Abstract
Objectives
The present study assessed the changes in functional, biochemical, and echocardiographic measures following long‐term liothyronine therapy in heart failure (HF) patients with low‐triiodothyronine (T3) syndrome (LT3S).
Methods
In the present placebo‐controlled, double‐blind study, adult patients with clinically stable New York Heart Association functional class I–III systolic HF and LT3S receiving standard HF therapy were randomly assigned 1:1 to receive oral liothyronine or placebo for 6 weeks. Low‐T3 syndrome was defined as a serum free T3 of less than the lower limit of normal (<2.4 pg/mL) with normal thyroid‐stimulating hormone (thyrotropin) and free thyroxin values.
Results
Fifty patients, including 39 (78%) men with a mean ± standard deviation age of 60 ± 15 years were included. The 6‐min walk distance increased in the liothyronine group by 93 ± 16 m and in the placebo group by 67 ± 28 m, resulting in a treatment effect of 26 m (P = 0.003). A higher decrease of high‐sensitivity C‐reactive protein level was seen in the liothyronine group than in the placebo group (P = 0.009). Liothyronine markedly decreased serum N‐terminal pro‐brain natriuretic peptide level compared with the placebo (P = 0.01). A significant increase was also seen in the left ventricular ejection fraction by liothyronine as compared with the placebo (<0.001).
Conclusion
Triiodothyronine replacement by chronic liothyronine therapy seems to safely benefit stable HF patients with LT3S receiving optimal HF medications.
Keywords: Congestive heart failure, Liothyronine, Low‐T3 syndrome
Introduction
Heart failure (HF) is a major public health problem and is one of the most common causes of hospitalization and death in the western world. Despite recent advances in early HF diagnosis along with the extensive use of neurohormonal blockade agents, that is, beta‐blockers, angiotensin, and aldosterone inhibitors, HF‐related morbidity and mortality are steadily increasing. This suggests the presence of other less‐understood pathophysiological processes in this chronic systemic disease.
It has been presumed that changes in thyroid hormones may constitute an important pathophysiological component in the development and/or progression of HF.1, 2, 3, 4 Low‐triiodothyronine (T3) syndrome (LT3S), a type of sick euthyroid syndrome (SES), is the initial and most common alteration in the metabolism of thyroid hormones in patients with cardiac diseases, with an incidence of up to 30% in HF patients.5, 6, 7 This syndrome is characterized by a reduction in serum total and free‐T3 concentrations in the presence of normal thyroxin (T4) and thyroid‐stimulating hormone (TSH, thyrotropin) levels.8 In HF, patients with LT3S are associated with higher New York Heart Association (NYHA) functional class, alterations in cardiac index and ventricular filling pressures, and impaired left ventricular function.6, 9 The negative prognostic impact of LT3S in patients with HF has also been demonstrated in several clinical studies.5, 6, 7, 10, 11, 12
However, the benefits of thyroid hormone replacement in HF patients with LT3S have not been ascertained yet. Moreover, selection of the appropriate type of hormone to replace is another challenge. The majority of previous investigations have used levothyroxin (L‐T4) preparations, and questions regarding the benefits of T3 replacement are still unresolved. As a result, the present study was intended to evaluate the beneficial effects of long‐term T3 replacement with the synthetic form of T3 hormone (i.e. L‐T3 sodium or liothyronine sodium), if any, on the functional, biochemical, and echocardiographic measures in HF patients with LT3S.
Method
Study design and population
The study design was a single‐centre, randomized, prospective, double‐blind, and placebo‐controlled study. Patients were recruited consecutively from the Heart Failure and Transplant Clinic of Rajaei Cardiovascular, Medical and Research Center, Tehran, Iran from March 2013 to May 2014 to evaluate the efficacy of treatment with liothyronine for 6 weeks in adult patients (>18 years of age) with clinically stable NYHA classes I–III systolic HF (echocardiography‐derived left ventricular ejection fraction of <40%) and LT3S receiving standard HF therapy. Patients with history of any type of primary thyroid disease were excluded. Patients were also excluded if they were taking thyroid hormones or derivatives, steroids, non‐steroidal anti‐inflammatory drugs, lithium, phenobarbital, iodine‐containing compounds, clofibrate, carbamazepine, anti‐thyroid agents, or heparin. Patients who had received radiographic contrast medium within 4 weeks before study were also excluded. Additional exclusion criteria included amiodarone therapy during the past 6 months, concomitant severe systemic disease, ventricular arrhythmias, severe obesity (body mass index of >35 kg/m2), and pregnant women or women undergoing estroprogestinic therapy.
