Abstract
A prospective study based on serial liver biopsies was performed to investigate the efficacy of sodium‐glucose cotransporter 2 inhibitor for nonalcoholic fatty liver disease complicated with type 2 diabetes mellitus. Conclusion: Treatment for 24 weeks resulted in improvement in histopathologic features in all 5 patients. (Hepatology Communications 2017;1:46–52)
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- cel‐miR‐39
Caenorhabditis elegans microRNA 39
- DM
diabetes mellitus
- miRNA
microRNA
- miR‐122
microRNA 122
- NAFLD
nonalcoholic fatty liver disease
- NAS
nonalcoholic fatty liver disease activity score
- NASH
nonalcoholic steatohepatitis
- RT
reverse transcription
- SGLT2I
sodium‐glucose cotransporter 2 inhibitor
Introduction
Nonalcoholic fatty liver disease (NAFLD) is currently the most common liver disease worldwide across different ethnicities1, 2, 3, 4 and is associated with serious health care issues. NAFLD includes a wide spectrum of liver pathologies ranging from nonalcoholic fatty liver, which is usually benign, to nonalcoholic steatohepatitis (NASH), which may lead to liver cirrhosis, hepatocellular carcinoma, and liver failure without excessive alcohol intake.5 Treatment with vitamin E and farnesoid X nuclear receptor ligand obeticholic acid is reported to improve the histologic features of NAFLD.6, 7
Canagliflozin, a sodium‐glucose cotransporter 2 inhibitor (SGLT2I), improves hyperglycemia in patients with type 2 diabetes mellitus (T2DM) by enhancing urinary glucose excretion.8, 9, 10, 11, 12 A recent randomized, double‐blind, phase III noninferiority clinical trial (CANTATA‐SU) on patients with T2DM inadequately controlled with metformin concluded that canagliflozin reduced fasting plasma glucose, hemoglobin A1c, body weight, and blood pressure.13 Canagliflozin also improved liver function tests (e.g., aspartate aminotransferase [AST], alanine aminotransferase [ALT], and gamma‐glutamyl transpeptidase) and reduced visceral adipose tissue.13 These findings suggest a therapeutic potential of canagliflozin for NAFLD patients complicated with T2DM.
Recent studies have demonstrated high serum levels of various micro(mi)RNAs in patients with NAFLD and that high levels of serum miRNA 122 (miR‐122) correlate with histopathologic disease severity.14, 15, 16, 17 These findings highlight the potential usefulness of serum miR‐122 in the prediction of SGLT2I‐induced histologic improvement of NAFLD. The aim of the present preliminary study was to determine the efficacy of canagliflozin in NAFLD patients complicated with T2DM and the usefulness of serum miR‐122 in predicting histologic improvement in such patients.
Materials and Methods
A prospective study (single center, single arm, nonrandomized, open, and uncontrolled clinical trial) was performed at our hospital to determine the efficacy of a SGLT2I (canagliflozin 100 mg once daily for 24 weeks) in NAFLD patients complicated with T2DM. Treatment efficacy was evaluated by determining changes from baseline to the end of the 24‐week treatment in various histopathologic components of NASH (e.g., steatosis, lobular inflammation, ballooning, and fibrosis stage) and clinical parameters. Between November 2015 and May 2016, 5 Japanese patients were enrolled in the present study. Histopathologic evidence of definite NAFLD was based on liver biopsies obtained ≤30 days before the start of the SGLT2I. NAFLD was diagnosed based on liver histopathologic findings of steatosis in ≥5% of hepatocytes. The study protocol was in compliance with the Good Clinical Practice Guidelines (E6) and the 2013 Declaration of Helsinki and was approved by the institutional review board. All patients provided written informed consent. This trial was registered as clinical trial UMIN000018166 (https://upload.umin.ac.jp/cgi-open-bin/ctr/index.cgi).
The enrolled patients were consecutive patients aged 20‐64 years at the time of screening and had T2DM with fatty liver as diagnosed by abdominal ultrasonography. The following patients were excluded from the study: patients with an alcohol consumption of <20 g/day; patients with other liver diseases (e.g., primary biliary cirrhosis, autoimmune hepatitis, drug‐induced liver disease, viral hepatitis, hemochromatosis, biliary obstruction, α‐1‐antitrypsin deficiency‐associated liver disease, and Wilson disease); patients with contraindications to treatment with SGLT2I; patients considered to be ineligible for inclusion in the study as determined by the family physician; patients who did not consent to the 24‐week course of treatment with SGLT2I as outlined in the study protocol, including the need for an evaluation by liver biopsy before treatment and at the end of the 24‐week treatment; pregnant or lactating female patients.
