Abstract
The present minireview of the place of cystatin C in clinical medicine emphasizes, and discuss the evidence, that cystatin C-based GFR-estimating equations do not require the use of vague terms like race and sex, that cystatin C-based GFR-esti mating equations are useful for both children and adults, including the elderly, that the best GFR-estimation requires simultaneous use of both cystatin C- and creatinine-based equations, that cystatin C-based GFR-estimating equations are superior to creatinine-based equations in predicting end-stage renal disease, cardiovascular manifestations, hospitalisation and death, and, finally that cystatin C is required to diagnose the new syndrome “Shrunken Pore Syndrome” with its high mortality and morbidity, even in the absence of reduced GFR. When automated laboratory equipment is available, the cost of cystatin C is comparable to that of enzymatically determined creatinine.
The conclusion is that cystatin C should be used at least as often as creatinine in clinical medicine.
Key words: cystatin C, GFR, morbidity, mortality, renal, shrunken-pore-syndrome
INTRODUCTION
The introduction of creatinine as a marker of GFR started in 1926 with the publication of an article by Poul Brandt Rehberg: “Studies on kidney function. The rate of filtration and reabsorption in the human kidney” (1). Since then the use of creatinine has been a vital element of clinical medicine. Cystatin C was suggested to be a marker of GFR in 1979(2) and a few articles published before 1994 supported its use as a GFR-marker (3-5). In 1994 an article with the title “Serum cystatin C, determined by a rapid, automated particle-enhanced turbidimetric method, is a better marker than serum creatinine for glomerular filtration rate” was published (6), which initiated widespread studies of cystatin C as a marker of GFR. Today, October 2017, the search string in PubMed “Cystatin C AND (renal OR kidney)” produces more than 3500 titles. The information obtained in these 3500 investigations allows the conclusion that the low-cost analysis of cystatin C should be an integral part of the analysis spectrum for optimal evaluation of the kidney status of a patient.
This is because:
Cystatin C-based GFR-estimating equations do not require the use of vague terms like race and sex
Cystatin C-based GFR-estimating equations are useful for both children and adults, including the elderly
The best GFR-estimation requires simultaneous use of both cystatin C- and creatinine-based equations
Cystatin C-based GFR-estimating equations are superior to creatinine-based equations in predicting end-stage renal disease, cardiovascular manifestations, hospitalisation and death
Cystatin C is required to diagnose the new syndrome “Shrunken Pore Syndrome” with its high mortality and morbidity, even in the absence of reduced GFR.
CYSTATIN C-BASED GFR-ESTIMATING EQUATIONS DO NOT REQUIRE THE USE OF VAGUE TERMS LIKE RACE AND SEX
One of the main advantages of cystatin C compared to creatinine as a GFR-marker is that it is less dependent upon the body composition of a patient. For example, while muscle mass strongly influences creatinine, it does not, or only marginally, affect cystatin C (7-11).
Creatinine-based GFR-estimating equations therefore contain terms aiming at evaluating the muscle mass of a specific patient. These terms refer to the race and sex of the patient. Specific race-factors have been suggested for Afro-Americans (12), Japanese (13,14), Chinese (15), Koreans (16), and native Americans and Hispanics (17). But “race” is a very vague term, difficult to define and does not consider the problem that a major part of the world population represents persons with mixed ethnicity. In contrast, the cystatin C concentration varies only marginally with ethnicity and no vague race terms are therefore required in cystatin C-based GFR-estimating equations (18-20).
The mean muscle mass of females is lower than that of males and creatinine-based GFR-estimating equations therefore requires significant sex-related factors for females (21). However, the world is less and less sex-dichotomized and the existence of more than two sexes is now acknowledged in several countries (22). This ambiguity in applying creatinine-based GFR-estimating equations does not apply for some cystatin C-based GFR-estimating equations, since muscle mass only marginally, or not at all, influences the cystatin C-level and thus cystatin C-based GFR-estimating equations do not require factors for sex (20).
