Abstract
Determination of cirrhosis in nonalcoholic fatty liver disease (NAFLD) is important as it alters prognosis and management. We aimed to examine whether cirrhosis was diagnosed incidentally or intentionally in patients with NAFLD. We reviewed 100 patients with NAFLD cirrhosis to determine mode of cirrhosis diagnosis (incidental or by intent), severity of liver disease at diagnosis, diagnostician, and previous clinical imaging or laboratory evidence of unrecognized cirrhosis. The majority (66/100) of patients with NAFLD cirrhosis were diagnosed incidentally, with the majority of these (74%) diagnosed with NAFLD simultaneously. Those with incidental cirrhosis diagnoses had more deranged platelet and international normalized ratio levels (P < 0.05) and were more likely to have concomitant hepatocellular carcinoma (HCC) (12% versus 0%, P < 0.05). Incidental cirrhosis was diagnosed following imaging (32%) or liver tests (26%) performed for reasons unrelated to liver disease, following unexpected endoscopic finding of varices (21%) or an unexpected surgical finding (14%). Diagnoses by intent were predominantly made by gastroenterologists/hepatologists, whereas general practitioners, surgeons, and physicians tended to diagnose cirrhosis incidentally (P < 0.001). The majority of patients diagnosed incidentally (n = 48/66, 73%) had previous thrombocytopenia, splenomegaly, or high noninvasive fibrosis scores. Following diagnosis, patients diagnosed incidentally were less likely to undergo HCC screening. Conclusion: The majority of patients with NAFLD cirrhosis are diagnosed incidentally. These patients are more likely to have advanced liver disease and HCC. Increased awareness of screening for cirrhosis is needed in patients with NAFLD. (Hepatology Communications 2017;1:53–60)
Abbreviations
- FIB‐4
fibrosis‐4
- HCC
hepatocellular carcinoma
- INR
international normalized ratio
- MELD
Model for End‐Stage Liver Disease
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NFS
nonalcoholic fatty liver disease fibrosis score
Introduction
Worldwide, nonalcoholic fatty liver disease (NAFLD) has emerged as the most common cause of chronic liver disease.1, 2 Consequently, NAFLD is frequently encountered in primary care where the diagnosis may be incidental during investigation of other health problems. Importantly, a subset of individuals with NAFLD may progress to liver cirrhosis, which can be complicated by hepatocellular carcinoma (HCC), or liver failure requiring liver transplantation or resulting in death.3, 4 Liver cirrhosis due to NAFLD is currently an important and increasing indication for liver transplantation in the United States.5 The prevalence of cirrhosis in patients with NAFLD is not insignificant; 12%‐17% of patients selected for biopsy have cirrhosis, whereas 3%‐4% of community‐dwelling NAFLD subjects have advanced fibrosis.
The importance of cirrhosis as a prognostic factor predicting outcomes in patients with NAFLD has been highlighted in several recent studies, with overall and liver‐related mortality in these patients approaching 80% and 55%, respectively, after 12 years.6, 7 Aside from prognostic implications, the diagnosis of cirrhosis has management implications regarding screening for varices, surveillance for HCC, and closer monitoring.2 Recognition of NAFLD patients who have cirrhosis and are at the highest risk of harm is the first essential step toward the goal of reducing NAFLD‐related morbidity and mortality.
Despite the poor prognosis associated with NAFLD cirrhosis, these patients are frequently asymptomatic until they develop complications of liver decompensation, which heralds a rapid decline and poor survival.8 Routine liver function tests in compensated cirrhosis may be normal and thus are not reliable for fibrosis staging among patients with NAFLD.9 Thus, the diagnosis of cirrhosis requires a deliberate diagnostic approach using noninvasive methods, such as the NAFLD fibrosis score (NFS), transient elastography, or invasive liver biopsy.10, 11 The decision to screen patients with NAFLD for cirrhosis may be hampered by an underestimation of disease prevalence or a lack of regard for NAFLD as a clinically important condition.12 Correspondingly, patients with NAFLD cirrhosis are at risk of remaining undiagnosed, with only 0%‐3% of patients with NAFLD in primary care with a high NFS either recognized or referred for specialist review.13, 14 Anecdotal clinical experience also suggests that a significant proportion of patients with NAFLD are unintentionally (or incidentally) discovered to be cirrhotic during the investigation of alternative medical conditions unrelated to their liver disease.
