in both staging and OS.
MATERIALS AND METHODS
The NCDB contains records of 168,377 patients diagnosed with GC between 2004 and 2014. Multiple steps of inclusion/exclusion were implemented to ensure proper selection for the purposes of the study as follows:
1) All patients with metastatic disease identified through data items CS_METS_AT_DX and ANALYTIC_GROUP_STAGE were excluded from the study. Selected patients were confirmed not to have any evidence of metastatic disease through data items CS_METS_LIVER, CS_METS_LUNG, CS_METS_BRAIN, and CS_METS_BONE.
2) All patients who were reported to receive any treatment modality with a palliative intent (data item PALLIATIVE_CARE) were excluded.
3) Patients who did not receive surgical treatment or “limited” resection such as “local destruction” or “local excision” as reported in data item RX_SUMM_SURG_PRIM_SITE were excluded. Only patients who received surgical intervention reported to be at least partial gastrectomy were included in the analysis.
4) Only patients who underwent R0 resection confirmed microscopically were included in the analysis (data item RX_SUMM_SURGICAL_MARGINS).
5) Only patients confirmed to have gastric adenocarcinoma on final pathology were included. Other pathologies were excluded (data item HISTOLOGY using the International Classification of Diseases for Oncology-3).
6) All patients who had a missing T stage (missing pathology report) or N stage (unknown number of resected nodes or positive nodes) were excluded (data items TNM_PATH_T and TNM_PATH_N).
7) Patients with stage 0 gastric adenocarcinoma were removed from the dataset.
8) All patients were staged according to the 7th edition of the American Joint Committee on Cancer (AJCC) staging classification of GC.
SPSS version 22.0 (IBM Corp., Armonk, NY, USA) was used to conduct the statistical analysis. Cox univariate and multivariate logistic regressions were utilized to determine significant predictors of survival. Joinpoint analysis was applied using the Joinpoint regression software 4.4.0.0 produced by the surveillance research program of the National Cancer Institute (Calverton, MD, USA). The median survival for each number of resected nodes, the number of resected nodes, and tumor, node, and metastasis (TNM) stage were incorporated in the model as the dependent factor, independent factor, and by-variable, respectively. The analysis was conducted to the node resection range between 1 and 40. For nodal resections >40, the number of cases at each point was <0.5% of the entire dataset. This limitation in size rendered the medians “unreached,” thus inapplicable in the analysis. Kaplan-Meier method was used to determine patients' survival according to the TNM stage and the extent of lymphadenectomy. Bayesian information criterion (BIC) was used to determine the optimal nodal cutoff in the models created based on the results of multivariate regression. Statistical significance was set at P<0.05 throughout the analysis.
RESULTS
Dataset description
After the application of the inclusion/exclusion criteria, 40,281 patients with stage 1–3 gastric adenocarcinoma and having a known number of resected and positive lymph nodes, approached with no palliative intent, who underwent at least partial gastrectomy as a surgical treatment with R0 resection were included. The mean patient age was 66.33±12.63 years, and 64.9% of the patients were men. Table 1 summarizes the patients' demographic and perioperative characteristics.
Table 1. Summary of the patients' demographic and perioperative characteristics as reported in the NCDB.
| Characteristics | Values | |
|---|---|---|
| Age | 66.33±12.63 | |
| Sex | Male | 26,137 (64.9) |
| Female | 14,144 (35.1) | |
| Race | White | 29,639 (73.6) |
| Black | 6,047 (15.0) | |
| Other | 4,595 (11.3) | |
| Charlson score | 0 | 27,112 (67.3) |
| 1 | 9,735 (24.2) | |
| ≥2 | 3,434 (8.5) | |
| Grade | Well-differentiated | 2,616 (6.5) |
| Moderately differentiated | 11,879 (29.5) | |
| Poorly differentiated | 23,386 (58.0) | |
| Not reported | 2,400 (6.0) | |
| Location | Cardia (C16.0) | 13,793 (34.2) |
| Fundus (C16.1) | 1,308 (3.2) | |
| Corpus (C16.2) | 3,149 (7.8) | |
| Antrum (C16.3) | 8,842 (22.0) | |
| Pylorus (C16.4) | 1,255 (3.1) | |
| Lesser curvature (C16.5) | 3,786 (9.4) | |
| Greater curvature (C16.6) | 1,681 (4.2) | |
| Overlapping lesion (C16.8) | 2,343 (5.8) | |
| NOS (C16.9) | 4,124 (10.2) | |
| Mean No. of resected nodes (median) | 16.04±10.92 (14) | |
| No. of patients with ≥15 nodes retrieved | 20,815 (51.7) | |
| Mean No. of positive LN (median) | 2.68±4.56 (1) | |
| Mean LOH | 11.23±10.99 | |
| 30-day mortality | 1,302 (3.2) | |
| Chemotherapy | 20,858 (51.8) | |
| Radiation | 14,682 (36.4) | |
| AJCC Stage | I | 12,655 (31.4) |
| II | 19,116 (47.5) | |
| III | 8,510 (21.1) | |
| T stage (7th edition) | T1 | 9,941 (24.7) |
| T2 | 11,590 (28.8) | |
| T3 | 14,444 (35.9) | |
| T4 | 4,306 (10.7) | |
| N stage (7th edition) | N0 (0 positive nodes) | 19,564 (48.6) |
| N1 (1–2 positive nodes) | 8,189 (20.3) | |
| N2 (3–6 positive nodes) | 6,695 (16.6) | |
| N3 (>7 positive nodes) | 5,833 (14.5) |
All values are expressed as mean±standard deviation or number (%) not otherwise specified.