CYSTATIN C-BASED GFR-ESTIMATING EQUATIONS ARE USEFUL FOR BOTH CHILDREN AND ADULTS, INCLUDING THE ELDERLY
The strong correlation between muscle mass and creatinine poses a special problem concerning the use of creatinine-based GFR-estimating equations in childhood, since the muscle mass strongly increases with age. As a consequence, different equations generally have to be used for adults and children (23-25). In contrast, since the muscle mass does not influence cystatin C significantly many cystatin C-based equations work for both children and adults (20, 23-25). One of them is the CAPA-equation which has been shown to work from 1 – 50 years of age (20, 25 and unpublished observations by Grubb A, et al.). Another problem related to the use of creatinine-based equations is that the muscle mass in the elderly is often considerably reduced, so that it negatively affects the ability of these equations to demonstrate a reduced GFR in the elderly. In contrast, cystatin C-based equations are not significantly influenced by muscle mass and therefore useful in identifying reduced GFR also in the elderly with low muscle mass (26).
THE BEST GFR-ESTIMATION REQUIRES SIMULTANEOUS USE OF BOTH CYSTATIN C- AND CREATININE-BASED EQUATIONS
Although creatinine-based GFR-estimating equations are inferior in diagnostic performance compared to cystatin C-based equations for several populations, it has generally been shown that the best GFR-estimation requires use of both cystatin C and creatinine in the equation (27-31). The best estimates of GFR, produced by cystatin C-based equations, eGFRcystatin C, produce values of which 80-85% are within ±30% of GFR measured by invasive gold-standard methods and similar figures are valid for the corresponding estimates, eGFRcreatinine, obtained by creatinine-based equations (27-31). Equations using both cystatin C and creatinine might produce values of which 90-91% are within ±30% of GFR measured by invasive gold-standard methods (30,32). Still better results are obtained if the mean, eGFRmean =(eGFRcystatin C + eGFRcreatinine)/2 of the estimate obtained by a cystatin C-and a creatinine-based equation are used, rather than complex equations containing both cystain C and creatinine (32-34). This is due to that combined equations do not perform optimally in a number of clinical situations, for example, if the patient has an abnormally low muscle mass or is treated with a high dose of glucocorticoids. A strategy for GFR estimation based on the automatic use of a combined cystatin C and creatinine-based equation will, in these cases, have a worse diagnostic performance than a strategy that only uses the cystatin C- or creatinine-based GFR-estimating equation not influenced by the specific patient characteristics (33,34). Such a strategy thus requires that GFR is estimated by both a cystatin C- and a creatinine-based equation, producing eGFRcystatin C or eGFRcreatinine, and that the results are compared. If the two equations produce similar estimates, their average is a very reliable estimate of GFR. If the estimates do not agree and a specific factor known to disturb either the cystatin C- or creatinine-based estimate is present, only the estimate produced by the equation not disturbed by this factor, is used (33,34). As a matter of fact, since 1994, when cystatin C-based estimations of GFR were introduced in Lund in parallel with creatinine-based estimations, we have had 20-30 cases for which eGFRcystatin C and eGFRcreatinine. agreed, but disagreed with GFR measured by our invasive gold-standard procedure (plasma clearance of iohexol). In all cases, in which relevant information was available, the error was caused by technical problems in the execution of the gold-standard procedure. We therefore consider that, in practice, eGFRmean based upon agreeing eGFRcystatin C and eGFRcreatinine is at least as reliable as GFR measured by invasive gold-standard procedures (33,34). This strategy is described at the multilingual site www.egfr.se (35), which can also be implemented to calculate absolute GFR from relative GFR, which might be required in, e.g., dosing of medicines cleared by the kidneys.
