Abstract
Purpose
This study evaluated the clinical characteristics of bilateral leg edema during follow‐up of heart failure (HF) patients and determined the added value of monitoring fluid weight gain for deciding whether this non‐specific sign is a more clinically relevant sign.
Methods
Retrospective analysis was performed on 1826 visits from 83 ambulatory patients with established mild‐to‐moderate HF. Evaluated HF‐related signs included leg edema, pulmonary crackles, S3, weight gain, and ultrasound pleural effusion.
Results
During follow‐up, 75 patients with 161 visits had at least one of the following HF‐related events: weight gain (n = 107), leg edema (n = 90), ultrasound pleural effusion (n = 85), pulmonary crackles (n = 29), and S3 (n = 16). Compared with the events of sole leg edema (n = 23), leg edema events with additional HF‐related sign(s) (n = 67) accompanied more symptomatic worsening (7% vs. 55%, P < 0.0001), and a higher incidence (61% vs. 96%, P = 0.0002) and magnitude of increased serum B‐type natriuretic peptide. Sole leg edema events rarely progressed to worsening HF before the next regular clinic visit. Patients with the event of both leg edema and weight gain more often experienced worsening HF requiring extra clinic visits and/or hospitalization. Amongst a total of 67 leg edema events with additional HF‐related signs, 56 (84%) coexisted with weight gain. Therefore, additional monitoring of weight gain efficiently distinguished the clinically significant leg edema events from insignificant sole leg edema events.
Conclusions
During follow‐up of mild‐to‐moderate HF patients, sole leg edema appeared around 30% of the leg edema events, which is considered clinically insignificant. Additional checking for weight gain could be useful for determining whether this sign is a clinically relevant HF‐related sign. The appearance of these both signs during follow‐up of established HF patients should be intentionally watched or treated by extra diuretics and/or drug adjustment to prevent worsening of HF.
Keywords: Heart failure, Physical sign, Leg edema, Body weight, Monitoring
Introduction
Signs of heart failure (HF) include right heart congestion (e.g. lower leg edema, raised jugular venous pressure, ascites, and hepatomegaly), left heart congestion [pulmonary crackles, wheezing and the third heart sound (S3)], and fluid weight gain.1, 2, 3, 4, 5 Amongst these signs, bilateral leg edema and changes in body weight are easily monitored and may be important for caring of patients with HF, but both of these physical signs are often not specific for worsening HF status.4, 5, 6, 7, 8, 9 In suspected HF patients, the use of lower leg edema as a predictor of HF is reported to be the simplest sign of right‐sided congestion, but it is not considered specific enough to use as the sole basis for an accurate diagnosis of worsening HF status.8, 10, 11, 12 To date, the role of monitoring bilateral leg edema for detecting HF worsening and the clinical validity of monitoring body weight change during follow‐up of established HF patients are still controversial. Thus, the aim of the present study was to thoroughly examine the clinical characteristics of the appearance of bilateral leg edema, and to determine the added value of monitoring fluid weight gain for determining whether this non‐specific sign is a more clinically relevant sign for worsening HF in established HF patients during follow‐up.
Materials and methods
Study protocol
The present study was a sub‐study of a recently published study5 focusing on monitoring body composition changes in established HF patients performed in the cardiology clinic of Nishida Hospital. Eligible patients had at least one decompensated HF episode that resulted in hospitalization or treatment with intravenous loop diuretics. Informed consent was obtained from all patients before study enrollment.
At study entry, patient characteristics, history, and primary aetiology were recorded. Study patients were instructed to visit to the outpatient clinic every 2 to 6 weeks according to their condition. The patients enrolled in this study were interviewed regarding changes in symptoms and examined for the appearance of physical signs of fluid retention upon each visit to the clinic by a clinician (H. K.). Additional routine tests included searching for the ultrasound pleural effusion (US‐PLE),13, 14 monitoring changes in the fluid status using a digital body weight scale incorporating a bioelectrical impedance analyzer4, 5 and measuring B‐type natriuretic peptide (BNP) levels.15
In the present study, data from the clinical records for HF monitoring were retrospectively reviewed to search for events of the presence of one or more of the HF‐related sign(s) described in the succeeding text. For each event, the following issues were examined: accompanying symptoms, precipitating causes, patient's response to the event (e.g. regular or extra/urgent clinic visit), physician's decision, and attitude to the event (e.g. sole education/observation or drug treatment and outpatient clinic or hospitalization) and clinical course during hospitalization or up to the next regular outpatient clinic visit after the event.
Evaluation of heart failure‐related symptoms and signs
Evaluated HF‐related symptoms included dyspnea on exertion or at rest, orthopnea, and paroxysmal nocturnal dyspnea. Clinically significant HF‐related signs adopted in the present study included the appearance of S3, bilateral crackles beyond the basal lung, bilateral leg edema around, or above the ankle, fluid weight gain (defined as body weight gain of ≥1.5 kg with decreased body fat percentage compared with the most recent previous clinic visit with clinical stability),4, 5 and the presence of US‐PLE.13, 14 Significant leg edema was graded as mild (around the ankle) or moderate/severe (higher than the ankle or knee.9 Efforts were made to investigate the precipitating factors that led to the worsening HF.16
Chest ultrasound
Chest ultrasound was performed on each patient using a commercially available real‐time, wide‐angle phased‐array system.13, 14 The patient was placed in a sitting position on a bed or chair, and chest ultrasound was performed on each hemithorax using a 3.5‐MHz sector transducer through the intercostal space and scanning along the paravertebral, scapular, and posterior axillary lines. The presence of pleural effusion was diagnosed by the appearance of an anechoic space between the parietal pleura and the highly reflective visceral pleura‐lung interface (positive US‐PLE sign).