NCDB = National Cancer Database; NOS = Not Otherwise Specified; LN = lymph node; LOH = length of hospitalization; AJCC = American Joint Committee on Cancer.
Dataset validation
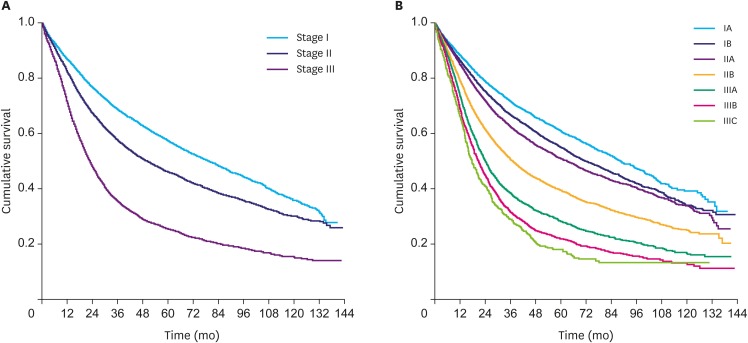
To validate the selection, a survival analysis was conducted on the selected group of patients based on their AJCC TNM stages. The analysis showed a median survival of 79.64±1.66, 50.01±0.98, and 22.57±0.37 months for stages I, II, and III, respectively. Fig. 1 demonstrates the results of the survival analysis. The 5-year survival rates in the selected population were: stage IA: 66.0%; stage IB: 48.2%; stage IIA: 44.1%; stage IIB: 34.5%; stage IIIA: 24.8%; stage IIIB: 16.8%; and stage IIIC: 11.9%, which is in accordance with the survival rates of patients with GC following surgery as reported by the American Cancer Society [15].
Fig. 1.
Kaplan-Meier for OS according to the AJCC stage. (A) Stage I (median, 79.64±1.66 months); stage II (median, 50.01±0.98 months); and stage III (median, 22.57±0.37 months). (B) Stage IA (median, 89.07±2.80 months); stage IB (median, 72.74±2.02 months); stage IIA (median, 63.47±1.59 months); stage IIB (median, 37.32±0.89 months); stage IIIA (24.25±0.54 months); stage IIIB (median, 20.73±0.58 months); and stage IIIC (median, 17.81±1.06 months).
OS = overall survival; AJCC = American Joint Committee on Cancer.
