Abstract
Human red blood cells (RBC), which are the cells most commonly used in the study of biological membranes, have some glycoproteins in their cell membrane. These membrane proteins are band 3 and glycophorins A–D, and some substoichiometric glycoproteins (e.g., CD44, CD47, Lu, Kell, Duffy). The oligosaccharide that band 3 contains has one N-linked oligosaccharide, and glycophorins possess mostly O-linked oligosaccharides. The end of the O-linked oligosaccharide is linked to sialic acid. In humans, this sialic acid is N-acetylneuraminic acid (NeuAc). Another sialic acid, N-glycolylneuraminic acid (NeuGc) is present in red blood cells of non-human origin. While the biological function of band 3 is well known as an anion exchanger, it has been suggested that the oligosaccharide of band 3 does not affect the anion transport function. Although band 3 has been studied in detail, the physiological functions of glycophorins remain unclear. This review mainly describes the sialo-oligosaccharide structures of band 3 and glycophorins, followed by a discussion of the physiological functions that have been reported in the literature to date. Moreover, other glycoproteins in red blood cell membranes of non-human origin are described, and the physiological function of glycophorin in carp red blood cell membranes is discussed with respect to its bacteriostatic activity.
Keywords: red blood cell, glycoproteins, biological function, glycophorin, band 3, O-linked oligosaccharides, N-acetylneuraminic acid, N-glycolylneuraminic acid
1. Introduction
The blood of mammals, such as humans, as well as of birds, reptiles, and teleosts, contains red blood cells (erythrocytes). Human red blood cells, which are the most commonly studied by researchers, are approximately 7 μm in diameter, and their centre has a dented discoid shape.
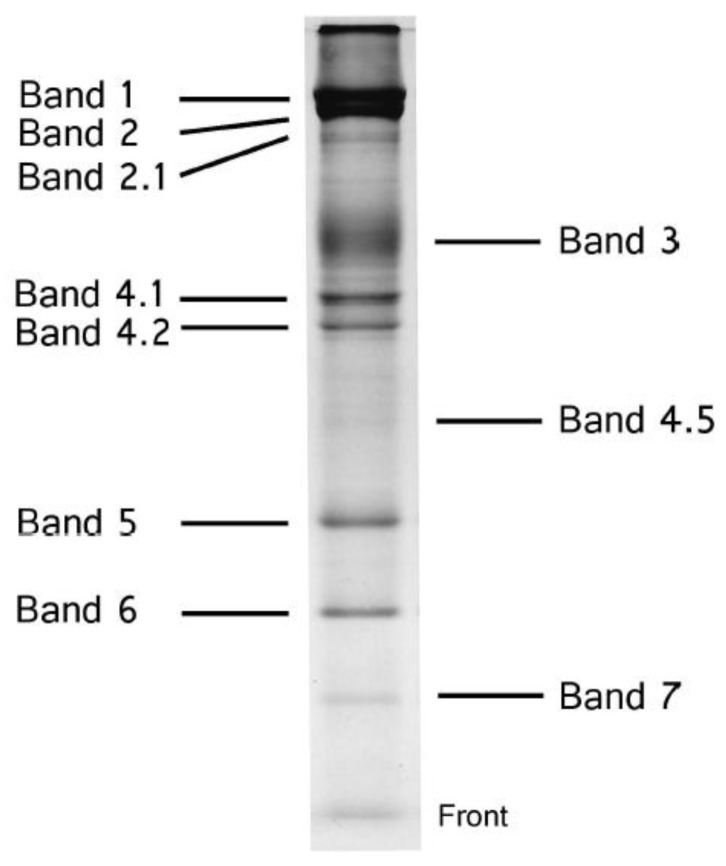
Studies on biological membranes normally use human red blood cells (RBC) because these erythrocytes have no nuclei and are easy to obtain for cell membrane preparation. For the preparation of membrane proteins, it is necessary to solubilize the phospholipid bilayer by using detergents. Fairbanks et al. [1] developed a method in which red blood cell membranes were solubilized by sodium dodecyl sulfate (SDS), and then the extracted membrane proteins were separated by acrylamide gel electrophoresis (SDS-PAGE). As a result, membrane proteins and glycoproteins could be detected on SDS gels. Figure 1 shows the typical human red blood cell membrane proteins separated by SDS-PAGE using the method of Laemmli [2], which was later improved by Fairbanks et al. [1]. Table 1 depicts the nomenclature of each cell membrane protein according to Fairbanks et al. [1], along with apparent molecular mass (kDa) and physiological function. Band 3, band 4.1, and band 4.2 are called the designated names at present.
Figure 1.
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis of human red blood cell (RBC) membranes. Healthy human RBC membranes stained with Coomassie brilliant blue. Electrophoresis was performed according to the method of Laemmli [2].
Table 1.
Red blood cell membrane proteins.
| Band | Molecular Mass (kDa) | Designation | Function |
|---|---|---|---|
| 1 | 240 | spectrin (α chain) | components of cytoskeleton |
| 2 | 220 | spectrin (β chain) | |
| 2.1 | 210 | ankylin | |
| 3 | 100 | band 3 (AE1) | anion transporter |
| 4.1 | 82 | band 4.1 | components of cytoskeleton |
| 4.2 | 76 | band 4.2 | |
| 4.5 | 55 | band 4.5 (GLUT1) | glucose transporter |
| 5 | 43 | actin | components of cytoskeleton |
| 6 | 35 | glyceraldehyde-3-phoshate dehydrogenase (GAPDH) | glycolytic enzyme |
| 7 | 31 | stomatin | – |
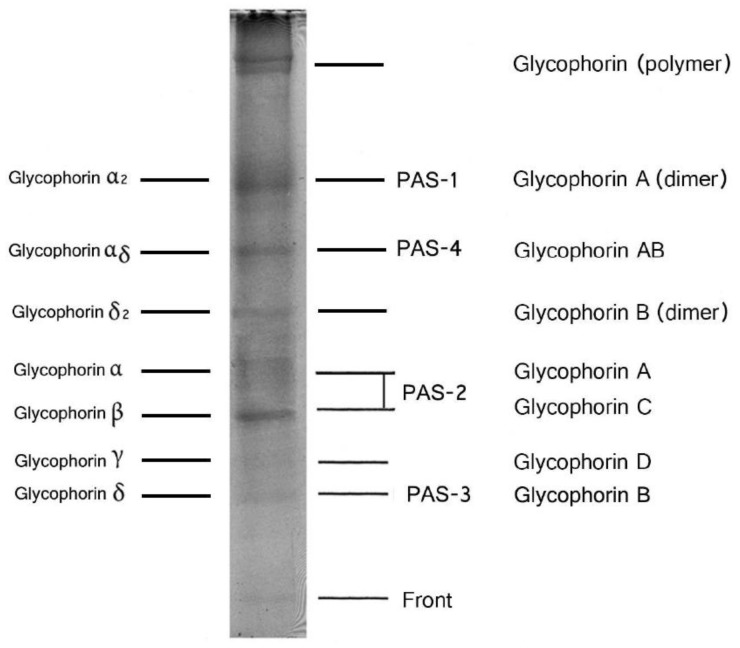
Band 3 is a glycoprotein and is detected as a diffuse band on SDS gel due to the microheterogeneity of the attached oligosaccharides [3]. Band 3 is detectable on the SDS gel by using protein staining with Coomassie Brilliant Blue (CBB) because band 3 has a small amount of oligosaccharides. Band 3 contains approximately 7% carbohydrate and contributes to approximately 10% of the total membrane carbohydrate [4]. By contrast, the sialooligosaccharide-rich glycoproteins are detectable by staining with the periodic acid-Schiff’s (PAS) reagent [1,5]. The glycoproteins detected by PAS staining are called glycophorins. Figure 2 shows the nomenclature of human red cell membrane sialoglycoproteins. The designation of glycophorins is confusing because different nomenclatures have been used by different researchers. In the first part of the 1970s, the glycophorins were called as PAS 1–4 and, at present, they are termed glycophorins A–D, respectively [6]. Glycophorin α is the same as glycophorin A, and glycophorin δ, β, and γ are glycophorins B, C, and D, respectively [6,7,8].
Figure 2.
SDS-polyacrylamide gel electrophoresis of human red blood cell (RBC) membranes. Healthy human RBC membranes stained with periodic acid-Schiff (PAS) stain [1]. Electrophoresis was performed according to the method of Laemmli [2].
Glycophorin A (dimer) is observed below band 3 on SDS-polyacrylamide gels (Figure 2), is a major component of red cell membrane glycoproteins, and is reported to have an apparent molecular mass of 29 [9] to 36 kDa [10,11].
The electrophoretic migration of glycophorin is relatively low when compared to other membrane components because glycophorin is heavily glycosylated. Although the molecular mass of the other membrane proteins can estimated by migration on SDS-PAGE, the molecular mass of each glycophorin cannot be estimated in this manner.
2. Structure of the Human Red Blood Cell Membrane
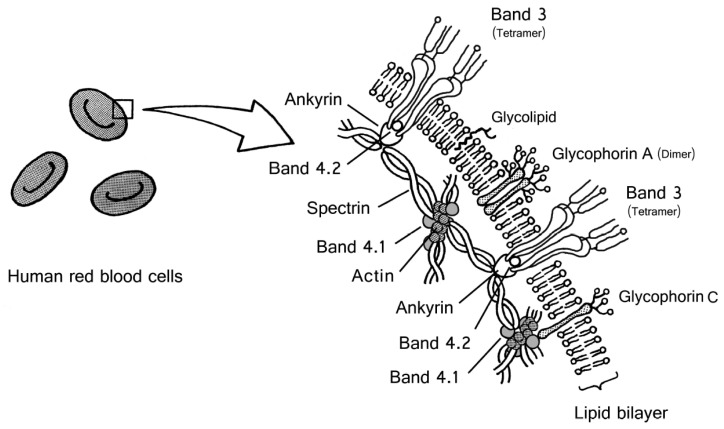
Red blood cell membrane proteins [12,13,14] can be classified into transmembrane proteins and peripheral membrane proteins that associate with the cytoplasmic side of the lipid bilayer. Band 3 and glycophorins are transmembrane proteins. Transmembrane proteins associate with the peripheral proteins that constitute the protein meshwork (cytoskeleton). Figure 3 shows a schematic drawing of the site of band 3 and glycophorins in human red blood cell membrane structure, with reference to the review on the red cell membrane skeleton [14,15]. The main component of the cytoskeleton is a spectrin tetramer. Its ends are linked by binding to the actin filament and band 4.1, and it forms a junctional complex. These junctions are linked to the end of some spectrin tetramers and form a netlike meshwork.