Liver biopsy specimens were obtained using a 14‐gauge modified Vim Silverman needle (Tohoku University style; Kakinuma Factory, Tokyo, Japan). The biopsy tissue sample was fixed in 10% formalin, and sections were stained with hematoxylin and eosin, Masson trichrome, silver impregnation, and periodic acid‐Schiff after diastase digestion. Each specimen was evaluated by all four pathologists (Dr. Keiichi Kinowaki, Dr. Fukuo Kondo, Dr. Takeshi Fujii, and Dr. Toshio Fukusato), who were blinded to the clinical findings, and the final assessment of histopathologic findings was reported by consensus. An adequate liver biopsy sample was defined as a specimen more than 1.5 cm in length and/or containing more than 11 portal tracts. Specimens with steatosis of <5%, ≥ 5% to < 33%, ≥ 33% to < 66%, and ≥66% were scored as steatosis grade 0, 1, 2, and 3, respectively. Lobular inflammation of no foci, <2 foci, ≥ 2 to < 4 foci, and ≥4 foci per 200 × field was scored as 0, 1, 2, and 3, respectively. Hepatocyte ballooning of none, few cells, and many cells was scored as 0, 1, and 2, respectively. NAFLD activity score (NAS) represented the sum of steatosis, lobular inflammation, and hepatocyte ballooning scores (range, 0‐8 points; 5‐8 points as the definition of NASH, 3 or 4 points as borderline, and 0‐2 points as non‐NASH). A fibrosis stage of none, zone 3 perisinusoidal fibrosis (stage 1), zone 3 perisinusoidal fibrosis with portal fibrosis (stage 2), zone 3 perisinusoidal fibrosis and portal fibrosis with bridging fibrosis (stage 3), and cirrhosis (stage 4) was scored as 0, 1, 2, 3, and 4, respectively.18, 19 Patients were also classified into four categories by histopathology according to the classification by Matteoni et al.20 as follows: type 1, fatty liver alone; type 2, fat accumulation and lobular inflammation; type 3, fat accumulation and ballooning degeneration; type 4, fat accumulation, ballooning degeneration, and either a Mallory‐Denk body or fibrosis (type 3 or 4 as the definition of NASH and type 1 or 2 as non‐NASH).
The primary outcome measure included histopathologic changes in individual histopathologic components of NASH from baseline to the end of the 24‐week treatment. Histopathologic improvement was defined as a decrease in NAS of 1 point or more without worsening of the fibrosis stage. The secondary outcomes included changes in clinical parameters during the same period (e.g., physical examination, laboratory data, and transient elastography).
The normal ranges of AST and ALT at our hospital are 13‐33 IU/L and 8‐42 IU/L for men and 6‐27 IU/L for women, respectively. Obesity was defined as a body mass index (BMI) of more than 25.0 kg/m2. The severity of liver stiffness, which correlates directly with liver fibrosis and inflammation, was estimated at baseline and at the end of the 24‐week treatment. Measurements were performed using the FibroScan‐502 (Echosens, Paris, France) with the M‐probe and XL‐probe. Patients were placed in a supine position with the right hand at the most abducted position for right‐lobe liver scanning. When at least 10 measurements were obtained with valid measurements at ≥60% and an interquartile range of <30%, such measurements were considered valid; the median value of these measurements was used for analysis.21, 22
Serum miR‐122 levels were evaluated at three time points: baseline, at day 1, and at the end of the 24‐week SGLT2I treatment. Serum samples were frozen at –80°C within 4 h of collection until used for testing. Circulating miR‐122 levels were collectively assayed using the stored frozen serum samples. Circulating miRNA was extracted from 200 µL of serum samples using the QIAGEN miRNeasy serum‐plasma kit (QIAGEN, Tokyo, Japan) according to the instructions provided by the manufacturer. RNA was reverse transcribed using the TaqMan MicroRNA Reverse Transcription (RT) kit (Life Technologies