Joinpoint regression
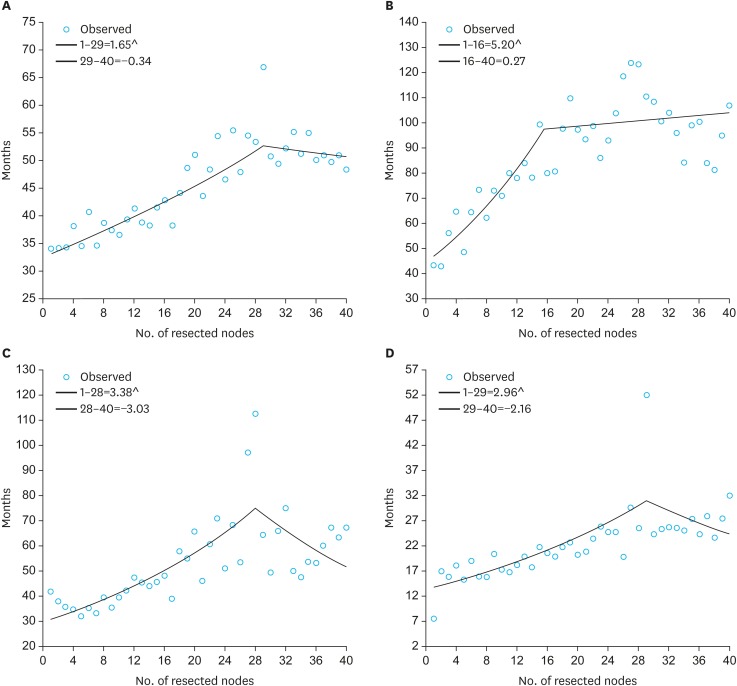
Joinpoint analysis was applied as described above to determine the optimal number of nodal resection for improved median survival. Regression analysis showed that the resection of 29 nodes yielded the optimal survival benefit for the entire population. Subgroup analysis indicated that 16, 28, and 29 nodes were the optimal numbers of retrieved nodes for improved survival in stages I, II, and III, respectively. Fig. 2 shows the results of the Joinpoint analysis.
Fig. 2.
Joinpoint analysis to determine the optimal node resection for median survival. (A) Overall: Joinpoint determined at 29 nodes. (B) Stage I: Joinpoint determined at 16 nodes. (C) Stage II: Joinpoint determined at 28 nodes. (D) Stage III: Joinpoint determined at 29 nodes.
Regression analysis
The results of Cox univariate and multivariate regression analyses showed that age, presence of ≥2 comorbidities according to the Charlson score, tumor grade, higher nodal stage, and cardiac tumors were poor prognostic factors, whereas female sex, chemotherapy, radiation, and the number of resected nodes (as a continuous variable) were favorable prognostic predictors of OS. Table 2 demonstrates the results of the Cox univariate and multivariate regression analyses with the reported hazard ratios.
Table 2. Results of univariate and multivariate Cox regression analyses for significant predictors of survival in gastric adenocarcinoma.
| Characteristics | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (CI) | P | HR (CI) | P | ||
| Age | 1.021 (1.019–1.024) | <0.001 | 1.025 (1.023–1.026) | <0.001 | |
| Sex | |||||
| Male | Reference | Reference | |||
| Female | 0.911 (0.882–0.968) | <0.001 | 0.908 (0.880–0.937) | <0.001 | |
| Race | |||||
| White | Reference | ||||
| Black | 0.999 (0.994–1.061) | 0.152 | NS | NS | |
| Other | 0.990 (0.964–1.044) | 0.118 | NS | NS | |
| Charlson score | |||||
| 0 | Reference | Reference | |||
| 1 | 1.122 (1.091–1.163) | <0.001 | 1.083 (1.046–1.120) | <0.001 | |
| ≥2 | 1.455 (1.407–1.496) | <0.001 | 1.407 (1.340–1.478) | <0.001 | |
| Grade | |||||
| Well-differentiated | Reference | Reference | |||
| Moderately differentiated | 1.588 (1.439–1.766) | <0.001 | 1.304 (1.211–1.404) | <0.001 | |
| Poorly differentiated | 2.111 (1.883–2.241) | <0.001 | 1.624 (1.511–1.746) | <0.001 | |
| Tumor location | |||||
| Non-cardiac adenocarcinoma | Reference | Reference | |||
| Cardiac adenocarcinoma | 1.128 (1.100–1.151) | <0.001 | 1.396 (1.352–1.441) | <0.001 | |
| Chemotherapy | 0.859 (0.838–0.875) | <0.001 | 0.844 (0.809–0.880) | <0.001 | |
| Radiation | 0.931 (0.907–0.944) | <0.001 | 0.921 (0.844–0.960) | <0.001 | |
| No. of resected nodes | 0.989 (0.988–0.991) | <0.001 | 0.978 (0.977–0.980) | <0.001 | |
| Nodal stage | |||||
| N0 | Reference | Reference | |||
| N1 | 1.742 (1.678–1.808) | <0.001 | 1.896 (1.821–1.975) | <0.001 | |
| N2 | 2.379 (2.292–2.469) | <0.001 | 2.841 (2.724–2.963) | <0.001 | |
| N3 | 3.301 (3.181–3.426) | <0.001 | 4.511 (4.313–4.719) | <0.001 | |
HR = hazard ratio; CI = confidence interval; NS = not significant.
