Abstract
Aims
Non‐invasive positive pressure ventilation rapidly improves the symptoms of acute heart failure (AHF). A portion of patients, however, are forced to be intubated even though intubation is associated with serious complications, and hypercapnia is often observed in AHF requiring intubation. The purpose of this study is to examine the clinical profile and management of hypercapnia in AHF patients.
Methods and results
We examined the arterial blood gas analysis in 193 consecutive AHF patients (73 ± 12 years, 61% men) at admission. Many patients (n = 129, 66.8%) had already been treated with oxygen by the ambulance staff. Hypercapnia (PaCO2 at admission >45 mmHg) and hypocapnia (PaCO2 < 35 mmHg) were observed in 33.7% and 32.6%, respectively. Whereas 16 (24.6%) hypercapnic patients were intubated, there were only one (1.5%) normocapnic and no hypocapnic patients intubated. Patients with hypercapnia are more likely to be in the New York Heart Association Class IV (96.9% vs. 78.9%, P < 0.001), to have acute onset within 6 h (50.8% vs. 25.0%, P < 0.001), and to have radiographic pulmonary oedema (84.6% vs. 57.8%, P < 0.001) than those with hypo‐normocapnia. Hypercapnia was more frequent in patients with acute cardiogenic pulmonary oedema than in those with acute decompensated heart failure (51.9% vs. 23.6%, P < 0.001). At discharge, hypercapnia was observed in 17.8% of patients who were hypercapnic at admission.
Conclusion
Hypercapnia emerged in AHF acutely and transiently, was associated with immediate airway intervention, and was possibly involved in the pathophysiology of acute pulmonary oedema. Patients with acute onset dyspnoea should have their respiratory status carefully managed. These pathophysiological findings are expected to be utilized in treating or preventing AHF.
Keywords: Hypercapnia, Acute heart failure, Heart failure, Carbon dioxide
Introduction
Acute heart failure (AHF) is a common cause of hospitalization in older patients with a high mortality rate.1 Non‐invasive positive pressure ventilation (NPPV) rapidly improves the symptoms of AHF including acute cardiogenic pulmonary oedema (APE) than oxygen alone.2, 3, 4 A portion of patients, however, is forced to be intubated due to unconsciousness or other reasons, even though intubation is associated with serious complications.5 Hypercapnia is often observed in AHF requiring intubation and is considered to be associated with severe respiratory distress. However, the prevalence, clinical background, and roles in emergent airway management of hypercapnia are uncertain because blood gas data in unselected consecutive AHF patients have been lacking. Therefore, the purpose of this study is to examine the clinical profile and management of hypercapnia in AHF patients.
Methods
Study patients
From September 2011 to February 2013, 287 patients with AHF and without concomitant ST elevation acute myocardial infarction (AMI) were consecutively admitted to Yokohama City University Medical Centre. AHF was defined by the Framingham criteria with the variables on admission.6 Patients with a reduced ejection fraction and a preserved ejection fraction were included. We prospectively enrolled 197 patients who provided written consent and subsequently assessed arterial blood gases (ABG) in all. For unconscious patients on admission, informed consent was obtained after the recovery of consciousness. We analysed the data of 193 patients after excluding those with sampling error (n = 4). The European Society of Cardiology definitions were used to classify APE and acute decompensated heart failure (ADHF).7, 8 APE was defined as the acute onset of dyspnoea within the preceding 6 h and radiographic alveolar oedema.9 The allocation of airway intervention was left to the discretion of the doctor in accordance with established guidelines.10, 11 The study was approved by the ethics review committee of our institution, and signed informed consent was obtained from each patient before participation. This study was registered with the UMIN protocol registration system with the identification number UMIN000010161.
Measurement
Data regarding patient medical history, symptoms, and treatments were collected for each patient. Night‐time admission was defined as admission from 8 pm to 9 am. Radiographic alveolar oedema was defined as ill‐defined semi‐confluent shadows present in any part of the lung.12 ABG, haematology, and biochemistry tests were obtained upon admission. Hypercapnia and hypocapnia were defined as PaCO2 > 45 mmHg and PaCO2 < 35 mmHg, respectively.13 Those with hypercapnia on admission underwent the second assessment of ABG at discharge. Underlying diseases were diagnosed at discharge according to the American College of Cardiology/American Heart Association clinical data standards.14 The estimated glomerular filtration rate was calculated by the modified formula described in the Modification of Diet in Renal Disease study, which was conducted by the Japanese Society of Nephrology.15 Echocardiography was performed with standard parasternal and apical views in the emergency department.
Statistical analysis
The data are presented as the means ± standard deviation, as well as medians with inter‐quartile ranges and frequencies. The unpaired Student's t‐test and the Mann–Whitney U test were used to compare two groups as appropriate. Differences in categorical variables were analysed by the chi‐square test. Univariate and multivariate forward logistic regression analyses were used to assess the association between clinical characteristics and the mode of airway intervention (NPPV, intubation, and NPPV or intubation). The odds ratio (OR) and 95% confidence interval (CI) for NPPV, intubation, and NPPV/ intubation were calculated. Multivariate analyses included age, gender, body mass index, clinical background (i.e. coronary artery disease, hypertension, valvular heart disease, atrial fibrillation, prior hospitalization for heart failure, diabetes mellitus, and pack‐years of smoking), pulse oximeter saturation with oxygen, radiographic alveolar oedema, left ventricular ejection fraction on echocardiography, serum creatinine, and the presence of hypercapnia using stepwise selection of covariates. The Hosmer–Lemeshow statistic was applied to assess model calibration. The significance level to include and exclude in the model was set to 0.05 and 0.10, respectively. Two‐tailed P values of less than 0.05 were considered to indicate statistically significant differences. The analyses were performed using SPSS 17.0 J for Windows (SPSS Inc., Tokyo, Japan).