Japan, Tokyo, Japan). Each sample was spiked with Caenorhabditis elegans miR‐39 (cel‐miR‐39) as a control for extraction and amplification steps. The reaction mixture contained 5 µL of RNA solution, 1.5 µL of 10 × RT buffer, 0.15 µL of 100 mM deoxyribonucleotide triphosphate mixture, 1 µL of MultiScribe reverse transcriptase enzyme, 3 µL of 5 × RT primer, 0.19 µL of RNase inhibitor, and 4.16 µL of nuclease free water in a total volume of 15 µL. The reaction was performed at 16°C for 30 minutes followed by 42°C for 30 minutes. The reaction was terminated by heating the solution at 85°C for 5 minutes. Serum miR‐122 was amplified using primers and probes provided by Applied Biosystems (Foster City, CA) using TaqMan miRNA assays according to the instructions supplied by the manufacturer. The reaction mixture contained 10 µL of 2 × TaqMan Universal Master Mix II, 1 µL of 20 × TaqMan assay solution, 1.3 µL of RT product, and 7.7 µL of nuclease free water in a total volume of 20 µL. Amplification conditions were 95°C for 10 minutes followed by 40 denaturing cycles for 15 seconds at 95°C and annealing and extension for 60 seconds at 60°C in an ABI7300 thermal cycler. The relative expression of serum miR‐122 was calculated using the comparative cycle threshold method (2−ΔΔCT)23, 24 with spiked cel‐miR‐39 as the normalized internal control. The expression levels of miRNA were calibrated relative to the levels of serum miR‐122 measured in 286 clinical samples.14 The serum miR‐122 ratio represented the serum miR‐122 level measured at baseline, day 1, and week 24 of treatment, divided by the level at baseline
The Wilcoxon test was used for the comparison of paired samples. All P values less than 0.05 by the two‐tailed test were considered significant. Statistical analyses were performed using SPSS software Version 2 (SPSS Inc., Chicago, IL).
Results
The changes in histopathologic scores between the first and second liver biopsies are summarized in Table 1. In all 5 patients, the rates of hepatocyte steatosis and NAS improved at 24 weeks compared to the pretreatment. Two of the 5 patients (cases 4 and 5) showed decreases in the fibrosis stage score, with large decreases in the rates of hepatocyte steatosis (50% in case 4, 30% in case 5). Based on the NAS, 4 of 5 patients (cases 1, 2, 4, and 5) improved from NASH to borderline, while the other patient (case 3) improved from borderline to non‐NASH. Furthermore, according to the Matteoni classification, 1 of the 5 patients (case 3) improved from NASH to non‐NASH. In conclusion, the 24‐week treatment with the SGLT2I resulted in histopathologic improvement (defined as a decrease in NAS of 1 point or more without worsening of the fibrosis stage) in all 5 patients compared to pretreatment.
Table 1.
Histopathologic Findings at the Time of the First and Second Liver Biopsies
| Case 1 (64‐year‐old. male) | Case 2 (44‐year‐old. male) | Case 3 (60‐year‐old. female) | Case 4 (63‐year‐old. female) | Case 5 (60‐year‐old. male) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st biopsy | 2nd biopsy | 1st vs. 2nda | 1st biopsy | 2nd biopsy | 1st vs. 2nda | 1st biopsy | 2nd biopsy | 1st vs. 2nda | 1st biopsy | 2nd biopsy | 1st vs. 2nda | 1st biopsy | 2nd biopsy | 1st vs. 2nda | |
| Steatosis (%) | 2 (50%) | 1 (30%) | ↓ | 2 (40%) | 1 (20%) | ↓ | 1 (30%) | 1 (5‐10%) | ↓ | 3 (80%) | 1 (30%) | ↓ | 2 (60%) | 1 (30%) | ↓ |
| Lobular inflammation | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 1 | ↓ | ||||
| Ballooning | 1 | 1 | 1 | 1 | 1 | 0 | ↓ | 2 | 1 | ↓ | 1 | 1 | |||
| Stage | 1 | 1 | 2 | 2 | 1 | 1 | 4 | 3 | ↓ | 2 | 1 | ↓ | |||
| NAFLD activity score | 5 | 4 | ↓ | 5 | 4 | ↓ | 3 | 2 | ↓ | 7 | 4 | ↓ | 5 | 3 | ↓ |
| Matteoni classification | 4 | 4 | 4 | 4 | 4 | 2 | ↓ | 4 | 4 | 4 | 4 | ||||
Factors that tended to decrease at second biopsy relative to first biopsy, are indicated by black arrow.