BIC
Based on the results of regression analysis, 2 models were created including the entire population and using the significant survival predictors, in addition to 2 nodal dissection cutoffs: ≥15 nodes per the current NCCN guidelines and ≥29 nodes based on our Joinpoint analysis. A total of 20,815 patients (51.7%) had ≥15 nodes retrieved as recommended by the NCCN, whereas 4,640 (11.5%) had ≥29 nodes resected. The BIC for the model with ≥15 nodes was 21,860.426. This was significantly higher than the one with the cutoff of ≥29 nodes, which was at 19,705.648, indicating a better fit when the latter cutoff is used.
Survival benefit
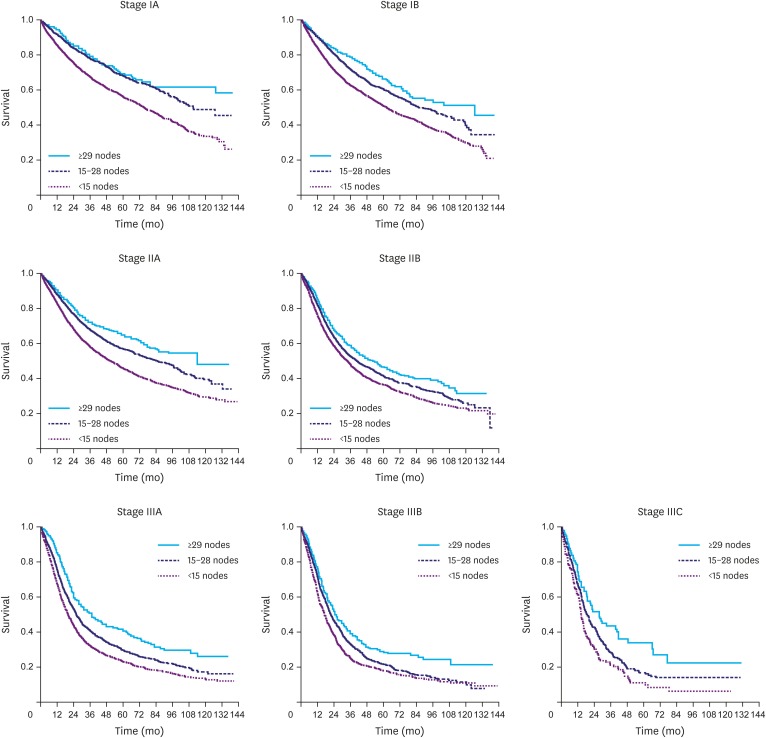
The survival benefit was compared for each AJCC stage based on the 2 dissection cutoffs: ≥15 nodes in compliance with the current NCCN guidelines and ≥29 nodes in compliance with the present NCDB Joinpoint. Notably, using ≥15 nodes as a cutoff did not improve survival in late-stage disease compared to the immediately lower stage. However, compliance with the ≥29 node cutoff provided a comparable median survival toward the immediately lower stage in IB (compared to IA), IIA (compared to IB), IIIC (compared to IIIB), and a trend in IIIA (compared to IIB), suggesting that the tumor is surgically downgraded in these stages when ≥29 nodes are retrieved during lymphadenectomy. The results are summarized in Table 3. Moreover, Kaplan-Meier curves demonstrated an increased survival between noncompliance with the nodal dissection guidelines (<15 nodes), compliance with the NCCN guidelines but not the NCDB Joinpoint (15–28 nodes), and compliance with both (≥29 nodes) as shown in Fig. 3.
Table 3. Survival benefit based on the AJCC TNM stages and compliance with either the current NCCN guidelines (≥15 nodes) or with the NCDB Joinpoint (≥29 nodes).
| AJCC TNM stages | Stage IA | Stage IB | Stage IIA | Stage IIB | Stage IIIA | Stage IIIB | Stage IIIC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compliance with the NCCN guidelines (≥15 nodes retrieved) | |||||||||||||
| Median survival (mon) | 126.95±9.56 | 94.26±4.83 | 91.17±3.65 | 42.58±1.82 | 28.12±0.97 | 22.67±0.79 | 20.01±1.43 | ||||||
| P | <0.001 | 0.019 | <0.001 | <0.001 | <0.001 | 0.029 | |||||||
| Compliance with the current NCDB Joinpoint (≥29 nodes retrieved) | |||||||||||||
| Median survival (mon) | 127.51±33.82 | 126.26±26.13 | 114.46±22.95 | 52.93±4.34 | 43.22±3.09 | 25.13±1.97 | 23.56±4.26 | ||||||
| P | 0.116 | 0.340 | <0.001 | 0.030 | <0.001 | 0.881 | |||||||
AJCC = American Joint Committee on Cancer; TNM = tumor, node, and metastasis; NCCN = National Comprehensive Cancer Network; NCBD = National Cancer Database.