Due to concerns regarding the lack of awareness of NAFLD cirrhosis, we sought to examine whether patients with NAFLD‐related cirrhosis reviewed at a tertiary hospital clinic had been diagnosed incidentally or by intent with cirrhosis. Furthermore, we wished to assess whether an incidental diagnosis of cirrhosis was associated with complications, such as HCC or more severe liver disease, and to retrospectively determine whether noninvasive strategies of fibrosis assessment would have been diagnostic of cirrhosis.
Patients and Methods
STUDY COHORT
Subjects with a diagnosis of cirrhosis related to NAFLD who attended the Department of Hepatology, Sir Charles Gairdner Hospital, Nedlands, Western Australia, between 2009 and 2015 were reviewed. Data were extracted from a prospectively collected clinical NAFLD database, electronic medical records, and general practitioner referrals. Inclusion criteria required age 18 years or older, diagnosis of NAFLD liver cirrhosis based on liver histology or validated imaging criteria (requiring two of the following: capsule nodularity, intra‐abdominal varices, splenomegaly),15, 16 and alcohol ingestion < 120 g/week for women and <210 g/week for men.17 Exclusion criteria included other causes of liver cirrhosis, including viral hepatitis B and C, autoimmune hepatitis, primary sclerosing cholangitis, primary biliary cirrhosis, hemochromatosis, alpha‐1‐antitripsin disease, or Wilson's disease, according to routine clinical, biochemical, and histologic criteria. Patients with cryptogenic cirrhosis and secondary causes of NAFLD were also excluded.
CIRRHOSIS DIAGNOSIS
Information was abstracted using a standardized data collection form, including: 1) mode of diagnosis of cirrhosis (incidental or by intent [defined below]); 2) specialty of diagnostician (general practitioner, gastroenterologist/hepatologist, surgeon, general physician, or other); 3) previous clinical, radiologic, or laboratory evidence of unrecognized liver cirrhosis, including unexplained thrombocytopenia, hyperbilirubinemia, or splenomegaly; and 4) timing of diagnosis of NAFLD with regards to diagnosis of cirrhosis. Additional variables examined at the time of cirrhosis diagnosis included liver function and presence of portal hypertension (varices, splenomegaly, thrombocytopenia, ascites), presence of HCC diagnosed according to standard radiologic criteria,18 and evidence of decompensation, which was defined by the presence of one or more of the following conditions: ascites, hepatic encephalopathy, spontaneous bacterial peritonitis, variceal hemorrhage, or jaundice. Following the diagnosis of cirrhosis, the inclusion into an HCC‐screening program (defined as a liver ultrasound every 6 months) and subsequent development of HCC were also recorded.
A diagnosis of cirrhosis was considered to be “incidental” according to established criteria, namely if it was previously undiagnosed, discovered unintentionally, and was unrelated to the medical condition being treated or investigated.19 Patients were required to have no previous record of cirrhosis or advanced liver disease in their hospital or family physician medical records and to have no personal knowledge of cirrhosis prior to the diagnosis. The medical indication and investigation request that led to the incidental diagnosis was also reviewed and was required to have no mention of liver disease, NAFLD, or cirrhosis. Incidental diagnoses were categorized according to the initial test that led to investigation, review, and cirrhosis diagnosis. For example, an incidental finding of varices during an upper endoscopy indicated for another reason, such as dyspepsia or abdominal pain; an incidental finding of cirrhosis on imaging performed for reasons unrelated to liver disease, such as abdominal pain; an incidental finding of abnormal liver function tests that were performed for reasons unrelated to liver disease; and an incidental finding at surgery. A cirrhosis diagnosis “by intent” was defined when a staging investigation was used for the purpose of assessing fibrosis severity. This included FibroScan, imaging, noninvasive biomarker panels, and/or liver biopsy performed in patients with NAFLD with the aim of diagnosing or ruling out liver cirrhosis.