The changes in clinical parameters between the first and second liver biopsies are summarized in Table 2. In all 5 patients, the 24‐week SGLT2I treatment significantly reduced BMI, waist circumference, fasting plasma glucose, and markers of liver dysfunction (gamma‐glutamyl transpeptidase, ferritin, and type IV collagen 7S) and improved the findings of transient elastography (liver stiffness measurement).
Table 2.
Clinical parameters at die time of the first and second liver biopsies
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st biopsy | 2nd biopsy | lst biopsy | 2nd biopsy | 1st biopsy | 2nd biopsy | lst biopsy | 2nd biopsy | lst biopsy | 2nd biopsy | P a | |
| Physical examination | |||||||||||
| Body mass index (kg/m2) | 23.5 | 22.3 | 25.3 | 25.0 | 27.9 | 26.3 | 27.8 | 25.5 | 29.0 | 27.4 | 0.042 |
| Waist circumference (cm) | 76.6 | 73.6 | 96.9 | 94.4 | 88.1 | 81.2 | 89.2 | 86.3 | 102.3 | 98.5 | 0.043 |
| Laboratory data | |||||||||||
| Serum aspartate aminotransferase (IU/L) | 22 | 20 | 10 | 10 | 19 | 18 | 39 | 21 | 32 | 23 | 0.068 |
| Serum alanine aminotransferase (IU/L) | 24 | 17 | 17 | 17 | 23 | 27 | 63 | 19 | 50 | 29 | 0.144 |
| Alkaline phosphatase (IU/L) | 249 | 261 | 226 | 233 | 235 | 211 | 363 | 269 | 191 | 165 | 0.225 |
| Gamma‐glutamyl transpeptidase (IU/L) | 42 | 35 | 42 | 27 | 23 | 19 | 36 | 21 | 46 | 22 | 0.042 |
| Fasting plasma glucose (mg/dL) | 126 | 106 | 265 | 182 | 180 | 140 | 106 | 97 | 134 | 119 | 0.043 |
| C‐peptide (ng/mL) | 2.30 | 1.86 | 2.10 | 1.76 | 3.47 | 1.53 | 3.84 | 1.70 | 3.79 | 3.31 | 0.102 |
| HbAlc (%) | 6.6 | 6.6 | 12.0 | 10.3 | 7.5 | 7.5 | 7.9 | 6.4 | 7.3 | 6.7 | 0.109 |
| Total cholesterol (mg/dL) | 168 | 175 | 217 | 212 | 188 | 158 | 210 | 217 | 144 | 152 | 0.684 |
| Triglycerides (mg/dL) | 232 | 103 | 153 | 288 | 191 | 136 | 105 | 85 | 256 | 208 | 0.500 |
| High‐density lipoprotein cholesterol (mg/dL) | 40 | 55 | 37 | 44 | 45 | 44 | 37 | 38 | 57 | 56 | 0.336 |
| Low‐density lipoprotein cholesterol (mg/dL) | 84 | 96 | 149 | 122 | 97 | 79 | 145 | 146 | 52 | 59 | 0.686 |
| Uric acid (mg/dL) | 6.7 | 5.4 | 4.4 | 5.0 | 5.9 | 5.7 | 5.5 | 5.2 | 6.4 | 6.3 | 0.345 |
| Hyaluronic acid (μg/L) | 16 | 20 | 17 | 5 | 42 | 23 | 102 | 80 | 18 | 23 | 0.225 |
| Type IV collagen 7S (ng/mL) | 4.4 | 3.0 | 4.4 | 2.3 | 3.8 | 2.9 | 4.9 | 4.2 | 21.2 | 18.6 | 0.043 |
| Procollagen III peptide (U/mL) | 0.54 | 0.60 | 0.46 | <0.5 | 0.49 | 0.70 | 0.70 | 0.70 | 1.10 | 1.30 | 0.197 |
| High sensitive C‐reactive protein (mg/dL) | 0.081 | 0.150 | 0.026 | 0.026 | 0.062 | 0.084 | 0.075 | 0.063 | 0.020 | 0.015 | 0.465 |
| Serum ferritin (pg/L) | 233 | 123 | 259 | 183 | 202 | 99 | 602 | 298 | 1696 | 410 | 0.043 |
| Serum miR‐122 (Fold change) | 0.28 | 0.10 | 0.09 | 0.16 | 0.37 | 0.04 | 0.34 | 0.13 | 0.59 | 0.36 | 0.080 |
| Transient elastography | |||||||||||
| Liver Stiffness Measurement (kPa) | 5.8 | 4.9 | 4.2 | 2.9 | 5.1 | 3.6 | 9.9 | 6.8 | 5.2 | 3.2 | 0.039 |
| Controlled Attenuation Parameter (dB/m) | 267 | 184 | 271 | 249 | 222 | 291 | 249 | 234 | 339 | 318 | 0.345 |
Wilcoxon test was used for comparison of paired samples at the time of first and second liver biopsies.