Fig. 3.
Kaplan-Meier curves demonstrating the survival benefit for each TNM stage based on the cutoff of retrieved nodes.
TNM = tumor, node, and metastasis.
Stage migration
To demonstrate the effect of stage migration in these survival curves, the rate of each N stage and the mean number of metastatic nodes were compared for each T stage. Remarkably, the retrieval of <15 nodes resulted in an evident nodal understaging throughout the T stages.
Also, the results show that the nodal sampling of ≥15 nodes is adequate for nodal staging in T1–T3 given the correspondence in N stages compared to ≥29 nodes, which indicates that the survival benefit between groups is truly attributed to a better disease control and not stage migration.
In T4, however, more than 29 nodes are required to achieve a more accurate nodal staging, suggesting a combined effect of adequate dissection and stage migration on the survival difference. The results are shown in Table 4.
Table 4. Comparison of the 3 lymphadenectomy cutoffs of inadequate nodal sampling (<15 nodes), compliance with the NCCN guidelines but not NCDB Joinpoint (15–28 nodes), and compliance with both (≥29 nodes) to demonstrate the effect of nodal stage migration as the number of retrieved nodes increases.
| T stage | <15 nodes retrieved | 15–28 nodes retrieved | ≥29 nodes retrieved | P | ||
|---|---|---|---|---|---|---|
| <15 nodes retrieved vs. 15–28 nodes retrieved | 15–28 nodes retrieved vs. ≥29 nodes retrieved | |||||
| T1 | Rate of N0 (%) | 79.9 | 73.6 | 71.8 | <0.001 | 0.281 |
| Rate of N1 (%) | 11.4 | 15.2 | 15.3 | |||
| Rate of N2 (%) | 7.8 | 9.8 | 11.6 | |||
| Rate of N3 (%) | 0.9 | 1.4 | 1.3 | |||
| Positive nodes | 0.54±1.56 | 0.99±2.73 | 1.16±3.24 | <0.001 | 0.080 | |
| T2 | Rate of N0 (%) | 48.2 | 38.9 | 37.8 | <0.001 | 0.207 |
| Rate of N1 (%) | 26.5 | 23.2 | 21.6 | |||
| Rate of N2 (%) | 19.6 | 19.6 | 20.3 | |||
| Rate of N3 (%) | 5.7 | 18.3 | 20.3 | |||
| Positive nodes | 1.69±2.52 | 3.23±4.10 | 3.48±5.33 | <0.001 | 0.074 | |
| T3 | Rate of N0 (%) | 32.3 | 23.0 | 22.8 | <0.001 | 0.162 |
| Rate of N1 (%) | 30.7 | 23.8 | 22.0 | |||
| Rate of N2 (%) | 25.1 | 29.3 | 30.5 | |||
| Rate of N3 (%) | 11.9 | 23.9 | 24.7 | |||
| Positive nodes | 2.88±3.35 | 5.20±5.31 | 6.90±8.55 | <0.001 | <0.001 | |
| T4 | Rate of N0 (%) | 30.8 | 16.1 | 11.7 | <0.001 | 0.031 |
| Rate of N1 (%) | 34.9 | 19.0 | 18.3 | |||
| Rate of N2 (%) | 21.9 | 34.7 | 34.4 | |||
| Rate of N3 (%) | 12.4 | 30.2 | 35.6 | |||
| Positive nodes | 3.58±4.19 | 7.96±6.99 | 13.86±13.01 | <0.001 | <0.001 | |
All values are expressed as mean±standard deviation or percentage.
NCCN = National Comprehensive Cancer Network; NCBD = National Cancer Database.
Operative outcomes
The NCDB reports on the length of hospitalization (LOH), 30-day readmission, and 30-day mortality as the main surgical outcomes. The resection of ≥29 nodes was associated with a significantly higher 30-day mortality (4.3%) compared to resection of 15–28 nodes and <15 nodes (3.0% and 2.1%, respectively; P<0.001). Other outcomes were not different between the groups of node dissection (<15, 15–28, and ≥29 nodes), including 30-day readmission rates (6.6%, 6.0%, and 6.7%, respectively; P=0.097), and mean LOH (11.54, 10.63, and 11.15 days, respectively; P=0.198).