Among patients who were diagnosed with cirrhosis, three noninvasive fibrosis scores (NFS, fibrosis‐4 [FIB‐4], and HepaScore) were calculated within 6 months of cirrhosis diagnosis.11, 20 The NFS and FIB‐4 score were calculated according to the published algorithms.11, 21 The HepaScore and its four component serum markers (hyaluronic acid, bilirubin, gamma‐glutamyl transpeptidase, α2‐macroglobulin) were available for all patients. Validated cutoff points of the NFS (>0.676), FIB‐4 (>1.5), and HepaScore (>0.84) were considered to be predictive of advanced fibrosis.11, 20, 21
The study was approved by the Sir Charles Gairdner Hospital Human Research Ethics Committee as an audit with “minimal risk,” and thus the requirement for informed consent was waived in keeping with the National Health and Medical Research Council statement on ethical conduct in human research. The study was performed according to the principles of the Declaration of Helsinki.
STATISTICAL ANALYSIS
The baseline characteristics were summarized in percentages for categorical variables and as the median and interquartile range or mean and standard deviation for continuous variables. Comparisons were made between patients diagnosed in the category of incidental versus by intent, using the two‐tailed Fisher's exact test for categorical variables or the two‐sample t test or Mann‐Whitney U test for continuous variables. All confidence intervals, significance tests, and resulting P values were two sided, with an alpha level of 0.05. Statistical analyses were performed using SPSS software, release 22.
Results
PATIENT CHARACTERISTICS
We identified 100 patients with cirrhosis who fulfilled our inclusion and exclusion criteria. The baseline characteristics of the patients at the time of cirrhosis diagnosis are listed in Table 1. Overall, the mean age of these patients was 66 ± 10.9 years, with a male predominance (62%). Approximately three quarters of patients had diabetes, two thirds had hypertension, and half had hypertriglyceridemia. Median Child‐Turcotte‐Pugh and Model for End‐Stage Liver Disease (MELD) scores were 6 (range 5‐11) and 11 (range 6‐25), respectively.
Table 1.
Baseline Characteristics of Participants According to Type Of Diagnosis Of Cirrhosis (Incidental Versus By Intent)
| Overall (n = 100) | Incidental Diagnosis (n = 66) | By Intent Diagnosis (n = 34) | P Valuea | |
|---|---|---|---|---|
| Age in years | 66.4 (10.9) | 67 (11.8) | 65 (8.9) | 0.32 |
| Sex N, (%) | ||||
| Male | 62 (62) | 39 (59) | 23 (68) | 0.51 |
| BMI (kg/m2) | 32 (7.8) | 31 (8.2) | 32 (7.1) | 0.35 |
| Metabolic syndrome N, (%) | 42 (42) | 28 (42) | 14 (41) | 0.53 |
| Type 2 diabetes N, (%) | 77 (77) | 50 (76) | 27 (79) | 0.80 |
| Hyperlipidemia N (%) | 50 (50) | 32 (48) | 18 (53) | 0.83 |
| Hypertension N (%) | 69 (69) | 43 (65) | 26 (76) | 0.26 |
| Child‐Turcotte‐Pugh score | 5 (5‐11) | 6 (5‐11) | 6 (5‐11) | 0.84 |
| MELD score | 10 (6‐25) | 11(6‐25) | 9 (6‐20) | 0.08 |
| HepaScore | 0.90 (0.08‐1.00) | 0.93 (0.08‐1.00) | 0.83 (0.08‐1.00) | 0.07 |
| NAFLD fibrosis score | 1.86 (–3.557‐6.04) | 2.11 (–3.557‐6.04) | 1.38 (–2.500‐3.73) | 0.04 |