Monitoring of weight gain
Evaluation of fluid body‐weight gain by measuring body weight and percent body fat was described in detail previously.4, 5 Briefly, for monitoring definite HF patient, body weight and percent body fat were measured twice and averaged. Patients wore light clothes and were barefoot during weighing. Based on prior experience,4 the criterion of significant fluid weight gain was defined as a body weight gain of at least 1.5 kg with a concomitant decrease in percent body fat from the most recent visit with clinical stability to the time of target weight gain. Adjustments of reference dry weight, as determined by the evaluation of symptoms, physical exam, and chest ultrasound, were made at each clinic visit. In the case of worsening HF status, as diagnosed at regular or extra/urgent clinic visit, target dry weight was determined after decongestion therapy in the hospital or outpatient clinic.
Statistical analysis
Continuous variables are presented as mean values (±standard deviation). Categorical values are reported as numbers and percentages. For continuous variables, group data between two groups were compared using Student's unpaired t‐test and across more than three groups using the Kruskal–Wallis test with post hoc test. Categorical data were compared using Fisher's exact test or the chi‐square test. A P value of less than 0.05 was considered statistically significant. All analyses were performed using GraphPad Prism 4.0 (GraphPad Software, San Diego, CA).
Results
Baseline characteristics of the study patients
Table 1 shows the baseline characteristics of the study patients during clinical stability around the time of study entry. A total of 83 ambulatory patients (39% men, aged 77 ± 12 years) with mild‐to‐moderate HF (New York Heart Association functional class II or III) were enrolled. Over a mean follow‐up of 652 ± 456 days, 1826 visits (mean interval, 28 days) were evaluated. The mean frequency of a patient's visit to the outpatient clinic was 22 ± 14 times (range 5–63 times).
Table 1.
Baseline characteristics of the study patients
| Characteristics | Study patients (n = 83) |
|---|---|
| Age (yrs) | |
| Mean ± SD | 77 ± 12 |
| Range | 29–93 |
| Male | 32 (39%) |
| Body height (cm) (mean ± SD) | 151 ± 10 |
| Primary cause of HF | |
| Hypertension | 39 (47%) |
| Valvular | 18 (22%) |
| Cardiomyopathy | 12 (14%) |
| Ischemic | 6 (7%) |
| Arrhythmia | 5 (6%) |
| Others | 3 (4%) |
| Left ventricular ejection fraction | |
| Mean ± SD | 53 ± 14 |
| Range | 20–77 |
| Atrial fibrillation | 29 (35%) |
| Lower leg varicose veins | 8 (10%) |
| B‐type natriuretc peptide (pg/mL) | |
| Mean ± SD | 147 ± 135 |
| Range | 37–1110 |
| NYHA‐FC at stable period | |
| II | 62 (75%) |
| III | 21 (25%) |
| Medication | |
| Diuretics | 81 (98%) |
| ACE inhibitors/ARB | 54 (65%) |
| Beta‐blockers | 38 (46%) |
| Calcium antagonists | 31 (37%) |
| Digitalis | 7 (8%) |
| Nitrates | 6 (7%) |
Data present are values in mean ± standard deviation or number (percent).
ACE, angiotensin‐converting enzyme; ARB, angiotensin II receptor blocker; NYHA‐FC, New York Heart Association functional class; HF, heart failure.
Appearance of heart failure‐related signs
Table 2 shows the classification of outpatient visits by cumulative number of HF‐related signs and the relation to the appearance of each worsening HF‐related sign and worsening of symptoms. In 161 visits of 75 patients amongst 83 study patients, the patient had at least 1 of the following events: fluid weight gain (n = 107), leg edema (n = 90), US‐PLE (n = 85), pulmonary crackles (n = 29) and S3 gallop (n = 16) (Figure 1 and Table 2).
Table 2.
Classification of 161 visits (75 patients) with the presence of heart failure‐related sign(s) and their relation to the appearance of other heart failure sign(s) and worsening symptoms
| Isolated HF sign (n = 67) | Coexistence of leg edema and weight gain (n = 56) | Leg edema and HF sign(s) other than weight gain (n = 11) | Weight gain and HF sign(s) other than leg edema (n = 20) | ≥2 HF signs other than leg edema and weight gain (n = 7) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Leg edema | Weight gain | Others | Additional HF sign(s) | ||||||
| None | 1 | ≥2 | |||||||
| Classification of each visit with HF sign(s) (n = 161) | 23 | 31 | 13 | 13 | 27 | 16 | 11 | 20 | 7 |
| Appearance of each HF sign | |||||||||
| Leg edema (n = 90) | 23 | 0 | 0 | 13 | 27 | 16 | 11 | 0 | 0 |
| Fluid weight gain (n = 107) | 0 | 31 | 0 | 13 | 27 | 16 | 0 | 20 | 0 |
| Positive US‐PLE (n = 85) | 0 | 0 | 12 | 0 | 23 | 16 | 9 | 19 | 6 |
| Pulmonary crackles (n = 29) | 0 | 0 | 1 | 0 | 3 | 11 | 2 | 8 | 4 |
| S3 (n = 16) | 0 | 0 | 0 | 0 | 1 | 7 | 1 | 3 | 4 |
| Worsening symptoms (n = 56) | 2 | 0 | 4 | 4 | 17 | 11 | 5 | 9 | 4 |
HF, heart failure; S3, the third heart sound; US‐PLE, ultrasound pleural effusion.
Data present are values in number.
Figure 1.
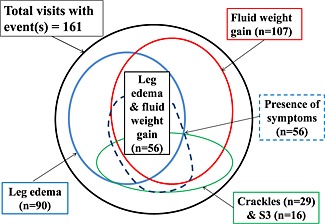
Appearance of symptom and signs amongst a total of 161 clinic visits by patients presenting with more than one heart failure‐related sign(s). Ultrasound pleural effusion. Note: There appeared combined appearance of leg edema and fluid weight gain in 56 (84%) of a total of 67 leg edema events.
Clinical characteristics of leg edema
Severity of bilateral leg edema was mild in 58, moderate in 31, and severe in 1 patient(s). Table 3 shows the relation of the severity of leg edema with the other HF‐associated signs. The appearance of each HF‐related sign and cumulative number of HF‐related signs was not different (P = 0.49) between events of mild (1.36 ± 1.09; n = 58) and moderate/severe bilateral leg edema (1.53 ± 1.11; n = 32), but more symptomatic changes appeared in events of moderate/severe bilateral leg edema (31% vs. 66%, P = 0.002).