Abbreviation: HbA1c, glycated hemoglobin type A1C.
The logarithmically transformed serum miR‐122 ratios at baseline, day 1, and 24 weeks after the start of the SGLT2I are shown in Fig. 1. Three patients (cases 1, 3, and 4) who showed a reduced ratio at day 1 also showed a reduction at week 24. In case 3, the Matteoni classification at 24 weeks improved from type 4 to type 2, which was mainly due to the resolution of ballooning, and the serum miR‐122 ratios at both day 1 and week 24 were the lowest among all patients. These findings suggest that the reduction of serum miR‐122 ratio at day 1 was a marker of histopathologic improvement at week 24.
Figure 1.
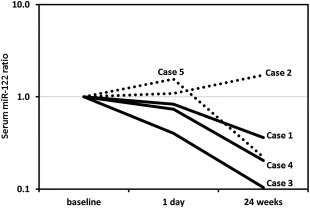
Logarithmically transformed serum miR‐122 ratio at baseline, day 1, and week 24 of treatment with an SGLT2I. The serum miR‐122 ratio represents the serum miR‐122 level at the above three time points divided by that at baseline. The 3 patients (cases 1, 3, and 4; solid line) who showed a reduction of the miR‐122 ratio at day 1 also showed a reduction of the ratio at week 24. In case 3, the Matteoni classification at week 24 was type 4 compared with type 2 at baseline, and this change was due to the resolution of ballooning; the serum miR‐122 ratios at both day 1 and week 24 of this patient were the lowest among all patients.
Discussion
A recent study reported that ipragliflozin, an SGLT2I, prevented hepatic triglyceride accumulation and fibrosis in choline‐deficient l‐amino acid‐defined diet rats,25 suggesting a therapeutic potential of SGLT2I for patients with NAFLD. To our knowledge, the present prospective study based on serial liver biopsies is the first to demonstrate the usefulness of canagliflozin for NAFLD complicated with T2DM. The SGLT2I improved the rates of hepatocyte steatosis and NAS at 24 weeks in all patients together with improvement in histopathologic findings. Furthermore, the SGLT2I also improved BMI, waist circumference, glucose metabolism, liver serologic markers, and findings of transient elastography. These findings highlight the therapeutic potential of canagliflozin as an effective therapeutic option for NAFLD complicated with T2DM. However, the results of the present study do not allow drawing conclusions regarding the mechanism of action of an SGLT2I in NAFLD, i.e., whether the observed effects were mediated through improvement of the associated metabolic abnormalities or the direct effect of the SGLT2I. Further basic and clinical research is needed to determine the exact mechanism of action.
The present study has certain limitations. First, the study design was an uncontrolled before–after study. Second, the study included a relatively small number of patients and treatment with SGLT2I was arbitrarily ended at 24 weeks. Indeed, the present results showed decreases in the fibrosis stage score in only 2 of 5 patients (40%) at 24 weeks, whereas all 5 patients showed improvement in type IV collagen 7S and the liver stiffness measurement, representing markers of the fibrosis stage. The above differences in the results could be due to the short‐term evaluation of 24 weeks based on serial liver biopsies. A further large‐scale and long‐term randomized controlled trial should be performed to confirm the therapeutic potential of an SGLT2I and determine its effects on histopathologic features of NAFLD, including the fibrosis stage.
