Abstract
Background
Calcipotriol ointment has been demonstrated to be a very safe and effective topical drug for psoriasis. This study aims to investigate the effect of calcipotriol on IFN-γ-induced keratin 17 (K17) expression in a human keratinocyte cell line (HaCaT), which is a widely accepted as a mimic in vitro model for psoriasis.
Material/Methods
We used Western blot, immunofluorescence staining, and luciferase reporter system assays to evaluate the expression of K17 and the possible underlying mechanisms.
Results
Administration of IFN-γ (125–1000 U) increased K17 expression in a dose-dependent manner, and 250 U/ml IFN-γ significantly elevated K17 expression. The experimental results showed that calcipotriol at concentrations of 10−7 M and 10−5 M suppressed the IFN-γ-induced K17 expression by 58.10% and 70.68%, respectively. Through immunofluorescence staining and luciferase reporter assay, we found that Vitamin D Response Element (VDRE) affected IFN-activated site (Gamma-activated sequence, GAS) function at the transcriptional level and was involved in the inhibition of K17 expression.
Conclusions
Our data suggest that calcipotriol downregulates IFN-γ-mediated K17 expression in keratinocytes in a dose-dependent manner via VDRE effect GAS function. The inhibitory effect of calcipotriol on K17 expression may be a potential mechanism and function in the treatment psoriasis.
MeSH Keywords: Calcitriol, Keratin-17, Psoriasis
Background
Psoriasis is a common chronic skin disease characterized by inflammation, hyperproliferation, and high relapse rate, affecting 1–3% of the world population. Psoriasis leads to a low quality of life and reduces physical and mental functioning [1,2]. Evidence from clinical studies and experimental models support that psoriasis is a T cell-mediated autoimmune disease. An epitope of keratin 17 (K17) has recently been described as a putative crucial target for psoriasis [3].
Previous investigations indicated that K17 was overexpressed in psoriatic lesions but was undetectable in healthy epidermis [4]. K17 may also function as an autoantigen in the immunopathogenesis of psoriasis. Some restricted T cell epitope regions were found on the K17 molecule, which was associated with the activation of the proliferation of T cells and the subsequent production of psoriasis-associated cytokines, such as IFN-γ, IL-17A, and IL-22. It has been shown that IFN-γ can increase the expression of K17 in a human keratinocyte cell line (HaCaT) [5,6]. Our group previously reported that the K17/T cells/cytokine autoimmune loop was involved in the pathogenesis of psoriasis, and IFN-γ is an important cytokine [6]. This loop could explain the positive feedback mechanism between IFN-γ activity and K17 expression in psoriasis.
Calcipotriol is a synthetic vitamin D3 analogue widely used for topical treatment of psoriasis. Previous studies have shown that calcipotriol binds to the vitamin D receptor (VDR), which is expressed in keratinocytes, and it inhibits proliferation and promote differentiation of keratinocytes in psoriasis [7,8]. VDR is a DNA-binding transcription factor that generates an active signal transduction complex and exerts its biological function by recognizing vitamin D-responsive elements (VDREs) in the DNA sequence [9].
Because IFN-γ and K17 participate in the pathogenesis of keratinocyte proliferation in psoriasis, and calcipotriol inhibits keratinocyte proliferation, we hypothesized that calcipotriol functions in inhibiting IFN-γ-induced K17 expression. We tested this hypothesis by observing the effect of calcipotriol on the IFN-γ-induced K17 expression in HaCaT cells and explored the molecular mechanism involved.
Material and Methods
Chemicals and reagents
Calcipotriol monohydrate, which is a 94.2% white crystalline powder, was provided by LEO Pharma Pty Ltd. Australia. The drug was dissolved in 100% isopropyl alcohol at 5 mM and stored in liquid nitrogen. Working concentrations for experiments were further diluted in isopropyl alcohol. Handling of calcipotriol was away from direct light. Recombinant human IFN-γ was purchased from PeproTech (Rocky Hill, NJ, USA) and dissolved in deionized water at 2×106 U/ml and kept at −80°C.