Table 3.
Relation of the severity of leg edema to other heart failure‐associated signs
| Mild leg edema | Moderate/severe leg edema | P‐valuea | |
|---|---|---|---|
| (n = 58) | (n = 32) | ||
| Appearance of HF‐related signs | |||
| Fluid weight gain (≥1.5 kg) | 34 (59%) | 22 (69%) | 0.37 |
| Positive US‐PLE | 29 (50%) | 19 (59%) | 0.51 |
| Pulmonary crackles | 11 (19%) | 5 (16%) | 0.78 |
| S3 | 6 (11%) | 3 (9%) | 1 |
| Distribution of cumulative number of HF signs | |||
| 0 | 16 (28%) | 7 (22%) | 0.89 |
| 1 | 16 (28%) | 8 (25%) | |
| 2 | 17 (29%) | 11 (34%) | |
| ≥3 | 9 (15%) | 6 (19%) | |
| Number of HF signs | 1.36 ± 1.09 | 1.53 ± 1.11 | 0.49 |
| Worsening of dyspnea | 18 (31%) | 21 (66%) | 0.002* |
[Correction added after online publication 2 July 2015: 69% changed to 59% in column Moderate/severe leg edema and row Positive US‐PLE].
HF, heart failure; S3, the third heart sound; US‐PLE, ultrasound pleural effusion.
Data present are values in mean ± standard deviation or number (percent).
P‐value according to Student's unpaired t‐test or Fisher's exact/chi‐square test.
Significance.
The appearance of sole leg edema isolated from the other HF‐related signs occurred in 23 visits (26% of 90 leg edema events; Table 4): amongst these visits, only 2 visits were accompanied by symptomatic worsening, and the BNP level from stable to worsening HF decreased in 9 and was only mildly elevated (50 pg/mL>) in 10 visits. Compared with events of the sole appearance of bilateral leg edema (n = 23), leg edema events with the presence of additional HF‐related signs (n = 67) had frequent symptomatic worsening (7% vs. 55%, P < 0.0001), and a higher incidence (61% vs. 96%, P = 0.0002) and magnitude of increased serum BNP.
Table 4.
Comparison between the events of isolated appearance of leg edema or fluid weight gain and those of the presence of additional heart failure sign(s)
| Presence of leg edema | P‐valuea | Presence of weight gain | P‐valuea | |||
|---|---|---|---|---|---|---|
| Additional HF sign(s) | Additional HF sign(s) | |||||
| No (n = 23) | Yes (n = 67) | No (n = 31) | Yes (n = 76) | |||
| Worsening of dyspnea | 2 (7%) | 37 (55%) | <0.0001* | 0 | 41 (54%) | <0.0001* |
| BNP level (pg/mL) at stability | ||||||
| <100 | 8 (35%) | 23 (34%) | 0.243 | 16 (52%) | 29 (38%) | 0.24 |
| 100 to <500 | 13 (56%) | 43 (64%) | 15 (48%) | 43 (57%) | ||
| 500≤ | 2 (9%) | 1 (2%) | 0 | 4 (5%) | ||
| BNP change from stability to worsening | ||||||
| Decreased | 9 (39%) | 3 (4%) | 0.0002* | 8 (26%) | 4 (5%) | 0.0047* |
| Increased | 14 (61%) | 64 (96%) | 23 (74%) | 72 (95%) | ||
| ΔBNP (pg/mL) from stability to worsening in increased BNP events | (n = 14) | (n = 64) | (n = 23) | (n = 72) | 0.029* | |
| <50 | 10 (72%) | 10 (16%) | 0.0002* | 10 (44%) | 12 (17%) | |
| 50 to <100 | 2 (14%) | 11 (17%) | 4 (17%) | 11 (15%) | ||
| 100 to <300 | 2 (14%) | 25 (39%) | 7 (30%) | 27 (37%) | ||
| 300≤ | 0 | 18 (28%) | 2 (9%) | 22 (31%) | ||
BNP, B‐type natriuretic peptide; HF, heart failure.
Data present are values in number (percent).
P‐value according to Fisher's exact/chi‐square test.
Significance.
Clinical characteristics of fluid weight gain
Sole fluid weight gain isolated from the other HF‐related signs occurred in 31 visits (29% of 107 fluid weight gain events; Table 4): not all of these visits were accompanied by symptomatic worsening, and the serum BNP from stable to worsening HF decreased in 8 and was only mildly elevated (50 pg/mL>) in 10 visits. Compared with events of sole appearance of fluid weight gain (n = 31), those events with the presence of additional HF‐related signs (n = 76) were often accompanied by symptomatic worsening (0% vs. 54%, P < 0.0001) and had a higher incidence (74% vs. 95%, P = 0.0047) and magnitude of increased serum BNP. When compared with sole appearance of bilateral leg edema, sole appearance of fluid weight gain presented with a trend towards higher elevation of BNP (50 > vs. ≥50 pg/mL) from stable to worsening of HF (P = 0.173).
Patient's behaviour, physician's decision, and clinical course between events of sole leg edema/weight gain vs. multiple heart failure signs
Table 5 shows the comparisons of patient's behaviour, physician' decision, and clinical course between events of sole leg edema/fluid weight gain vs. multiple HF‐related signs. Upon the occurrence of HF‐related events, there were only 3 (6%) extra/urgent clinic visits [hazard ratio (HR) 0.07, 95% confidence interval (CI) 0.02–0.24] and 1 (2%) HF‐related hospitalization [HR 0.03, 95% CI 0.003–0.19] amongst a total of 54 events of sole leg edema or fluid weight gain, although there happened as many as 43 (46%) extra/urgent clinic visits and 40 (43%) events of HF‐related hospitalization amongst a total of 94 events with two or more HF‐related signs (P < 0.0001 for each). None of the events with sole leg edema or fluid weight gain progressed to HF‐related death, but seven events with multiple signs resulted in HF‐related death during hospitalization.
Table 5.