DISCUSSION
The standard extent of lymphadenectomy in GC surgery is yet to be established among western surgeons. The current western practice is mostly influenced by the concern of the high morbidity and mortality following D2 lymphadenectomy, without a significant survival benefit based on the British [9] and Dutch trials [10], despite some positive evidence of the role of extensive node dissection in other studies [16,17]. Increasing evidence shows that the morbidity in D2 lymphadenectomy can be significantly reduced by performing spleen- and pancreas-sparing nodal dissections [6,18,19,20], causing an important shift in the modern practice since the completion of the above-mentioned trials.
For staging purposes, the retrieval of at least 15 nodes was reported to be adequate [21,22], a guideline currently adopted and recommended by the NCCN. Nevertheless, the impact of lymphadenectomy on survival is not clearly stated in the current guidelines.
In our analysis, we used the Joinpoint regression to detect the extent of lymphadenectomy that yields the optimal survival and concluded that the retrieval of 29 nodes is the “Joinpoint” for the overall population, which is in exact agreement with the results of a recent analysis of the combined SEER and Yonsei University databases [23].
Joinpoint is a statistical software for the analysis of trends using joinpoint models; it takes trend data (e.g., incidence, rates, survival, and among others) and fits the simplest joinpoint model that the data allow to detect a change or modification in the behavior of the trend [24]. Interestingly, our subgroup analysis demonstrated that the survival benefit of lymphadenectomy for stage I cancers relapses to an insignificant slope after 16 nodes. On the other hand, stages II and III demonstrate an obvious survival benefit from a more extensive nodal dissection of up to 28 and 29 nodes, respectively. In the randomized trial by the Italian Gastric Cancer Study Group, a similar conclusion was made, indicating that extensive lymphadenectomy may only provide longer survival in the late stages of GC [25].
With regard to staging, our results support that the retrieval of ≥15 nodes is adequate to establish an accurate nodal stage as recommended by the NCCN, despite a trend toward the need for more nodes in T4 cancers to avoid stage migration into a better prognosis category and a false inflation of the survival in this group of patients. Notably, the rate of compliance with the NCCN guidelines to retrieve at least 15 nodes in the NCDB was only 48%. Many national registry studies demonstrated that <40% of gastrectomies were compliant with these guidelines in the United States and the United Kingdom [12,26,27].
The same analysis also proved that the survival benefit associated with more extensive nodal dissection is attributed to aggressive disease control rather than stage migration. However, an aggressive approach in surgery for upper gastrointestinal cancer in western populations has been accompanied by a high rate of morbidity and mortality, mainly due to the low volume and limited surgeon experience. A “large-volume” center for GC in the United States is defined to perform >20 gastrectomies per year [28,29] compared to the Eastern experience where large tertiary care centers perform at least 200 GC surgeries yearly [30]. As a result, poorer surgical outcomes were noted in western populations, with inpatient mortality rates of 4%–9% per Medicare data [29] compared with the <1% mortality rate in Korean and Japanese trials [31,32]. The NCDB 2004–2014 reports a 30-day mortality of 3.4% in gastrectomies performed with curative intent for stages I–III gastric adenocarcinoma, with higher rates in more extensive lymphadenectomies.
The limitations of the current analysis originate from its retrospective nature, data loss and misreport, and lack of detailed surgical inputs and outcomes, all of which are inherent shortcomings of registry-based data banks. Therefore, we were not able to study the impact of certain factors known to influence the outcome, such as incidental splenectomy and distal pancreatectomy as part of the extensive lymphadenectomy. Another criticism often highlighted in such analyses is patient selection. We applied very strict inclusion/exclusion criteria to achieve a proper selection of the cases of interest and validated the dataset with epidemiologic and survival analyses that matched the nationally reported oncologic data. These steps were taken to ensure that the selected patients represent the population of the surgically treated gastric adenocarcinoma and that the conclusions of this analysis would provide a clinical relevance to the management of these patients.
The extent of lymphadenectomy in gastric adenocarcinoma is a marker of an improved disease control that results in longer OS. The results of our analysis indicated that retrieval of ≥15 nodes is adequate for staging. However, 29 nodes might be needed to achieve an optimal survival benefit throughout the AJCC TNM stages. Extensive lymphadenectomy in western populations continues to demonstrate high rates of postoperative mortality; thus, extrapolation of the current data should be done with caution with regard to patient selection for more extensive lymphadenectomy.