CYSTATIN C – BASED GFR-ESTIMATING EQUATIONS ARE SUPERIOR TO CREATININE-BASED EQUATIONS IN PREDICTING END-STAGE RENAL DISEASE, CARDIOVASCULAR MANIFESTATIONS, HOSPITALISATION AND DEATH
One important reason to estimate GFR in a patient is to decide whether the patient suffers from chronic kidney disease or not, and to classify the degree of the chronic kidney disease, if present. Both eGFRcystatin C and eGFRcreatinine work well for this purpose. However, another important aspect of the estimation is how well it predicts the consequences of kidney disease, e.g., end-stage renal disease, cardiovascular manifestations, hospitalisation and death, since this knowledge influences decisions concerning the intensity of the treatment modalities. In this respect, eGFRcystatin C and eGFRcreatinine differ, because the published scientific studies virtually unanimously show that eGFRcystatin C is significantly superior to eGFRcreatinine (36-39).
The cause for the superiority of eGFRcystatin C as a risk marker is unknown, but observational studies have shown that inflammation, old age, male gender, greater weight, and cigarette smoking correlate with higher cystatin C levels (40). But statistical correlations in observational studies do not prove causal connections. A study of elective surgery of patients demonstrated a postoperative sharp rise in inflammation of the patients, with large increases in the levels of CRP of all patients, but with no increase in the cystatin C levels, thus rejecting the hypothesis that inflammation causes a raise in the production of cystatin C (41). The correlations between inflammation, old age, male gender, greater weight, and cigarette smoking and cystatin C might be due to that all these factors promote the development of atherosclerosis, also in the renal arteries, thus producing a decrease in GFR and an increase in cystatin C (41). These correlations therefore speak in favour of cystatin C as a GFR-marker and not against it.
CYSTATIN C IS REQUIRED TO DIAGNOSE THE NEW SYNDROME “SHRUNKEN PORE SYNDROME” WITH ITS HIGH MORTALITY AND MORBIDITY, EVEN IN THE ABSENCE OF REDUCED GFR
The use of eGFRmean and the simultaneous comparison of eGFRcystatin C and eGFRcreatinine, as the best way to estimate GFR in clinical practice (32-34,42) identifies a number of patients with significant differences between eGFRcystatin C and eGFRcreatinine. Part of these differences can be explained by factors, such as muscle wasting or treatment with large doses of glucocorticoids, known to invalidate the GFR estimations based on creatinine or cystatin C (33). But the majority of the patients with such differences between eGFRcystatin C and eGFRcreatinine, do not display any known such factor and their eGFRmean is, despite the differences between eGFRcystatin C and eGFRcreatinine, still the best way to estimate GFR (41).
Most of the patients displaying these differences has a pattern of eGFRcystatin C and eGFRcreatinine in which eGFRcystatin C is lower than eGFRcreatinine (42,43). When the levels of low-molecular mass proteins other than cystatin C, e.g., β2-microglobulin, β-trace protein, and retinol-binding protein, were determined in patients with eGFRcystatin C ≤ 60% of eGFRcreatinine, it was observed that the concentration ratios of these proteins to creatinine were, like the cystatin C-creatinine ratio, higher, than in patients in whom eGFRcystatin C ≈eGFRcreatinine (43).
The genes for these proteins are located at different chromosomes and have different regulation elements and the synthesis of these proteins is not generally known to be influenced by factors affecting the production of cystatin C (43). This strongly indicates that the production of these proteins and cystatin C is not co-regulated and therefore cannot explain the concordant increases of their plasma levels. But the concurrent increase can be explained if the proteins have a common clearance mechanism by glomerular filtration and that this is reduced by shrinking of the glomerular pores (43). Therefore, the observation that eGFRcystatin C ≤ 60% of eGFRcreatinine in a patient indicates the presence of a new syndrome, tentatively called “Shrunken Pore Syndrome” (43). The explanation that creatinine and other small molecules do not simultaneously increase in concentration would then be, that their sieving coefficients are still close to unity (i.e., one) despite the shrunken pores resulting in reduced sieving coefficients for proteins similar in size to cystatin C (43-45).
It is noteworthy, that a similar mechanism previously has been suggested for the increase in plasma levels of low-molecular mass proteins in the third trimester of pregnancy (46-48) and for the development of still higher concentrations of low-molecular mass proteins in preeclampsia (49,50). This suggests that the (patho-)physiologic changes in late pregnancy and preeclampsia are similar to those occurring in patients with “Shrunken Pore Syndrome.”