Cell culture
HaCaT cells were maintained in our lab. The cells were cultured in RPMI 1640 medium (Gibco-Invitrogen, Carlsbad, CA, USA) with 10% FBS (Gibco-Invitrogen, Carlsbad, CA, USA) and incubated at 37°C, 5% CO2. For luciferase reporter assay, 2×103 cells/well were grown in 48-well plates (Corning, Tewksbury, MA, USA). For Western blot assay and quantitative real-time PCR (q-PCR) preparation, 2×105 cells/well were cultured in 6-well plates (Corning, Tewksbury, MA, USA). For fluorescence dye, 1×103 cells were seeded in 35 mm glass-bottom dishes (Corning, Tewksbury, MA, USA).
Reverse transcription and quantitative real-time PCR (q-PCR)
After 2×105 cells were attached for 24 h, cell culture medium was changed with serum-free RPMI 1640 for at least 8 h before treatment as indicated for each experiment. Total RNA was extracted using TRIzol reagent (Life Tech, Grand Island, NY, USA) following the manufacturer’s instructions. We used 1 ug of RNA for reverse transcription using the PrimeScript RT reagent kit (Takara, Ohtsu, Japan). Then, the cDNA was used to determine relative expression of target genes by SYBR premix Ex Taq II (Takara, Ohtsu, Japan), using the iQ™5 real-time PCR detection system (Bio-Rad, Hercules, CA, USA). The primers used in the amplification were as follows: K17: 5′-CCA TGC AGG CCT TGG AGA-3′ (F), 5′-GTC TTC ACA TCC AGC AGG A-3′ (R); β-actin 5′-CAC GAT GGA GGG GCC GGA CTC ATC-3′ (F), 5′-TAA AGA CCT CTA TGC CAA CAC AGT-3′ (R). The reaction was performed according to manufacturer’s instructions, with an initial temperature of 95°C for 10 min and followed by 95°C for 30 s, 55°C for 1 min, 72°C for 30 s for 40 cycles. All reactions were run in triplicate for at least 3 independent experiments. Data were analyzed using 2−ΔΔCT method.
Western blot analysis
Total proteins of HaCaT cells were prepared for Western blot analysis. The treated cells were incubated with RIPA buffer on ice for 20 min, then centrifuged at 4°C for 15 min before collection of the supernatant. The protein concentration of the supernatant was determined and 30 ug protein samples were separated by 10% SDS-PAGE gel, then transferred to a PVDF membrane (Life Tech, Grand Island, NY, USA) by semi-dry electrophoretic transfer procedure. The membrane was blocked in 5% dry milk in TBST for 1 h at room temperature, and then incubated overnight at 4°C with primary antibodies against K17 (Abcam, Cambridge, UK), STAT1 (Cell Signaling Technology, Danvers, MA, USA), phospho-STAT1 (Cell Signaling Technology, Danvers, MA, USA), or β-actin (Cell Signaling Technology, Danvers, MA, USA). Subsequently, the membrane was washing in TBST and incubated for 2 h at room temperature with HRP-labeled goat anti-mouse and goat anti-rabbit secondary antibodies (CWBIO, Beijing, China). Visualization of the protein bands was performed using the Pierce ECL Western Blotting Substrate kit (Thermo Scientific, Grand Island, NY, USA).
Transcription factor binding sites analysis
Potential binding sites of transcription factors within the promoter region spanning from −2000 to +20 bp of K17 gene were assessed using MatInspector Professional (MatInspector software version 8.1, Matrix Library 9.1 from the Genomatix suite v3.4). MatInspector is available on-line at http://www.genomatix.de/cgi-bin/matinspector_prof/mat_fam.pl.
Plasmid constructs and dual-luciferase reporter assay
For the construction of the pGL3-k and pGL3-v reporter vectors, full-length K17-promoter sequence and vitamin D receptor loci deleted K17-promoter sequence were synthesized by Sangon Biotech Co. Ltd. (Shanghai, China), then ligated respectively into the pGL3-Basic reporter vector (Promega, Madison, WI, USA). pGL3-K or pGL3-V-mediated transcription was assessed using lipofectamine 3000-based transfection. HaCaT cells were seeded in 48-well plates for 24 h, then transfected with 0.5 ug pGL3-K or pGL3-V, alone with 0.0125 ug Renilla luciferase plasmid as an internal control. The pGL3-basic and pGL3-control plasmids were the negative and positive control, respectively. After 4 h of transfection, cells were changed with fresh medium and treated with IFN-γ and/or calcipotriol (10−7 M). After 24 h, cells were lysed in passive lysis buffer. Firefly and Renilla luciferase activity was measured sequentially using the dual-luciferase reagent assay kit (Promega, Madison, WI, USA) on a luminometer according to the manufacturer’s instructions.