| FIB‐4 score | 5.6 (0.2‐16.9) | 6.1 (0.4‐16.9) | 4.4 (0.2‐14.3) | 0.02 |
| AST (U/L) | 68 (15‐496) | 72 (16‐496) | 60 (15‐157) | 0.26 |
| ALT (U/L) | 68 (8‐452) | 76 (12‐452) | 54 (8‐191) | 0.87 |
| GGT (U/L) | 191 (19‐951) | 196 (19‐951) | 182 (37‐629) | 0.85 |
| ALP (U/L) | 150 (37‐1130) | 151 (37‐1130) | 150 (39‐932) | 0.93 |
| Platelets ( × 109/L) | 141 (31‐686) | 150 (31‐427) | 161 (66‐686) | 0.03 |
| Albumin (g/L) | 36 (20‐49) | 36 (24‐49) | 37 (20‐47) | 0.42 |
| Creatinine (mmol/L) | 90 (43‐684) | 94 (43‐684) | 83 (51‐180) | 0.44 |
| INR | 1.2 (0.36) | 1.2 (0.36) | 1.1 (0.38) | 0.01 |
| Total bilirubin (µmol/L) | 25 (4‐160) | 26 (4‐160) | 23 (5‐100) | 0.32 |
| Fasting glucose (mmol/L) | 6.9 (8‐22) | 7.2 (8‐22) | 6.9 (4.1‐21) | 0.28 |
| Cholesterol (mmol/L) | 3.8 (1.1‐7.2) | 3.8 (1.1‐7.2) | 3.9 (1.4‐6.7) | 0.75 |
| Triglycerides (mmol/L) | 1.7 (0.5‐4.3) | 1.6 (0.7‐4.2) | 1.8 (0.5‐4.3) | 0.22 |
P values are for the comparison between groups. Mann‐Whitney U test for continuous variables or Fisher's exact test for categorical variables. Quantitative data are expressed as mean (SD) or median (range).
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; GGT, gamma‐glutamyl transpeptidase; INR, international normalized ratio.
CIRRHOSIS DIAGNOSIS: INCIDENTAL VERSUS INTENTIONAL
Severity of Liver Disease
The majority (66%) of NAFLD cirrhosis subjects had been diagnosed incidentally. Clinical and biochemical characteristics comparing incidental and by intent groups at the time of diagnosis are shown in Table 1. Subjects with incidentally diagnosed cirrhosis tended to have more severe liver disease with significantly higher international normalized ratio (INR) levels (P = 0.01), lower platelet counts (P = 0.03), and a tendency toward higher MELD scores (P = 0.08). In addition, patients incidentally diagnosed with cirrhosis had a higher mean NFS (P = 0.04) and FIB‐4 (P = 0.02).
Concomitant HCC
At the time of diagnosis of cirrhosis, 8 patients (12%) who were diagnosed incidentally had concomitant HCC, whereas no patient who was diagnosed intentionally had concomitant HCC (P < 0.05).
Time of NAFLD Diagnosis
Overall, 63 patients had not had a prior diagnosis of NAFLD confirmed by either liver imaging or histology before the diagnosis of cirrhosis was made. A concomitant diagnosis of NAFLD was more commonly made in the incidental group (n = 49, 74%) when compared with the by intent group (n = 14, 41%) (P < 0.005) (Fig. 1).
Figure 1.
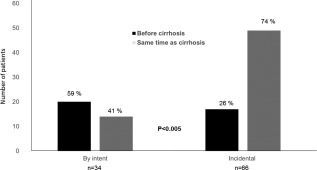
Time of NAFLD diagnosis by imaging or liver biopsy according to an incidental or intentional diagnosis of cirrhosis.
Diagnostician
The specialty of diagnostician varied significantly (P < 0.001) according to type of diagnosis (incidental or by intent) (Fig. 2). Patients with cirrhosis who were intentionally diagnosed were predominantly identified by gastroenterologists or hepatologists (n = 27/34, 79%), whereas general practitioners, surgeons, and internal medicine physicians were more likely to make incidental diagnoses (P < 0.001).
Figure 2.
