Abstract
Background
High expression of the RNA-binding motif protein 3 (RBM3) has previously been described as a favorable clinicopathological factor in several cancers, including ovarian cancer, colorectal cancer, prostate cancer, and breast cancer. The aim of this study was to examine the prognostic implications of RBM3 expression in gastric cancer.
Material/Methods
Immunohistochemical analysis of RBM3 expression from 123 patients showed that upregulated RBM3 was mainly found in intestinal-type (n=78, case=59) cancer compared to diffuse-type (n=15, case=8) and mixed-type (n=30, case=17). There were no significant differences in RBM3 expression in subgroups of clinicopathological parameters. RBM3 expression was strongly associated with p53 but not with Ki-67. Cox univariate analysis revealed that high RBM3 expression was closely associated with prolonged overall survival time (HR 0.504, 95% CI: 0.300–0.845, P=0.009). Multivariate analysis remained supporting these associations when adjusted for age, sex, tumor size, differentiation grade, TNM stage, lymphatic invasion, and Ki-67 and p53 expression (HR 0.541, 95% CI: 0.308–0.952, P=0.033), where Lauren grade was not included. Lauren grade was the only factor with independent prognostic significance in a model adjusted for all factors. These results were confirmed by Kaplan-Meier analysis.
Results
Therefore, together with the upregulated RBM3 expression observed in intestinal-type of Lauren grade, we suggest that upregulation of RBM3 is partially responsible for the favorable overall survival in cases with intestinal Lauren grade, which is demonstrated by the box diagram and Kaplan-Meier analysis. Our results showed that high RBM3 expression in gastric cancer is mainly found in intestinal-type of Lauren grade and is associated with longer overall survival time.
Conclusion
We found that RBM3 is a potential biomarker of good prognosis and deserves further validation.
MeSH Keywords: Intestinal Diseases, Prognosis, RNA-Binding Proteins, Stomach Neoplasms
Background
Worldwide, gastric cancer is one of the most common malignancies in the digestive system [1] and it is the second leading cause of cancer-related death [2]. The diagnosis and treatment of gastric cancer is one of the key points in clinical medicine research [3]. Because the clinical features of gastric cancer are often atypical and unclear, doctors are often confused and misled in the diagnostic process [4,5]. Currently, the Japanese gastric cancer protocol recommends D2 radical surgery as a standard surgical resection of gastric cancer, which is widely recognized by more and more international experts and scholars [5–7]. However, for most patients with gastric cancer, even when D2 radical surgery achieves R0 resection, it cannot effectively control the recurrence and metastasis of gastric cancer, and the 5-year survival rate is only 30% [8,9].
Studies have shown that if gastric cancer can be found in the early stages, the survival rate of patients with gastric cancer can be significantly improved [8,10]. There are many factors that affect the prognosis of gastric cancer, involving many clinical and pathological factors, such as tumor staging, histological differentiation, tumor type, depth and extent of invasion, and with or without metastasis and tumor resection [11–13]. In addition, research shows that tumor markers are inseparable from tumor occurrence and development, and tumor markers exist in the tumor, or are produced by the tumor, including ki-67, p53 [13].
RNA-binding protein (RBP) plays an important role in the regulation of post-transcriptional gene expression, and it regulates cell function by binding to RNA [14]. RBM3 protein is a member of the RNA-binding protein family and is a glycine-rich RNA-binding protein [14,15]. RNA-binding motif protein 3 is an important cold shock protein, and environmental stimuli such as hypothermia, ischemia, and hypoxia can increase its expression [15]. In several types of cancer cells, upregulated RBM3 expression promotes cell differentiation and prevents apoptosis [16–20].
In addition, RBM3 is considered to be a potential proto-oncogene, for 3 reasons: (1) RBM3 expression level is closely correlated with tumor staging, indicating that it may be related to the occurrence of tumors [21]; (2) RBM3 silence can increase the number of cells in G2/M phase and eventually lead to apoptosis [21,22]; and (3) RBM3 can improve the stability and translation of mRNA of cyclooxygenase-2, IL-8, and vascular endothelial growth factor [23,24]. It is well known that changes in the level of RBM3 mRNA play an important role in cell transformation and tumorigenesis [25]. RBM3 binds directly to mRNA, altering the secondary structure of mRNA and affecting the access of mRNA initiation factor to the ribosome subunit [26], which modulates the potential activity of kinases in tumors. Recently studies showed that high expression of RBM3 is found in various types of human malignancies. RBM3 has also been demonstrated to be an independent factor of good prognosis in several cancers, including ovarian, prostate, breast, bladder, and colorectal cancer [16–20].