Results
Characteristics of participants
Of the 193 patients, ADHF presented with a frequency of 65.8%, APE 26.9%, cardiogenic shock 1.6%, hypertensive heart failure 3.6%, right ventricular failure 1.6%, and high output heart failure 0.5%. Table 1 presents the characteristics of participants. Mean age was 73 ± 12 years, and 36.6% had prior hospitalization for heart failure. Mean ejection fraction on admission was 38 ± 16%, 64.2% were patients with reduced ejection fraction (<45%), and 35.7% were those with preserved ejection fraction (≥45%). Sixty‐seven per cent of patients had already been treated with oxygen by ambulance staff, and an additional 7% was treated immediately after arriving at the hospital, in which ABG was then assessed under oxygen supplementation in 74% of patients. In‐hospital mortality was 3.1%.
Table 1.
Characteristics of the overall cohort and in patients with hypercapnia and hypo‐normocapnia
| Overall cohort | Hypercapnia | Hypo‐normocapnia | ||
|---|---|---|---|---|
| Characteristics | n = 193 | n = 65 | n = 128 | P |
| Mean age, years | 73 (12) | 74 (9) | 73 (13) | 0.63 |
| Sex, male, % | 60.6 | 58.5 | 61.7 | 0.76 |
| Body mass index, kg/m2 | 23.7 (4.8) | 23.3 (4.6) | 23.9 (4.9) | 0.42 |
| Background | ||||
| Coronary artery disease, % | 60.1 | 64.6 | 57.8 | 0.44 |
| Hypertension, % | 75.1 | 80.0 | 72.7 | 0.29 |
| Valvular heart disease, % | 29.5 | 24.6 | 32.0 | 0.32 |
| Atrial fibrillation, % | 30.6 | 21.5 | 35.2 | 0.07 |
| Prior hospitalization for heart failure, % | 36.6 | 37.5 | 36.2 | 0.87 |
| Diabetes mellitus, % | 43.5 | 49.2 | 40.6 | 0.28 |
| Current smoker, % | 29.6 | 37.1 | 26.0 | 0.13 |
| Pack‐years of smoking | 30 (37) | 28 (29) | 31 (41) | 0.62 |
| Medications prior to admission | ||||
| Loop diuretic, % | 39.2 | 39.7 | 38.9 | >0.99 |
| Spironolactone, % | 17.5 | 19.0 | 16.7 | 0.69 |
| ACE inhibitor or ARB, % | 45.5 | 42.9 | 46.8 | 0.64 |
| Beta blocker, % | 34.0 | 37.1 | 32.5 | 0.62 |
| Digoxin, % | 10.5 | 4.7 | 13.5 | 0.08 |
| Night‐time admission (8 pm–9 am), % | 48.7 | 66.2 | 39.8 | <0.001 |
| Admission by ambulance, % | 70.2 | 89.2 | 60.3 | <0.001 |
| Acute onset within 6 h, % | 33.7 | 50.8 | 25.0 | <0.001 |
| Shock, % | 1.6 | 4.6 | 0.0 | 0.04 |
| NYHA Class IV, % | 85.0 | 96.9 | 78.9 | <0.001 |
| Disturbance of consciousness, % | 25.9 | 55.7 | 11.3 | <0.001 |
| Systolic blood pressure, mmHg | 167 (41) | 188 (43) | 156 (36) | <0.001 |
| Diastolic blood pressure, mmHg | 94 (29) | 105 (30) | 89 (27) | <0.001 |
| Heart rate, beats/min | 110 (27) | 116 (27) | 108 (27) | 0.03 |
| Respiratory rate, times/min (n = 130) | 27 (7) | 28 (8) | 26 (7) | 0.19 |
| Pulse oximeter saturation with oxygen, % | 94 (7) | 92 (10) | 95 (6) | 0.01 |
| Oxygen supplementation, % | 66.8 | 84.4 | 57.9 | <0.001 |
| Radiographic alveolar oedema, % | 66.8 | 84.6 | 57.8 | <0.001 |
| Echocardiography | ||||
| Left ventricular ejection fraction, (%) | 38 (16) | 40 (17) | 37 (16) | 0.28 |
| Moderate/severe mitral regurgitation, % | 53.9 | 46.2 | 57.8 | 0.13 |
| Laboratory findings | ||||
| Haemoglobin, g/dL | 12.4 (2.3) | 12.5 (2.2) | 12.4 (2.4) | 0.97 |
| Serum creatinine, mg/dL | 1.59 (1.74) | 1.55 (1.57) | 1.61 (1.82) | 0.81 |
| Estimated GFR, mL/min/1.72 m2 | 48 (23) | 49 (26) | 47 (22) | 0.54 |
| B‐type natriuretic peptide, pg/mL* | 849 (480–1495) | 913 (590–1604) | 745 (427–1429) | 0.12 |
| In‐hospital management | ||||
| NPPV or intubation, % | 34.7 | 78.5 | 12.5 | <0.001 |
| NPPV, % | 27.5 | 58.5 | 11.7 | <0.001 |
| Intubation, % | 8.8 | 24.6 | 0.8 | <0.001 |
| Pulmonary artery catheter, % | 4.7 | 6.2 | 3.9 | 0.49 |
| Coronary angiography, % | 35.2 | 36.9 | 34.4 | 0.75 |
| Percutaneous coronary intervention, % | 9.4 | 9.2 | 9.4 | >0.99 |
| Coronary artery bypass grafting, % | 5.7 | 6.2 | 5.5 | >0.99 |
| Intra‐aortic balloon pump, % | 1.0 | 1.5 | 0.8 | >0.99 |
| Length of hospital stay, days* | 17 (10–26) | 18 (12–28) | 17 (9–26) | 0.25 |
ACE, angiotensin converting enzymes; ARB, angiotensin 2 receptor blockers; NYHA, New York Heart Association; GFR, glomerular filtration rate; NPPV, non‐invasive positive pressure ventilation.