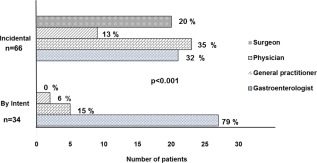
Diagnostician according to an incidental or intentional diagnosis of cirrhosis.
Methods of Diagnosis
An incidental diagnosis of cirrhosis was most commonly made on imaging that had been performed for reasons unrelated to liver diseases (n = 21/66, 32%) or following abnormal liver function tests performed for reasons unrelated to NAFLD or liver diseases (n = 17/66, 26%). Fourteen patients (21%) had incidental findings of varices on endoscopy, and 9 patients (14%) had an unexpected finding of cirrhosis at surgery. Of concern, 5 patients had cirrhosis diagnosed because of a new onset of hepatic complication (i.e., ascites and or encephalopathy).
Of the 34 patients with intentionally diagnosed cirrhosis, 7 (21%) underwent liver biopsy, 3 (9%) were diagnosed by FibroScan, and 24 (70%) were diagnosed by cross‐sectional imaging.
Prior Evidence of Cirrhosis
Review of laboratory and radiology results from electronic and clinical files over a median of 7 years (range 1‐17 years) prior to the cirrhosis diagnosis revealed that a high proportion of patients incidentally diagnosed with cirrhosis had previous evidence of cirrhosis as compared with patients diagnosed by intent (73% versus 30%, P < 0.001). Among patients with incidentally diagnosed cirrhosis, previous unexplained thrombocytopenia and splenomegaly were present in 22 (46%) and 18 (37%) patients, respectively (Fig. 3). The remaining 8 patients had previous evidence of liver cirrhosis based on imaging (reported nodularity and coarse echotexture of the liver). Despite this, no documentation of liver disease or cirrhosis was present in the investigation request form, medical notes, or subsequent correspondence from the ordering physician.
Figure 3.
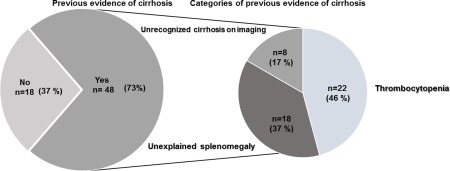
Previous evidence of cirrhosis in patients with incidentally diagnosed cirrhosis (n = 66).
NONINVASIVE FIBROSIS SCORES IN INCIDENTAL CIRRHOSIS SUBJECTS
The NFS and FIB‐4 were retrospectively calculated for incidentally diagnosed patients by using the variables available at the time of referral or at the first laboratory evaluation. A high proportion of incidental patients had an NFS >0.676 (80%) and a FIB‐4 > 1.5 (80%), consistent with advanced hepatic fibrosis. At the time of the cirrhosis diagnosis, the majority of patients in the incidental group had HepaScore values >0.84 (54, 81%), consistent with advanced hepatic fibrosis
SCREENING FOR HEPATOCELLULAR CARCINOMA
Following the diagnosis of cirrhosis, the majority of patients who were diagnosed by intent entered into an HCC‐screening program (25/34, 74%), whereas only a minority of subjects incidentally diagnosed and without HCC at baseline were subsequently entered into a screening program (24/58, 41%, P = 0.01). Consequently, during 70.5 months (interquartile range, 8.5‐276 months) of follow‐up, HCC was less likely to be diagnosed during screening in the incidental group compared to the by intent group (40% versus 69%, P = 0.01). Patients not in an HCC‐screening program were more likely to have a higher Barcelona Clinic Liver Cancer stage than those in a screening program (stage C, 52% versus 20%, P = 0.01) (Fig. 4).
Figure 4.
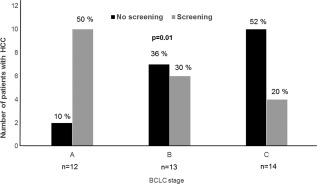
BCLC stage at the time of HCC diagnosis in patients who had and had not undergone screening. Abbreviation: BCLC, Barcelona Clinic Liver Cancer.