Figure 3.
Illustration showing the location of band 3 and glycophorins in human red blood cell membranes. This illustration is based on several reviews [14,15,22]. According to Lux [14], the location of substoichiometric proteins (e.g., CD44, CD47, Lu, Kell, Duffy) remains unclear. In this illustration, these glycoproteins are omitted, the actin junctional complex (4.1R complex) is simplified, and the topology of band 3 and glycophorins is defined.
The N-terminal cytoplasmic domain of band 3 attaches to an intracellular protein (ankyrin), which binds to a spectrin tetramer. By connecting band 3 to ankyrin, band 3 links the cytoskeleton through the spectrin network. This cytoskeleton enables deformability and thereby maintains the integrity of the biconcave-shaped red blood cells.
Glycophorins C and D are linked to the band 4.1 protein and connect the cytoskeleton structure [16,17,18,19]. Glycophorins C, D, and band 3 are associated with the cytoskeleton, and the maintenance of shape and mechanical properties of the red blood cell after passing through capillary vessels [20].
This information indicates that glycophorins C and D are anchored to the membrane by the cytoskeleton. In contrast, there is only one report that glycophorin A connects to band 4.1 protein [21]. It is believed that glycophorins A and B are not associated with the cytoskeleton, thus enabling them to be easily released from the red cell membrane.
3. Structure of Band 3
The band 3 protein [3,23] is the most abundant of the red cell membrane proteins and composes approximately 25% of these proteins. For Band 3, approximately 1.0 × 106 copies are present in each red blood cell. The molecular mass of band 3 is estimated at approximately 100 kDa by SDS-PAGE. By mild proteolysis, the band 3 protein, which consists of 911 amino acid residues, can be divided into a hydrophilic cytoplasmic fragment of 41 kDa and a hydrophobic membrane fragment of 52 kDa [24]. It forms a dimer or tetramer in the phospholipid bilayer, and this complex connects to the cytoskeleton through ankyrin.
The elongated part of the N-terminal domain facing the cytosol consists of a hydrophilic domain whose ends connect to the hydrophobic domain, and this unit crosses the membrane several times. The number of passes of the polypeptide unit has been established, and there may be 14 membrane spans based on cDNA analysis. The C-terminal domain is assumed to be on the cytoplasmic side of the membrane; however, the physiological function of this domain remains unclear.
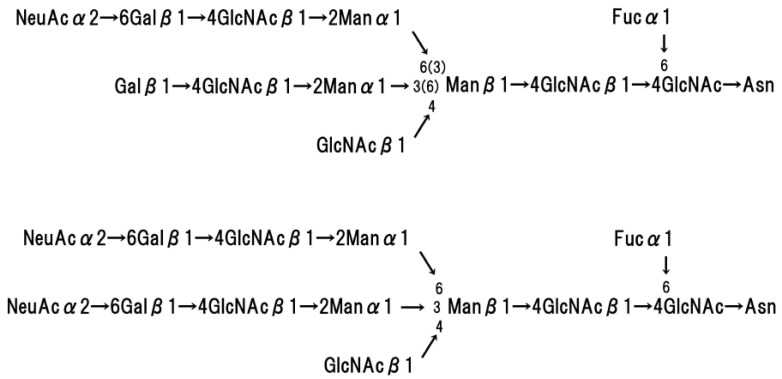
Recently, the crystal structure of the band 3 anion exchanger domain has been analysed in detail [25]. Band 3 is composed of amino peptides and a single N-linked oligosaccharide, and the molecular mass of the carbohydrate is approximately 8% of band 3. This N-linked oligosaccharide is linked at Asn-649, which is located at a site approximately 28 amino acids from the C-terminus of the band 3 polypeptide. This oligosaccharide is exposed on the external side of the membrane and is heterogeneous in size on different band 3 molecules, as determined by the number of repeating N-acetyllactosamine units (Galβ1→4 GlcNAcβ1→3) [26] (Figure 4). The end of the band 3 oligosaccharide is linked to sialic acid. This sialic acid is N-acetylneuraminic acid (5-acetamido-3,5-dideoxy-α-d-glycero-d-galacto-2-nonulopyranosidonic acid; Neu5Ac, NeuAc, NANA) and occurs broadly in humans [27].
Figure 4.
N-linked oligosaccharides of human band 3.
4. Structure of Human Glycophorins
Glycophorins [10,28] A and B represent approximately 85% and 10% of the PAS-positive membrane components, respectively, whereas, glycophorins C and D are minor species, contributing only 4% and 1% to the PAS-positive components, respectively [29].
For the major sialo-glycoprotein, glycophorin A, it was deduced that 5 × 105–10 × 105 copies are present in each red blood cell [30], which corresponds to 1.6% of the total human red blood cell proteins [31]. By contrast, 0.2 × 105–1.0 × 105 copies of glycophorin B are present [32]. Glycophorin A consists of 131 amino acid residues as a single polypeptide chain [33] and contains 16 oligosaccharides attached to the N-terminus, accounting for a third of the molecule [34].
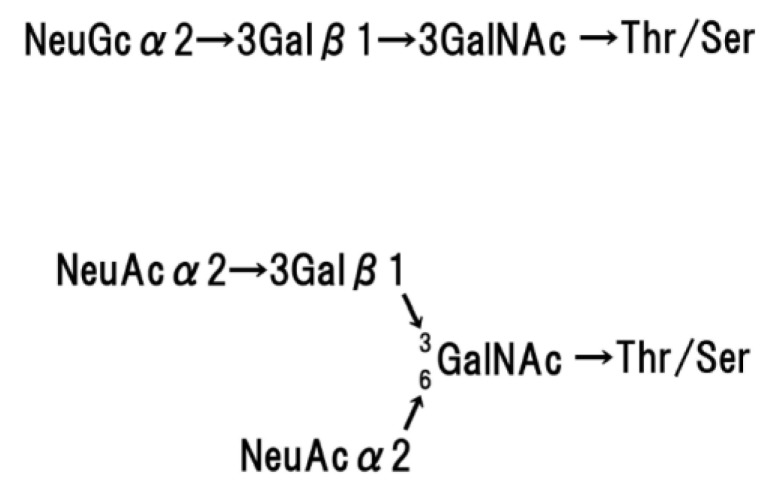
Glycophorin A homodimerizes in the red cell membrane [35,36,37], and this molecular structure has been analysed using NMR [38,39], polarized FTIR [40], and FRET measurements [41]. Glycophorin A is comprised of two motifs, an N-terminal moiety on the extracellular surface and a tethered C-terminal moiety, which consists of an α-helix (hydrophobic transmembrane domain) and a hydrophilic cytoplasmic domain. Glycophorin A is surrounded by approximately 34 phospholipids [42]. Fifteen O-linked oligosaccharide chains and a single N-linked oligosaccharide chain are attached to the extracellular N-terminal moiety. Figure 5 and Figure 6 show the basic structure of each oligosaccharide. The basic structure of the O-linked oligosaccharide consists of two NeuAc, Gal, and GalNAc, which form a tetra-saccharide, and the terminal GalNAc residue is attached to The/Ser of the polypeptide chain [43,44,45]. The basic structure of the N-linked oligosaccharide consists of NeuGc, Gal, Man, Fuc, and GlcNAc, and the terminal GlcNAc residue is attached to Asp 26 of the glycophorin polypeptide [46]. The amount of sialic acid contained in glycophorin A corresponds to approximately 75% of the total sialic acid of the red blood cell membrane [47].
Figure 5.
Basic structure of O-linked oligosaccharides of glycophorin A.
Figure 6.
Basic structure of N-linked oligosaccharides of glycophorin A.
Glycophorin B was isolated by Furthmayr et al. [31,48] using the lithium diiodosalicynate (LIS)-phenol method [49]. The molecular mass of glycophorin B is estimated at approximately 20 kDa, and the sequence of N-terminal domain is identical to that of glycophorin A [11,50] because both of the glycophorins are generated from the same gene cluster [51,52,53]. Glycophorins C and D are also generated from the same glycophorin gene cluster, and their molecular weights are estimated at approximately 32 kDa and approximately 23 kDa, respectively [54,55]. Glycophorins C and D have been deduced to occur at approximately 5.0 × 104–10 × 104 and approximately 2.0 × 104 copies in each red blood cell, respectively [56].
Glycophorin B has no N-linked oligosaccharide and 11 O-linked oligosaccharides bound to the protein moiety; glycophorin C has a single N-linked oligosaccharide and 12 O-linked oligosaccharide chains; and, glycophorin D contains six O-linked chains [56,57]. The structures of the oligosaccharides of the other glycophorins are similar to those of glycophorin A [48]. In 1990, a gene for a fifth glycophorin (glycophorin E) was identified by the isolation of genomic clones, and its nucleotide sequence is almost identical to that of the glycophorin B gene [58,59]. However, the expression and the function of glycophorin E have not been clarified [60].
5. Other Glycoprotein in the Human Red Blood Cell Membrane
Although they have not been detected by PAS staining of SDS-PAGE gels, some glycoproteins in human red blood cell membranes have been identified by using monoclonal antibodies.
There are reports that a band 4.5 protein (GLUT1) contains N-glycan, and increasing the N-glycosylation of GLUT1 may influence its function as a glucose transporter [61].
The Na-H exchanger (NHE1), which associates with actin-binding proteins (ERM: ezrin, radixin, and moesin), contains both N-linked and O-linked oligosaccharides [62]. Its isoform, NHE2, has only O-linked oligosaccharides [63]. However, glycosylation of NHFs had no apparent effect on the rate of ion exchange [64]. CD44 and CD47 are also glycoproteins. The former binds to protein band 4.1 (4.1R), and the latter binds to band 4.2 (protein 4.2) and to the ankyrin-linked band 3 complex [14]. In non-erythroid cells, CD44 binds directly to ERM proteins [65]. CD44, which is involved in cell-cell communication, is known to be the cell surface receptor for hyaluronan (HA) and contains N-glycans, O-glycans, and glycosaminoglycans [66]. It has been reported that the glycosylation of CD44 affects its HA affinity [67,68]. CD47, which is also involved in cell-cell communication, carries N-glycans [69] but does not require glycans to interact with signal regulatory protein α (SIRPα) [70]. The physiological function of these glycans seems to be providing structural integrity, similar to LPS attached to the outer cell membranes of gram-negative bacteria.