Median (inter‐quartile range).
Blood gas and airway management
Average pH, PaO2, PaCO2, HCO3− were 7.33 ± 0.16, 141 ± 113 mmHg, 47.0 ± 23.9 mmHg, and 22.1 ± 4.0 mEq/L, respectively. Hypercapnia, hypocapnia, and normocapnia were observed in 33.7%, 32.6%, and 33.7% of patients, respectively. PaCO2 was 52.1 ± 27.0 mmHg in patients who had been treated with oxygen and 36.3 ± 9.2 mmHg in those without oxygen (P < 0.001). Logistic regression analysis revealed that hypercapnia but not hypocapnia was a significant predictor of NPPV [univariate OR with 95% CI of hypercapnia vs. hypo‐normocapnia; 10.63 (5.10–22.22), P < 0.001, and OR of hypocapnia vs. normo‐hypercapnia; 0.08 (0.02–0.27), P < 0.001] and NPPV/intubation [OR of hypercapnia; 25.64 (11.63–55.56), P < 0.001, and OR of hypocapnia; 0.05 (0.02–0.17), P < 0.001, Table 2]. Whereas 16 (24.6%) hypercapnic patients were intubated, there were only one normocapnic and no hypocapnic patients intubated. Therefore, ORs for intubation were not assessed both in univariate and multivariate analyses. After multivariate adjustment, hypercapnia was still a significant predictor of NPPV and NPPV/intubation (Table 3). These models were reliable (P = 0.99 for NPPV and P = 0.34 for NPPV or intubation by the Hosmer–Lemeshow test). The incidence of immediate airway management in hypercapnic, normocapnic, and hypocapnic patients is also shown in Figure 1.
Table 2.
Univariate logistic regression analyses of possible factors associated for NPPV, intubation, and NPPV or intubation
| For NPPV | For intubation | For NPPV or intubation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Univariate OR | 95% CI | P | Univariate OR | 95% CI | P | Univariate OR | 95% CI | P |
| Age, per 5 years | 0.99 | 0.87–1.13 | 0.88 | 0.93 | 0.76–1.14 | 0.48 | 0.98 | 0.87–1.11 | 0.77 |
| Sex, male | 0.80 | 0.42–1.51 | 0.48 | 1.21 | 0.43–3.42 | 0.72 | 0.94 | 0.52–1.73 | 0.85 |
| Body mass index, per kg/m2 | 1.02 | 0.96–1.09 | 0.53 | 0.94 | 0.84–1.06 | 0.34 | 1.00 | 0.93–1.06 | 0.88 |
| Background | |||||||||
| Coronary artery disease | 0.98 | 0.53–1.94 | 0.96 | 0.60 | 0.20–1.78 | 0.36 | 0.85 | 0.46–1.56 | 0.60 |
| Hypertension | 1.18 | 0.56–2.50 | 0.66 | 2.65 | 0.58–12.05 | 0.21 | 1.40 | 0.69–2.84 | 0.35 |
| Valvular heart disease | 0.46 | 0.21–1.00 | 0.049 | 0.99 | 0.33–2.96 | 0.99 | 0.45 | 0.22–0.91 | 0.03 |
| Atrial fibrillation | 0.50 | 0.24–1.06 | 0.07 | 0.68 | 0.21–2.17 | 0.52 | 0.48 | 0.24–0.95 | 0.04 |
| Prior hospitalization for heart failure | 1.24 | 0.65–2.39 | 0.51 | 1.23 | 0.45–3.40 | 0.69 | 1.20 | 0.65–2.21 | 0.57 |
| Diabetes mellitus | 1.36 | 0.72–2.57 | 0.34 | 1.97 | 0.72–5.41 | 0.19 | 1.57 | 0.86–2.85 | 0.14 |
| Current smoker | 0.95 | 0.47–1.92 | 0.88 | 0.85 | 0.26–2.80 | 0.79 | 0.90 | 0.46–1.74 | 0.75 |
| Pack‐years of smoking, per 10 pack‐years | 0.96 | 0.88–1.06 | 0.43 | 0.95 | 0.81–1.13 | 0.59 | 0.96 | 0.88–1.05 | 0.39 |
| Medications prior to admission | |||||||||
| Loop diuretic | 1.00 | 0.52–1.94 | 0.99 | 0.83 | 0.30–2.36 | 0.73 | 0.96 | 0.52–1.77 | 0.89 |
| Spironolactone | 1.02 | 0.44–2.37 | 0.97 | 0.61 | 0.13–2.79 | 0.52 | 0.80 | 0.36–1.80 | 0.59 |
| ACE inhibitor or ARB | 0.98 | 0.51–1.87 | 0.95 | 0.63 | 0.22–1.77 | 0.38 | 0.86 | 0.47–1.58 | 0.63 |