Discussion
NAFLD is the most common cause of liver test abnormalities in the community; approximately 1 in 25 patients having advanced fibrosis, which is associated with a significantly worse outcome and need for focused care.7, 22, 23 Our results show that the majority of patients with NAFLD cirrhosis and referred to a tertiary hospital clinic had a diagnosis of cirrhosis obtained incidentally and had not previously undergone fibrosis assessment despite having previous clinical or biochemical evidence of advanced liver disease. Subjects diagnosed incidentally had more severe liver disease with higher INR and MELD levels and lower platelet counts, were more likely to have HCC at the time of diagnosis, and were less likely to enter an HCC‐screening program. The lack of HCC screening was subsequently associated with a higher Barcelona Clinic Liver Cancer stage in those who developed HCC compared to those detected in a screening program. Notably, the application of simple noninvasive fibrosis scores (NFS, FIB‐4, or HepaScore) would have detected 80% of subjects as having advanced fibrosis.
To the best of our knowledge, this is the first study examining how NAFLD‐related cirrhosis is diagnosed and whether patients had previously been diagnosed with NAFLD. In our study, the majority of patients had not previously been diagnosed with NAFLD by imaging or liver biopsy prior to being diagnosed with cirrhosis. Under‐recognition of NAFLD appears to be common, with two studies finding the majority of patients in primary care who had incidental findings of elevated alanine aminotransferase levels or hepatic steatosis on imaging were not subsequently evaluated for NAFLD.13, 14 Similarly, a recent study of patients with NAFLD cirrhosis who were listed for liver transplant showed that the majority of these patients were not aware of an underlying NAFLD diagnosis until they presented with complications of portal hypertension.24 This may in part be due to an underestimation of disease prevalence by primary care and specialist physicians. Survey data demonstrates that the perceived prevalence of NAFLD is significantly underestimated and that the majority of patients with risk factors, such as obesity and diabetes, are not screened for NAFLD.25, 26 Under‐recognition of NAFLD and in particular NAFLD‐related cirrhosis may also be attributed to the misconception that NAFLD is not a clinically significant diagnosis.12
It is particularly notable that three quarters of our cirrhotic cohort had diabetes. Diabetes is considered one of the most important predictors of advanced fibrosis in NAFLD,27 with up to 18% of unselected diabetic patients having increased liver stiffness consistent with advanced fibrosis or cirrhosis.28, 29 Thus, patients with diabetes who are managed at primary care or specialist endocrinology clinics should receive screening for liver fibrosis as currently recommended by clinical guidelines.30 A recent viewpoint reported that 6%‐7% of the adult general population have liver fibrosis, with most of the cases associated with NAFLD.31 This supports the concept of screening the general population, which may reduce incidental diagnoses of cirrhosis in the future; however, further data on the accuracy and cost effectiveness of this approach are needed.
We found a high proportion of NAFLD‐related cirrhosis was diagnosed incidentally despite the majority of subjects having previous evidence of cirrhosis either clinically or biochemically. Notably, use of a noninvasive fibrosis score (i.e., NFS, FIB‐4, HepaScore) could have detected 80% of patients in our study at an earlier stage. Underutilization of noninvasive panels was noted in a Veteran's Affair cohort; only 3% of NAFLD patients with a high NFS and thus at risk of fibrosis were referred for specialist evaluation.14 The lack of fibrosis evaluation in NAFLD or recognition of cirrhosis may be hampered by the over‐reliance on liver enzyme abnormalities to guide further evaluation or referral for specialist care, given that alanine aminotransferase is a poor predictor of cirrhosis in NAFLD.9, 12
It is important to acknowledge the limitations of our study, including being a retrospective review with the possibility of selection bias related to patients all being reviewed at a tertiary academic center. It is possible that incidentally diagnosed subjects with NASH cirrhosis remain in the community if they have no evidence of decompensation, although in our experience, a diagnosis of cirrhosis usually precipitates referral for specialist care. In addition, although an extensive review of electronic and paper medical records, referral letters, imaging, and laboratory reports was undertaken, these may have been incomplete, leading to potential misclassification of an intentional or incidental diagnosis. However, a diagnosis of cirrhosis is significant and typically included in a patient's medical history. Furthermore, the incidental diagnosis precipitated referral to a tertiary hepatology clinic, strongly suggesting it had not been entertained previously.