In addition to glycophorins, several glycoproteins in human red cell membranes have blood group antigens [71]. DARC/Duffy blood group antigens have been reported in proteins that contain N-glycan [72] and in the receptor for the malaria parasite [73]. Lu (Lutheran) blood antigen, which enables binding to α-spectrin, appears to contain small amounts of both N-glycan and O-glycan [74]. These glycans are affected by the expression of Lub antigenic activity [75]. Although present in only small amounts, the Kell blood group is a major antigenic system in human RBCs, and it interacts with band 3 protein [76]. This antigen is carried by a 93 kDa glycoprotein, and it has six N-linked polysaccharides that do not contain NeuAc [77,78]. However, the physiological function of Kell antigen is related to its amino acid composition, not polysaccharides [79], in contrast to I blood type antigen (the poly-N-acetyllactosamine structure) [80]. The diversity of blood group antigens, as mentioned above, which is lacking in carp sera (in the Section 9), may be related to the existence of various anti-carbohydrate antibodies in human sera [81]. Researchers are interested in the reactions of these antibodies with oligosaccharides containing mainly NeuAc, Gal, and Fuc at the termini of polysaccharides, but these antibodies do not react with Man, which is the primary component of N-glycan.
6. Physiological Function of Band 3
Band 3 [3,82,83,84] is well known to function as an anion exchanger. When red blood cells pass through the lung blood vessels, band 3 protein has a function in collecting CO2 from human tissues in exchange for Cl− as the form of HCO3−. Moreover, such an anion exchange function is also involved in pH regulation within cells. There are several reports on the band 3-like membrane protein having these functions in various cell membranes [84,85].
The role of the N-linked oligosaccharide of band 3 has not been established. There is a report that the oligosaccharide of band 3 does not affect the anion transport function in red blood cells [86]. Other reports indicate a role for the oligosaccharide as an antigen, and these reactions are related to the ageing phenomenon [87,88,89]. In this reaction, when the red cell membrane is damaged by oxidation through the ageing process, the localization of band 3 changes to bind anti-band 3 IgG (senescent antigen), and this binding site is at the oligosaccharide that has terminal NeuAc residue. The anti-band 3 IgG that binds band 3 is recognized in the spleen by macrophages, and macrophage autolysis digests the senescent red blood cell [90,91,92,93]. However, other reports demonstrate that the anti-band 3 IgG was bound to the band 3 protein itself, not the carbohydrate moieties [94,95].
7. Physiological Function of Human Glycophorins
Glycophorins [30,96] are extracted from red cell membrane preparations in the aqueous phase using organic solvents with some detergents because of their high content of sialic acid and highly hydrophobic protein moiety. Several organic solvents have been used as extract solutions, mainly chloroform/methanol [97], pyridine [98], and LIS-phenol [49]. The obtained glycophorin preparation is highly prone to aggregate, even in the presence of SDS [99,100,101,102]. Glycophorin A (PAS 1) forms a dimer in the SDS gel in SDS-PAGE [100,103,104]. Moreover, it forms a polymer [50] or hybridizes with glycophorin B (PAS-4, α-δ-glycophorin) [105,106]. This dimerization is caused by the α-helix of the transmembrane domain of glycophorin A interacting with another glycophorin in the intact red cell membrane [37,40]. Glycophorin B also forms a dimer, similar to glycophorin A [18].
As mentioned above (in Section 2), glycophorins C and D are associated with the cytoskeleton and the maintenance of the shape and mechanical properties of red blood cells [21], and this function contributes to the negative surface charge caused by glycophorins containing sialic acid. Without its negative charge, the mobility of the red blood cell is greatly reduced [107].
The major glycophorin, glycophorin A, is a single polypeptide chain and is linked to several sialo-oligosaccharides, which carry some blood group antigens. These blood group antigens are located at the O-linked oligosaccharides and the nearby amino acid sequence of these oligosaccharides binding site. Glycophorin A carries the M and N blood group antigens, as determined by the amino acid sequence at residue 1 and residue 5 of the mature polypeptide (Ser1/Gly5 for M and Leu1/Glu5 for N). Glycophorin B, on the other hand, has only an N form, and its amino acid sequence is identical to that of N-type glycophorin A. Glycophorin B carries other blood group antigens, the Ss blood group, as determined by the amino acid sequences at Met 29 for S or Thr 29 for s specificity [108].
Both glycophorin polypeptides possess 3 O-linked oligosaccharides containing NeuAc linked at amino acid residues 2, 3, and 4. Both glycophorins lose blood group activities by desialylation treatment [109]. The Wright (Wr) antigens are also reported to be associated with band 3 and glycophorin A [110]. Glycophorin C and its shorter form, glycophorin D, are antigenically distinct from glycophorins A and B. Glycophorin C carries several blood group antigens (Gerbich, Yus, Wb, Ana, and Dha, and others) [111,112,113]. Glycophorins are also reported to carry the ABH blood group antigens [114,115]. According to Podbielska et al. [116], O-linked oligosaccharides isolated from glycophorin A carried the A, B, or H blood group antigen. Although these oligosaccharides reacted with ABH blood group antigens, the reaction was estimated at a relatively low level. It is thought that the oligosaccharides containing Fuc are minor components in the total amount of oligosaccharides from glycophorin A.
Another physiological function of glycophorins involves the lectin receptors. Glycophorins are linked to several sialo-oligosaccharides, so several lectins, such as Psathyrella velutina lectin (PVL), wheat germ agglutinin (WGA), and others, interact with the oligosaccharide moiety of glycophorin [117,118,119].
Other functions of glycophorins as receptors are reported to be involved with the vital functions of interaction of red blood cells and the influenza virus [120,121], Sendai virus [122,123], malaria parasite [124,125,126,127], and Escherichia coli α-haemolysin [128]. Although the relation between influenza virus and human red blood cells was known as early as the 1940s [129], there was difficulty proving this reaction because glycophorin is prone to aggregation, and the use of organic solvents and contaminating glycolipids in the purification process need to be considered. In the 1990s, the vital function became clear after the development of new experimental methods (e.g., using detergents [130], reconstruction of glycophorin-bearing liposome by using egg phosphatidylcholine [120], quick-freezing electron microscopy [121], and elastic light scattering [131]). Recently, experiments performed with stem cell lines revealed the interaction of glycophorin A with band 3 [132], and glycophorin C as a receptor for the rodent malaria parasite [133].
Several lines of evidence have suggested an interaction between glycophorin A and band 3 in their biosynthesis and processing [3,134]. However, glycophorin A knockdown had no effect on the cell surface expression of band 3 [110]. Moreover, glycophorin A-deficient red blood cells did not clearly demonstrate the physiological role of glycophorin A [135].
8. Glycoproteins in Red Cell Membranes of Non-Human Origin
Although glycoproteins in the human red cell membrane have been researched extensively, there are few reports on glycoproteins in other mammalian or avian red cell membranes. Some researchers have reported the electrophoretic patterns of mammalian red cell membranes (ox, horse, swine, sheep, goat, rabbit, guinea pig, rat, and mouse) [136,137]. According to these reports, these main membrane proteins are similar to those of human red cell membranes. Each membrane component (ankyrin, spectrin, band 3, band 4.1, and actin) was detected using SDS gels. The electrophoretic pattern of the cat red blood cell membrane (Felis catus) was also similar to that of human [138].
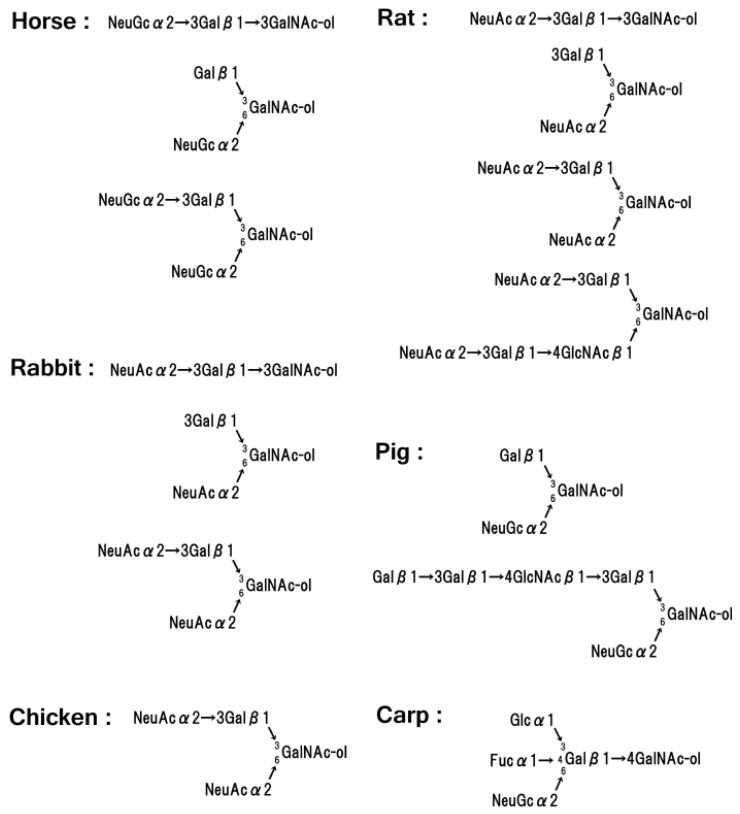
On the other hand, glycophorin patterns (PAS-stained band patterns) are different in humans [136], and these differences are caused by the component containing oligosaccharides. The glycophorins from horse [139], bovine [140], pig [141], and goat contain sialic acid as NeuGc (N-glycolylneuraminic acid), not as NeuAc [142]. While the sialic acid of dog [143] and mouse [144] is NeuAc, canine individuals containing only NeuGc have been reported. The monkey glycophorin demonstrates MN blood group activities and contains a single O-linked oligosaccharide that is composed of NeuGc, Gal, and GalNAc [145]. The core structural unit of these O-linked sialo-oligosaccharides from various mammalian sources is sialic acidα2→3Galβ1→3(sialic acidα2→6)GalNAc-ol or sialic acid α2→[3Galβ1→4GlcNacβ1]n→3Galβ1→3GalNAc-ol [146] (Figure 7).
Figure 7.
O-linked sialo-oligosaccharides of glycoproteins of mammalian, avian and teleost origins.