Recent studies demonstrated the presence of high serum levels of various miRNAs in patients with NAFLD and that miR‐122 serum levels are particularly associated with histopathologic disease severity.14, 15, 16, 17 To our knowledge, the present study is the first to demonstrate the association of serum miR‐122 with SGLT2I‐induced histopathologic changes in NAFLD. In this regard, early prediction of the effectiveness of SGLT2I is important, and whether the reduction in the serum miR‐122 ratio at day 1 of treatment can be used to predict the histopathologic improvement at treatment week 24 is an interesting question. In the present study, the 3 patients who showed a reduction of the ratio at day 1 also showed a reduction of the same ratio at week 24. Admittedly, the results of case 2 differed from those of the other patients. This patient showed an increase in the serum miR‐122 ratio and a reduction of the hepatocyte steatosis score, despite only a 20% decrease in the rate of hepatocyte steatosis. Further large‐scale studies should be performed to investigate the usefulness of serum miR‐122 as an early predictor of histopathologic response to treatment with SGLT2I.
In conclusion, we have demonstrated in this preliminary study the potential effectiveness of a 24‐week treatment with an SGLT2I in 5 NAFLD patients complicated with T2DM. Further long‐term prospective studies of large population samples are needed to confirm both the clinical and histopathologic effects of an SGLT2I in NAFLD.
Acknowledgment
We thank Dr. Keiichi Kinowaki and Dr. Takeshi Fujii (Department of Pathology, Toranomon Hospital) and Dr. Fukuo Kondo and Dr. Toshio Fukusato (Department of Pathology, Teikyo University School of Medicine) for assistance in pathological diagnosis.
This study was conducted without any external or internal funding.
Conflicts of interest: Dr. Kumada has received honoraria from MSD K.K., Bristol‐Myers Squibb, Gilead Sciences, AbbVie Inc., GlaxoSmithKline K.K., and Dainippon Sumitomo Pharma. Dr. Fumitaka Suzuki and Dr. Yoshiyuki Suzuki have received an honorarium from Bristol‐Myers Squibb. Dr. Arase has received an honorarium from MSD K.K. Dr. Ikeda has received honoraria from Dainippon Sumitomo Pharma and Eisai Co., Ltd. All other authors declare no conflict of interest.
REFERENCES
- 1. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 2002;346:1221‐1231. [DOI] [PubMed] [Google Scholar]
- 2. Williams R. Global changes in liver disease. Hepatology 2006;44:521‐526. [DOI] [PubMed] [Google Scholar]
- 3. Sumida Y, Nakajima A, Itoh Y. Limitations of liver biopsy and non‐invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol 2014;20:475‐485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akuta N, Kawamura Y, Suzuki F, Saitoh S, Arase Y, Kunimoto H, et al. Correlation of histopathological features and genetic variations with prognosis of Japanese patients with nonalcoholic fatty liver disease. J Hep 2015;2:1. [Google Scholar]
- 5. Kleiner DE, Brunt EM. Nonalcoholic fatty liver disease: pathologic patterns and biopsy evaluation in clinical research. Semin Liver Dis 2012;32:3‐13. [DOI] [PubMed] [Google Scholar]
- 6. Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al; NASH CRN . Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010;362:1675‐1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Neuschwander‐Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al.; NASH Clinical Research Network . Farnesoid X nuclear receptor ligand obeticholic acid for non‐cirrhotic, non‐alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo‐controlled trial. Lancet 2015;385:956‐965. Erratum in: Lancet 2015;385:946. Lancet 2016;387:1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nomura S, Sakamaki S, Hongu M, Kawanishi E, Koga Y, Sakamoto T, et al. Discovery of canagliflozin, a novel C‐glucoside with thiophene ring, as sodium‐dependent glucose cotransporter 2 inhibitor for the treatment of type 2 diabetes mellitus. J Med Chem 2010;53:6355–6360. [DOI] [PubMed] [Google Scholar]