ACKNOWLEDGMENTS
We acknowledge Dr. Jinsong Chen from the Department of Biostatistics, University of Illinois College of Medicine, for his guidance during the statistical analysis of this work.
Footnotes
- Conceptualization: N.S.A, S.G.I.
- Data curation: N.S.A, S.G.I.
- Formal analysis: N.S.A.
- Investigation: N.S.A, S.G.I.
- Methodology: N.S.A, S.G.I.
- Project administration: S.G.I.
- Resources: N.S.A, S.G.I.
- Supervision: S.G.I.
- Validation: N.S.A, S.G.I.
- Visualization: N.S.A, S.G.I.
- Writing - original draft: N.S.A.
- Writing - review & editing: N.S.A, S.G.I.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Lin Y, Ueda J, Kikuchi S, Totsuka Y, Wei WQ, Qiao YL, et al. Comparative epidemiology of gastric cancer between Japan and China. World J Gastroenterol. 2011;17:4421–4428. doi: 10.3748/wjg.v17.i39.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 4.International Agency for Research on Cancer; World Health Organization. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012 [Internet] Lyon: International Agency for Research on Cancer; 2014. [cited 2017 Oct 20]. Available from: http://globocan.iarc.fr. [Google Scholar]
- 5.Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Prediction of cancer incidence and mortality in Korea, 2013. Cancer Res Treat. 2013;45:15–21. doi: 10.4143/crt.2013.45.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu CW, Hsiung CA, Lo SS, Hsieh MC, Chen JH, Li AF, et al. Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol. 2006;7:309–315. doi: 10.1016/S1470-2045(06)70623-4. [DOI] [PubMed] [Google Scholar]
- 7.Maruyama K, Okabayashi K, Kinoshita T. Progress in gastric cancer surgery in Japan and its limits of radicality. World J Surg. 1987;11:418–425. doi: 10.1007/BF01655804. [DOI] [PubMed] [Google Scholar]
- 8.de Aretxabala X, Konishi K, Yonemura Y, Ueno K, Yagi M, Noguchi M, et al. Node dissection in gastric cancer. Br J Surg. 1987;74:770–773. doi: 10.1002/bjs.1800740904. [DOI] [PubMed] [Google Scholar]
- 9.Cuschieri A, Fayers P, Fielding J, Craven J, Bancewicz J, Joypaul V, et al. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial. The Surgical Cooperative Group. Lancet. 1996;347:995–999. doi: 10.1016/s0140-6736(96)90144-0. [DOI] [PubMed] [Google Scholar]
- 10.Bonenkamp JJ, Songun I, Hermans J, Sasako M, Welvaart K, Plukker JT, et al. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet. 1995;345:745–748. doi: 10.1016/s0140-6736(95)90637-1. [DOI] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network (US) NCCN Clinical Practice Guidelines in Oncology. Gastric Cancer, Version 3. 2016. Fort Washington (PA): National Comprehensive Cancer Network; 2016. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Dang P, Raut CP, Pandalai PK, Maduekwe UN, Rattner DW, et al. Comparison of a lymph node ratio-based staging system with the 7th AJCC system for gastric cancer: analysis of 18,043 patients from the SEER database. Ann Surg. 2012;255:478–485. doi: 10.1097/SLA.0b013e31824857e2. [DOI] [PubMed] [Google Scholar]
- 13.Bunt AM, Hermans J, Smit VT, van de Velde CJ, Fleuren GJ, Bruijn JA. Surgical/pathologic-stage migration confounds comparisons of gastric cancer survival rates between Japan and Western countries. J Clin Oncol. 1995;13:19–25. doi: 10.1200/JCO.1995.13.1.19. [DOI] [PubMed] [Google Scholar]
- 14.Putchakayala K, Difronzo LA. D2 lymph node dissection improves staging in patients with gastric adenocarcinoma. Am Surg. 2011;77:1326–1329. [PubMed] [Google Scholar]
- 15.American Cancer Society. Survival Rates for Stomach Cancer, by Stage. Atlanta (GA): American Cancer Society; 2016. [Google Scholar]
- 16.Lee HK, Yang HK, Kim WH, Lee KU, Choe KJ, Kim JP. Influence of the number of lymph nodes examined on staging of gastric cancer. Br J Surg. 2001;88:1408–1412. doi: 10.1046/j.0007-1323.2001.01875.x. [DOI] [PubMed] [Google Scholar]