As “Shrunken Pore Syndrome” was identified recently (43), only a few studies of its clinical consequences have been performed. The first investigation showed, that the long-term mortality in patients undergoing elective coronary artery bypass grafting was much higher in patients suffering from “Shrunken Pore Syndrome” than in patients without the syndrome (51). This was true both when the preoperative GFR was normal or reduced (Figure 1A and B). In this study, the cystatin C-based CAPA-equation was used to produce eGFRcystatin C and the creatinine-based LMrev-equation to produce eGFRcreatinine, as both these equations work not only for adults, but also for children (20,52,53). Interestingly, an increase in mortality was not only observed when eGFRcystatin C ≤ 60% of eGFRcreatinine, but also when eGFRcystatin C ≤ 70% of eGFRcreatinine (Figure 1 A and B). Ongoing studies demonstrate that the long-term mortality in “Shrunken Pore Syndrome” increases inversely with the eGFRcystatin C/eGFRcreatinine-ratio, starting at 0.90. Recently published and ongoing studies in several different types of populations corroborate, that “Shrunken Pore Syndrome” is associated with significantly increased mortality and morbidity (54,55) and indicate that the syndrome also predicts higher risks for development of end-stage renal disease, cardiovascular manifestations and for hospitalisation.
Figure 1A.
Survival after coronary artery bypass surgery for patients with GFR > 60 mL/min per 1.73 m2 with and without Shrunken Pore Syndrome (SPS)
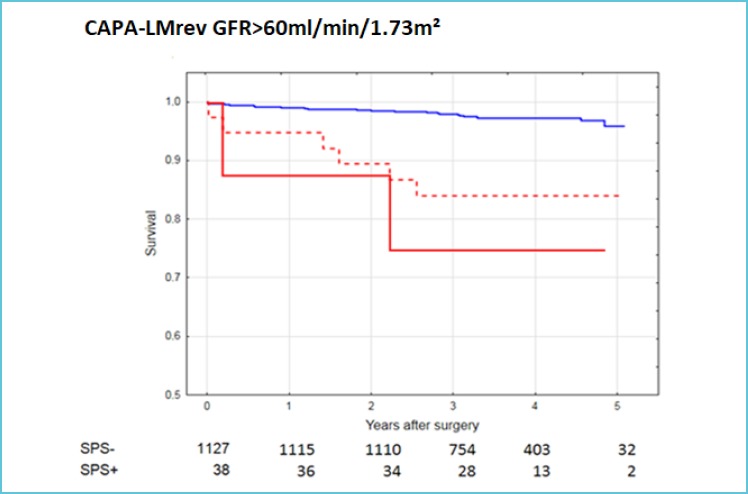
eGFRcystatin C was estimated using the CAPA equation and eGFRcreatinine using the LMrev equation.
The cut-on li level for SPS was eGFRcystatin C ≤ 70% of eGFRcreatinine (red’”broken line) or eGFRcystatin C< 60% of eGFRcreatinine (red unbroken line).
The unbroken blue line indicates the mortality of patients without SPS (0.90<eGFRcystatin C/eGFRcreatinine <1.10).
The numbers below indicate patients with and without SPS, when the cut-off level was 70%.
Figure 1B.
Survival after coronary artery bypass surgery for patients with GFR < 60 mL/min per 1.73 m2 with and without Shrunken Pore Syndrome (SPS)
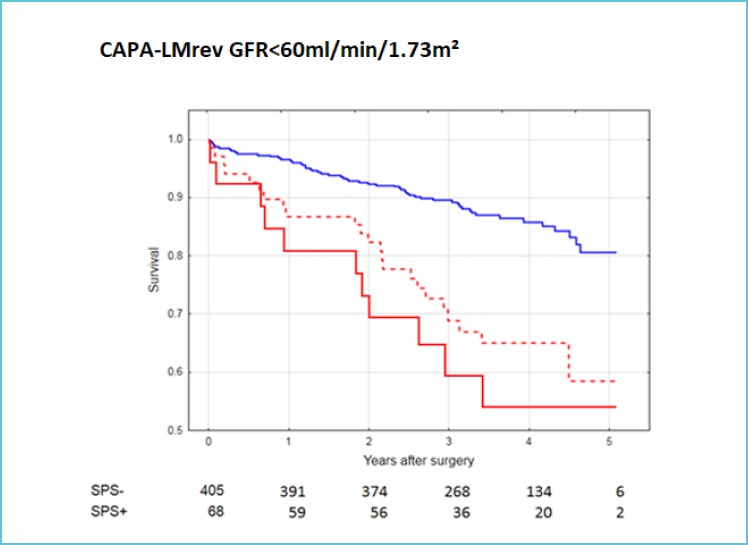
eGFRcystatin C was estimated using the CAPA equation and eGFRcreatinine using the LMrev equation.