| Beta blocker | 1.36 | 0.70–2.66 | 0.36 | 0.79 | 0.27–2.35 | 0.67 | 1.22 | 0.65–2.28 | 0.54 |
| Digoxin | 0.87 | 0.30–2.53 | 0.80 | — | 0.60 | 0.21–1.72 | 0.34 | ||
| Night‐time admission (8 pm–9 am) | 4.31 | 2.14–8.66 | <0.001 | 2.75 | 0.93–8.14 | 0.07 | 4.90 | 2.55–9.40 | <0.001 |
| Admission by ambulance | 10.71 | 3.18–36.08 | <0.001 | — | 16.46 | 4.90–55.25 | <0.001 | ||
| Acute onset within 6 h | 2.49 | 1.30–4.79 | <0.01 | 2.41 | 0.88–6.58 | 0.09 | 3.16 | 1.69–5.91 | <0.001 |
| Shock | — | — | — | ||||||
| NYHA Class IV | — | — | — | ||||||
| Disturbance of consciousness | 5.12 | 2.50–10.44 | <0.001 | 30.68 | 6.69–140.83 | <0.001 | 14.36 | 6.47–31.87 | <0.001 |
| Systolic blood pressure, per 10 mmHg | 1.10 | 1.01–1.18 | 0.02 | 1.13 | 1.00–1.27 | 0.049 | 1.13 | 1.05–1.22 | <0.01 |
| Diastolic blood pressure, per 10 mmHg | 1.08 | 0.97–1.20 | 0.18 | 1.11 | 0.94–1.30 | 0.21 | 1.10 | 0.99–1.22 | 0.07 |
| Heart rate, per 10 beats/min | 1.17 | 1.04–1.32 | <0.01 | 1.21 | 1.01–1.45 | 0.04 | 1.20 | 1.07–1.35 | <0.01 |
| Respiratory rate, per times/min | 1.07 | 1.01–1.13 | 0.01 | 1.06 | 0.99–1.15 | 0.11 | 1.09 | 1.03–1.15 | <0.01 |
| Pulse oximeter saturation with oxygen, per % | 0.97 | 0.93–1.01 | 0.10 | 0.92 | 0.88–0.97 | <0.01 | 0.94 | 0.90–0.98 | <0.01 |
| Oxygen supplementation | 9.27 | 3.17–27.12 | <0.001 | 8.30 | 1.07–64.38 | 0.04 | 10.72 | 4.03–28.50 | <0.001 |
| Radiographic alveolar oedema | 12.82 | 3.83–43.48 | <0.001 | 8.93 | 1.16–66.67 | 0.04 | 14.29 | 4.90–41.67 | <0.001 |
| Echocardiography | |||||||||
| Left ventricular ejection fraction, per 5% | 0.96 | 0.87–1.06 | 0.45 | 0.86 | 0.72–1.04 | 0.11 | 1.00 | 0.85–1.03 | 0.18 |
| Moderate/severe mitral regurgitation | 1.29 | 0.68–2.45 | 0.43 | 0.57 | 0.21–1.57 | 0.27 | 0.90 | 0.50–1.64 | 0.74 |
| Laboratory findings | |||||||||
| Haemoglobin, per g/dL | 0.96 | 0.84–1.10 | 0.57 | 1.09 | 0.88–1.35 | 0.42 | 1.00 | 0.88–1.14 | 0.97 |
| Serum creatinine, per mg/dL | 1.03 | 0.87–1.23 | 0.72 | 1.13 | 0.91–1.40 | 0.28 | 1.09 | 0.92–1.28 | 0.32 |
| Estimated GFR, per mL/min/1.72 m2 | 0.95 | 0.83–1.10 | 0.50 | 0.93 | 0.74–1.16 | 0.51 | 0.94 | 0.82–1.07 | 0.32 |
| Ln [BNP (pg/mL)], per 1 | 1.34 | 0.90–2.01 | 0.15 | 1.71 | 0.88–3.34 | 0.12 | 1.51 | 1.03–2.22 | 0.04 |
| Arterial blood gas | |||||||||
| pH, per 0.1 | 0.48 | 0.37–0.62 | <0.001 | 0.29 | 0.18–0.47 | <0.001 | 0.11 | 0.05–0.21 | <0.001 |
| PaO2, per 10 mmHg | 1.08 | 1.05–1.12 | <0.001 | 1.03 | 0.99–1.06 | 0.15 | 1.11 | 1.06–1.15 | <0.001 |
| PaCO2, per 5 mmHg | 1.24 | 1.13–1.36 | <0.001 | 1.30 | 1.17–1.46 | <0.001 | 2.03 | 1.61–2.56 | <0.001 |
| Hypercapnia | 10.63 | 5.10–22.22 | <0.001 | 41.47 | 5.35–321.23 | <0.001 | 25.64 | 11.63–55.56 | <0.001 |
| Hypocapnia | 0.08 | 0.02–0.27 | <0.001 | — | 0.05 | 0.02–0.17 | <0.001 | ||
| HCO3 −, per mEq/L | 1.00 | 0.99–1.02 | 0.90 | 0.88 | 0.79–0.99 | 0.03 | 1.00 | 0.98–1.01 | 0.70 |
OR, odds ratio; CI, confidence interval; ACE, angiotensin converting enzymes; ARB, angiotensin 2 receptor blockers; BNP, B‐type natriuretic peptide; NYHA, New York Heart Association; GFR, glomerular filtration rate; NPPV, non‐invasive positive pressure ventilation.