Immunofluorescence staining and confocal microscopy
HaCaT cells were grown on 35-mm glass-bottom dishes, treated with IFN-γ (250U) and/or 100 nM calcipotriol for 24 h based on the experimental design. K17 and Phospho-STAT1 fluorescence intensity was determined as follows. HaCaT cells were washed in phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde for at least 15 min. After being fixed and subsequent washing in PBS, the cells were treated in 0.3% Triton X-100 for 10 min and incubated with anti-human K17 mAb (Abcam, Cambridge, UK) or anti-human phospho-STAT1 Rabbit mAb (Cell Signaling Technology, Danvers, MA, USA) overnight at 4°C, and then with CY3-labeled goat anti-rabbit IgG (KPL, Gaithersburg, MD, USA) or Alexa fluor 488 goat anti-rabbit IgG (Abcam, Cambridge, UK) for 1 h at 37°C and counterstained with DAPI in the dark. Samples were analyzed using a FV-1000/ES confocal microscope (Olympus, Tokyo, Japan).
Statistical analyses
All analyses were performed using GraphPad Prism 7.0 (GraphPad Software, San Diego, CA, USA). For statistical analyses, an unpaired Student’s t-test (two-tailed) was used. Data are expressed as mean ±SEM. P-value <0.05 was considered statistically significant.
Results
Calcipotriol alleviated IFN-γ-induced expression of K17 in HaCaT cells
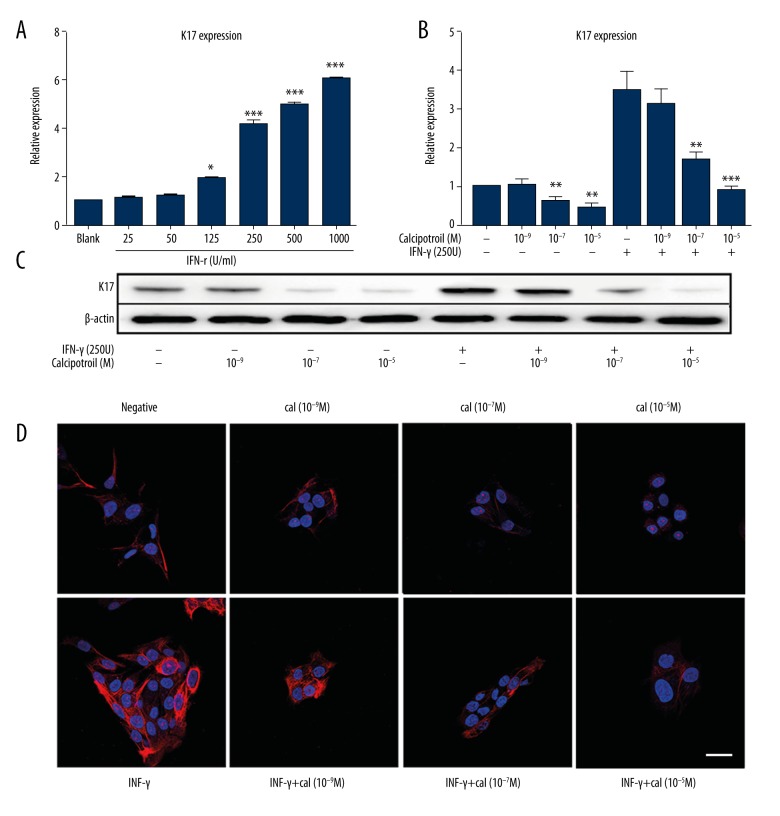
HaCaT cells were incubated in 6-well plates for 24 h to a subconfluent state. Then, 25–1000 U/ml IFN-γ was added into different groups of wells for 48 h, and total RNA of each group was obtained. K17 mRNA levels were determined by q-PCR and all data were normalized to the control group (0 U/ml IFN-γ). As showed in Figure 1A, administration of IFN-γ (125–1000 U) increased K17 expression in a dose-dependent manner, and 250 U/ml IFN-γ elevated K17 expression significantly (P<0.001).