The cut-off level for SPS was eGFRcystatin C ≤ 70% of eGFRcreatinine (red broken line) or eGFRcystatin C≤ 60% of eGFRcreatinine (red unbroken line).
The unbroken blue line indicates the mortality of patients without SPS (0.90<eGFRcystatin C/eGFRcreatinine <1.10).
The numbers below indicate patients with and without SPS, when the cut-off level was 70%.
CONCLUSION
The use of cystatin C (or eGFRcystatin C) in addition to creatinine improves the estimation of GFR, makes it independent of vague terms like race and sex, and facilitates its use for children and the elderly. It also allows the identification of a new syndrome (Shrunken Pore Syndrome) associated with a high morbidity and mortality. When automated laboratory equipment is available, the cost of cystatin C is comparable to that of enzymatically determined creatinine. Cystatin C should therefore be used at least as often as creatinine in the clinical routine.
REFERENCES
- 1.Rehberg PB. Studies on kidney function. The rate of filtration and reabsorption in the human kidney. Biochem J 1926;20:447-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Löfberg H, Grubb A. Quantitation of γ-trace in human biological fluids: indications for production in the central nervous system. Scand J Clin Lab Invest 1979;39:619–626. [DOI] [PubMed] [Google Scholar]
- 3.Grubb A, Simonsen O, Sturfelt G, Truedsson L, Thysell H. Serum concentration of cystatin C, factor D and beta-2-microglobulin as a measure of glomerular filtration rate. Acta Med Scand 1985;218:499–503. [DOI] [PubMed] [Google Scholar]
- 4.Simonsen O, Grubb A, Thysell H. The blood serum concentration of cystatin C (gamma-trace) as a measure of the glomerular filtration rate. Scand J Clin Lab Invest 1985;45:97–101. [DOI] [PubMed] [Google Scholar]
- 5.Grubb A, Löfberg H. Human γ-trace. Structure, function and clinical use of concentration measurements. Scand J Clin Lab Invest 1985;45(Suppl. 177):7-13. [PubMed] [Google Scholar]
- 6.Kyhse-Andersen J, Schmidt C, Nordin G, Andersson B, Nilsson-Ehle P, Lindström V, Grubb A. Serum cystatin C, determined by a rapid, automated particle-enhanced turbidimetric method, is a better marker than serum creatinine for glomerular filtration rate. Clin Chem 1994;40:1921–1926. [PubMed] [Google Scholar]
- 7.Vinge E, Lindergård B, Nilsson-Ehle P, Grubb A. Relationships among serum cystatin C, serum creatinine, lean tissue mass and glomerular filtration rate in healthy adults. Scand J Clin Lab Invest 1999;59:1–6. [DOI] [PubMed] [Google Scholar]
- 8.Seronie-Vivien S, Delanaye P, Pieroni L, Mariat C, Froissart M, Cristol JP. SFBC ‘Biology of renal function and renal failure’ working group. Cystatin C: current position and future prospects. Clin Chem Lab Med 2008;46:1664–1686. [DOI] [PubMed] [Google Scholar]
- 9.Chew JSC, Saleem M, Florkowski CM, George PM. Cystatin C – a paradigm of evidence based laboratory medicine. Clin Biochem Rev 2008;29:47–62. [PMC free article] [PubMed] [Google Scholar]
- 10.Thomassen SA, Johannesen IL, Erlandsen EJ, Abrahamsen J, Randers E. Serum cystatin C as a marker of the renal function in patients with spinal cord injury. Spinal Cord 2002;40:524–528. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins MA, Brown DJ, lerino FL, Ratnaike SI. Cystatin C for estimation of glomerular filtration rate in patients with spinal cord injury. Ann Clin Biochem 2003;40:364–368. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Greene T, Kusek JW, Beck GJ. A simplified equation to predict glomerular filtration rate from serum creatinine [Abstract]. J Am Soc Nephrol 2000;11:A0828. [Google Scholar]