Patients had been receiving optimized anti‐HF therapy (adequate doses of diuretics and neurohormonal blockades including beta‐blockers and angiotensin converting enzyme inhibitors or angiotensin receptor blockers) and aldosterone antagonists according to the latest guidelines on HF,13, 14 and none of them had been hospitalized, had emergency visits, or changed medical therapy during the 3 preceding months. Moreover, patients were kept on the same medical regimen (including the type and dose of the medications) during all the study period. In the present study, LT3S is defined as a serum free T3 (fT3) of less than the lower limit of normal (<2.4 pg/mL) with normal TSH and free T4 (fT4) values. Stable thyroid function pattern with low fT3 levels was confirmed on the basis of two consecutive determinations within the last month before study.
Study protocol
At study entry, the subjects' clinical history was reviewed, and physical examination, echocardiography, 6‐min walk test, and biochemical measurements were performed. Eligible patients were randomized 1:1 to receive double‐blinded liothyronine or placebo. Patients were randomized in a consecutive order, starting with the lowest provided medication number. The investigators, patients, and laboratory personnel remained blinded to the treatment until the closure of the clinical database. In addition to their background therapy for HF, patients received 25 microgram PO per day liothyronine sodium (Cynomel® 0.025 mg, Sanofi‐Aventis, France) or matching placebo (lactose). Active drugs and placebo were prepared in the identical opaque capsules by a pharmacist who was not involved in any other parts of the study. Patients were visited every 2 weeks, and drug toleration as well as probable side effects was evaluated. At the end of the 6th week, all patients were re‐evaluated by the same investigators with examining biomedical indices, exercise parameters, and echocardiographic assessment. Primary outcome measure was the change in the 6‐min walk distance (6MWD) over a 6‐week period of liothyronine administration. The secondary outcome measures included the effects of liothyronine on NYHA function class, N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) level, high‐sensitivity (hs) C‐reactive protein level (as a pro‐inflammatory marker), and the echocardiographically derived left ventricular ejection fraction (LVEF) and LV dimensions and volumes. The safety of T3 replacement was assessed throughout the study by careful monitoring of haemodynamic measures (including heart rate and systolic/diastolic/mean blood pressures) and undesirable adverse events.
The present study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki. Institutional Review Boards at Iran University of Medical Science approved the study protocol, and written informed consent was obtained from all patients.
Assessment of exercise capacity
New York Heart Association function class of patients was assessed by the same investigator evaluating patients at rest, dressing, walking, and climbing the stairs. NYHA classes range from I (no symptoms) to IV (symptoms at rest). Functional capacity of patients was also assessed using the 6‐min walk test, conducted in a flat straight 30 m corridor according to the latest guideline provided by the American thoracic society.15 The distance walked in 6 min is expressed in metres.
Blood sampling and analysis
Basal blood samples were taken at 08:00 h from an antecubital vein after a 30‐min rest in supine position. Blood samples were collected in the morning after an overnight fast and immediately processed on the same day. Plasma NT‐proBNP levels were measured by enzyme immunoassay (IMMULITE® 2000 XPi, Siemens Healthcare Diagnostic Inc., USA), intra‐assay and inter‐assay Coefficient Variations (CVs) being 1% and 2.1%, respectively (normal values < 100 pg/mL). fT3, fT4, and TSH were all measured by a chemiluminescence assay (Architect, Abbott Laboratories, North Chicago, IL, USA). The reference intervals for our laboratory are the following: fT3: 2.4–4.7 pg/mL, fT4: 7–18 pg/mL, and TSH: 0.27–4.2 mU/L.
Echocardiography
All patients underwent a complete echocardiographic examination with a commercially available system (Vivid™ 7, GE Healthcare, Horten, Norway). The LV end‐systolic and end‐diastolic volume and LVEF were measured by the modified biplane Simpson's method from the apical four‐chamber and two‐chamber views.16 M‐mode left ventricular dimensions were obtained from the parasternal long‐axis view. The velocity time integral of the aortic annular flow was obtained by tracing the pulse Doppler profile and multiplied by the area of the aortic annulus.17 Deceleration time of early filling was measured from transmitral flow. All measurements were averaged over three consecutive beats.