Table 3.
Multivariate logistic regression analyses of possible factors associated for NPPV and NPPV or intubation
| For NPPV | For NPPV or intubation | |||||
|---|---|---|---|---|---|---|
| Variable | Multivariate OR | 95% CI | P | Multivariate OR | 95% CI | P |
| Age, per 5 years | Not selected | Not selected | ||||
| Sex, male | Not selected | Not selected | ||||
| Body mass index, per kg/m2 | Not selected | Not selected | ||||
| Coronary artery disease | Not selected | Not selected | ||||
| Hypertension | Not selected | Not selected | ||||
| Valvular heart disease | Not selected | Not selected | ||||
| Atrial fibrillation | Not selected | Not selected | ||||
| Prior hospitalization for heart failure | Not selected | Not selected | ||||
| Diabetes mellitus | Not selected | Not selected | ||||
| Pack‐years of smoking, per 10 pack‐years | Not selected | Not selected | ||||
| Pulse oximeter saturation with oxygen, per % | Not selected | Not selected | ||||
| Radiographic alveolar oedema | 13.68 | 3.05–61.41 | <0.01 | 18.51 | 4.52–75.76 | <0.001 |
| Left ventricular ejection fraction, per 5% | Not selected | 0.83 | 0.72–0.97 | 0.02 | ||
| Serum creatinine, per mg/dL | Not selected | Not selected | ||||
| Hypercapnia | 7.90 | 3.53–17.71 | <0.001 | 29.63 | 10.59–82.86 | <0.001 |
NPPV, non‐invasive positive pressure ventilation.
Figure 1.
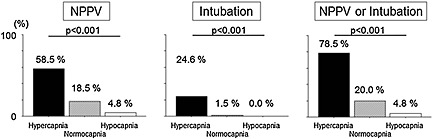
The incidence of immediate airway management in hypercapnic, normocapnic, and hypocapnic patients. The incidence of NPPV, intubation, and NPPV/intubation was higher in hypercapnic patients. NPPV, non‐invasive positive pressure ventilation.
The difference between patients with hypercapnia and hypo‐normocapnia
The difference of characteristics between those with hypercapnia and hypo‐normocapnia can be observed in Table 1. Baseline characteristics, including age, sex, hypertension, diabetes, ejection fraction, ischaemic aetiology, smoking status, and prior medications, were comparable between those with hypercapnia and hypo‐normocapnia. However, patients with hypercapnia were more likely to be in the New York Heart Association (NYHA) Class IV (96.9% vs. 78.9%, P < 0.001), to have acute onset within 6 h (50.8% vs. 25.0%, P < 0.001), and to have radiographic pulmonary oedema (84.6% vs. 57.8%, P < 0.001) than were those with hypo‐normocapnia, suggesting stronger and more acute pulmonary congestion in hypercapnic patients (Figure 2). Disturbances of consciousness (DOC) were also more frequently observed in hypercapnic patients than those with hypo‐normocapnia (55.7% vs. 11.3%, P < 0.001, Figure 2). In‐hospital mortality was comparable between those with hypercapnia and hypo‐normocapnia (3.1% vs. 3.1%, P > 0.99). We could assess the second ABG at discharge in 45 of 65 patients who were initially hypercapnic on admission. At discharge, hypercapnia and hypocapnia were observed in eight (17.8%) and 11 (24.4%) patients, respectively.
Figure 2.
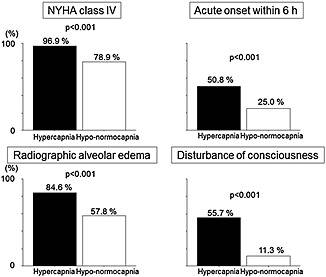
The differences in clinical findings at admission between patients with hypercapnia and hypo‐normocapnia. The patients with hypercapnia are more likely to be in NYHA Class IV, to have acute onset within 6 h, and to have radiographic pulmonary oedema than those with hypo‐normocapnia. Disturbance of consciousness was also more frequently observed in hypercapnic patients than those with hypo‐normocapnia. NYHA, New York Heart Association.
The difference between acute cardiogenic pulmonary oedema and acute decompensated heart failure
We compared the value of ABG between patients with APE and ADHF. The pH was lower (7.26 ± 0.15 vs. 7.38 ± 0.12, P < 0.001), PaCO2 was higher (55.4 ± 25.1 vs. 41.4 ± 19.0 mmHg, P < 0.001), and PaO2 was higher (175 ± 143 vs. 129 ± 100 mmHg, P = 0.02) in APE than ADHF. The HCO3− was comparable between the two groups (21.8 ± 4.0 vs. 22.4 ± 3.9 mEq/L, P = 0.32). Patients with APE were more frequently hypercapnic than were those with ADHF (51.9% vs. 23.6%, P < 0.001).