- 13.Imai E, Horio M, Nitta K, Yamagata K, Iseki K, Tsukamoto Y, Ito S, Makino H, Hishida A, Matsuo S. Modification of the modification of diet in renal disease (MDRD) study equation for Japan. Am J Kidney Dis 2007;50:927–937. [DOI] [PubMed] [Google Scholar]
- 14.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009;53:982-992. [DOI] [PubMed] [Google Scholar]
- 15.Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, Xu JS, Huang SM, Wang LN, Huang W, Wang M, Xu GB, Wang HY. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 2006;17:2937-2944. [DOI] [PubMed] [Google Scholar]
- 16.Lee CS, Cha RH, Lim YH, Kim H, Song KH, Gu N, Yu KS, Lim CS, Han JS, Kim S, Kim YS. Ethnic coefficients for glomerular filtration rate estimation by the Modification of Diet in Renal Disease study equations in the Korean population. J Korean Med Sci 2010;25:1616-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevens LA, Claybon MA, Schmid CH, Chen J, Horio M, Imai E, Nelson RG, Van Deventer M, Wang HY, Zuo L, Zhang YL, Levey AS. Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int 2011;79:555-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uhlmann EJ, Hock KG, Issitt C, Sneeringer MR, Cervelli DR, Gorman RT, Scott MG. Reference intervals for plasma cystatin C in healthy volunteers and renal patients, as measured by the Dade Behring BN II system, and correlation with creatinine. Clin Chem 2001;47:2031-2033. [PubMed] [Google Scholar]
- 19.Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S. GFR estimating equation based on standardized serum cystatin C. Am J Kidney Dis 2013;61:197-203. [DOI] [PubMed] [Google Scholar]
- 20.Grubb A, Horio M, Hansson LO, Björk J, Nyman U, Flodin M, Larssson A, Bökenkamp A, Yasuda Y, Blufpand H, Lindström V, Zegers I, Althaus H, Blirup-Jensenl S, Itoh Y, Sjöström P, Nordin G, Christensson A, Klima H, Sunde K, Hjort-Christensen P, Armbruster D, Ferrrero C: Generation of a new cystatin C-based estimating equation for glomerular filtration rate using seven assays standardized to the international calibrator. Clin Chem 2014;60:974-986. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, van Lente F. Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006;145:247–254. [DOI] [PubMed] [Google Scholar]
- 22.Ainsworth C. Sex redefined. The idea of two sexes is simplistic. Biologists now think there is a wider spectrum than that. Nature 2015;518:288-291. [DOI] [PubMed] [Google Scholar]
- 23.Bökenkamp A, Domanetzki M, Zinck R, Schumann G, Byrd D, Brodehl J. Cystatin C – a new marker of glomerular filtration rate in children independent of age and height. Pediatrics 1998;101:875-881. [DOI] [PubMed] [Google Scholar]
- 24.Bökenkamp A, Domanetzki M, Zinck R, Schumann G, Brodehl J. Reference values for cystatin C serum concentrations in children. Pediatr Nephrol 1998;12:125-129. [DOI] [PubMed] [Google Scholar]
- 25.Leion F, Hegbrant J, den Bakker E, Jonsson M, Abrahamson M, Nyman U, Björk J, Lindström V, Larsson A, Bökenkamp A, Grubb A. Estimating glomerular filtration rate (GFR) in children. The average between a cystatin C- and a creatinine-based equation improves estimation of GFR in both children and adults and enables diagnosing Shrunken Pore Syndrome. Scand J Clin Lab Invest 2017;77:338-344. [DOI] [PubMed] [Google Scholar]