Non-mammalian red blood cell membranes have not been researched extensively. A major reason for this is that avian and teleost red blood cells contain a nucleus, and this causes difficulty during cell membrane preparation. The red blood cell membranes of pigeon [147], chicken [148], and turkey [149] for avian species and rainbow trout [150] for teleosts have been reported, and the main membrane proteins are similar to those of human red blood cells. Research on chicken band 3 [151] and rainbow trout band 3 [152,153] has been reported, while detection of glycophorins (PAS-stained bands on SDS-gel) has been reported based on chicken membrane preparation, and the core structure of the O-linked oligosaccharide is the tetraose NeuAcα2→3Galβ1→3(NeuAcα2→6)GalNAc-ol [154,155] (Figure 7).
There were no reports on teleost glycophorin until Aoki et al. reported the detection of glycophorins in carp and rainbow trout red cell membranes [156].
9. Glycophorin in Carp Red Blood Cell Membranes
Aoki et al. reported the presence of glycophorins in red blood cell membranes of carp on SDS electrophoresis gels by PAS staining [156]. While membrane proteins from carp membrane preparations are similar to those of human red cell membranes, carp and trout showed different glycophorin patterns. The major glycophorin from carp membrane preparations is positioned near the human glycophorin A (dimer). According to the amino acid composition of carp glycophorin, there was no striking difference from that of human glycophorin A [157]. Although human glycophorins A and B carry the MN and Ss blood group antigens, it is unclear whether carp glycophorin carries these blood group antigens, as no blood group antigen reaction has been observed by titration (Aoki et al., unpublished materials).
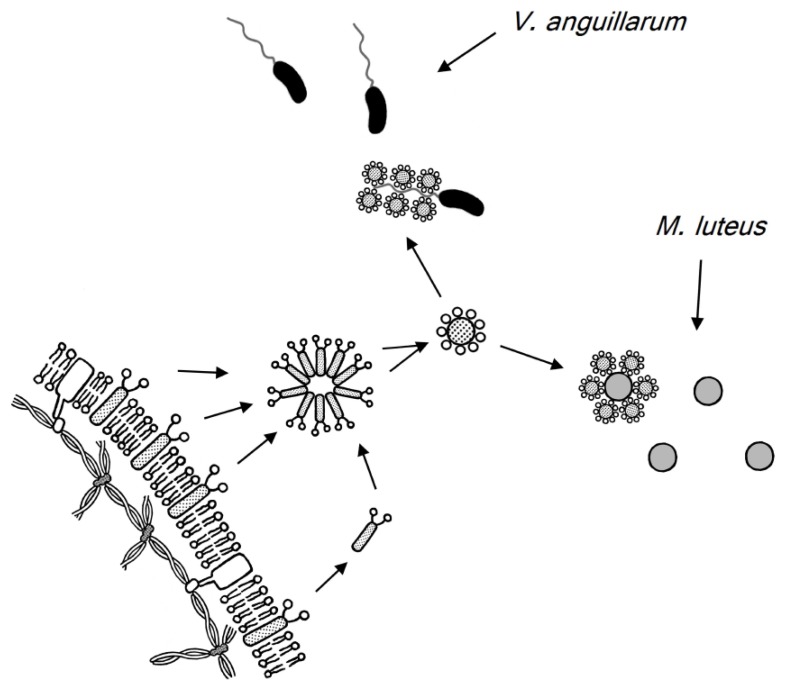
The O-linked oligosaccharide of carp glycophorin exhibited bacteriostatic activity, and this activity is observed on all tested bacteria (Gram-positive bacteria: Micrococcus luteus and Bacillus subtilis, Gram-negative bacteria: Vibrio anguillarum, Edwardsiella tarda, Aeromonas hydrophila, Escherichia coli, and Pseudomonas fluorescens) [157,158]. In the blood of diseased carp infected by P. fluorescens, carp glycophorin is released from red blood cell membranes and interacts with the bacterium [158]. By electron microscopic observations, the released carp glycophorin molecule from the cell membrane attaches to the flagellum of V. anguillarum or the cell surface of M. luteus and inhibits bacterial growth [157].
These bacteriostatic activities are caused by the sialo-oligosaccharide from carp glycophorin and are attributed to the nature of the lectin receptor. It is thought that some lectin-like proteins exist on the surface of Gram-positive bacteria or the flagellum of Gram-negative bacteria. These observations indicate that carp glycophorin is released from red cell membranes and adsorbed onto the surface of invading bacteria in the blood (Figure 8).
Figure 8.
Schematic representation of the carp glycophorin interaction with invading bacteria in carp blood.
In the Edo period of Japan, drinking carp blood was well known to the people as a folk remedy for tuberculosis. As tuberculous is caused by the bacterium (Mycobacterium tuberculosis), the bacteriostatic activity of carp glycophorin is suggested to relate to the efficacy of drinking carp blood.
The structure of the bacteriostatic sialo-oligosaccharide of carp glycophorin was determined as NeuGcα2→6(Fucα1→4)(Glcα1→3)Galβ1→4GalNAc-ol [159] (Figure 7). The 1→4 linkage of GalNAc is unique as compared with other O-linked oligosaccharides of mammalian origin. Interestingly, the β1→3 glycosidic linkage of xylan, which is a component of seaweed cell walls, is unlike the standard β1→4 linkage of land plants [160]. It is possible to detect the β1→4 linkage of N-acetylgalactosamine in marine organisms.
The sialo-oligosaccharides from carp glycophorin that have bacteriostatic activity are pentoses. This may be related to the finding that penta- or hexasaccharides obtained from chitin have bacteriostatic activity [161]. In the bacteriostatic reaction, it is supposed that the size of the oligosaccharide would correspond to the dimension of the cleft that occurs in the lectin-like protein, and might contribute to the charge of sialic acid. In teleost blood, IgG does not exist, and other antibodies exist in low levels [162]. It is suggested that glycophorin may exist as a substitute for antibodies in teleost blood. Although the physiological function of human glycophorin has not yet been clarified, the structure of the human glycophorin O-linked tetra oligosaccharide is a simpler form than that of the carp’s pentose. It is considered that IgG became a major component in the human immune system and that the bacteriostatic activity of human glycophorins has been lost in the process of evolution.
10. Conclusions
The research on glycosylation of RBC membrane proteins has not been fully investigated when compared to the research on the protein moiety of RBC glycoproteins. Moreover, the target of research on RBC is mainly human blood. Although the preparation of human RBC membranes is relatively easy, the component of human RBC is more complicated than non-mammalian RBC, especially in regard to the blood group antigens.
On the contrary, the preparation of teleostei RBC membranes is fairly difficult because containing cell nucleus, the component of RBC is simpler than that of human. In particular, the glycosylation of carp RBC is simpler than that of human glycophorins A, B, C, and D. As mentioned above, accordingly, it is suggested that the collection of information on the non-mammalian RBCs leads the comprehension on the role of human RBC glycosylation.
Acknowledgments
The author is indebted to Atsushi Ooi, Mie University, for the preparation of this work. I am also grateful to Yasuko Mizuno, Toray Research Center, Inc. (Kamakura, Japan).
Conflicts of Interest
The author declares no conflict of interest.
References
- 1.Fairbanks G., Steck T.L., Wallach D.F.H. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971;10:2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- 2.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 3.Tanner M.J.A. Molecular and cellular biology of the erythrocyte anion exchanger (AE 1) Semin. Hematol. 1993;30:34–57. [PubMed] [Google Scholar]
- 4.Steck T. The organization of proteins in the human red blood cell membrane: A review. J. Cell Biol. 1974;62:1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doerner K.C., White B.A. Detection of glycoproteins separated by nondenaturing polyacrylamide gel electrophoresis using the periodic acid-Schiff stain. Anal. Biochem. 1990;187:147–150. doi: 10.1016/0003-2697(90)90433-A. [DOI] [PubMed] [Google Scholar]
- 6.Anstee D.J., Tanner M.J.A. Structure and function of the red cell membrane sialoglycoproteins. Br. J. Haematol. 1986;64:211–215. doi: 10.1111/j.1365-2141.1986.tb04113.x. [DOI] [PubMed] [Google Scholar]
- 7.Anstee D.J., Mawby W.J., Tanner M.J.A. Abnormal blood-group-Ss-active sialoglycoproteins in the membrane of Miltenberger class III, IV and V human erythrocytes. Biochem. J. 1979;183:193–203. doi: 10.1042/bj1830193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Owens J.W., Mueller T.J., Morrison M. A minor sialoglycoprotein of the human erythrocyte membrane. Arch. Biochem. Biophys. 1980;204:247–254. doi: 10.1016/0003-9861(80)90030-2. [DOI] [PubMed] [Google Scholar]
- 9.Grefrath S.P., Reynolds J.A. The molecular weight of the major glycoprotein from the human erythrocyte membrane. Proc. Nat. Acad. Sci. USA. 1974;71:3913–3916. doi: 10.1073/pnas.71.10.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukuda M. Molecular-genetics of the glycophorin A gene cluster. Semin. Hematol. 1993;30:138–151. [PubMed] [Google Scholar]
- 11.Blanchard D., Dahr W., Hummel M., Lartron F., Beyreuther K., Cartron J.-P. Glycophorins B and C from human erythrocyte membranes. Purification and sequence analysis. J. Biol. Chem. 1987;262:5808–5811. [PubMed] [Google Scholar]
- 12.Mohandas N., Chasis J.A. Red blood cell deformability, membrane material properties and shape: Regulation by transmembrane, skeletal and cytosolic proteins and lipids. Semin. Hematol. 1993;30:171–192. [PubMed] [Google Scholar]
- 13.Bennet V. The membrane skeleton of human erythrocytes and its implications for more complex cells. Ann. Rev. Biochem. 1985;54:273–304. doi: 10.1146/annurev.bi.54.070185.001421. [DOI] [PubMed] [Google Scholar]
- 14.Lux S.E., IV Anatomy of the red cell membrane skeleton: Unanswered questions. Blood. 2016;127:187–199. doi: 10.1182/blood-2014-12-512772. [DOI] [PubMed] [Google Scholar]
- 15.Cordat E., Reithmeier R.A.F. Structure, function, and trafficking of SLC4 and SLC26 anion transporters. Curr. Top. Membr. 2014;73:3–15. doi: 10.1016/B978-0-12-800223-0.00001-3. [DOI] [PubMed] [Google Scholar]