- 17.Siewert JR, Böttcher K, Stein HJ, Roder JD. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg. 1998;228:449–461. doi: 10.1097/00000658-199810000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu W, Choi GS, Chung HY. Randomized clinical trial of splenectomy versus splenic preservation in patients with proximal gastric cancer. Br J Surg. 2006;93:559–563. doi: 10.1002/bjs.5353. [DOI] [PubMed] [Google Scholar]
- 19.Biffi R, Chiappa A, Luca F, Pozzi S, Lo Faso F, Cenciarelli S, et al. Extended lymph node dissection without routine spleno-pancreatectomy for treatment of gastric cancer: low morbidity and mortality rates in a single center series of 250 patients. J Surg Oncol. 2006;93:394–400. doi: 10.1002/jso.20495. [DOI] [PubMed] [Google Scholar]
- 20.Uyama I, Ogiwara H, Takahara T, Kikuchi K, Iida S, Kubota T, et al. Spleen- and pancreas-preserving total gastrectomy with superextended lymphadenectomy including dissection of the para-aortic lymph nodes for gastric cancer. J Surg Oncol. 1996;63:268–270. doi: 10.1002/(SICI)1096-9098(199612)63:4<268::AID-JSO10>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 21.Karpeh MS, Leon L, Klimstra D, Brennan MF. Lymph node staging in gastric cancer: is location more important than Number? An analysis of 1,038 patients. Ann Surg. 2000;232:362–371. doi: 10.1097/00000658-200009000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077–3079. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 23.Woo Y, Goldner B, Ituarte P, Lee B, Melstrom L, Son T, et al. Lymphadenectomy with optimum of 29 lymph nodes retrieved associated with improved survival in advanced gastric cancer: a 25,000-patient international database study. J Am Coll Surg. 2017;224:546–555. doi: 10.1016/j.jamcollsurg.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Cancer Institute (US) Joinpoint Regression Program, Version 4.4.0.0 (Jan 4, 2017) Rockville (MD): National Cancer Institute; 2017. [Google Scholar]
- 25.Degiuli M, Sasako M, Ponti A, Vendrame A, Tomatis M, Mazza C, et al. Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br J Surg. 2014;101:23–31. doi: 10.1002/bjs.9345. [DOI] [PubMed] [Google Scholar]
- 26.Reid-Lombardo KM, Gay G, Patel-Parekh L, Ajani JA, Donohue JH Gastric Patient Care Evaluation Group from the Commission on Cancer. Treatment of gastric adenocarcinoma may differ among hospital types in the United States, a report from the National Cancer Data Base. J Gastrointest Surg. 2007;11:410–419. doi: 10.1007/s11605-006-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mullaney PJ, Wadley MS, Hyde C, Wyatt J, Lawrence G, Hallissey MT, et al. Appraisal of compliance with the UICC/AJCC staging system in the staging of gastric cancer. Br J Surg. 2002;89:1405–1408. doi: 10.1046/j.1365-2168.2002.02262.x. [DOI] [PubMed] [Google Scholar]
- 28.Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 29.Smith DL, Elting LS, Learn PA, Raut CP, Mansfield PF. Factors influencing the volume-outcome relationship in gastrectomies: a population-based study. Ann Surg Oncol. 2007;14:1846–1852. doi: 10.1245/s10434-007-9381-0. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt B, Yoon SS. D1 versus D2 lymphadenectomy for gastric cancer. J Surg Oncol. 2013;107:259–264. doi: 10.1002/jso.23127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sano T, Sasako M, Yamamoto S, Nashimoto A, Kurita A, Hiratsuka M, et al. Gastric cancer surgery: morbidity and mortality results from a prospective randomized controlled trial comparing D2 and extended para-aortic lymphadenectomy--Japan Clinical Oncology Group study 9501. J Clin Oncol. 2004;22:2767–2773. doi: 10.1200/JCO.2004.10.184. [DOI] [PubMed] [Google Scholar]
- 32.Park DJ, Lee HJ, Kim HH, Yang HK, Lee KU, Choe KJ. Predictors of operative morbidity and mortality in gastric cancer surgery. Br J Surg. 2005;92:1099–1102. doi: 10.1002/bjs.4952. [DOI] [PubMed] [Google Scholar]