Comparison of patient behaviour, physician decision, and clinical course between the events of isolated leg edema or weight gain vs. those of the appearance of multiple heart failure‐related signs
| Events of isolated appearance | Events of multiple HF‐related signs (n = 94) | All isolated vs. ≥2 HF signs | ||||
|---|---|---|---|---|---|---|
| All (n = 54) | Leg edema | Weight gain | Hazard ratio | P‐valuea | ||
| (n = 23) | (n = 31) | (95% CI) | ||||
| Extra/urgent clinic visit | 3 (6%) | 2 (7%) | 1 (3%) | 43 (46%) | 0.07 (0.02–0.24) | <0.0001* |
| Worsening of symptoms | 2 (4%) | 2 (7%) | 0 | 50 (53%) | 0.03 (0.08–0.15) | <0.0001* |
| Both of the above | 2 (4%) | 2 (7%) | 0 | 36 (38%) | 0.06 (0.014–0.27) | <0.0001* |
| Precipitating cause of worsening HF | ||||||
| Poor adherence to drugs | 6 (11%) | 2 (7%) | 4 (13%) | 14 (15%) | 0.71 (0.26–1.98) | 0.62 |
| Inappropriate drug prescription | ||||||
| Reduction of diuretics | 3 (6%) | 2 (7%) | 1 (3%) | 5 (5%) | 1.05 (0.24–4.57) | 1 |
| Use of NSAIDs | 0 | 0 | 0 | 9 (10%) | 0.08 (0.005–1.45) | 0.03* |
| Physical/dietary/alcoholic excess | 3 (6%) | 1 (4%) | 2 (6%) | 16 (17%) | 0.29 (0.08–1.03) | 0.07 |
| Unknown | 42 (78%) | 18 (78%) | 24 (77%) | 50 (53%) | 3.08 (1.44–6.58) | 0.005* |
| Drug treatment | ||||||
| No (education and observation) | 46 (85%) | 18 (78%) | 28 (90%) | 12 (13%) | 39.3 (15–103) | <0.0001* |
| Re‐starting of regular prescription | 6 (11%) | 2 (7%) | 4 (13%) | 14 (15%) | 0.71 (0.26–1.98) | 0.62 |
| Extra diuretics for several days | 6 (11%) | 4 (17%) | 2 (6%) | 81 (86%) | 0.02 (0.007–0.06) | <0.0001* |
| Adjustments of recent regular drugs | 5 (9%) | 3 (13%) | 2 (6%) | 74 (79%) | 0.03 (0.01–0.08) | <0.0001* |
| HF‐related hospitalization | 1 (2%) | 1 (4%) | 0 | 40 (43%) | 0.03 (0.003–0.19) | <0.0001* |
| HF‐related death | 0 | 0 | 0 | 7 (7%) | 0.11 (0.006–1.9) | 0.048* |
CI, confidence interval; HF, heart failure; NSAIDs, non‐steroidal anti‐inflammatory drugs.
Data present are values in number (percent).
P‐value, hazard ratio (all isolated to ≥2 HF signs) and 95% CI according to Fisher's exact/chi‐square test.
Significance.
Added value of fluid weight gain to leg edema
Amongst a total of 67 leg edema events with the presence of one or more additional HF‐related signs, 56 events (84%) coexisted with fluid weight gain (Figure 1 , Table 2). Therefore, additional signs of fluid weight gain for distinguishing substantial number of the clinically significant leg edema events (n = 56) from the insignificant sole leg edema events (n = 23). When taking the symptoms into consideration, five additional events of leg edema with coexistent HF sign(s) other than fluid weight gain (n = 11) were differentiated from the isolated leg edema events.
The clinical course of the 56 events with the presentation of both leg edema and weight gain was almost similar to that of the 38 events with the presentation of ≥2 HF‐related signs other than events fulfilling such criterion (Supporting Information, Table S1 ). However, patients with the event of both leg edema and weight gain more often experienced extra diuretic usage and prescription adjustment, and tended to have a higher incidence of extra‐ or urgent clinic visits.
As presented in Table 6, events requiring an extra clinic visit and/or hospitalization (n = 63) presented with more symptomatic worsening of dyspnea and higher serum BNP levels compared with other regular clinic visits and the presentation of HF‐related sign(s). Amongst these 63 events of worsening HF, 37 (59%) were associated with both leg edema and fluid weight gain.
Table 6.
Comparison of heart failure‐related events requiring an extra clinic visit/hospitalization and those noted during regular clinic visits not requiring hospitalization
| Events requiring an extra clinic visit and/or hospitalization (n = 63) | Events noted during regular clinic visits requiring no hospitalization (n = 98) | |||
|---|---|---|---|---|
| Both leg edema and weight gain | Leg edema or weight gain | Others | ||
| (n = 19) | (n = 68) | (n = 11) | ||
| HF‐related sign | ||||
| Leg edema | 45 (71%) | 19 (100%) | 26 (38%) | 0 |
| Fluid weight gain | 46 (73%) | 19 (100%) | 42 (62%) | 0 |
| Positive US‐PLE | 53 (84%) | 7 (37%) | 14 (21%) | 11 (100%) |
| Pulmonary crackles | 17 (30%) | 5 (26%) | 5 (7%) | 2 (18%) |
| S3 | 15 (24%) | 1 (5%) | 0 | 0 |
| Cumulative number of HF signsa | 2.81 ± 1.03 | 2.68 ± 0.67 | 1.28 ± 0.51* | 1.18 ± 0.41* |
| Worsening of dyspneab | 50 (79%) | 2 (11%) | 4 (6%) | 0 |
| Both fluid weight gain and leg edema | 37 (59%) | 19 (100%) | — | 0 |
| With worsening of symptomsc | 30/37 (81%) | 2/19 (11%) | — | — |
| Leg edema or fluid weight gain | 16 (25%) | — | 4 (6%) | — |
| With worsening of symptomsc | 12/16 (75%) | — | 4/68 (6%) | 0 |
| Serum B‐type natriuretic peptide (pg/mL) | ||||
| Recent clinic visit without HF signs | 281 ± 460 | 131 ± 110 | 141 ± 117 | 194 ± 135 |
| Clinic visit presenting with HF sign(s)d | 595 ± 565 | 233 ± 153† | 199 ± 156* | 269 ± 226 |
| Extra diuretics and/or drug adjustment | 63 (100%) | 15 (79%) | 3 (4%) | 3 (27%) |
| Clinical course | ||||
| Worsening HF before next clinic visit | — | 1 (5%) | 2 (3%) | 0 |
| HF‐related death | 7 | 0 | 0 | 0 |
HF, heart failure; S3, the third heart sound; US‐PLE, ultrasound pleural effusion.