- 16.Hemming N.J., Anstee D.J., Mawby W.J., Reid M.E., Tanner M.J.A. Localization of the protein 4.1-binding site on human erythrocyte glycophorins C and D. Biochem. J. 1994;299:191–196. doi: 10.1042/bj2990191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hemming N.J., Anstee D.J., Staricoff M.A., Tanner M.J.A., Mohandas N. Identification of the membrane attachment sites for protein 4.1 in the human erythrocyte. J. Biol. Chem. 1995;270:5360–5366. doi: 10.1074/jbc.270.10.5360. [DOI] [PubMed] [Google Scholar]
- 18.Reid M.E., Takakuwa Y., Conboy J., Tchernia G., Mohandas N. Glycophorin C content of human erythrocyte membrane is regulated by protein 4.1. Blood. 1990;11:2229–2234. [PubMed] [Google Scholar]
- 19.Alloisio N., Dalla Venezia N., Rana A., Andrabi K., Texier P., Gilsanz F., Cartron J.-P., Delaunay J., Chishti A.H. Evidence that red blood cell protein p55 may participate in the skeleton-membrane linkage that involves protein 4.1 and glycophorin C. Blood. 1993;82:1323–1327. [PubMed] [Google Scholar]
- 20.Staricoff M.A., Tanner M.J.A. Role of band 3 and glycophorin C in the maintenance of the shape and mechanical properties of the human red blood cell. Cell. Mol. Biol. Lett. 1996;1:151–161. [Google Scholar]
- 21.Lovrien R.E., Anderson R.A. Glycophorin is linked by band 4.1 protein to the human erythrocyte membrane skeleton. Nature. 1984;307:655–658. doi: 10.1038/307655a0. [DOI] [PubMed] [Google Scholar]
- 22.Mohandas N., Gallagher P.G. Red cell membrane: Past, present, and future. Blood. 2008;112:3939–3948. doi: 10.1182/blood-2008-07-161166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okubo K., Hamasaki N. Recent progress on band 3 protein. Seikagaku. 1992;64:1116–1120. (In Japanese) [PubMed] [Google Scholar]
- 24.Steck T.L., Koziarz J.J., Singh M.K., Reddy G., Köhler H. Preparation and analysis of seven major, topographically defined fragments of band 3, the predominant transmembrane polypeptide of human erythrocyte membranes. Biochemistry. 1978;17:1216–1222. doi: 10.1021/bi00600a013. [DOI] [PubMed] [Google Scholar]
- 25.Arakawa T., Kobayashi-Yurugi T., Alguel Y., Iwanari H., Hatae H., Iwata M., Abe Y., Hino T., Ikeda-Suno C., Kuma H., et al. Crystal structure of the anion exchanger domain of human erythrocyte band 3. Science. 2015;350:680–684. doi: 10.1126/science.aaa4335. [DOI] [PubMed] [Google Scholar]
- 26.Tsuji T., Irimura T., Osawa T. The carbohydrate moiety of band 3 glycoprotein of human erythrocyte membranes. Structures of lower molecular weight oligosaccharides. J. Biol. Chem. 1981;256:10497–10502. [PubMed] [Google Scholar]
- 27.Fukuda M., Dell A., Oates J.E., Fukuda M.N. Structure of branched lactosaminoglycan, the carbohydrate moiety of band 3 isolated from adult human erythrocytes. J. Biol. Chem. 1984;259:8260–8273. [PubMed] [Google Scholar]
- 28.Chasis J.A., Mohandas N. Red blood cell glycophorins. Blood. 1992;80:1869–1879. [PubMed] [Google Scholar]
- 29.Cartron J.-P., Colin Y., Kudo S., Fukuda M. Molecular Genetics of Human Erythrocyte Sialoglycoproteins Glycophorins A, B, C, and D. Springer; Boston, MA, USA: 1990. pp. 299–335. [Google Scholar]
- 30.Blanchard D. Human red cell glycophorins: Biochemical and antigenic properties. Transfus. Med. Rev. 1990;4:170–186. doi: 10.1016/S0887-7963(90)70263-5. [DOI] [PubMed] [Google Scholar]
- 31.Furthmayer H. Glycophorins A, B, and C: A family of sialoglycoproteins. Isolation and preliminary characterization of trypsin derived peptides. J. Supramol. Struct. 1978;9:79–95. doi: 10.1002/jss.400090109. [DOI] [PubMed] [Google Scholar]
- 32.De Isla N.G., Riquelme B.D., Rasia R.J., Valverde J.R., Stoltz J.F. Quantification of glycophorin A and glycophorin B on normal human RBCs by flow cytometry. Transfusion. 2003;43:1145–1152. doi: 10.1046/j.1537-2995.2003.00471.x. [DOI] [PubMed] [Google Scholar]
- 33.Tomita M., Marchesi V.T. Amino-acid sequence and oligosaccharide attachment sites of human erythrocyte glycophorin. Proc. Natl. Acad. Sci. USA. 1975;72:2964–2968. doi: 10.1073/pnas.72.8.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomita M., Furthmayr H., Marchesi V.T. Primary structure of human erythrocyte glycophorin A. Isolation and characterization of peptides and complete amino acid sequence. Biochemistry. 1978;17:4756–4770. doi: 10.1021/bi00615a025. [DOI] [PubMed] [Google Scholar]
- 35.Bormann B.-J., Knowles W.J., Marchesi V.T. Synthetic peptides mimic the assembly of transmembrane glycoproteins. J. Biol. Chem. 1989;264:4033–4037. [PubMed] [Google Scholar]
- 36.Lemmon M.A., Flanagan J.M., Hunt J.F., Adair B.D., Bormann B.J., Dempsey C.E., Engelman D.M. Glycophorin A dimerization is driven by specific interactions between transmembrane α-helices. J. Biol. Chem. 1992;267:7683–7689. [PubMed] [Google Scholar]
- 37.Brosig B., Langosch D. The dimerization motif of the glycophorin A transmembrane segment in membranes: Importance of glycine residues. Protein Sci. 1998;7:1052–1056. doi: 10.1002/pro.5560070423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacKenzie K.R., Prestegard J.H., Engelman D.M. A transmembrane helix dimer: Structure and implications. Science. 1997;276:131–133. doi: 10.1126/science.276.5309.131. [DOI] [PubMed] [Google Scholar]
- 39.Smith S.O., Song D., Shekar S., Groesbeek M., Ziliox M., Aimoto S. Structure of the transmembrane dimer interface of glycophorin A in membrane bilayers. Biochemistry. 2001;40:6553–6558. doi: 10.1021/bi010357v. [DOI] [PubMed] [Google Scholar]
- 40.Smith S.O., Eilers M., Song D., Crocker E., Ying W., Groesbeek M., Metz G., Ziliox M., Aimoto S. Implications of threonine hydrogen bonding in the glycophorin A transmembrane helix dimer. Biophys. J. 2002;82:2476–2486. doi: 10.1016/S0006-3495(02)75590-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anbazhagan V., Schneider D. The membrane environment modulates self-association of the human GpA TM domain—Implications for membrane protein folding and transmembrane signaling. Biochim. Biophys. Acta. 2010;1798:1899–1907. doi: 10.1016/j.bbamem.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 42.Shan X., Davis J.H., Chu J.W.K., Sharom F.J. 2H-NMR investigation of DMPC/glycophorin bilayers. Biochim. Biophys. Acta. 1994;1193:127–137. doi: 10.1016/0005-2736(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 43.Thomas D.B., Winzler R.J. Structural studies on human erythrocyte glycoproteins. Alkali-labile oligosaccharides. J. Biol. Chem. 1969;244:5943–5946. [PubMed] [Google Scholar]
- 44.Fukuda M., Lauffenburger M., Sasaki H., Rogers M.E., Dell A. Structures of novel sialylated O-linked oligosaccharides isolated from human erythrocyte glycophorins. J. Biol. Chem. 1987;262:11952–11957. [PubMed] [Google Scholar]
- 45.Pisano A., Redmond J.W., Williams K.L., Gooley A.A. Glycosylation sites identified by solid-phase Edman degradation: O-linked glycosylation motifs on human glycophorin A. Glycobiology. 1993;3:429–435. doi: 10.1093/glycob/3.5.429. [DOI] [PubMed] [Google Scholar]
- 46.Yoshima H., Furthmayr H., Kobata A. Structures of the asparagine-linked sugar chains of glycophorin A. J. Biol. Chem. 1980;255:9713–9718. [PubMed] [Google Scholar]
- 47.Marchesi V.T., Furthmayr H., Tomita M. The red cell membrane. Ann. Rev. Biochem. 1976;45:667–698. doi: 10.1146/annurev.bi.45.070176.003315. [DOI] [PubMed] [Google Scholar]
- 48.Furthmayr H., Tomita M., Marchesi V.T. Fractionation of the major sialoglycopeptides of the human red blood cell membrane. Biochem. Biophys. Res. Commun. 1975;65:113–121. doi: 10.1016/S0006-291X(75)80068-4. [DOI] [PubMed] [Google Scholar]
- 49.Marchesi V.T., Andrews E.P. The use of lithium diiodosalicylate (LIS) to isolate glycoproteins from cell membranes. Science. 1971;174:1247–1248. doi: 10.1126/science.174.4015.1247. [DOI] [PubMed] [Google Scholar]
- 50.Furthmayr H. Structural comparison of glycophorins and immunochemical analysis of genetic variants. Nature. 1978;271:519–524. doi: 10.1038/271519a0. [DOI] [PubMed] [Google Scholar]
- 51.Siebert P.D., Fukuda M. Isolation and characterization of human glycophorin A cDNA clones by a synthetic oligonucleotide approach: Nucleotide sequence and mRNA structure. Proc. Natl. Acad. Sci. USA. 1986;83:1665–1669. doi: 10.1073/pnas.83.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siebert P.D., Fukuda M. Molecular cloning of a human glycophorin B cDNA: Nucleotide sequence and genomic relationship to glycophorin A. Proc. Natl. Acad. Sci. USA. 1987;84:6735–6739. doi: 10.1073/pnas.84.19.6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tate C.G., Tanner M.J.A. Isolation of cDNA clones for human erythrocyte membrane sialoglycoproteins α and δ. Biochem. J. 1988;254:743–750. doi: 10.1042/bj2540743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mattei M.G., Colin Y., Le Van Kim C., Mattei J.F., Cartron J.-P. Localization of the gene for human erythrocyte glycophorin C to chromosome 2, q14–q21. Hum. Genet. 1986;74:420–422. doi: 10.1007/BF00280497. [DOI] [PubMed] [Google Scholar]