Figure 1.
Calcipotriol reduced IFN-γ-induced K17 expression in HaCaT cells. (A, B) Real-time PCR analysis of K17 mRNA level. Data are expressed as 2−ΔΔCT relative to untreated HaCaT cells. The data presented as means ±S.D. of 3 independent experiments. * P<0.05, *** P<0.001. (C) Protein expression of K17 was examined using Western blot. (D) Immunofluorescence was performed on HaCaT cells to measure K17 expression in different group. Bars, 30 μm.
After identifying 250 U/ml as a suitable concentration of IFN-γ for the experiment, we then determined whether calcipotriol mediates IFN-γ-induced K17 expression. HaCaT cells were pretreated with IFN-γ (250 U) or H2O control for 2 h, followed by an additional treatment with different concentrations of calcipotriol (10−5 M, 10−7 M, 10−9 M) or isopropyl alcohol as solvent control. In addition, HaCaT cells were treated with calcipotriol alone (10−5 M, 10−7 M, 10−9 M) as the drug control. K17 mRNA levels were determined by q-PCR at 24 h after treatment from total RNA extracts. According to the results, treatment with calcipotriol at 10−9 M did not significantly inhibit K17 expression (P>0.05). However, calcipotriol consistently inhibited K17 mRNA levels in a dose-dependent manner as compared with blank control. This result was consistent with a previous study showing that calcipotriol inhibits keratinocytes proliferation. K17 was highly expressed in HaCaT cells after being treated with IFN-γ, which is similar to psoriasis in vivo. Calcipotriol at the concentrations of 10−7 M and 10−5 M suppressed IFN-γ-induced K17 expression by 58.10% and 70.68%, respectively (Figure 1B). To further confirm this finding, HaCaT cells were treated the same as aforementioned, and then were collected in lysis buffer and subjected to Western blot to separate proteins. Similarly, IFN-γ-induced K17 protein levels were decreased by calcipotriol treatment in a dose-dependent manner, but no significant difference was shown when cells were treated with 10−9 M calcipotriol (Figure 1C). Two-color immunofluorescence immunostaining revealed weak K17 staining in the cytoplasm of negative control cells and strong staining of IFN-γ-treated cells. There was a gradual decrease in staining intensity of K17 with the different concentration of calcipotriol stimulation after 48 h (Figure 1D). These data demonstrate that calcipotriol suppressed the IFN-γ induced K17 expression in HaCaT cells in a dose-dependent manner.
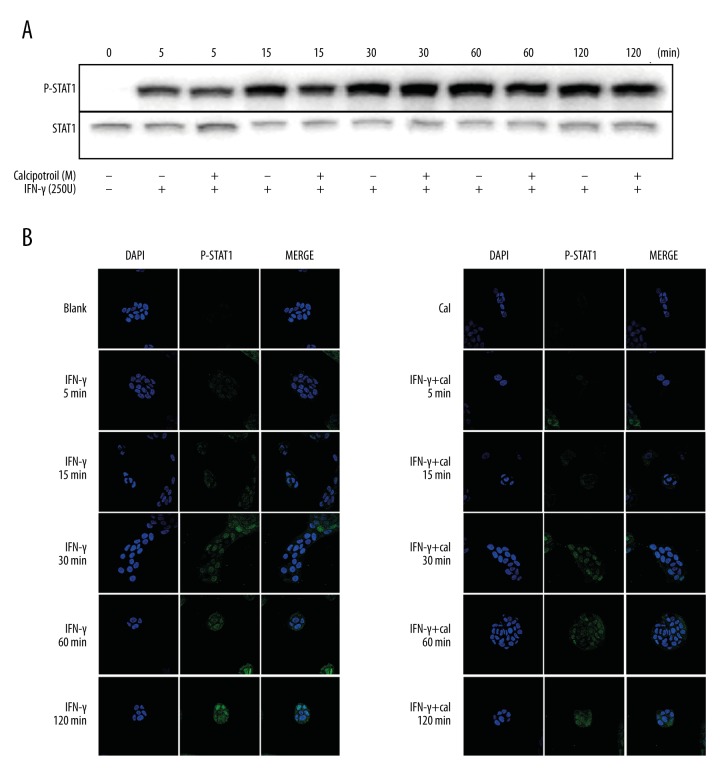
Calcipotriol inhibited IFN-γ-induced K17 expression in a STAT1 signaling-independent manner
IFN-γ primarily signals through the activation of Jak-Stat pathway to affect gene regulation. To determine at which level calcipotriol regulated IFN-γ-induced K17 expression, phosphorylation of STAT1 was observed in IFN-γ-treated HaCaT cells in the presence and absence of calcipotriol. In experimental groups, HaCaT cells were pretreated with IFN-γ (250 U), followed by additional treatment with calcipotriol (10−7 M) for 5 min, 15 min, 30 min, 60 min, and 120 min. The control groups were cells stimulated with