Statistical analysis
All analyses were conducted by Statistical Package For Social Sciences (SPSS) software, version 19 (SPSS Inc., Chicago, IL, USA). All data were initially analyzed using the Kolmogorov–Smirnov test to assess for normality. Categorical variables are presented as numbers and percentages and quantitative ones as means ± standard deviation (SD). Categorical data were compared by the X2 test, quantitative variables by the Student's t‐test, the Mann–Whitney U test, and the Kruskal–Wallis test, as appropriate. For comparative analysis of quantitative variables between the study subgroups, Student's t‐test was used. Relationships were assessed using Pearson, Spearman, or Kendall tests depending on their distribution. Changes in all of the outcome measures were normally distributed and were analyzed using 2‐sample Student t‐test. All P‐values were two‐tailed, and P < 0.05 was considered statistically significant.
Results
Patient demographics
Fifty patients with ischemic [n = 28 (56%)] and idiopathic dilated cardiomyopathy [n = 22 (44%)] including 39 (78%) men and 11 (22%) women with a mean ± SD age of 60 ± 15 (range 20–85) years, and a mean ± SD left ventricular ejection fraction of 26 ± 12 were included in the present study. The two study groups (each n = 25) were well matched in terms of age, sex, underlying causes of HF, and other clinical characteristics, NYHA class, 6MWD, NT‐proBNP level, and other biochemical measures, as well as echocardiographic parameters. Baseline patient characteristics are depicted in Table 1.
Table 1.
Baseline characteristics of the study population
| Variable | All | Liothyronine | Placebo | P‐value |
|---|---|---|---|---|
| (n = 50) | (n = 25) | (n = 25) | ||
| Male gender | 43 (86.0) | 24 (96.0) | 19 (76.0) | 0.098 |
| Age (years) | 60.1 ± 15.0 | 63.0 ± 12.7 | 57.4 ± 16.8 | 0.228 |
| LVEF, % | 30.83 ± 5.85 | 31.42 ± 4.30 | 29.91 ± 5.96 | 0.063 |
| NYHA class, n | 0.096 | |||
| I | 1 (2) | 1 (4) | 0 | |
| II | 19 (38) | 6 (24) | 13 (52) | |
| III | 30 (60) | 18 (72) | 12 (48) | |
| Aetiology of HF, n | 0.254 | |||
| Ischemic | 28 (56.0) | 16 (64.0) | 12 (48.0) | |
| Idiopathic | 22 (44.0) | 9 (36.0) | 13 (52.0) | |
| Risk factors, n | ||||
| DM | 6 (12.0) | 2 (8.0) | 4 (16.0) | 0.384 |
| HTN | 9 (18.0) | 3 (12.0) | 6 (24.0) | 0.270 |
| DLP | 5 (10.0) | 2 (8.0) | 3 (12.0) | 0.637 |
| Cigarette smoking, n | 5 (10.0) | 2 (8.0) | 3 (12.0) | 0.637 |
| Treatments, n | 0.084 | |||
| Diuretic | 46 (92) | 24 (96) | 22 (88) | |
| ACEI/ARB | 48 (96) | 23 (92) | 25 (100) | |
| Beta‐blocker | 47(94) | 24 (96) | 23 (92) | |
| Spirinolactone | 43 (86) | 23 (92) | 20 (80) | |
| Digoxin | 24 (48) | 13 (52) | 11 (44) | |
| CCB | 14 (28) | 5 (20) | 9 (36) | |
| CRT | 4 (8.0) | 3 (12) | 1 (4.0) | |
| ICD | 5 (10) | 2 (8.0) | 3 (12) | |
| VAD | 0 | 0 | 0 | |
| Creatinine, mg/dL | 1.68 ± 0.67 | 1.54 ± 0.77 | 1.55 ± 0.55 | 0.747 |
| Haemoglobin, mg/dL | 13.21 ± 2.74 | 13.97 ± 4.85 | 13.69 ± 3.42 | 0.843 |
| Hs‐CRP, mg/dL | 22.34 ± 10.75 | 21.69 ± 11.42 | 23.54 ± 12.45 | 0.725 |
| NT‐proBNP, ng/dL | 5075.74 ± 1197.96 | 4995.43 ± 1281.39 | 5145.06 ± 1368.83 | 0.94 |
| 6MWD, m | 360 ± 168 | 350 ± 100 | 369 ± 118 | 0.069 |
Data are presented as mean ± standard deviation and number (%).