- 26.Colantonio LD, Tanner RM, Warnock DG, Gutiérrez OM, Judd S, Muntner P, Bowling CB. The role of cystatin-C in the confirmation of reduced glomerular filtration rate among the oldest old. Arch Med Sci 2016;12:55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouvet Y, Bouissou F, Coulais Y, Séronie-Vivien S, Tafani M, Decramer S, Chatelut E. GFR is better estimated by considering both serum cystatin C and creatinine levels. Pediatr Nephrol 2006;21:1299–1306. [DOI] [PubMed] [Google Scholar]
- 28.Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, Xu JS, Huang SM, Wang LN, Huang W, Wang M, Xu GB, Wang HY; Chinese eGFR Investigation Collaboration. Improved GFR estimation by combined creatinine and cystatin C measurements. Kidney Int 2007;72:1535–1542. [DOI] [PubMed] [Google Scholar]
- 29.Tidman M, Sjöström P, Jones I. A Comparison of GFR estimating formulae based upon s-cystatin C and s-creatinine and a combination of the two. Nephrol Dial Transplant 2008;23:154–160. [DOI] [PubMed] [Google Scholar]
- 30.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS. Estimating GFR using cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 2008;51:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol 2009;20:629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nyman U, Grubb A, Sterner G, Björk J. Different equations to combine creatinine and cystatin C to predict GFR. Arithmetic mean of existing equations performs as well as complex equations. Scand J Clin Lab Invest 2009;69:619–627. [DOI] [PubMed] [Google Scholar]
- 33.Grubb A. Non-invasive estimation of glomerular filtration rate (GFR). The Lund model: simultaneous use of cystatin C- and creatinine-based GFR-prediction equations, clinical data and an internal quality check. Scand J Clin Lab Invest 2010;70:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grubb A, Nyman U, Björk J. Improved estimation of glomerular filtration rate (GFR) by comparison of eGFRcystatin C and eGFRcreatinine. Scand J Clin Lab Invest 2012;72:73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.www.egfr.se (availability tested November 6, 2017).
- 36.Jernberg T, Lindahl B, James S, Larsson A, Hansson LO, Wallentin L, Cystatin C: a novel predictor of outcome in suspected or confirmed non-ST-elevation acute coronary syndrome. Circulation 2004;110:2342-2348. [DOI] [PubMed] [Google Scholar]
- 37.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 2005;352:2049–2060. [DOI] [PubMed] [Google Scholar]
- 38.Peralta CA, Shlipak MG, Judd S, Cushman M, McClellan W, Zakai NA, Safford MM, Zhang X, Muntner P, Warnock D. Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA 2011;305:1545–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shlipak MG, Matsushita K, Ärnlöv J, Inker LA, Katz R, Polkinghorne KR, Rothenbacher D, Sarnak MJ, Astor BC, Coresh J, Levey AS, Gansevoort RTCKD Prognosis Consortium. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med 2013;369:932–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knight EL, Verhave JC, Spiegelman D, Hillege HL, de Zeeuw D, Curhan GC, de Jong PE. Factors influencing cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int 2004;65:1416–1421. [DOI] [PubMed] [Google Scholar]
- 41.Grubb A, Björk J, Nyman U, Pollak J, Bengzon J, Östner G, Lindström V. Cystatin C, a marker for successful aging and glomerular filtration rate, is not influenced by inflammation. Scand J Clin Lab Invest 2011;71:145–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Björk J, Grubb A, Larsson A, Hansson LO, Flodin M, Sterner G, Lindström V, Nyman U. Accuracy of GFR estimating equations combining standardized cystatin C and creatinine assays: A cross-sectional study in Sweden. Clin Chem Lab Med 2015;53:403–414. [DOI] [PubMed] [Google Scholar]