IFN-γ (250 U) for the same time as in the experimental group. Western blot analysis showed that STAT1 phosphorylation was increased over time in the experimental and control groups, but no significant difference was indicated at each time point between the experimental group and control group (Figure 2A). This result was further confirmed by immunofluorescence staining. The fluorescence of phosphorylated STAT1 (p-STAT1) under confocal fluorescence microscopy gradually increased from 5 min to 120 min after IFN-γ with or without calcipotriol stimulation. Immunofluorescence staining revealed no substantial change of nuclear fluorescence of p-STAT1 staining between the experimental group and control group (Figure 2B). The above results suggest that calcipotriol does not affect STAT1 activation, IFN-γ-induced STAT1 activation, or activated STAT1 nuclear translocation in HaCaT cells.
Figure 2.
The effect of calcipotriol on STAT1 signaling pathway. HaCaT cells were pretreated with IFN-γ (250 U) for 10 min, followed by an additional treatment with 10−7 M calcipotriol for 5 min, 15 min, 30 min, 60 min and 120 min. Control group was cells stimulated with IFN-γ (250 U) or calcipotriol (10−7 M) for same time duration as the experimental group. (A) Western blot analysis of P-STAT1 expression. (B) Immunofluorescence was performed to measure P-STAT1 expression. DAPI staining for nuclei is blue. Bars, 30 μm.
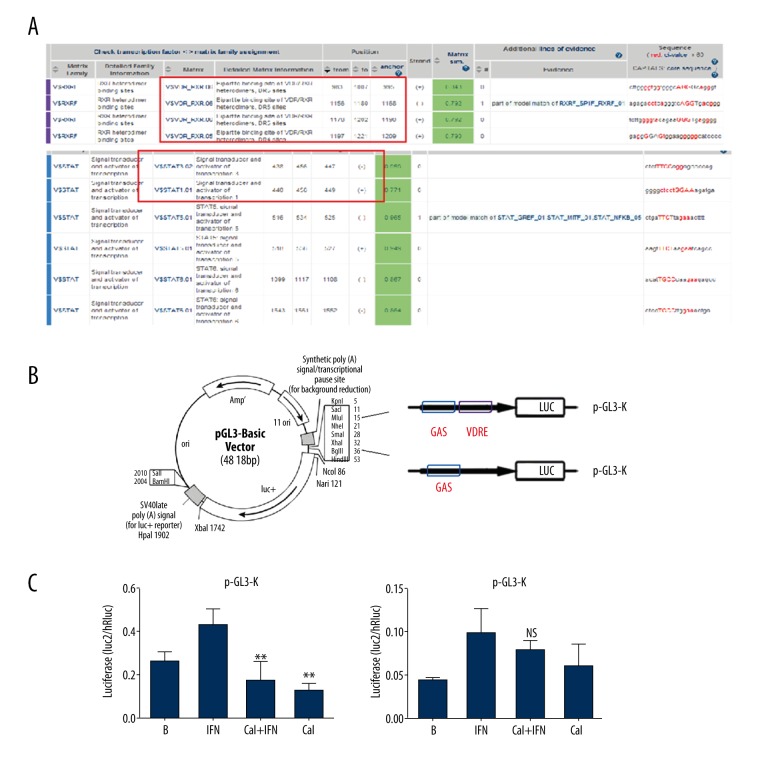
Calcipotriol regulates IFN-γ induced K17 expression via activation of VDRE sites
To identify whether the K17 gene is a direct target of VDRE that depresses IFN-γ-induced K17 expression, a transfection assay was designed. Two reporter vectors containing full-length K17 promoter region (pGL3-K) or a mutant VDRE site (pGL3-V) were constructed and transfected into HaCaT cells, followed by IFN-γ and/or calcipotriol treatment for 24 h. Then, the cells were processed as samples for luciferase activity measurement, following protocols of the dual-luciferase reagent assay kit (Promega). Cells transfected with the pGL3-basic and pGL3-control plasmids were set as the negative and positive controls, respectively. Results showed that the cells transfected with pGL3-K vector upregulated K17 expression as an effect of IFN-γ stimulus, but calcipotriol lowered K17 level significantly despite the presence of IFN-γ. On the other hand, neither IFN-γ nor calcipotriol significantly affected the expression of K17 when HaCaT cells were transfected with the pGL3-V vector containing a mutant VDRE site (Figure 3). Collectively, the data of the transfection assay suggest that VDRE site plays a critical role in regulating IFN-γ induced K17 expression.
Figure 3.
Calcipotriol regulated IFN-g induced K17 expression via activation of VDRE sites. (A) Analysis of VDRE and GAS binding sites within the K17 promoter using MatInspector Professional software. (B) Construction of the luciferase expression plasmid: full length of K17 promoter (pGLE-K) or mutated VDRE site plasmid (pGL3-V). (C) The luciferase activity in pGL3-K or pGL3-V transfected HaCaT cells. ** P<0.005.