- 55.Le Van Kim C., Colin Y., Blanchard D., Dahr W., London J., Cartron J.-P. Gerbich blood group deficiency of the Ge:-1,-2,-3 and Ge:-1,-2, 3 types. Immunochemical study and genomic analysis with cDNA probes. Eur. J. Biochem. 1987;165:571–579. doi: 10.1111/j.1432-1033.1987.tb11478.x. [DOI] [PubMed] [Google Scholar]
- 56.Cartron J.-P., Le Van Kim C., Colin Y. Glycophorin C and related glycophorins: Structure, function, and regulation. Semin. Hematol. 1993;30:152–168. [PubMed] [Google Scholar]
- 57.Dahr W., Beyreuther K., Kordowicz M., Krüger J. N-terminal amino acid sequence of sialoglycoprotein D (glycophorin C) from human erythrocyte membranes. Eur. J. Biochem. 1982;125:57–62. doi: 10.1111/j.1432-1033.1982.tb06650.x. [DOI] [PubMed] [Google Scholar]
- 58.Kudo S., Fukuda M. Identification of a novel human glycophorin, glycophorin E, by isolation of genomic clones and complementary DNA clones utilizing polymerase chain reaction. J. Biol. Chem. 1990;265:1102–1110. [PubMed] [Google Scholar]
- 59.Vignal A., Rahuel C., London J., Zahar B.C., Schaff S., Hattab C., Okubo Y., Cartron J.-P. A novel gene member of the human glycophorin A and B gene family. Eur. J. Biochem. 1990;191:619–625. doi: 10.1111/j.1432-1033.1990.tb19166.x. [DOI] [PubMed] [Google Scholar]
- 60.Kudo S., Fukuda M. Contribution of gene conversion to the retention of the sequence for M blood group type determinant in glycophorin E gene. J. Biol. Chem. 1994;269:22969–22974. [PubMed] [Google Scholar]
- 61.Onetti R., Baulida J., Bassols A. Increased glucose transport in ras-transformed fibroblasts: A possible role for N-glycosylation of GLUT1. FEBS Lett. 1997;407:267–270. doi: 10.1016/S0014-5793(97)00340-2. [DOI] [PubMed] [Google Scholar]
- 62.Counillon L., Pouysségur J., Reithmeier R.A.F. The Na+/H+ exchanger NHF-1 possesses N- and O-linked glycosylation restricted the first N-terminal extracellular domain. Biochemistry. 1994;33:10463–10469. doi: 10.1021/bi00200a030. [DOI] [PubMed] [Google Scholar]
- 63.Tse C.-M., Levine S.A., Yun C.H.C., Khurana S., Donowitz M. The plasma membrane Na+/H+ exchanger 2 is an O-linked but not an N-linked sialoglycoprotein: Use of a polyclonal antibody to identify and characterize glycosylation. Biochemistry. 1994;33:12954–12961. doi: 10.1021/bi00248a003. [DOI] [PubMed] [Google Scholar]
- 64.Orlowski J., Grinstein S. Na+/H+ exchangers of mammalian cells. J. Biol. Chem. 1997;272:22373–22376. doi: 10.1074/jbc.272.36.22373. [DOI] [PubMed] [Google Scholar]
- 65.Denker S.P., Huang D.C., Orlowski J., Furthmayr H., Barber D.L. Direct binding of the Na–H exchanger NHE1 to ERM proteins regulates the cortical cytoskeleton and cell shape independently of H+ translocation. Mol. Cell. 2000;6:1425–1436. doi: 10.1016/S1097-2765(00)00139-8. [DOI] [PubMed] [Google Scholar]
- 66.Ponta H., Wainwright D., Herrlich P. Molecules in focus The CD44 protein family. Int. J. Biochem. Cell Biol. 1998;30:299–305. doi: 10.1016/S1357-2725(97)00152-0. [DOI] [PubMed] [Google Scholar]
- 67.Skelton T.P., Zeng C., Nocks A., Stamenkovic I. Glycosylation provides both stimulatory and inhibitory effects on cell surface and soluble CD44 binding to hyaluronan. J. Cell Biol. 1998;140:431–446. doi: 10.1083/jcb.140.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Isacke C.M., Yarwood H. The hyaluronan receptor, CD44. Int. J. Biochem. Cell Biol. 2002;34:718–721. doi: 10.1016/S1357-2725(01)00166-2. [DOI] [PubMed] [Google Scholar]
- 69.Mawby W.J., Holmes C.H., Anstee D.J., Spring F.A., Tanner M.J.A. Isolation and characterization of CD47 glycoprotein: A multispanning membrane protein which is the same as integrin-associated protein (IAP) and the ovarian tumour marker OA3. Biochem. J. 1994;304:525–530. doi: 10.1042/bj3040525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Subramanian S., Boder E.T., Discher D.E. Phylogenetic divergence of CD47 interactions with human signal regulatory protein α reveals locus of species specificity. Implications for the binding site. J. Biol. Chem. 2007;282:1805–1818. doi: 10.1074/jbc.M603923200. [DOI] [PubMed] [Google Scholar]
- 71.Anstee D.J. Blood group-active surface molecules of the human red blood cell. Vox Sang. 1990;58:1–20. doi: 10.1111/j.1423-0410.1990.tb02049.x. [DOI] [PubMed] [Google Scholar]
- 72.Grodecka M., Bertrand O., Karolak E., Lisowski M., Waśniowska K. One-step immunopurification and lectinochemical characterization of the Duffy atypical chemokine receptor from human erythrocytes. Glycoconj. J. 2012;29:93–105. doi: 10.1007/s10719-011-9367-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ryan J.R., Stoute J.A., Amon J., Dunton R.F., Mtalib R., Koros J., Owour B., Luckhart S., Wirtz R.A., Barnwell J.W., et al. Evidence for transmission of Plasmodium vivax among a Duffy antigen negative population in western Kenya. Am. J. Trop. Med. Hyg. 2006;75:575–581. [PubMed] [Google Scholar]
- 74.Daniels G., Khalid G. Identification, by immunoblotting, of the structures carrying Lutheran and para-Lutheran blood group antigens. Vox Sang. 1989;57:137–141. doi: 10.1111/j.1423-0410.1989.tb01151.x. [DOI] [PubMed] [Google Scholar]
- 75.Parson S.F., Mallinson G., Judson P.A., Anstee D.J., Tanner M.J., Daniels G.L. Evidence that the Lub blood group antigen is located on red cell membrane glycoproteins of 85 and 78 kd. Transfussion. 1987;27:61–63. doi: 10.1046/j.1537-2995.1987.27187121477.x. [DOI] [PubMed] [Google Scholar]
- 76.Redman C.M., Avellino G., Pfeffer S.R., Mukherjee T.K., Nichols M., Rubinstein P., Marsh W.L. Kell blood group antigens are part of a 93,000-dalton red cell membrane protein. J. Biol. Chem. 1986;261:9521–9525. [PubMed] [Google Scholar]
- 77.Jaber A., Blanchard D., Goossens D., Bloy C., Lambin P., Rouger P., Salmon C., Cartron J.-P. Characterization of the blood group Kell (K1) antigen with a human monoclonal antibody. Blood. 1989;73:1597–1602. [PubMed] [Google Scholar]
- 78.Lee S., Zambas E.D., Marsh W.L., Redman C.M. Molecular cloning and primary structure of Kell blood group protein. Proc. Natl. Acad. Sci. USA. 1991;88:6353–6357. doi: 10.1073/pnas.88.14.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee S., Russo D., Redman C. Functional and structural aspects of the Kell blood group system. Transfus. Med. Rev. 2000;14:93–103. doi: 10.1016/S0887-7963(00)80001-2. [DOI] [PubMed] [Google Scholar]
- 80.Yu L.-C., Twu Y.-C., Chou M.-L., Reid M.E., Gray A.R., Moulds J.M., Chang C.-Y., Lin M. The molecular genetics of the human I locus and molecular background explain the partial association of the adult i phenotype with congenital cataracts. Blood. 2003;101:2081–2088. doi: 10.1182/blood-2002-09-2693. [DOI] [PubMed] [Google Scholar]
- 81.Huflejt M.E., Vuskovic M., Vasiliu D., Xu H., Obukhova P., Shilova N., Tuzikov A., Galanina O., Arun B., Lu K., et al. Anti-carbohydrate antibodies of normal sera: Findings, surprises and challenges. Mol. Immunol. 2009;46:3037–3049. doi: 10.1016/j.molimm.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 82.Beppu M., Kikugawa K. Senescent cell antigens in the clearance of senescent cells. Seikagaku. 1995;67:303–307. (In Japanese) [PubMed] [Google Scholar]
- 83.Reithmeier R.A.F., Casey J.R., Kalli A.C., Sansom M.S.P., Alguel Y., Iwata S. Band 3, the human red cell chloride/bicarbonate anion exchanger (AE1, SLC4A1), in a structural context. Biochim. Biophys. Acta. 2016;1858:1507–1532. doi: 10.1016/j.bbamem.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 84.Alper S.L., Kopito R.R., Libresco S.M., Lodish H.F. Cloning and characterization of a murine band 3-related cDNA from kidney and from a lymphoid cell line. J. Biol. Chem. 1988;263:17092–17099. [PubMed] [Google Scholar]
- 85.Kopito R.R., Lee B.S., Simmons D.M., Lindsey A.E., Morgans C.W., Schneider K. Regulation of intracellular pH by a neuronal homolog of the erythrocyte anion exchanger. Cell. 1989;59:927–937. doi: 10.1016/0092-8674(89)90615-6. [DOI] [PubMed] [Google Scholar]
- 86.Casey J.R., Pirraglia C.A., Reithmeier R.A.F. Enzymatic deglycosylation of human Band 3, the anion transport protein of the erythrocyte membrane. Effect on protein structure and transport properties. J. Biol. Chem. 1992;267:11940–11948. [PubMed] [Google Scholar]
- 87.Kay M.M.B. Location of senescent cell antigen on band 3. Proc. Natl. Acad. Sci. USA. 1984;81:5753–5757. doi: 10.1073/pnas.81.18.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kay M.M.B. Isolation of the phagocytosis-inducing IgG-binding antigen on senescent somatic cells. Nature. 1981;289:491–494. doi: 10.1038/289491a0. [DOI] [PubMed] [Google Scholar]