An important finding of this study was that an incidental finding of cirrhosis was associated with more severe liver disease, a higher rate of concomitant HCC, and a lower rate of subsequent HCC screening. Subjects not enrolled in screening programs were more likely to present with more advanced HCC. Ultrasound surveillance for HCC in patients with cirrhosis remains an important tool to identify cancer at early stages and is correlated with curative treatments and long‐term survival.32
In conclusion, while NAFLD‐related cirrhosis is uncommon, it is frequently an incidental diagnosis and is associated with more advanced liver disease and HCC. These patients are unlikely to undergo HCC surveillance that may have otherwise reduced NAFLD cirrhosis‐related morbidity and mortality. The utilization of easily available noninvasive fibrosis markers can readily identify patients at advance stages or at high risk of progression and should be standard in the routine care of NAFLD patients in primary care, nonhepatology centers, and hepatology specialist centers. Increased awareness of screening for cirrhosis and HCC is needed in patients with NAFLD.
Conflict of interest: Leon A. Adams and Gary P. Jeffrey are employed by the University of Western Australia, which has a licensing agreement with Quest Diagnostics regarding the commercialization of HepaScore. Other co‐authors have no conflict of interest.
REFERENCES
- 1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease‐meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2015; 64:73‐84. [DOI] [PubMed] [Google Scholar]
- 2. Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263‐2273. [DOI] [PubMed] [Google Scholar]
- 3. Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 2013;10:686‐690. [DOI] [PubMed] [Google Scholar]
- 4. Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population‐based cohort study. Gastroenterology. 2005;129:113‐121. [DOI] [PubMed] [Google Scholar]
- 5. Siow W, van der Poorten D, George J. Epidemiological trends in NASH as a cause for liver transplant. Curr Hepatol Rep 2016;15:67‐74. [Google Scholar]
- 6. Younossi ZM, Stepanova M, Rafiq N, Makhlouf H, Younoszai Z, Agrawal R, et al. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver‐related mortality. Hepatology 2011;53:1874‐1882. [DOI] [PubMed] [Google Scholar]
- 7. Angulo P, Kleiner DE, Dam‐Larsen S, Adams L, Einar BS, Charatcharoenwitthaya P, et al. The prognostic relevance of liver histology features in nonalcoholic fatty liver disease: the PRELHIN study. Hepatol 2014;60:226A‐227A. [Google Scholar]
- 8. Sanyal AJ, Banas C, Sargeant C, Luketic VA, Sterling RK, Stravitz RT, et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology 2006;43:682‐689. [DOI] [PubMed] [Google Scholar]
- 9. Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology 2003;37:1286‐1292. [DOI] [PubMed] [Google Scholar]
- 10. Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454‐462. [DOI] [PubMed] [Google Scholar]
- 11. Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846‐854. [DOI] [PubMed] [Google Scholar]
- 12. Wieland AC, Quallick M, Truesdale A, Mettler P, Bambha KM. Identifying practice gaps to optimize medical care for patients with nonalcoholic fatty liver disease. Dig Dis Sci 2013;58:2809‐2816. [DOI] [PubMed] [Google Scholar]
- 13. Wright AP, Desai AP, Bajpai S, King LY, Sahani DV, Corey KE. Gaps in recognition and evaluation of incidentally identified hepatic steatosis. Dig Dis Sci 2015;60:333‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blais P, Husain N, Kramer JR, Kowalkowski M, El‐Serag H, Kanwal F. Nonalcoholic fatty liver disease is underrecognized in the primary care setting. Am J Gastroenterol 2015;110:10‐14. [DOI] [PubMed] [Google Scholar]