Discussion
The present study demonstrated that hypercapnia was frequently observed in AHF, as were hypo‐ and normocapnia. Hypercapnia but not hypocapnia was a strong predictor of immediate airway intervention. Patients with hypercapnia had stronger and more acute pulmonary congestion, as indicated by higher presence of the NYHA Class IV, acute onset within 6 h, and radiographic pulmonary oedema, than those with hypo‐normocapnia. A large majority of patients who had been initially hypercapnic on admission became hypo‐normocapnic during hospitalization. Patients with APE were more frequently hypercapnic in comparison with ADHF patients.
To the best of our knowledge, the prevalence of hypercapnia in an unselected AHF population including APE has never been reported. Miñana et al. retrospectively investigated arterial blood gas in 588 patients with ADHF, but they excluded 258 APE patients.16 These researchers also did not show an indication of ABG and might have assessed only those who were considered to need ABG. In these authors' cohort, 9.2% of patients had hypercapnia (defined as PaCO2 at admission >50 mmHg), which was even fewer than in our population [i.e. 23.6% in non‐APE (ADHF) patients], partially due to the difference in the definition of hypercapnia. In the present study, hypercapnia but not hypocapnia was a strong predictor of immediate airway intervention. Regarding intubation, our finding was expected because recent guidelines recommend endotracheal intubation if such symptoms as worsening hypoxemia, failing respiratory effort, and increasing confusion are present.10, 11 In previous studies of APE, hypercapnia is not a constant finding, as several investigators have reported higher,17, 18 and others reported normal,19, 20 PaCO2 levels, although none of these studies directly compared APE and non‐APE. Hypocapnia in APE was also noted as a negative prognostic factor in a report by Valopour.13 However, Masip insisted that hypocapnia might be either due to tachypnea in response to hypoxemia in non‐severe cases or compensation for metabolic acidosis, and its clinical significance is not homogeneous among patients.21 In the present study, hypocapnic patients were much less frequently treated by NPPV/intubation than were hypercapnic patients.
Mechanisms underlying hypercapnia and disturbance of consciousness
Although DOC was associated with hypercapnia in the present study, it is not simple to determine the mechanisms underlying hypercapnia and DOC in AHF because both hypercapnia and DOC could be both the cause and the result. These two factors could constitute a vicious cycle. As triggers to DOC, preceding hypoxia or cardiogenic shock might be possible. Other reasons for DOC (i.e. dysglycaemia, uraemia, electrolytes abnormality, sepsis, and stroke) could not be completely excluded but deemed less likely in the setting of AHF. In the present study, hypoxia was more frequently observed in hypercapnic patients. Cardiogenic shock was too rare to be estimated as a main reason for DOC. On the other hand, as triggers to hypercapnia, alveolar hypoventilation might be possible. Reduced dilution capacity might not be a typical cause of hypercapnia because carbon dioxide can be diluted much faster than oxygen. As a result, hypocapnia, rather than hypercapnia, was reported to coexist frequently in those with lung injury.22, 23 As reasons for alveolar hypoventilation, airway obstruction and obesity‐associated hypoventilation could be considered. In our study, there was no difference in smoking status and body mass index between those with and without hypercapnia, and hypercapnia was not observed at discharge in most initially hypercapnic patients. These results did not support the presence of airway obstruction by underlying pulmonary disease or obesity‐associated hypoventilation and were in accordance with previous reports.24, 25 On the contrary, hypercapnic patients were more likely to be in NYHA Class IV, to have acute onset within 6 h, and to have radiographic pulmonary oedema than were those with hypo‐normocapnia in our cohort. Respiratory muscle fatigue due to increased airway pressure, especially in acute heaviness of the lung, rather than long‐lasting dyspnoea, could trigger hypoventilation, although all of these explanations are nothing more than speculations. The airway obstruction by bronchial oedema also could lead to respiratory muscle fatigue.26 Valipour also advocated that hypercapnia in the absence of chronic obstructive pulmonary disease (COPD) is a consequence of respiratory muscle fatigue.13 Although respiratory rates were comparable between the two groups, this finding might indicate relatively insufficient respiratory response in those with hypercapnia, who should have a much higher respiratory rate. Those who have fallen in hypercapnia with DOC would not be able to recover without the help of immediate airway intervention, and a portion of these patients might have lapsed into sudden death before arriving at the hospital. Takahashi reported that longer pre‐hospital transportation time was associated with higher mortality in AHF.27 As such patients might be under‐represented in clinical research and even not be diagnosed as AHF, the present study may have clinical significance by characterising AHF patients with hypercapnia. Patients who had been treated by oxygen had significantly higher PaCO2 levels than those without oxygen. Although oxygen supplementation is less likely to be a cause of hypercapnia because a reduction in ventilation associated with removal of a hypoxic stimulus was observed in COPD patients but not typically in AHF patients,28 we cannot exclude the influence of oxygen supplementation on PaCO2 at admission.