Data present are values in mean ± standard deviation or number (percent).
P‐value < 0.0001 according to Kruskal–Wallis test. Post hoc testing:
P < 0.001, for comparison with extra clinic visit/hospitalization.
P‐value < 0.0001 according to Fisher's exact/chi‐square test.
P‐value < 0.001 according to Fisher's exact/chi‐square test.
P‐value < 0.0001 according to Kruskal–Wallis test. Post hoc testing: *P < 0.001; †P < 0.01, for comparison with extra clinic visit/hospitalization.
Amongst 19 regular clinic visits in which patients presented with both leg edema and weight gain, extra diuretics and/or drug adjustment was prescribed at 15 clinic visits, and outpatient follow‐up without hospitalization or changes in medication was prescribed at four clinic visits. All of these visits except one were subsequently uneventful until the next regular clinic visit.
With regard to events of either leg edema or body weight gain at a regular clinic visit with an uneventful clinical course (n = 68), serum BNP levels were lower (P < 0.001), the cumulative number of HF‐related signs was smaller (P < 0.001), and the incidence of worsening dyspnea was lower compared with the events requiring an extra clinic visit and/or hospitalization (n = 63). Amongst these 68 events, only two progressed to worsening of HF before the next regular clinic visit.
Discussion
The present study evaluated the clinical significance of bilateral leg edema in detail during follow‐up of patients with mild‐to‐moderate HF and evaluated the added value of monitoring fluid weight gain to this frequently non‐specific HF sign. The results demonstrated that the appearance of bilateral leg edema is one of the leading presentations of HF‐related signs during follow‐up of mild‐to‐moderate HF patients, but ~30% of the leg edema events were the only physical presentation isolated from the other HF‐related signs, which usually seems to be clinically insignificant because of its clinical characteristics and the lack of progress to the worsening HF‐related events or death. Furthermore, the present study indicated that additional monitoring of fluid weight gain and symptoms could enhance the somewhat non‐specific physical sign of leg edema as a more clinically relevant sign of evidence of HF worsening.
Revisits of bilateral leg edema in heart failure
Bilateral leg edema is one of the important factors in diagnosing, monitoring, and managing HF status.1, 2, 3 This sign is a common physical finding with numerous etiologies, however, including venous insufficiency, adverse reaction to medication, chronic obstructive pulmonary disease, sleep apnea or hypopnea syndrome, obesity, and elevated central venous pressure because of HF or renal failure.6, 7, 8, 9
Amongst adult patients with stage A cardiovascular disease status (i.e. patients at high risk for congestive HF, but without structural heart disease or symptoms of HF), the incidence of bilateral leg edema is around 10%, and its severity is usually mild.9 According to recent studies evaluating HF‐related physical findings, Mueller et al. 10 observed the appearance of lower extremity edema in a moderate percentage (23%) of patients with non‐cardiac cause of dyspnea compared with those with a cardiac cause of dyspnea (47%) in an emergency department setting. Kelder et al. 11 reported a similar observation in the primary care setting for diagnosing new‐onset HF (bilateral ankle edema of 22% in patients without HF vs. 41% in patients with HF). In an assessment of geriatric patients with suspected HF, Oudejans et al. 8 reported that the appearance of bilateral ankle swelling is not different between those diagnosed as not having HF (36%) and those having new, slow‐onset HF (43%). Damy et al. 12 noted that a third of the patients with ankle swelling alone, and a similar proportion of those with lung crackles alone, do not have HF.
The present study demonstrated that the appearance of bilateral leg edema is one of the leading presentations of HF‐related signs during follow‐up of mild‐to‐moderate HF patients, but ~30% of the leg edema events were sole appearance isolated from the other HF‐related signs, which usually seems to be clinically insignificant. Recently, the prognostic value of peripheral edema in HF patients was reconfirmed.17 Indeed, one recent study18 indicated that adherence only to this common, and frequently non‐specific, HF‐related sign could enhance cardiac event‐free survival in HF patients. Therefore, health care participants and HF patients should realize the nature and limitation (non‐specificity) of physical signs of leg edema as described here and readily utilize this important clinical sign in conjunction with searching for other HF‐related signs upon the evaluation of HF status during follow‐up.
Monitoring of body weight in heart failure
Heart failure guidelines19, 20 recommend that patients experiencing an increase of 2 kg over stable body weight over a period of 48 to 72 h should initiate contact with medical or nursing personnel. Contrary to this recommendation, Lewin et al. 21 indicate that rapid weight gain is not a sensitive measure for assessing clinical deterioration: a gain of greater than 2 kg within 48 to 72 h predicted deterioration only 9% of the time, and a gain of greater than 2% was sensitive only 17% of the time. A recent report by Chaudhry et al. 22 showed that increases in the weight gain of patients hospitalized for HF become apparent about 30 days before hospitalization and accelerate markedly in the week preceding admission. Our recent study5 also indicates that a gradual weight gain correlates with a worsening status in chronic HF patients over a median duration of 30 days. Several years prior to these publications, Schiff et al. 23 reported the existence of slow (over several weeks to months) and rapid (<8 days) worsening of HF. In the rapidly progressive type, the main mechanism is suggested to be pulmonary congestion due to fluid redistribution from the peripheral circulation to the pulmonary circulation with modest body fluid accumulation.24 In such a pathophysiologic situation, worsening HF might be accompanied by a negligible change in body weight. In the slowly progressive type of worsening HF, the HF onset is thought to be more gradual with significant body fluid retention. In such a situation, the lag of symptomatic changes behind weight gain might provide an opportunity for physicians and/or patients to interrupt the spiralling course of worsening HF by monitoring the body weight and/or changes in body composition.