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; CRT, cardiac resynchronization therapy; DLP, dyslipidemia; DM, diabetes mellitus; HF, heart failure; Hs‐CRP, high‐sensitivity C‐reactive protein; HTN, hypertension; ICD, implantable cardiac device; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; VAD, ventricular assisted device; 6MWD, 6‐min walk distance.
At presentation, 30 patients (60%) were in NYHA class III, 19 patients (38%) were in NYHA class II, and only one patient (2%) was in NYHA class I HF. Mean LVEF was 31 ± 6%, and mean 6MWD was 360 ± 168 m.
Effects of liothyronine on exercise tolerance
No significant difference was seen in the baseline NYHA function class of the study groups (P = 0.09). The NYHA class significantly declined in liothyronine and placebo groups (both P < 0.001). Fifteen patients (60%) in the liothyronine group improved from NYHA class III to NYHA class II compared with five patients (20%) in the placebo group. Two patients (8%) in the liothyronine group improved from NYHA class III to NYHA class I compared with six patients (24%) in the placebo group. Four patients (16%) in the liothyronine group and eight patients (32%) in the placebo group improved from NYHA class II to NYHA class I. One patient (4%) in the placebo group deteriorated from NYHA class III to class IV. All other patients (four patients: 16% in the liothyronine and five patients: 20% in the placebo groups) remained in their previous NYHA class. However, these changes in both groups were statistically comparable (P = 0.69).
Baseline mean 6MWD was comparable with the two study groups (350 ± 100 m, in the liothyronine group vs. 369 ± 218 m in the placebo group, P = 0.69). The 6MWD increased in the liothyronine group by 93 ± 16 m and in the placebo group by 67 ± 28 m, resulting in a treatment effect of 26 m (P = 0.003, Figure 1 A).
Figure 1.
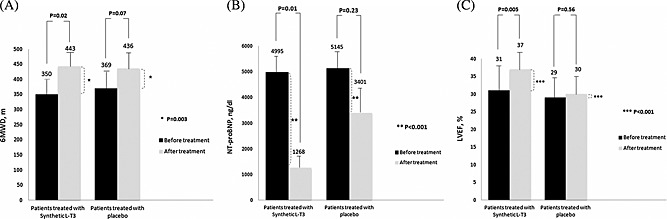
The changes in (A) the 6‐min walk distance (6MWD), (B) plasma N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) level, and (C) left ventricular ejection fraction (LVEF) in patients treated with synthetic L‐T3 (liothyronine) or placebo over a period of 6 weeks.
At the follow‐up, two patients in the liothyronine group and two patients in placebo group died with no difference in the mortality rate between the two study groups.
Effects of liothyronine on haemodynamics
No significant differences were seen in the changes of haemodynamic measures, i.e., heart rate, systolic; diastolic and mean blood pressures, between the liothyronine and placebo groups (P = 0.64, 0.56, 0.85, and 0.73, respectively, Table 2).
Table 2.