- 89.Kay M.M.B., Goodman S.R., Sorensen K., Whitfield C.L., Wong P., Zaki L., Rudloff V. Senescent cell antigen is immunologically related to band 3. Proc. Natl. Acad. Sci. USA. 1983;80:1631–1635. doi: 10.1073/pnas.80.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Beppu M., Mizukami A., Nagoya M., Kikugawa K. Binding of anti-band 3 autoantibody to oxidatively damaged erythrocytes. Formation of senescent antigen on erythrocyte surface by an oxidative mechanism. J. Biol. Chem. 1990;265:3226–3233. [PubMed] [Google Scholar]
- 91.Beppu M., Mizukami A., Nagoya M., Kikugawa K. Generation of senescent antigen on erythrocytes by partial blocking of SH groups of the membrane proteins. J. Pharmacobio-Dyn. 1992;15:353–358. doi: 10.1248/bpb1978.15.353. [DOI] [PubMed] [Google Scholar]
- 92.Beppu M., Mizukami A., Ando K., Kikugawa K. Antigenic determinants of senescent antigen of human erythrocytes are located in sialylated carbohydrate chains of Band 3 glycoprotein. J. Biol. Chem. 1992;267:14691–14696. [PubMed] [Google Scholar]
- 93.Ando K., Kikugawa K., Beppu M. Involvement of sialylated poly-N-acetyllactosaminyl sugar chains of band 3 glycoprotein on senescent erythrocytes in anti-band 3 autoantibody binding. J. Biol. Chem. 1994;269:19394–19398. [PubMed] [Google Scholar]
- 94.Kay M.M.B., Marchalonis J.J., Hughes J., Watanabe K., Schluter S.F. Definition of a physiologic aging autoantigen by using synthetic peptides of membrane protein band 3: Localization of the active antigenic sites. Proc. Natl. Acad. Sci. USA. 1990;87:5734–5738. doi: 10.1073/pnas.87.15.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lutz H.U., Gianora O., Nater M., Schweizer E., Stammler P. Naturally occurring anti-band 3 antibodies bind to protein rather than to carbohydrate on band 3. J. Biol. Chem. 1993;268:23562–23566. [PubMed] [Google Scholar]
- 96.Blumenfeld O.O., Huang C.-H. Molecular genetics of the glycophorin gene family, the antigens for MNSs blood groups: Multiple gene rearrangements and modulation of splice site usage result in extensive diversification. Hum. Mutat. 1995;6:199–209. doi: 10.1002/humu.1380060302. [DOI] [PubMed] [Google Scholar]
- 97.Hamaguchi H., Cleve H. Solubilization of human erythrocyte membrane glycoproteins and separation of the MN glycoprotein from a glycoprotein with I, S, and A activity. Biochim. Biophys. Acta. 1972;278:271–280. doi: 10.1016/0005-2795(72)90232-2. [DOI] [PubMed] [Google Scholar]
- 98.Blumenfeld O.O., Zvilichovsky B. Isolation of glycoproteins from red cell membranes using pyridine. Methods Enzymol. 1972;28:245–252. [Google Scholar]
- 99.Schulte T.H., Marchesi V.T. Self-association of human erythrocyte glycophorin A. Appearance of low mobility bands on sodium dodecyl sulfate gels. Biochim. Biophys. Acta. 1978;508:425–430. doi: 10.1016/0005-2736(78)90089-5. [DOI] [PubMed] [Google Scholar]
- 100.Furthmayr H., Marchesi V.T. Subunit structure of human erythrocyte glycophorin A. Biochemistry. 1976;15:1137–1144. doi: 10.1021/bi00650a028. [DOI] [PubMed] [Google Scholar]
- 101.Janado M., Azuma J., Onodera K. Heterogeneity of a human erythrocyte membrane glycoprotein. J. Biochem. 1973;74:881–887. [PubMed] [Google Scholar]
- 102.Lemmon M.A., Flanagan J.M., Treutlein H.R., Zhang J., Engelman D.M. Sequence specificity in the dimerization of transmembrane α-helixes. Biochemistry. 1992;31:12719–12725. doi: 10.1021/bi00166a002. [DOI] [PubMed] [Google Scholar]
- 103.Marton L.S.G., Garvin J.E. Subunit structure of the major human erythrocyte glycoprotein: Depolymerization by heating ghosts with sodium dodecyl sulfate. Biochem. Biophys. Res. Commun. 1973;52:1457–1462. doi: 10.1016/0006-291X(73)90664-5. [DOI] [PubMed] [Google Scholar]
- 104.Tuech J.K., Morrison M. Human erythrocyte membrane sialoglycoproteins: A study of interconversion. Biochem. Biophys. Res. Commun. 1974;59:352–360. doi: 10.1016/S0006-291X(74)80214-7. [DOI] [PubMed] [Google Scholar]
- 105.Johe K.K., Smith A.J., Vengelen-Tyler V., Blumenfeld O.O. Amino acid sequence of an α-δ-glycophorin hybrid. A structure reciprocal to Sta δ-α-glycophorin hybrid. J. Biol. Chem. 1989;264:17486–17493. [PubMed] [Google Scholar]
- 106.Merry A.H., Hodson C., Thomson E., Mallinson G., Anstee D.J. The use of monoclonal antibodies to quantify the levels of sialoglycoproteins α and δ and variant sialoglycoproteins in human erythrocyte membranes. Biochem. J. 1986;233:93–98. doi: 10.1042/bj2330093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ada G.L., Stone J.D. Electrophoretic studies of virus-red cell interaction: Mobility gradient of cells treated with viruses of the influenza group and the receptor-destroying enzyme of V. cholerae. Brit. J. Exptl. Pathol. 1950;31:263–274. [PMC free article] [PubMed] [Google Scholar]
- 108.Dahr W., Beyreuther K., Steinbach H., Gielen W., Krüger J. Structure of the Ss blood group antigens, II. A methionine/threonine polymorphism within the N-terminal sequence of the Ss glycoprotein. Hoppe-Seyler Z. Physiol. Chem. 1980;361:895–906. doi: 10.1515/bchm2.1980.361.1.895. [DOI] [PubMed] [Google Scholar]
- 109.Prohaska R., Koerner T.A.W., Jr., Armitage I.M., Furthmayr H. Chemical and carbon-13 nuclear magnetic resonance studies of the blood group M and N active sialoglycopeptides from human glycophorin A. J. Biol. Chem. 1981;256:5781–5791. [PubMed] [Google Scholar]
- 110.Pang A.J., Reithmeier R.A.F. Interaction of anion exchanger 1 and glycophorin A in human erythroleukaemic K562 cells. Biochem. J. 2009;421:345–356. doi: 10.1042/BJ20090345. [DOI] [PubMed] [Google Scholar]
- 111.Anstee D.J., Ridgwell K., Tanner M.J.A., Daniels G.L., Parsons S.F. Individuals lacking the Gerbich blood-group antigen have alterations in the human erythrocyte membrane sialoglycoproteins β and γ. Biochem. J. 1984;221:97–104. doi: 10.1042/bj2210097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dahr W., Moulds J., Baumeister G., Moulds M., Kiedrowski S., Hummel M. Altered membrane sialoglycoproteins in human erythrocytes lacking the Gerbich blood group antigens. Biol. Chem. Hoppe-Seyler. 1985;366:201–211. doi: 10.1515/bchm3.1985.366.1.201. [DOI] [PubMed] [Google Scholar]
- 113.Lisowska E. The Molecular Immunology of Complex Carbohydrates—2. Springer; Boston, MA, USA: 2001. Antigenic properties of human glycophorins—An update; pp. 155–169. [DOI] [PubMed] [Google Scholar]
- 114.Takasaki S., Kobata A. Chemical characterization and distribution of ABO blood group active glycoprotein in human erythrocyte membrane. J. Biol. Chem. 1976;251:3610–3615. [PubMed] [Google Scholar]
- 115.Wilczyńska Z., Miller-Podraza H., Kościelak J. The contribution of different glycoconjugates to the total ABH blood group activity of human erythrocytes. EFBS Lett. 1980;112:277–279. doi: 10.1016/0014-5793(80)80197-9. [DOI] [PubMed] [Google Scholar]
- 116.Podbielska M., Fredriksson S.-Å., Nilsson B., Lisowska E., Krotkiewski H. ABH blood group antigens in O-glycans of human glycophorin A. Arch. Biochem. Biophys. 2004;429:145–153. doi: 10.1016/j.abb.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 117.Sueyoshi S., Tsuji T., Osawa T. Carbohydrate-binding specificities of five lectins that bind to O-glycosyl-linked carbohydrate chains. Quantitative analysis by frontal-affinity chromatography. Carbohydr. Res. 1988;178:213–224. doi: 10.1016/0008-6215(88)80113-7. [DOI] [PubMed] [Google Scholar]
- 118.Krotkiewska B., Pasek M., Krotkiewski H. Interaction of glycophorin A with lectins as measured by surface plasmon resonance (SPR) Acta Biochim. Pol. 2002;49:481–490. [PubMed] [Google Scholar]
- 119.Anderson R., Paquette S., Lovrien R. Lectin-erythrocyte interaction with external transmembrane glycophorin saccharides controlling membrane internal cytoskeleta. J. Agric. Food Chem. 2002;50:6599–6604. doi: 10.1021/jf020261n. [DOI] [PubMed] [Google Scholar]
- 120.Ellens H., Bentz J., Mason D., Zhang F., White J.M. Fusion of influenza hemagglutinin-expressing fibroblasts with glycophorin-bearing liposomes: Role of hemagglutinin surface density. Biochemistry. 1990;29:9697–9707. doi: 10.1021/bi00493a027. [DOI] [PubMed] [Google Scholar]
- 121.Kanaseki T., Kawasaki K., Murata M., Ikeuchi Y., Ohnishi S. Structural features of membrane fusion between influenza virus and liposome as revealed by quick-freezing electron microscopy. J. Cell Biol. 1997;137:1041–1056. doi: 10.1083/jcb.137.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Suzuki Y., Suzuki T., Matsumoto M. Isolation and characterization of receptor sialoglycoprotein for hemagglutinating virus of Japan (Sendai virus) from bovine erythrocyte membrane. J. Biochem. 1983;93:1621–1633. doi: 10.1093/oxfordjournals.jbchem.a134301. [DOI] [PubMed] [Google Scholar]
- 123.Wybenga L.E., Epand R.F., Nir S., Chu J.W.K., Sharom F.J., Flanagan T.D., Epand R.M. Glycophorin as a receptor for Sendai virus. Biochemistry. 1996;35:9513–9518. doi: 10.1021/bi9606152. [DOI] [PubMed] [Google Scholar]