Many controversies exist, however, about the clinical usefulness of remote monitoring of body weight changes in established HF patients. Some recent studies25, 26, 27 have denied the usefulness of the monitoring body weight at home, and others28, 29, 30 indicate a substantial impact on reducing hospitalization or mortality in HF patients. Based on the results of the present study, it is possible that the presence of many (~30% of body weight gain events) sole fluid weight gain events (normally considered clinically insignificant) could have interfered with the meaningful information of clinically significant body weight change (probably accompanying additional HF‐related symptoms and signs/tests) during the follow‐up of HF patients by body weight monitoring. This finding could partly explain the recent observations by Zhang et al. 31 in which many false positive alerts of weight gain for predicting the worsening of HF occurred when using a moving average convergence divergence algorithm.
Moreover, the present study, using intensive and individualized follow‐up of HF patients at an outpatient clinic, suggests that frequent adjustments of target dry weight might be required to prevent worsening of HF. In the present study, we adjusted the target dry weight, as determined by symptomatic change, physical examination, and US‐PLE, at approximately 30‐day intervals, even in the absence of worsening HF events. In the case of worsening HF, target dry weight was determined after the decongestion therapy. Indeed, when and how to adjust the target dry weight during follow‐up of HF patients might be an important methodological problem because body weight/composition corresponding to eu‐volemic or optimal fluid volume status may not be stable over time.32 Thus, further study is required to develop better methods and algorithms for determining the target dry weight to detect and treat worsening HF by monitoring changes in body weight.31, 32
Utility of combined monitoring of leg edema and body weight in heart failure
As described previously, diagnosing and monitoring HF based on the physical signs of bilateral leg edema or body weight in isolation is difficult, in part because of the nebulous concept of continued assessment of a complex and ill‐defined disease process that is associated with significant disability and mortality. Physicians should use all possible information, however, to make a final adjudicated diagnosis of worsening HF. Combining two or more physical signs of right‐sided and left‐sided congestion, if present, would provide higher predictive value of the presence of HF, despite the lack of sensitivity for its identification.12, 33 In the present study, additional data regarding fluid weight gain allowed for clinically insignificant events to be distinguished from clinically significant events of leg edema (Figure 1 and Table 2). Although a strict standard treatment protocol does not exist in our hospital, patients presenting at clinic visits with two or more HF‐related signs are, in general, targeted for treatment. As such, administration of extra diuretics and/or drug adjustment at the clinic visit (n = 15) during which both leg edema and fluid weight gain present (i = 19) might have prevented these clinic visits to ultimately progress to worsening HF and/or hospitalization in this study (Table 6).
Leg edema and fluid weight gain, however, do not always appear concomitantly during worsening of HF. Therefore, an event of either leg edema or body weight gain should be carefully evaluated and/or monitored to determine the presence of other HF‐related symptom(s)/sign(s) or whether further deterioration might occur in conjunction with the appearance of other HF‐related symptom(s) and sign(s), because (1) each clinical event could be an indicator of the early stages of worsening HF in established HF patients and (2) there are daily fluctuations in dyspnea, edema, and body weight in HF patients.34 Readily available clinical tests could improve the ability to diagnose worsened HF, such as measurement of serum BNP levels8, 11 and notably by using chest ultrasound examinations for US‐PLE.13, 14 The addition of these tests might be useful for determining the clinical significance of the separate appearance of either leg edema or body weight gain (Table 6); patients showing abnormalities of the tests at clinic visits should be carefully managed and closely followed after an event presenting with only one of these signs.
Study limitations
The present study had certain limitations. First, the present study was undertaken in a population of mild‐to‐moderate HF patients. Therefore, the findings of the present study could not be generalized to more advanced HF patients. Second, the criteria to assess leg edema might somewhat affect the grading of the edema. The severity of edema, from slight to very marked, is traditionally reported on a four‐point scale based on the depth of indentation at the ankle.10 Because this scale is subjective, noting the height of the edema may be more practical and reproducible6 and is currently used universally, as in the present study. Third, in the present study, we did not search for specific local cause(s) (e.g. venous insufficiency and thrombosis) of leg edema events accompanying HF signs at each clinic visit. Finally, this study evaluated clinical events having one or more of HF‐related signs during follow‐up. Such events as presented with only symptoms, for example, arrhythmia or ischemia without accompanying HF‐related signs, are not included in the analysis.
Conclusions
Advanced technology for monitoring HF, using continuous measures of the intra‐thoracic impedance35 or intra‐cardiac pressure36 by an implanted device, could become the ultimate systems for patients with HF, but it is reasonable to speculate that not all HF patients, particularly those with mild‐to‐moderate HF status, could be covered by such an ideal health care system.37 Even in the advanced technology era, self‐caring of HF‐related symptoms/signs is the first and fundamental step to prevent worsening of HF events.38, 39 Optimal self‐care includes adherence to multi‐drug pharmacologic therapy, patient self‐assessment of symptoms and signs, and appropriate decision‐making about therapeutic and care‐seeking actions.38, 39, 40 However, insistence of a lot of parameters for individuals caring for HF at home decreases the number of compliant HF patients from 81% to 55%,28 indicating that compliance rates in HF patients might decrease as more activities are required from the patients. As shown in the present study, the appearance of bilateral leg edema and body weight gain are leading signs of worsening of HF during follow‐up, both of which are easily accessible by HF patients or the caretaker, but are often non‐specific for HF worsening. The non‐specificity of each HF‐related sign could partly be resolved by the utility of these two physical signs combined with monitoring symptoms,41, 42 which could enhance the predictive value of the presence of HF worsening. The appearance of these both signs during follow‐up of established HF patients should be intentionally watched or treated by extra diuretics and/or drug adjustment43 to prevent worsening of HF. To enhance the medical adherence and efficacy, educational efforts should pay more attention to understanding the usefulness and limitation of these important physical signs for those taking care of patients with HF.
The author declares that they have no conflict of interest.
None declared.
Supporting information
Comparison of the clinical course between two groups of the events of ≥2 HF‐related signs, one with the presentation of both leg edema and weight gain and the other lacking this criterion.