Discussion
In this study, we investigated the association between calcipotriol and IFN-γ-mediated K17 expression and demonstrated that IFN-γ-mediated K17 expression was reduced by calcipotriol in keratinocytes in a dose-dependent manner via VDRE effect on GAS function. IFN-γ is a key cytokine in innate and adaptive immunity for viral and intracellular bacterial infections. Psoriasis lesions have long been known to present high levels of IFN-γ, and in human keratinocytes IFN-γ could regulate K17 gene expression by activating the transcription factor STAT1 [10–12]. Research has shown that K17 plays an important role in pathogenesis of psoriasis because it shares similar epitopes with streptococci and promotes the proliferation and cytokines secretion of T cells [5,13]. In psoriatic skin, T cells play a central role in the subsequent production and activation of inflammatory cytokines. These cytokines activate the keratinocytes and promote their proliferation, which shifts expression profiles from K1 and K10 to K6, K16, and K17. Moreover, K17 is the only keratin reported to be induced by IFN-γ [6]. Hence, we conclude that a K17/T-cell/cytokine autoimmune loop is involved in the psoriatic pathogenesis, and IFN-γ is as an important cytokine member.
Calcipotriol ointment has been demonstrated to be a very safe and effective topical treatment for psoriasis. It is a vitamin D3 analogue that binds to VDR to regulate transcription of target genes [8]. 1,25(OH)2D3 is an active form of vitamin D and its receptor VDR is involved in regulating keratinocyte proliferation, promoting differentiation, and modulating both the humoral and cell-induced immune systems in the skin [14]. It has been shown that treatment with vitamin D or analogues in vivo exerts anti-proliferative and pro-differentiating effects in epidermal keratinocytes [15]. Moreover, Vitamin D analogues inhibit the production of cytokines needed for Th1 and Th17 differentiation, and stimulate T cells to produce anti-inflammatory Th2 cytokines [16]. Such effects are thought to be one of mechanism by which vitamin D treatment improves psoriasis. In psoriasis, vitamin D plays an important role due to its ability to modulate innate and adaptive immunity, it is implicated in keratinocyte turnover, and is involved in the integrity of cutaneous barriers [17–19]. At present, the molecular mechanisms by which vitamin D analogues regulate epidermal proliferation and differentiation are not completely understood. In this work, we explored the effect of calcipotriol on K17 expression using q-PCR, Western blot technique, and immunofluorescence as quantitative and qualitative approaches. We revealed that calcipotriol significantly downregulated IFN-γ-induced K17 expression in HaCaT cells (Figure 1). However, as shown in the Figure 1, calcipotriol consistently inhibited K17 expression in a dose-dependent manner as compared with blank control. In this regard, the effect of calcipotriol on K17 expression might be one of mechanisms by which calcipotriol inhibits keratinocytes proliferation in psoriasis.