- 15. Kudo M, Zheng RQ, Kim SR, Okabe Y, Osaki Y, Iijima H, et al. Diagnostic accuracy of imaging for liver cirrhosis compared to histologically proven liver cirrhosis. Intervirology 2008;51 Suppl 1:17‐26. [DOI] [PubMed] [Google Scholar]
- 16. Bonekamp S, Kamel I, Solga S, Clark J. Can imaging modalities diagnose and stage hepatic fibrosis and cirrhosis accurately? J Hepatol 2009;50:17‐35. [DOI] [PubMed] [Google Scholar]
- 17. European Association for the Study of the Liver; European Association for the Study of Diabetes; European Association for the Study of Obesity . EASL‐EASD‐EASO Clinical Practice Guidelines for the management of non‐alcoholic fatty liver disease. J Hepatol 2016;64:1388‐1402. [DOI] [PubMed] [Google Scholar]
- 18. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wolf SM, Paradise J, Caga‐anan C. The law of incidental findings in human subjects research: establishing researchers' duties. J Law Med Ethics 2008;36:361‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Adams LA, George J, Bugianesi E, Rossi E, De Boer WB, van der Poorten D, et al. Complex non‐invasive fibrosis models are more accurate than simple models in non‐alcoholic fatty liver disease. J Gastroenterol Hepatol 2011;26:1536‐1543. [DOI] [PubMed] [Google Scholar]
- 21. Vallet‐Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin‐Venier V, et al. FIB‐4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007;46:32‐36. [DOI] [PubMed] [Google Scholar]
- 22. Wong VW, Chu WC, Wong GL, Chan RS, Chim AM, Ong A, et al. Prevalence of non‐alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton‐magnetic resonance spectroscopy and transient elastography. Gut 2012;61:409‐415. [DOI] [PubMed] [Google Scholar]
- 23. Ioannou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999‐2002. Am J Gastroenterol 2006;101:76‐82. [DOI] [PubMed] [Google Scholar]
- 24. Nagpal SJS, Kabbany MN, Mohamad B, Lopez R, Zein NN, Alkhouri N. Portal hypertension complications are frequently the first presentation of NAFLD in patients undergoing liver transplantation evaluation. Dig Dis Sci 2016;61:2102‐2107. [DOI] [PubMed] [Google Scholar]
- 25. Said A, Gagovic V, Malecki K, Givens ML, Nieto FJ. Primary care practitioners survey of non‐alcoholic fatty liver disease. Ann Hepatol 2013;12:758‐765. [PubMed] [Google Scholar]
- 26. Bergqvist CJ, Skoien R, Horsfall L, Clouston AD, Jonsson JR, Powell EE. Awareness and opinions of non‐alcoholic fatty liver disease by hospital specialists. Inter Med J 2013;43:247‐253. [DOI] [PubMed] [Google Scholar]
- 27. Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology 1999;30:1356‐1362. [DOI] [PubMed] [Google Scholar]
- 28. Kwok R, Choi KC, Wong GL, Zhang Y, Chan HL, Luk AO, et al. Screening diabetic patients for non‐alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut 2016:65:1359‐1368. [DOI] [PubMed] [Google Scholar]
- 29. Arab JP, Barrera F, Gallego C, Valderas JP, Uribe S, Tejos C, et al. High prevalence of undiagnosed liver cirrhosis and advanced fibrosis in type 2 diabetic patients. Ann Hepatol 2016;15:721‐728. [DOI] [PubMed] [Google Scholar]
- 30. European Association for the Study of the Liver; Asociacion Latinoamericana para el Estudio del Higado . EASL‐ALEH Clinical Practice Guidelines: non‐invasive tests for evaluation of liver disease severity and prognosis. J Hepatol 2015;63:237‐264. [DOI] [PubMed] [Google Scholar]
- 31. Ginès P, Graupera I, Lammert F, Angeli P, Caballeria L, Krag A, et al. Screening for liver fibrosis in the general population: a call for action. Lancet Gastroenterol Hepatol 2016;1:256‐260. [DOI] [PubMed] [Google Scholar]
- 32. European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer . EASL–EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908‐943. [DOI] [PubMed] [Google Scholar]