The haemodynamic and laboratory parameters of the two study groups
| Variable | Liothyronin | Placebo | Liothyronin vs. placebo | ||||
|---|---|---|---|---|---|---|---|
| (n = 25) | (n = 25) | ||||||
| Before | After | P‐value | Before | After | P‐value | P‐value | |
| HR, b.p.m | 67 ± 25 | 82 ± 21 | 0.52 | 65 ± 14 | 76 ± 16 | 0.76 | 0.64 |
| SBP, mmHg | 125 ± 15 | 123 ± 18 | 0.43 | 117 ± 14 | 112 ± 18 | 0.37 | 0.56 |
| DBP, mmHg | 75 ± 16 | 73 ± 21 | 0.86 | 69 ± 25 | 74 ± 15 | 0.96 | 0.85 |
| MAP, mmHg | 90 ± 8 | 86 ± 11 | 0.54 | 87 ± 12 | 89 ± 9 | 0.84 | 0.73 |
| TSH, mU/L | 3.01 ± 0.94 | 2.90 ± 1.06 | 0.21 | 2.46 ± 1.81 | 2.02 ± 1.08 | 0.73 | 0.35 |
| Free T3, pg/mL | 1.15 ± 0.29 | 2.37 ± 0.84 | <0.001 | 1.24 ± 0.17 | 1.44 ± 0.13 | 0.62 | <0.001 |
| Free T4, pg/mL | 15.33 ± 3.96 | 13.97 ± 3.71 | 0.76 | 14.76 ± 3.14 | 15.53 ± 3.73 | 0.45 | 0.85 |
| NT‐proBNP, ng/dL | 4995.43 ± 1281.39 | 1268.36 ± 847.83 | 0.01 | 5145.06 ± 1368.83 | 3401.50 ± 1846.12 | 0.23 | <0.001 |
| Hs‐CRP, mg/dL | 21.69 ± 11.42 | 9.67 ± 3.56 | 0.02 | 23.54 ± 12.45 | 15.33 ± 9.45 | 0.44 | <0.001 |
| Haemoglobin, g/dL | 12.63 ± 2.00 | 13.39 ± 2.32 | 0.49 | 11.73 ± 2.27 | 12.53 ± 1.69 | 0.19 | 0.85 |
| Uric acid, mg/dL | 6.24 ± 2.96 | 4.91 ± 2.73 | 0.34 | 7.09 ± 2.55 | 5.30 ± 1.37 | 0.29 | 0.03 |
| Creatinine, mg/dL | t1.54 ± 0.77 | 1.52 ± 0.70 | 0.47 | 1.55 ± 0.55 | 1.49 ± 0.43 | 0.76 | 0.63 |
Data are presented as means ± standard deviation.
DBP, diastolic blood pressure; HR, heart rate; Hs‐CRP, high‐sensitivity C‐reactive protein; MAP, mean arterial pressure; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; SBP, systolic blood pressure; TSH; thyroid‐stimulating hormone, T3; triiodothyronine, T4; thyroxin.
Effects of liothyronine on laboratory measurements
In patients receiving liothyronine, TT3, and fT3, levels significantly decreased, while total and fT4 and TSH levels remained at the statistically similar values. However, thyroid function measures were relatively constant during the study period in the placebo group. Liothyronine therapy markedly decreased serum NT‐proBNP level, as compared with the placebo (P = 0.01, Figure 1 B). Furthermore, a higher decrease of hs‐C‐reactive protein level was seen in patients in the liothyronine group than those in the placebo group (P = 0.009). The changes in the laboratory measurements during the study period are provided in Table 2.
Effects of liothyronine on echocardiography parameters
The changes in the assessed echocardiographic parameters are depicted in Table 3. The LVEF (Figure 1 C) and LV end‐systolic volume improved significantly in liothyronine group as compared with the patient taking placebo (both P < 0.001).
Table 3.
The changes in echocardiographic measures of the two study groups
| Liothyronine (n = 25) | Placebo (n = 25) | Liothyronine vs. placebo | |||||
|---|---|---|---|---|---|---|---|
| Variable | Before | After | P‐value | Before | After | P‐value | P‐value |
| LVEF | 31.42 ± 14.30 | 37.12 ± 12.21 | 0.005 | 29.21 ± 11.14 | 30.12 ± 10.44 | 0.564 | <0.001 |
| LVEDD | 7.54 ± 1.13 | 6.21 ± 1.07 | 0.012 | 7.21 ± 1.16 | 7.19 ± 1.12 | 0.232 | <0.001 |
| LVESD | 6.40 ± 1.13 | 5.14 ± 0.59 | 0.039 | 6.26 ± 1.29 | 6.14 ± 1.19 | 0.475 | 0.032 |
| LVOTD | 1.96 ± 0.21 | 1.32 ± 0.14 | 0.121 | 2.10 ± 0.36 | 1.96 ± 0.54 | 0.112 | 0.073 |
| LVEDV | 196.72 ± 78.13 | 147.20 ± 41.19 | 0.002 | 247.24 ± 122.73 | 241.25 ± 120.13 | 0.245 | 0.059 |
| LVESV | 181.52 ± 35.48 | 169.59 ± 21.40 | <0.001 | 196.04 ± 69.79 | 187.44 ± 35.12 | 0.546 | <0.001 |
| LVOT/VTI | 15.02 ± 4.09 | 14.26 ± 2.12 | 0.156 | 14.29 ± 2.86 | 14.14 ± 2.32 | 0.788 | 0.851 |
| DT | 178.60 ± 79.05 | 127.52 ± 45.20 | 0.026 | 151.16 ± 55.27 | 144.17 ± 52.17 | 0.145 | 0.693 |
Data are presented as means ± standard deviation.