We also investigated the mechanism by which calcipotriol affects IFN-γ-induced K17 expression. As a vitamin D3 analogue, the genomic effects of calcipotriol are mediated through binding to the VDR. Exogenous vitamin D directly crosses the plasma membrane and interacts with VDR; the vitamin D-VDR complex translocates to the nucleus and modulates expression of VDR gene, where it binds to the VDRE [9]. IFN-γ binds to interferon gamma receptor (IFNAR) at the plasma membrane through janus activated kinase (JAK), and triggers a cascade of tyrosine phosphorylation leading to the activation of STAT1 homodimers known as GAF (Gamma-activated factor). GAF translocates into the nucleus and initiates gene transcription, where it binds to GAS sites [20]. Based upon these signaling pathways, we hypothesize that calcipotriol downregulates K17 expression as a result of repressed STAT1 activation. We first using Western blot and immunofluorescence staining to examine the level of p-STAT1, and we found that STAT1 phosphorylation was increased over time, but there was no difference compared the calcipotriol (10−7 M)-treated or untreated (IFN-γ-pretreated-HaCaT) cells. Moreover, immunofluorescence staining revealed that calcipotriol does not affect the nuclear translocation of p-STAT1. The above results suggest that calcipotriol does not affect IFN-γ-induced STAT1 activation and activated STAT1 transportation to the nucleus in HaCaT cells. To further ascertain whether decreased IFN-γ-induced K17 expression caused by calcipotriol is through transcriptional level, we studied whether calcipotriol can affect GAS-mediated transcription of the K17 promoter, using a luciferase reporter assay. We constructed 2 reporter vectors containing full-length K17 promoter region (pGL3-K), which contains GAS sites and VDRE sites, or the same sequence without VDRE sites (pGL3-V). Calcipotriol downregulated the activity of pGL3-K promoter in HaCaT cells pretreated with IFN-γ, whereas pGL3-V did not respond to calcipotriol, suggesting that calcipotriol decreased IFN-γ-mediated K17 expression through VDRE effect on GAS function (Figure 3).
Conclusions
We found that calcipotriol downregulates IFN-γ-mediated K17 expression in keratinocytes in a dose-dependent manner via VDRE effect on GAS function. Our findings further elucidate the molecular mechanisms underlying vitamin D analogues in regulating epidermal proliferation and differentiation, which suggests that the inhibitory effect of calcipotriol on K17 expression is a potential mechanism and function in the treatment psoriasis.
Footnotes
Conflict of interest
None.
Source of support: This study was financially supported by a grant from the National Natural Science Foundation of China (81430073)
References
- 1.Enamandram M, Kimball AB. Psoriasis epidemiology: The interplay of genes and the environment. J Invest Dermatol. 2013;133:287–89. doi: 10.1038/jid.2012.434. [DOI] [PubMed] [Google Scholar]
- 2.Boehncke WH, Schon MP. Psoriasis. Lancet. 2015;386:983–94. doi: 10.1016/S0140-6736(14)61909-7. [DOI] [PubMed] [Google Scholar]
- 3.Diani M, Altomare G, Reali E. T helper cell subsets in clinical manifestations of psoriasis. J Immunol Res. 2016;2016:7692024. doi: 10.1155/2016/7692024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bockelmann R, Horn T, Gollnick H, Bonnekoh B. Interferon-gamma-dependent in vitro model for the putative keratin 17 autoimmune loop in psoriasis: exploration of pharmaco- and gene-therapeutic effects. Skin Pharmacol Physiol. 2005;18:42–54. doi: 10.1159/000081685. [DOI] [PubMed] [Google Scholar]