Limitations
There are several limitations in the present study that warrant discussion. First, as we did not analyse outcome data after discharge, and only six deaths were observed during hospitalization, the impact of APE on short‐ and long‐term prognosis should be addressed in future studies. For the same reason, we could not answer the question of whether the presence or absence of hypercapnia might guide respiratory management. Another limitation was the uncertainty in the definition of APE, which was discussed in detail by our previous study.29 According to a recent review, APE represented 3% of all types of clinical presentations of AHFS,1, 30 which was less than the incidence (i.e. 26.9%) in the present study and considerably less than previous research, such as that of Parissis (37%).7 The distinction between APE and ADHF, as well as APE and hypertensive heart failure, is not clear and has no clear cutoffs. Therefore, the significance of combined analyses for APE and ADHF in the present study may be unclear. Given that AMI represents 30% of APE and hypercapnia was an independent predictor of intubation in APE,24 exclusion of ST elevation AMI in the present study is also a limitation. Because of the very small number of intubated patients under some of characteristics listed in Table 2, the univariate OR and CI were too high to be interpreted practically, and we could not analyse the multivariate analysis for intubation. The lack of data about the diagnosis of COPD as clinical background is also a large limitation to discuss hypercapnia, although we used the data of pack‐years of smoking instead.
In summary, hypercapnia emerged in AHF acutely and transiently, was associated with immediate airway intervention, and was possibly involved in the pathophysiology of APE. Patients with acute onset dyspnoea should have their respiratory status carefully managed. These pathophysiological findings may be beneficial in understanding how to treat or prevent AHF.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors.
Declaration of interest
S. Umemura reports that he has received unrestricted grant funding from Pfizer, Dainippon Sumitomo, Astellas, Shionogi, Daiichi Sankyo, MSD, AstraZeneca, Novartis, Nihon Boehringer Ingelheim, and Torii, and honoraria from Shionogi, MSD, and Kyowa Hakko Kirin. K. Kimura reports that he has received unrestricted grant funding from Toa Eiyo Ltd, Bayer, MSD, Astellas, AstraZeneca, Sanofi, Eli Lilly Japan, Research Institute for Production Development, Novartis, Pfizer, Shionogi, Kowa Souyaku, Daiichi Sankyo, Mitsubishi Tanabe, Nihon Boehringer Ingelheim, Takeda, Otsuka, and Ono, and honoraria from MSD and AstraZeneca. No other disclosures were reported.
Konishi, M. , Akiyama, E. , Suzuki, H. , Iwahashi, N. , Maejima, N. , Tsukahara, K. , Hibi, K. , Kosuge, M. , Ebina, T. , Sakamaki, K. , Matsuzawa, Y. , Endo, M. , Umemura, S. , and Kimura, K. (2015), Hypercapnia in patients with acute heart failure. ESC Heart Failure, 2: 12–19. doi: 10.1002/ehf2.12023.
Clinical trial registration: https://center.umin.ac.jp ID: UMIN000010161
References
- 1. Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol 2009;53:557–573. [DOI] [PubMed] [Google Scholar]
- 2. Peter JV, Moran JL, Phillips‐Hughes J, Graham P, Bersten AD. Effect of non‐invasive positive pressure ventilation (NIPPV) on mortality in patients with acute cardiogenic pulmonary oedema: a meta‐analysis. Lancet 2006;367:1155–1163. [DOI] [PubMed] [Google Scholar]
- 3. Gray A, Goodacre S, Newby DE, Masson M, Sampson F, Nicholl J. Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med 2008;359:142–151. [DOI] [PubMed] [Google Scholar]
- 4. Masip J, Roque M, Sanchez B, Fernandez R, Subirana M, Exposito JA. Noninvasive ventilation in acute cardiogenic pulmonary edema: systematic review and meta‐analysis. JAMA 2005;294:3124–3130. [DOI] [PubMed] [Google Scholar]
- 5. Kramer N, Meyer TJ, Meharg J, Cece RD, Hill NS. Randomized, prospective trial of noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med 1995;151:1799–1806. [DOI] [PubMed] [Google Scholar]
- 6. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971;285:1441–1446. [DOI] [PubMed] [Google Scholar]
- 7. Parissis JT, Nikolaou M, Mebazaa A, Ikonomidis I, Delgado J, Vilas‐Boas F, Paraskevaidis I, Mc Lean A, Kremastinos D, Follath F. Acute pulmonary oedema: clinical characteristics, prognostic factors, and in‐hospital management. Eur J Heart Fail 2010;12:1193–1202. [DOI] [PubMed] [Google Scholar]
- 8. Nieminen MS, Bohm M, Cowie MR, Drexler H, Filippatos GS, Jondeau G, Hasin Y, Lopez‐Sendon J, Mebazaa A, Metra M, Rhodes A, Swedberg K, Priori SG, Garcia MA, Blanc JJ, Budaj A, Cowie MR, Dean V, Deckers J, Burgos EF, Lekakis J, Lindahl B, Mazzotta G, Morais J, Oto A, Smiseth OA, Garcia MA, Dickstein K, Albuquerque A, Conthe P, Crespo‐Leiro M, Ferrari R, Follath F, Gavazzi A, Janssens U, Komajda M, Morais J, Moreno R, Singer M, Singh S, Tendera M, Thygesen K. Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology. Eur Heart J 2005;26:384–416. [DOI] [PubMed] [Google Scholar]