- 9. Sha S, Devineni D, Ghosh A, Polidori D, Chien S, Wexler D, et al. Canagliflozin, a novel inhibitor of sodium glucose co‐transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metab 2011;13:669–672. [DOI] [PubMed] [Google Scholar]
- 10. Devineni D, Morrow L, Hompesch M, Skee D, Vandebosch A, Murphy J, et al. Canagliflozin improves glycemic control over 28 days in subjects with type 2 diabetes not optimally controlled on insulin. Diabetes Obes Metab 2012;14:539–545. [DOI] [PubMed] [Google Scholar]
- 11. Liang Y, Arakawa K, Ueta K, Matsushita Y, Kuriyama C, Martin T, et al. Effect of canagliflozin on renal threshold for glucose, glycemia, and body weight in normal and diabetic animal models. PLoS One 2012;7:e30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosenstock J, Aggarwal N, Polidori D, Zhao Y, Arbit D, Usiskin K, et al.; Canagliflozin DIA 2001 Study Group . Dose‐ranging effects of canagliflozin, a sodium‐glucose cotransporter 2 inhibitor, as add‐on to metformin in subjects with type 2 diabetes. Diabetes Care 2012;35:1232–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA‐SU): 52 week results from a randomised, double‐blind, phase 3 non‐inferiority trial. Lancet 2013;382:941‐950. [DOI] [PubMed] [Google Scholar]
- 14. Akuta N, Kawamura Y, Suzuki F, Saitoh S, Arase Y, Kunimoto H, et al. Impact of circulating miR‐122 for histological features and hepatocellular carcinoma of nonalcoholic fatty liver disease in Japan. Hepatol Int 2016;10:647‐656. [DOI] [PubMed] [Google Scholar]
- 15. Cermelli S, Ruggieri A, Marrero JA, Ioannou GN, Beretta L. Circulating microRNAs in patients with chronic hepatitis C and non‐alcoholic fatty liver disease. PLoS One 2011;6:e23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yamada H, Suzuki K, Ichino N, Ando Y, Sawada A, Osakabe K, et al. Associations between circulating microRNAs (miR‐21, miR‐34a, miR‐122 and miR‐451) and non‐alcoholic fatty liver. Clin Chim Acta 2013;424:99‐103. [DOI] [PubMed] [Google Scholar]
- 17. Pirola CJ, Fernández Gianotti T, Castaño GO, Mallardi P, San Martino J, Mora Gonzalez Lopez Ledesma M, et al. Circulating microRNA signature in non‐alcoholic fatty liver disease: from serum non‐coding RNAs to liver histology and disease pathogenesis. Gut 2015;64:800‐812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al.; Nonalcoholic Steatohepatitis Clinical Research Network . Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313‐1321. [DOI] [PubMed] [Google Scholar]
- 19. Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander‐Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 1999;94:2467‐2474. [DOI] [PubMed] [Google Scholar]
- 20. Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 1999;116:1413‐1419. [DOI] [PubMed] [Google Scholar]
- 21. Boursier J, Zarski JP, de Ledinghen V, Rousselet MC, Sturm N, Lebail B, et al.; Multicentric Group from ANRS/HC/EP23 FIBROSTAR Studies . Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology 2013;57:1182‐1191. [DOI] [PubMed] [Google Scholar]
- 22. Kumagai E, Korenaga K, Korenaga M, Imamura M, Ueyama M, Aoki Y, et al. Appropriate use of virtual touch quantification and FibroScan M and XL probes according to the skin capsular distance. J Gastroenterol 2016;51:496‐505. [DOI] [PubMed] [Google Scholar]
- 23. Kroh EM, Parkin RK, Mitchell PS, Tewari M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription‐PCR (qRT‐PCR). Methods 2010;50: 298‐301. Erratum in: Methods 2010;52:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yu S, Liu Y, Wang J, Guo Z, Zhang Q, Yu F, et al. Circulating microRNA profiles as potential biomarkers for diagnosis of papillary thyroid carcinoma. J Clin Endocrinol Metab 2012;97:2084‐2092. [DOI] [PubMed] [Google Scholar]
- 25. Hayashizaki‐Someya Y, Kurosaki E, Takasu T, Mitori H, Yamazaki S, Koide K, et al. Ipragliflozin, an SGLT2 inhibitor, exhibits a prophylactic effect on hepatic steatosis and fibrosis induced by choline‐deficient l‐amino acid‐defined diet in rats. Eur J Pharmacol 2015;754:19‐24. [DOI] [PubMed] [Google Scholar]