Kataoka, H. (2015) Clinical significance of bilateral leg edema and added value of monitoring weight gain during follow‐up of patients with established heart failure. ESC Heart Failure, 2: 106–115. doi: 10.1002/ehf2.12043.
References
- 1. Leier CV, Chatterjee K. The physical examination in heart failure—Part I. Congest Heart Fail 2007;13: 41–47. [DOI] [PubMed] [Google Scholar]
- 2. Leier CV, Chatterjee K. The physical examination in heart failure—Part II. Congest Heart Fail 2007;13: 99–104. [DOI] [PubMed] [Google Scholar]
- 3. Gheorghiade M, Follath F, Ponikowski P, Barsuk JH, Blair JEA, Cleland JG, Dickstein K, Drazner MH, Fonarow GC, Jaarsma T, Jondeau G, Lopez Sendon J, Mebazaa A, Metra M, Nieminen M, Pang PS, Seferovic P, Stevenson LW, van Veldhuisen DJ, Zannad F, Anker SD, Rhodes A, McMurray JJV, Filippatos G. Assessing and grading congestion in acute heart failure: a scientific statement from the Acute Heart Failure Committee of the Heart Failure Association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail 2010;12: 423–433. [DOI] [PubMed] [Google Scholar]
- 4. Kataoka H. A new monitoring method for the estimation of body fluid status by digital weight scale incorporating bioelectrical impedance analyzer in definite heart failure patients. J Card Fail 2009;15: 410–418. [DOI] [PubMed] [Google Scholar]
- 5. Kataoka H. Detection of preclinical body fluid retention in established heart failure patients during follow‐up by a digital weight scale incorporating a bioelectrical impedance analyzer. Congest Heart Fail 2012;18: 37–42. [DOI] [PubMed] [Google Scholar]
- 6. Cho S, Atwood JE. Peripheral edema. Am J Med 2002;113: 580–586. [DOI] [PubMed] [Google Scholar]
- 7. Shah MG, Cho S, Atwood JE, Heidenreich PA. Peripheral edema due to heart disease: diagnosis and outcome. Clin Cardiol 2006;29: 31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oudejans I, Mosterd A, Bloemen JA, Valk MJ, van Velzen E, Wielders JP, Zuithoff NP, Rutten FH, Hoes AW. Clinical evaluation of geriatric outpatients with suspected heart failure: value of symptoms, signs, and additional tests. Eur J Heart Fail 2011;13: 518–527. [DOI] [PubMed] [Google Scholar]
- 9. Kataoka H. Clinical characteristics of lower‐extremity edema in stage A cardiovascular disease status defined by the ACC/AHA 2001 chronic heart failure guidelines. Clin Cardiol 2013;36: 555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mueller C, Frana B, Rodriguez D, Laule‐Kilian K, Perruchoud AP. Emergency diagnosis of congestive heart failure: impact of signs and symptoms. Can J Cardiol 2005;21: 921–924. [PubMed] [Google Scholar]
- 11. Kelder JC, Cramer MJ, van Wijngaarden J, van Tooren R, Mosterd A, Moons KGM, Lammers JW, Cowie MR, Grobbee DE, Hoes AW. The diagnostic value of physical examination and additional testing in primary care patients with suspected heart failure. Circulation 2011;124: 2865–2873. [DOI] [PubMed] [Google Scholar]
- 12. Damy T, Kallvikbacka‐Bennett A, Zhang J, Goode K, Buga L, Hobkirk J, Yassin A, Dubois‐Randé JL, Hittinger L, Cleland JGF, Clark AL. Does the physical examination still have a role in patients with suspected heart failure? Eur J Heart Fail 2011;13: 1340–1348. [DOI] [PubMed] [Google Scholar]
- 13. Kataoka H, Takada S. The role of thoracic ultrasonography for evaluation of patients with decompensated chronic heart failure. J Am Coll Cardiol 2000;35: 1638–1646. [DOI] [PubMed] [Google Scholar]
- 14. Kataoka H. Ultrasound pleural effusion sign as a useful marker for identifying heart failure worsening in established heart failure patients during follow‐up. Congest Heart Fail 2012;18: 272–277. [DOI] [PubMed] [Google Scholar]
- 15. Kataoka H. Relation of body fluid status to B‐type natriuretic peptide levels in patients with chronic heart failure during long‐term follow‐up. Clin Cardiol 2006;29: 457–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Michalsen A, König G, Thimme W. Preventable causative factors leading to hospital admission with decompensated heart failure. Heart 1998;80: 437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lala A, Vader J, Dunlay S, Ravichandran A, AbouEzzadine O, Zakeri R, Khazanie P, McNulty S. A two‐symptom congestion score in relation to outcomes after discharge with acute decompensated heart failure. J Card Fail 2013;19(suppl. 8): S39. [Google Scholar]
- 18. Lee KS, Lennie TA, Dunbar SB, Pressler SJ, Heo S, Moser DK. Regular monitoring of lower extremity edema predicts cardiac event‐free survival in patients with heart failure. J Card Fail 2011;17(suppl. 8): S5. [Google Scholar]
- 19. Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). Developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation and endorsed by the Heart Rhythm Society. J Am Coll Cardiol 2005;46: e1–e82. [DOI] [PubMed] [Google Scholar]
- 20. Dickstein K, Cohen‐Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole‐Wilson PA, Stromberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail 2008;10: 933–989. [DOI] [PubMed] [Google Scholar]
- 21. Lewin J, Ledwidge M, O'Loughlin C, McNally C, McDonald K. Clinical deterioration in established heart failure: what is the value of BNP and weight gain in aiding diagnosis? Eur J Heart Fail 2005;7: 953–957. [DOI] [PubMed] [Google Scholar]
- 22. Chaudhry SI, Wang Y, Concato J, Gill TM, Krumholz HM. Patterns of weight change preceding hospitalization for heart failure. Circulation 2007;116: 1549–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schiff GD, Fung S, Speroff T, McNutt RA. Decompensated heart failure: symptoms, patterns of onset, and contributing factors. Am J Med 2003;114: 625–630. [DOI] [PubMed] [Google Scholar]