- 124.Templeton T.J., Keister D.B., Muratova O., Procter J., Kaslow D.C. Adherence of erythrocytes during exflagellation of Plasmodium falciparum microgametes is dependent on erythrocyte surface sialic acid and glycophorins. J. Exp. Med. 1998;187:1599–1609. doi: 10.1084/jem.187.10.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pasvol G. How many pathways for invasion of the red blood cell by the malaria parasite? Trends. Parasitol. 2003;19:430–432. doi: 10.1016/j.pt.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 126.Lobo C.-A. Babesia divergens and Plasmodium falciparum use common receptors, glycophorins A and B, to invade the human red blood cell. Infect. Immun. 2005;73:649–651. doi: 10.1128/IAI.73.1.649-651.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ghislaine Mayer D.C., Jiang L., Achur R.N., Kakizaki I., Gowda D.C., Miller L.H. The glycophorin C N-linked glycan is a critical component of the ligand for the Plasmodium falciparum erythrocyte receptor BAEBL. PNAS. 2006;103:2358–2362. doi: 10.1073/pnas.0510648103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cortajarena A.L., Goñi F.M., Ostolaza H. Glycophorin as a receptor for Escherichia coli α-hemolysin in erythrocytes. J. Biol. Chem. 2001;276:12513–12519. doi: 10.1074/jbc.M006792200. [DOI] [PubMed] [Google Scholar]
- 129.Hirst G.K. The agglutination of red cells by allantoic fluid of chick embryos infected with influenza virus. Science. 1941;94:22–23. doi: 10.1126/science.94.2427.22. [DOI] [PubMed] [Google Scholar]
- 130.Cochet S., Volet G., Cartron J.-P., Bertrand O. New procedures for glycophorin A purification with high yield and high purity. J. Chromatogr. B. 2001;750:109–119. doi: 10.1016/S0378-4347(00)00434-5. [DOI] [PubMed] [Google Scholar]
- 131.Lee S., Lu W. Using elastic light scattering of red blood cells to detect infection of malaria parasite. IEEE Trans. Biomed. Eng. 2012;59:150–155. doi: 10.1109/TBME.2011.2168398. [DOI] [PubMed] [Google Scholar]
- 132.Giger K., Habib I., Ritchie K., Low P.S. Diffusion of glycophorin A in human erythrocytes. Biochim. Biophys. Acta. 2016;1858:2839–2845. doi: 10.1016/j.bbamem.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yiangou L., Montandon R., Modrzynska K., Rosen B., Bushell W., Hale C., Billker O., Rayner J.C., Pance A. A stem cell strategy identifies glycophorin C as a major erythrocyte receptor for the rodent malaria parasite Plasmodium berghei. PLoS ONE. 2016;11:e0158238. doi: 10.1371/journal.pone.0158238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hasssoun H., Hanada T., Lutchman M., Sahr K.E., Palek J., Hanspal M., Chishti A.H. Complete deficiency of glycophorin A in red blood cells from mice with targeted inactivation of the band 3 (AE1) gene. Blood. 1998;91:2146–2151. [PubMed] [Google Scholar]
- 135.Lesley J.B., Groves J.D., Okubo Y., Thilaganathan B., Tanner J.A. Altered band 3 structure and function in glycophorin A- and B-deficient (MkMk) red blood cells. Blood. 1994;84:916–922. [PubMed] [Google Scholar]
- 136.Hamaguchi H., Cleve H. Solubilization and comparative analysis of mammalian erythrocyte membrane glycoproteins. Biochem. Biophys. Res. Commun. 1972;47:459–464. doi: 10.1016/0006-291X(72)90736-X. [DOI] [PubMed] [Google Scholar]
- 137.Ballas S.K. Comparative distribution of glyceraldehyde 3-phosphate dehydrogenase activity in human, guinea-pig, rabbit and mouse erythrocytes. Comp. Biochem. Physiol. B. 1987;87:837–842. doi: 10.1016/0305-0491(87)90398-1. [DOI] [PubMed] [Google Scholar]
- 138.Matsuyama R., Ueda T., Inoue F. SDS-PAGE analysis of membrane proteins on domestic cat (Felis catus) erythrocytes. Med. Biol. 1999;138:79–82. (In Japanese) [Google Scholar]
- 139.Fukuda K., Tomita M., Hamada A. Isolation and characterization of alkali-labile oligosaccharide units from horse glycophorin. J. Biochem. 1980;87:687–693. doi: 10.1093/oxfordjournals.jbchem.a132797. [DOI] [PubMed] [Google Scholar]
- 140.Fukuda K., Kawashima I., Tomita M., Hamada A. Structural studies of the acidic oligosaccharide units from bovine glycophorin. Biochim. Biophys. Acta. 1982;717:278–288. doi: 10.1016/0304-4165(82)90180-5. [DOI] [PubMed] [Google Scholar]
- 141.Kawashima I., Fukuda K., Tomita M., Hamada A. Isolation and characterization of alkali-labile oligosaccharide units from porcine erythrocyte glycophorin. J. Biochem. 1982;91:865–872. doi: 10.1093/oxfordjournals.jbchem.a133774. [DOI] [PubMed] [Google Scholar]
- 142.Klimas N.G., Caldwell K.E., Whitney P.L., Fletcher M.A. Comparison of receptor properties of erythrocyte membrane glycoproteins. Dev. Comp. Immunol. 1981;6:765–774. [PubMed] [Google Scholar]
- 143.Yamashita T., Murayama J., Utsumi H., Hamada A. Structural studies of O-glycosidic oligosaccharide units of dog erythrocyte glycophorin. Biochim. Biophys. Acta. 1985;839:26–31. doi: 10.1016/0304-4165(85)90177-1. [DOI] [Google Scholar]
- 144.Angel A.-S., Grönberg G., Krotkiewski H., Lisowska E., Nilsson B. Structural analysis of the N-linked oligosaccharides from murine glycophorin. Arch. Biochem. Biophys. 1991;291:76–88. doi: 10.1016/0003-9861(91)90107-T. [DOI] [PubMed] [Google Scholar]
- 145.Murayama J.-I., Utsumi H., Hamada A. Amino acid sequence of monkey erythrocyte glycophorin MK. Its amino acid sequence has a striking homology with that of human glycophorin A. Biochim. Biophys. Acta. 1989;999:273–280. doi: 10.1016/0167-4838(89)90009-5. [DOI] [PubMed] [Google Scholar]
- 146.Krotkiewski H. The structure of glycophorins of animal erythrocytes. Glycoconj. J. 1988;5:35–48. doi: 10.1007/BF01048330. [DOI] [Google Scholar]
- 147.Dockham P.A., Vidaver G.A. Comparison of human and pigeon erythrocyte membrane proteins by one-and two-dimensional gel electrophoresis. Comp. Biochem. Physiol. 1987;87B:171–177. doi: 10.1016/0305-0491(87)90486-X. [DOI] [PubMed] [Google Scholar]
- 148.Weise M.J., Ingram V.M. Proteins and glycoproteins of membranes from developing chick red cells. J. Biol. Chem. 1976;251:6667–6673. [PubMed] [Google Scholar]
- 149.Caldwell A.B. Proteins of the turkey erythrocyte membrane. Biochemistry. 1976;15:2711–2718. doi: 10.1021/bi00657a035. [DOI] [PubMed] [Google Scholar]
- 150.Romano L., Passow H. Characterization of anion transport system in trout red blood cell. Am. J. Physiol. 1984;246:C330–C338. doi: 10.1152/ajpcell.1984.246.3.C330. [DOI] [PubMed] [Google Scholar]
- 151.Jay D.G. Characterization of the chicken erythrocyte anion exchange protein. J. Biol. Chem. 1983;258:9431–9436. [PubMed] [Google Scholar]
- 152.Michel F., Rudloff V. Isolation and characterization of the rainbow trout erythrocyte band-3 protein. FEBS J. 1989;181:181–187. doi: 10.1111/j.1432-1033.1989.tb14709.x. [DOI] [PubMed] [Google Scholar]
- 153.Hübner S., Michel F., Rudloff V., Appelhans H. Amino acid sequence of band-3 protein from rainbow trout erythrocytes derived from cDNA. Biochem. J. 1992;285:17–23. doi: 10.1042/bj2850017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jackson R.C. The exterior surface of the chicken erythrocyte. J. Biol. Chem. 1975;250:617–622. [PubMed] [Google Scholar]
- 155.Duk M., Krotkiewski H., Stasyk T.V., Lutsik-Kordovsky M., Syper D., Lisowska E. Isolation and characterization of glycophorin from nucleated (chicken) erythrocytes. Arch. Biochem. Biophys. 2000;375:111–118. doi: 10.1006/abbi.1999.1637. [DOI] [PubMed] [Google Scholar]
- 156.Aoki T., Fukai M., Ueno R. Glycoproteins in red cell membranes from carp and rainbow trout. Fish. Sci. 1996;62:498–499. doi: 10.2331/fishsci.62.498. [DOI] [Google Scholar]
- 157.Aoki T., Chimura K., Nakao N., Mizuno Y. Isolation and characterization of glycophorin from carp red blood cell membranes. Membranes. 2014;4:491–508. doi: 10.3390/membranes4030491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Aoki T., Inoue T. Glycophorin in red blood cell membranes of healthy and diseased carp, Cyprinus carpio L. J. Fish Dis. 2011;34:573–576. doi: 10.1111/j.1365-2761.2011.01262.x. [DOI] [PubMed] [Google Scholar]
- 159.Aoki T., Chimura K., Sugiura H., Mizuno Y. Structure of a sialo-oligosaccharide from glycophorin in carp red blood cell membranes. Membranes. 2014;4:764–777. doi: 10.3390/membranes4040764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Araki T., Aoki T., Kitamikado M. Isolation and identification of a β-1,3-xylanase-producing bacterium. Nippon Suisan Gakkaishi. 1987;53:2077–2081. doi: 10.2331/suisan.53.2077. [DOI] [Google Scholar]
- 161.Uchida Y. In: Application of Chitin, Chitosan. Chitin, Chitosan research association, editor. Gihodo Shuppan Co., Ltd.; Tokyo, Japan: 1990. pp. 71–98. (In Japanese) [Google Scholar]
- 162.Wilson M., Bengtén E., Miller N.W., Clem L.W., Du Pasquier L., Warr G.W. A novel chimeric Ig heavy chain from a teleost fish shares similarities to IgD. Proc. Natl. Acad. Sci. USA. 1997;94:4593–4597. doi: 10.1073/pnas.94.9.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]