- 43.Grubb A, Lindström V, Jonsson M, Bäck SE, Åhlund T, Rippe B, Christensson A. Reduction in glomerular pore size is not restricted to pregnant women. Evidence for a new syndrome: “Shrunken pore syndrome”. Scand J Clin Lab Invest 2015;75:333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lund U, Rippe A, Venturoli D, Tenstad O, Grubb A, Rippe B. Glomerular filtration rate dependence of sieving of albumin and some neutral proteins in rat kidneys. Am J Physiol 2003;284:F1226–F1234. [DOI] [PubMed] [Google Scholar]
- 45.Norden AG, Lapsley M, Lee PJ, Pusey CD, Scheinman SJ, Tam FW, Thakker RV, Unwin RJ, Wrong O. Glomerular protein sieving and implications for renal failure in Fanconi syndrome. Kidney Int 2001;60:1885-1892. [DOI] [PubMed] [Google Scholar]
- 46.Grubb A, Lindström V, Kristensen K, Christensson A, Wide-Swensson D, Strevens H, Schmidt C, Blirup-Jensen S. Filtration quality: a new measure of renal disease. Clin Chem Lab Med 2007;45(Suppl.):S273–S274. [Google Scholar]
- 47.Strevens H, Wide-Swensson D, Torffvit O, Grubb A. Serum cystatin C for assessment of glomerular filtration rate in pregnant and non-pregnant women. Indications of altered filtration process in pregnancy. Scand J Clin Lab Invest 2002;62:141–147. [DOI] [PubMed] [Google Scholar]
- 48.Kristensen K, Lindström V, Schmidt C, Blirup-Jensen S, Grubb A, Wide-Swensson D, Strevens H. Temporal changes of the plasma levels of cystatin C, beta-trace protein, beta-2-microglobulin, urate and creatinine during pregnancy indicate continuous alterations in the renal filtration process. Scand J Clin Lab Invest 2007;67:612–618. [DOI] [PubMed] [Google Scholar]
- 49.Strevens H, Wide-Swensson D, Grubb A. Serum cystatin C is a better marker for preeclampsia than serum creatinine or serum urate. Scand J Clin Lab Invest 2001;61:575–580. [DOI] [PubMed] [Google Scholar]
- 50.Kristensen K, Wide-Swensson D, Schmidt C, Blirup-Jensen S, Lindström V, Strevens H, Grubb A. Cystatin C, beta-2-microglobulin and beta-trace protein in pre-ec-lampsia. Acta Obstet Gynecol Scand 2007;86:921–926. [DOI] [PubMed] [Google Scholar]
- 51.Dardashti A, Nozohoor S, Grubb A, Bjursten H. Shrunken Pore Syndrome is associated with a sharp rise in mortality in patients undergoing elective coronary artery by-pass grafting. Scand J Clin Lab Invest 2016;76:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Björk J, Grubb A, Sterner G, Nyman U. Revised equations for estimating glomerular filtration rate based on the Lund-Malmö study cohort. Scand J Clin Lab Invest 2011;71:232–239. [DOI] [PubMed] [Google Scholar]
- 53.Nyman U, Björk J, Lindström V, Grubb A. The Lund-Malmö creatinine-based glomerular filtration rate prediction equation for adults also performs well in children. Scand J Clin Lab Invest 2008;68:568–576. [DOI] [PubMed] [Google Scholar]
- 54.Purde MT, Nock S, Risch L, Medina Escobar P, Grebhardt C, Nydegger UE, Stanga Z, Risch M. The cystatin C/creatinine ratio, a marker of glomerular filtration quality: associated factors, reference intervals, and prediction of morbidity and mortality in healthy seniors. Transl Res 2016;169:80–90. [DOI] [PubMed] [Google Scholar]
- 55.Christensson A, Grubb A, Molvin J, Holm H, Gransbo K, Tasevska-Dinevska G, Bachus E, Jujic A, Magnusson M. The shrunken pore syndrome is associated with declined right ventricular systolic function in a heart failure population – the HARVEST study. Scand J Clin Lab Invest. 2016;76:568-574. [DOI] [PubMed] [Google Scholar]