DT, deceleration time; LVEDD, left ventricular end‐diastolic dimension; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic dimension; LVESV, left ventricular end‐systolic volume; LVOTD, left ventricular outflow tract dimension; VTI, velocity time integral.
Discussion
In the present study, we assessed the beneficial role of long‐term liothyronine therapy, if any, in HF patients with LT3S, that is, preserved TSH and T4 values in the presence of a low T3 level. The main finding of our study was that a 6‐week trial of liothyronine therapy in these patients seems to improve exercise capacity, as reflected by 6MWD, and also decreases NT‐proBNP level in clinically stable HF patients with LT3S receiving optimal anti‐failure medications.
Several clinical observational studies have shown that LT3S adversely affects long‐term prognosis in HF and found it as an independent predictor of death in this population, even independently of NT‐proBNP and other cardiovascular risk factors.7, 18, 19 A number of structural and functional abnormalities have been recognized in the cardiac muscles of HF patients in the presence of a low‐T3 level.20 On the other hand, it has been demonstrated that improvement of the underlying heart disease, through the use of inotropes or cardiac transplantation, is accompanied by the reversal of the SES with an increase in fT3 serum level in HF patients.21 Accordingly, because a bidirectional relation has been documented between the heart and thyroid gland, it might be possible that correction of the thyroid dysfunction in patients with LT3S may be beneficial in HF patients, via the same pathophysiologic processes that treatment of clinical hypothyroidism or hyperthyroidism benefits chronic HF patients. Although the previous literature has not precisely confirmed whether restoration of SES is of therapeutic values in either stable or decompensated HF patients, several attempts have been made to answer this question. In an animal model of HF, Henderson et al. showed that correction of low‐T3 phenotype with physiologic replacement of T3 is associated with improved ejection fraction and contractile performance.21 Aloia et al. demonstrated the important effects of T3 serum level on clinical and haemodynamic measures of decompensated HF patients, even in patients receiving optimized standard acute heart failure treatment.22 They also showed that LT3S reversibility with dobutamine therapy is associated with haemodynamic and neurohormonal improvement. Moreover, intravenous administration of T3 in advanced HF patients has been demonstrated to enhance cardiac output and reduce peripheral vascular resistance.23 Short‐term synthetic T3 administration in patients with dilated cardiomyopathy has been shown to be associated with significant ventricular performance improvement and modulation of neuroendocrine profile by reducing adrenaline, aldosterone, and NT‐proBNP levels.24 Similarly, improved cardiovascular remodelling and function by T3 replacement in HF patients with LT3S have also been demonstrated in other studies.25, 26 In accordance with these results, we showed that T3 increment is associated with a higher reduction in NT‐proBNP level in HF patients, even with ‘still under‐normal’ levels of serum fT3.
The 6MWD is believed to be more reflective of daily living activities than the other functional parameters such as NYHA function class.15 Currently, 6‐min walk test is in extensive use for the assessment of the response to medical treatments in moderate to severe heart disease patients. Our study showed that long‐term liothyronine therapy dramatically increases 6MWD in HF patients with LT3S as compared with patients receiving placebo.
Inflammation is associated with progression of chronic heart failure (CHF) and constitutes one of the major therapeutic targets in patients with HF. Our data also revealed that patients taking liothyronine had a higher reduction in hs‐C‐reactive protein level compared with the patients receiving placebo. This result is in agreement with those provided by Lubrano et al. that showed that impaired T3 production in patients with stable CHF is associated with enhanced proinflammatory cytokines, which may be responsible for pathogenic mechanisms resulting in HF progression.27
During the study, liothyronine was well tolerated, and undesirable effects consisting of arrhythmias, myocardial ischemia, or haemodynamic instability were not documented. No significant changes were also noted in the haemodynamic parameters including heart rate and blood pressure.
We study a relatively small number of HF patients, which might limit the generalizability of our findings. Perhaps it is the major limitation of the present study. Definitely, the results provided by a larger group of HF patients with LT3S would be more reliable to ascertain the beneficial role of T3 restoration in these patients.