- 24. Cotter G, Metra M, Milo‐Cotter O, Dittrich HC, Gheorghiade M. Fluid overload in acute heart failure: re‐distribution and other mechanisms beyond fluid accumulation. Eur J Heart Fail 2008;10: 165–169. [DOI] [PubMed] [Google Scholar]
- 25. Chaudhry SI, Mattera JA, Curtis JP, Spertus JA, Herrin J, Lin Z, Phillips CO, Hodshon BV, Cooper LS, Krumholz HM. Telemonitoring in patients with heart failure. N Engl J Med 2010;363: 2301–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koehler F, Winkler S, Schieber M, Sechtem U, Stangl K, Böhm M, Boll H, Baumann G, Honold M, Koehler K, Gelbrich G, Kirwan BA, Anker SD. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation 2011;123: 1873–1880. [DOI] [PubMed] [Google Scholar]
- 27. Lyngå P, Persson H, Hägg‐Martinell A, Hägglund E, Hagerman I, Langius‐Eklöf A, Rosenqvist M. Weight monitoring in patients with severe heart failure (WISH): a randomized controlled trial. Eur J Heart Fail 2012;14: 438–444. [DOI] [PubMed] [Google Scholar]
- 28. Cleland JGF, Louis AA, Rigby AS, Janssens U, Balk AHMM, On behalf of the TEN‐HMS Investigators. Noninvasive home telemonitoring for patients with heart failure at high risk of recurrent admission and death: the Trans‐European Network‐Home‐Care Management System (TEN‐HMS) Study. J Am Coll Cardiol 2005;45: 1654–1664. [DOI] [PubMed] [Google Scholar]
- 29. Giordano A, Scalvini S, Zanelli E, Corrà U, Longobardi GL, Ricci VA, Baiardi P, Glisenti F. Multicenter randomized trial on home‐based telemanagement to prevent hospital readmission of patients with chronic heart failure. Int J Cardiol 2009;131;192–199. [DOI] [PubMed] [Google Scholar]
- 30. Inglis SC, Clark RA, McAlister FA, Stewart S, Cleland JGF. Which components of heart failure programmes are effective? A systemic review and meta‐analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: Abridged Cochrane Review. Eur J Heart Fail 2011;13: 1028–1040. [DOI] [PubMed] [Google Scholar]
- 31. Zhang J, Goode KM, Cuddihy PE, Cleland JGF. On behalf of the TEN‐HMS Investigators. Predicting hospitalization due to worsening heart failure using daily weight measurement: analysis of the Trans‐European Network‐Home‐Care Management System (TEN‐HMS) study. Eur J Heart Fail 2009;11: 420–427. [DOI] [PubMed] [Google Scholar]
- 32. Ledwidge MT, O'Hanlon R, Lalor L, Travers B, Edwards N, Kelly D, Voon V, McDonald KM. Can individualized weight monitoring using the HeartPhone algorithm improve sensitivity for clinical deterioration of heart failure? Eur J Heart Fail 2013;15: 447–455. [DOI] [PubMed] [Google Scholar]
- 33. Ahmed A, Allman RM, Aronow WS, DeLong JF. Diagnosis of heart failure in older adults: predictive value of dyspnea at rest. Arch Gerontol Geriatr 2004;38: 297–307. [DOI] [PubMed] [Google Scholar]
- 34. Webel AR, Frazier SK, Moser DK, Lennie TA. Daily variability in dyspnea, edema and body weight in heart failure patients. Eur J Cardiovasc Nurs 2007;6: 60–65. [DOI] [PubMed] [Google Scholar]
- 35. Yu CM, Wang L, Chau E, Chan RHW, Kong SL, Tang MO, Christensen J, Stadler RW, Lau CP. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation 2005;112: 841–848. [DOI] [PubMed] [Google Scholar]
- 36. Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S, Shavelle D, Jeffries B, Yadav JS, for the CHAMPION Trial Study Group . Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011;377: 658–666. [DOI] [PubMed] [Google Scholar]
- 37. Akar JG, Bao H, Jones P, Wang Y, Chaudhry SI, Varosy P, Masoudi FA, Stein K, Saxon LA, Curtis JP. Use of remote monitoring of newly implanted cardioverter‐defibrillators: insights from the Patient Related Determinants of ICD Remote Monitoring (PREDICT RM) study. Circulation 2013;128: 2372–2383. [DOI] [PubMed] [Google Scholar]
- 38. DeWalt DA, Pignone M, Malone R, Rawls C, Kosnar MC, George G, Bryant B, Rothman RL, Angel B. Development and pilot testing of a disease management program for low literacy patients with heart failure. Patient Educ Couns 2004;55: 78–86. [DOI] [PubMed] [Google Scholar]
- 39. Lainscak M, Blue L, Clark AL, Dahlström U, Dickstein K, Ekman I, McDonagh T, McMurray JJ, Ryder M, Stewart S, Strömberg A, Jaarsma T. Self‐care management of heart failure: practical recommendations from the Patient Care Committee of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2011;13: 115–126. [DOI] [PubMed] [Google Scholar]
- 40. Bui AL, Fonarow GC. Home monitoring for heart failure management. J Am Coll Cardiol 2012;59: 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ekman I, Cleland JGF, Andersson B. Exploring symptoms in chronic heart failure. Eur J Heart Fail 2005;7: 699–703. [DOI] [PubMed] [Google Scholar]
- 42. Song EK, Moser DK, Rayens MK, Lennie TA. Symptom clusters predict event‐free survival in patients with heart failure. J Cardiovasc Nurs 2010;25: 284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jones CD, Holmes GM, DeWalt DA, Erman B, Broucksou K, Hawk V, Cene CW, Wu JR, Pignone M. Is adherence to weight monitoring or weight‐based diuretic self‐adjustment associated with fewer heart failure‐related emergency department visits or hospitalization? J Card Fail 2012;18: 576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of the clinical course between two groups of the events of ≥2 HF‐related signs, one with the presentation of both leg edema and weight gain and the other lacking this criterion.