- 9. Kramer K, Kirkman P, Kitzman D, Little WC. Flash pulmonary edema: association with hypertension and reoccurrence despite coronary revascularization. Am Heart J 2000;140:451–455. [DOI] [PubMed] [Google Scholar]
- 10. Guidelines for treatment of acute heart failure (JCS 2011). Circ J 2013;77:2157–2201. [DOI] [PubMed] [Google Scholar]
- 11. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 12. Sharma S, Bhargava A, Krishnakumar R, Rajani M. Can pulmonary venous hypertension be graded by the chest radiograph? Clin Radiol 1998;53:899–902. [DOI] [PubMed] [Google Scholar]
- 13. Valipour A, Cozzarini W, Burghuber OC. Non‐invasive pressure support ventilation in patients with respiratory failure due to severe acute cardiogenic pulmonary edema. Respiration; International Review of Thoracic Diseases 2004;71:144–151. [DOI] [PubMed] [Google Scholar]
- 14. Radford MJ. ACC/AHA Key data elements and definitions for measuring the clinical management and outcomes of patients with chronic heart failure: a report of the american college of cardiology/american heart association task Force on Clinical Data Standards (Writing Committee to Develop Heart Failure Clinical Data Standards). J Am Coll Cardiol 2005;46:1179–1207. [Google Scholar]
- 15. Imai E, Matsuo S, Makino H, Watanabe T, Akizawa T, Nitta K, Iimuro S, Ohashi Y, Hishida A. Chronic Kidney Disease Japan Cohort (CKD‐JAC) study: design and methods. Hypertens Res 2008;31:1101–1107. [DOI] [PubMed] [Google Scholar]
- 16. Minana G, Nunez J, Banuls P, Sanchis J, Nunez E, Robles R, Mascarell B, Palau P, Chorro FJ, Llacer A. Prognostic implications of arterial blood gases in acute decompensated heart failure. Eur J Intern Med 2011;22:489–494. [DOI] [PubMed] [Google Scholar]
- 17. Bersten AD, Holt AW, Vedig AE, Skowronski GA, Baggoley CJ. Treatment of severe cardiogenic pulmonary edema with continuous positive airway pressure delivered by face mask. N Engl J Med 1991;325:1825–1830. [DOI] [PubMed] [Google Scholar]
- 18. Mehta S, Jay GD, Woolard RH, Hipona RA, Connolly EM, Cimini DM, Drinkwine JH, Hill NS. Randomized, prospective trial of bilevel versus continuous positive airway pressure in acute pulmonary edema. Crit Care Med 1997;25:620–628. [DOI] [PubMed] [Google Scholar]
- 19. Rasanen J, Heikkila J, Downs J, Nikki P, Vaisanen I, Viitanen A. Continuous positive airway pressure by face mask in acute cardiogenic pulmonary edema. Am J Cardiol 1985;55:296–300. [DOI] [PubMed] [Google Scholar]
- 20. Lin M, Yang YF, Chiang HT, Chang MS, Chiang BN, Cheitlin MD. Reappraisal of continuous positive airway pressure therapy in acute cardiogenic pulmonary edema. Short‐term results and long‐term follow‐up. Chest 1995;107:1379–1386. [DOI] [PubMed] [Google Scholar]
- 21. Masip J, Paez J, Sanchez B, Cancio B. Hypocapnia in acute pulmonary edema. Respiration; International Review of Thoracic Diseases 2004;71:657. [DOI] [PubMed] [Google Scholar]
- 22. Laffey JG, Kavanagh BP. Hypocapnia. N Engl J Med 2002;347:43–53. [DOI] [PubMed] [Google Scholar]
- 23. Laffey JG, Kavanagh BP. Carbon dioxide and the critically ill‐‐too little of a good thing? Lancet 1999;354:1283–1286. [DOI] [PubMed] [Google Scholar]
- 24. Masip J, Paez J, Merino M, Parejo S, Vecilla F, Riera C, Rios A, Sabater J, Ballus J, Padro J. Risk factors for intubation as a guide for noninvasive ventilation in patients with severe acute cardiogenic pulmonary edema. Intensive Care Med 2003;29:1921–1928. [DOI] [PubMed] [Google Scholar]
- 25. Aberman A, Fulop M. The metabolic and respiratory acidosis of acute pulmonary edema. Ann Intern Med 1972;76:173–184. [DOI] [PubMed] [Google Scholar]
- 26. Berend K, de Vries AP, Gans RO. Physiological approach to assessment of acid‐base disturbances. N Engl J Med 2014;371:1434–1445. [DOI] [PubMed] [Google Scholar]
- 27. Takahashi M, Kohsaka S, Miyata H, Yoshikawa T, Takagi A, Harada K, Miyamoto T, Sakai T, Nagao K, Sato N, Takayama M. Association between prehospital time interval and short‐term outcome in acute heart failure patients. J Card Fail 2011;17:742–747. [DOI] [PubMed] [Google Scholar]
- 28. Jacono FJ. Control of ventilation in COPD and lung injury. Respir Physiol Neurobiol 2013;189:371–376. [DOI] [PubMed] [Google Scholar]
- 29. Konishi M, Matsuzawa Y, Suzuki H, Akiyama E, Iwahashi N, Maejima N, Endo M, Tsukahara K, Hibi K, Kosuge M, Ebina T, Sakamaki K, Morita S, Umemura S, Kimura K. Higher level at admission and subsequent decline in hemoglobin in patients with acute pulmonary edema. Circ J 2014;78:896–902. [DOI] [PubMed] [Google Scholar]
- 30. Gheorghiade M, Zannad F, Sopko G, Klein L, Pina IL, Konstam MA, Massie BM, Roland E, Targum S, Collins SP, Filippatos G, Tavazzi L. Acute heart failure syndromes: current state and framework for future research. Circulation 2005;112:3958–3968. [DOI] [PubMed] [Google Scholar]