- 5.Johnston A, Gudjonsson JE, Sigmundsdottir H, et al. Peripheral blood T cell responses to keratin peptides that share sequences with streptococcal M proteins are largely restricted to skin-homing CD8(+) T cells. Clin Exp Immunol. 2004;138:83–93. doi: 10.1111/j.1365-2249.2004.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin L, Wang G. Keratin 17: A critical player in the pathogenesis of psoriasis. Med Res Rev. 2014;34:438–54. doi: 10.1002/med.21291. [DOI] [PubMed] [Google Scholar]
- 7.Kim GK. The rationale behind topical vitamin d analogs in the treatment of psoriasis: Where does topical calcitriol fit in? J Clin Aesthet Dermatol. 2010;3:46–53. [PMC free article] [PubMed] [Google Scholar]
- 8.Paul C, Stein Gold L, Cambazard F, et al. Calcipotriol plus betamethasone dipropionate aerosol foam provides superior efficacy vs. gel in patients with psoriasis vulgaris: Randomized, controlled PSO-ABLE study. J Eur Acad Dermatol Venereol. 2017;31:119–26. doi: 10.1111/jdv.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haussler MR, Jurutka PW, Mizwicki M, Norman AW. Vitamin D receptor (VDR)-mediated actions of 1alpha,25(OH)(2)vitamin D(3): genomic and non-genomic mechanisms. Best Pract Res Clin Endocrinol Metab. 2011;25:543–59. doi: 10.1016/j.beem.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Bonish B, Jullien D, Dutronc Y, et al. Overexpression of CD1d by keratinocytes in psoriasis and CD1d-dependent IFN-gamma production by NK-T cells. J Immunol. 2000;165:4076–85. doi: 10.4049/jimmunol.165.7.4076. [DOI] [PubMed] [Google Scholar]
- 11.Jiang CK, Flanagan S, Ohtsuki M, et al. Disease-activated transcription factor: allergic reactions in human skin cause nuclear translocation of STAT-91 and induce synthesis of keratin K17. Mol Cell Biol. 1994;14:4759–69. doi: 10.1128/mcb.14.7.4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baliwag J, Barnes DH, Johnston A. Cytokines in psoriasis. Cytokine. 2015;73:342–50. doi: 10.1016/j.cyto.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gudmundsdottir AS, Sigmundsdottir H, Sigurgeirsson B, et al. Is an epitope on keratin 17 a major target for autoreactive T lymphocytes in psoriasis? Clin Exp Immunol. 1999;117:580–86. doi: 10.1046/j.1365-2249.1999.01013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bikle DD. Vitamin D metabolism and function in the skin. Mol Cell Endocrinol. 2011;347:80–89. doi: 10.1016/j.mce.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawatsubashi S. Current topics on Vitamin D. The vitamin D functions in keratinocytes and its therapeutic applications. Clin Calcium. 2015;25:367–71. [PubMed] [Google Scholar]
- 16.van der Aar AM, Sibiryak DS, Bakdash G, et al. Vitamin D3 targets epidermal and dermal dendritic cells for induction of distinct regulatory T cells. J Allergy Clin Immunol. 2011;127:1532–40. doi: 10.1016/j.jaci.2011.01.068. [DOI] [PubMed] [Google Scholar]
- 17.Mattozzi C, Paolino G, Richetta AG, Calvieri S. Psoriasis, vitamin D and the importance of the cutaneous barrier’s integrity: An update. J Dermatol. 2016;43:507–14. doi: 10.1111/1346-8138.13305. [DOI] [PubMed] [Google Scholar]
- 18.Consiglio M, Viano M, Casarin S, et al. Mitochondrial and lipogenic effects of vitamin D on differentiating and proliferating human keratinocytes. Exp Dermatol. 2015;24:748–53. doi: 10.1111/exd.12761. [DOI] [PubMed] [Google Scholar]
- 19.Love JF, Tran-Winkler HJ, Wessels MR. Vitamin D and the human antimicrobial peptide LL-37 enhance group a streptococcus resistance to killing by human cells. MBio. 2012;3(5) doi: 10.1128/mBio.00394-12. pii: e00394-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hervas-Stubbs S, Perez-Gracia JL, Rouzaut A, et al. Direct effects of type I interferons on cells of the immune system. Clin Cancer Res. 2011;17:2619–27. doi: 10.1158/1078-0432.CCR-10-1114. [DOI] [PubMed] [Google Scholar]