Conclusion
In conclusion, we demonstrated that T3 replacement with synthetic T3 (oral liothyronine) for 6 weeks results in significant improvement in LV systolic function, NT‐proBNP and hs‐C‐reactive protein serum levels, and exercise tolerance, suggesting potential mechanisms by which liothyronine could safely benefit stable HF patients with LT3S receiving optimal anti‐failure medications.
Declaration of interest
None declared.
Amin, A. , Chitsazan, M. , Taghavi, S. , and Ardeshiri, M. (2015), Effects of triiodothyronine replacement therapy in patients with chronic stable heart failure and low‐triiodothyronine syndrome: a randomized, double‐blind, placebo‐controlled study. ESC Heart Failure, 2: 5–11. doi: 10.1002/ehf2.12025.
References
- 1. Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med 2001;344:501–509. [DOI] [PubMed] [Google Scholar]
- 2. Tang YD, Kuzman JA, Said S, Anderson BE, Wang X, Gerdes AM. Low thyroid function leads to cardiac atrophy with chamber dilatation, impaired myocardial blood flow, loss of arterioles, and severe systolic dysfunction. Circulation 2005;112:3122–3130. [DOI] [PubMed] [Google Scholar]
- 3. Olivares EL, Marassi MP, Fortunato RS, da Silva AC, Costa‐e‐Sousa RH, Araujo IG, Mattos EC, Masuda MO, Mulcahey MA, Huang SA, Bianco AC, Carvalho DP. Thyroid function disturbance and type 3 iodothyronine deiodinase induction after myocardial infarction in rats a time course study. Endocrinology 2007;148:4786–4792. [DOI] [PubMed] [Google Scholar]
- 4. Wassen FW, Schiel AE, Kuiper GG, Kaptein E, Bakker O, Visser TJ, Simonides WS. Induction of thyroid hormone‐degrading deiodinase in cardiac hypertrophy and failure. Endocrinology 2002;143:2812–2815. [DOI] [PubMed] [Google Scholar]
- 5. Hamilton MA, Stevenson LW, Luu M, Walden JA. Altered thyroid hormone metabolism in advanced heart failure. J Am Coll Cardiol 1990;16:91–95. [DOI] [PubMed] [Google Scholar]
- 6. Opasich C, Pacini F, Ambrosino N, Riccardi PG, Febo O, Ferrari R, Cobelli F, Tavazzi L. Sick euthyroid syndrome in patients with moderate‐to‐severe chronic heart failure. Eur Heart J 1996;17:1860–1866. [DOI] [PubMed] [Google Scholar]
- 7. Pingitore A, Landi P, Taddei MC, Ripoli A, L'Abbate A, Iervasi G. Triiodothyronine levels for risk stratification of patients with chronic heart failure. Am J Med 2005;118:132–136. [DOI] [PubMed] [Google Scholar]
- 8. Utiger RD. Altered thyroid function in nonthyroidal illness and surgery. To treat or not to treat? N Engl J Med 1995;333:1562–1563. [DOI] [PubMed] [Google Scholar]
- 9. Ascheim DD, Hryniewicz K. Thyroid hormone metabolism in patients with congestive heart failure: the low triiodothyronine state. Thyroid 2002;12:511–515. [DOI] [PubMed] [Google Scholar]
- 10. Silva‐Tinoco R, Castillo‐Martinez L, Orea‐Tejeda A, Orozco‐Gutierrez JJ, Vazquez‐Diaz O, Montano‐Hernandez P, Flores‐Rebollar A, Reza‐Albarran A. Developing thyroid disorders is associated with poor prognosis factors in patient with stable chronic heart failure. Int J Cardiol 2011;147:e24–e25. [DOI] [PubMed] [Google Scholar]
- 11. Kozdag G, Ural D, Vural A, Agacdiken A, Kahraman G, Sahin T, Ural E, Komsuoglu B. Relation between free triiodothyronine/free thyroxine ratio, echocardiographic parameters and mortality in dilated cardiomyopathy. Eur J Heart Fail 2005;7:113–118. [DOI] [PubMed] [Google Scholar]
- 12. Passino C, Pingitore A, Landi P, Fontana M, Zyw L, Clerico A, Emdin M, Iervasi G. Prognostic value of combined measurement of brain natriuretic peptide and triiodothyronine in heart failure. J Card Fail 2009;15:35–40. [DOI] [PubMed] [Google Scholar]
- 13. Heart Failure Society of America , Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Katz SD, Klapholz M, Moser DK, Rogers JG, Starling RC, Stevenson WG, Tang WH, Teerlink JR, Walsh MN. HFSA 2010 comprehensive heart failure practice guideline. J Card Fail 2010;16:e1–e194. [DOI] [PubMed] [Google Scholar]
- 14. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, ESC Committee for Practice Guidelines . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the heart failure association (HFA) of the ESC. Eur Heart J 2012;33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 15. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories . ATS statement: guidelines for the six‐minute walk test. Am J Respir Crit Care Med 2002;166:111–117. [DOI] [PubMed] [Google Scholar]
- 16. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St John Sutton M, Stewart W. Recommendations for chamber quantification. Eur J Echocardiogr 2006;7:79–108. [DOI] [PubMed] [Google Scholar]
- 17. Sun JP, Pu M, Fouad FM, Christian R, Stewart WJ, Thomas JD. Automated cardiac output measurement by spatiotemporal integration of color Doppler data. In vitro and clinical validation. Circulation 1997;95:932–939. [DOI] [PubMed] [Google Scholar]
- 18. Pfister R, Strack N, Wielckens K, Malchau G, Erdmann E, Schneider CA. The relationship and prognostic impact of low‐T3 syndrome and NT‐proBNP in cardiovascular patients. Int J Cardiol 2010;144:187–190. [DOI] [PubMed] [Google Scholar]
- 19. Iervasi G, Pingitore A, Landi P, Raciti M, Ripoli A, Scarlattini M, L'Abbate A, Donato L. Low‐T3 syndrome: a strong prognostic predictor of death in patients with heart disease. Circulation 2003;107:708–713. [DOI] [PubMed] [Google Scholar]
- 20. Pingitore A, Iervasi G. Triiodothyronine (T3) effects on cardiovascular system in patients with heart failure. Recent Pat Cardiovasc Drug Discov. 2008;3:19–27. [DOI] [PubMed] [Google Scholar]
- 21. Henderson KK, Danzi S, Paul JT, Leya G, Klein I, Samarel AM. Physiological replacement of T3 improves left ventricular function in an animal model of myocardial infarction‐induced congestive heart failure. Circ Heart Fail 2009;2:243–252. [DOI] [PubMed] [Google Scholar]
- 22. D'Aloia A, Vizzardi E, Bugatti S, Rovetta R, Bonadei I, Del Magro F, Curnis A, Dei Cas L. Effect of short‐term infusive dobutamine therapy on thyroid hormone profile and hemodynamic parameters in patients with acute worsening heart failure and low‐triiodothyronine syndrome. J Investig Med 2012;60:907–910. [DOI] [PubMed] [Google Scholar]
- 23. Hamilton M, Lynne W. Safety and hemodynamic effects of intravenous triiodothyronine in advanced heart failure. Am J Cardiol 1998;81:443–447. [DOI] [PubMed] [Google Scholar]
- 24. Pingitore A, Galli E. Acute effects of triiodothyronine (T3) replacement therapy in patients with chronic heart failure and low‐T3 syndrome: a randomized, placebo‐controlled study. J Clin Endocrinol Metab 2008;93:1351–1358. [DOI] [PubMed] [Google Scholar]
- 25. Gerdes AM, Iervasi G. Thyroid replacement therapy and heart failure. Circulation 2010;122:385–393. [DOI] [PubMed] [Google Scholar]
- 26. Khalife WI, Tang YD, Kuzman JA, Thomas TA, Anderson BE, Said S, Tille P, Schlenker EH, Gerdes AM. Treatment of subclinical hypothyroidism reverses ischemia and prevents myocyte loss and progressive LV dysfunction in hamsters with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol 2005;289:H2409–H2415. [DOI] [PubMed] [Google Scholar]
- 27. Lubrano V, Pingitore A, Carpi A, Iervasi G. Relationship between triiodothyronine and proinflammatory cytokines in chronic heart failure. Biomed Pharmacother 2010;64:165–169. [DOI] [PubMed] [Google Scholar]