To the best of our knowledge, the role of RBM3 in gastric cancer has not yet been reported. In the present study, we investigated the prognostic significance of RBM3 expression in patients with gastric cancer and the expression levels of RBM3 in cancer cells.
Material and Methods
Patients
Paraffin-embedded specimens were obtained from 123 patients with gastric cancer who underwent surgery in the hospital from 16 Jan 2013 to 12 Feb 2014, including 95 males and 28 females, aged 30–85 years, with an average age of 61.0±13.0 years and a median age of 62. None of the patients had received preoperative chemotherapy and none had autoimmune disease. The follow-up period ended on 25 Feb 2017. Survival was defined as the interval between the date of surgery and the date of death or the last follow-up. Staging of gastric cancer tissue was conducted according to the seventh edition of the TNM staging standard jointly revised by the American Joint Committee on Cancer (AJCC) and Union for International Cancel Control (UICC) in 2010. The clinical data of the patients were complete and all patients were followed up. Follow-up data were missing for 3 patients.
Immunohistochemistry and staining evaluation
Immunohistochemical SP method was used. Briefly, 4-μm paraffin-embedded tissue continuous sections were dewaxed with xylene and hydrated with ethanol gradient. Endogenous peroxidase was eliminated with 3% peroxides and repaired with EDTA. The sections were blocked with normal sheep serum at room temperature. Subsequently, the sections were stained with mouse monoclonal anti-RBM3 (ab134946) (Abcam), anti-Ki-67, and p53 (Santa Cruz Biotechnology) antibodies overnight at 4°C. After being washed with PBS, secondary antibody was added, followed by incubation for 0.5 h. Finally, DAB was used for coloring, with hematoxylin as a contrasting stain, and sealed with a neutral gum. Two immunohistochemically-stained slices for each sample was evaluated independently by 1 pathology specialist. Five images were observed in each section, and 100 cells were counted for each field of view. The results of RBM3 expression were recorded according to the following staining scale of tumor cells. (−): no staining or <1% nuclear staining; (+): 1% to 10% of the nucleus staining, and weaker cytoplasmic staining; (++): 10% to <50% of the nucleus staining, and strong cytoplasmic staining; (+++): ≥50% of the nucleus staining, and strong cytoplasmic staining. For statistical analysis, (−) and (+) were regarded as negative expression, and (++)~(+++) were regarded as positive expression. For Ki-67 and p53 staining, the fraction of staining was routinely recorded as follows: 0–10% (−), 10%–25% (+), 26–50% (++), 51–75% (+++), >75% (++++). For statistical analysis, (−) and (+) were regarded as negative expression, and (++)~(+++) were regarded as positive expression.
RBM3 siRNA assay
RBM3 siRNA and negative siRNA control (Applied Biosystems, USA) was performed with Lipofectamine 2000 (Invitrogen). Briefly, SGC-7901 and MGC-803 cells were seeded in 6-well plates. After growing to 50% confluence, cells were transfected using Lipofectamine 2000 with a final concentration of 50 nM siRNA. The transfected cells were then incubated for 5 h at 37°C.
Western blot assay
The extract protein samples from cancer gastric cell lines and from cancer and normal tissues of patients with gastric cancer were separated by 10% SDS-PAGE gels and transferred to PVDF membranes. Membranes were then blocked with 5% non-fat dry milk at room temperature for 1 h. Subsequently, the membranes were incubated with primary antibodies (anti-RBM3 (ab134946) (1: 500, Abcam), anti-GAPDH (1: 5000; Santa Cruz Biotechnology) at 4°C overnight. After washing 3 times with TBS, the blots were incubated with HRP-conjugated secondary antibodies (1: 2000 dilution, DAKO) for 1 h. The protein-antibody complexes were visualized using an enhanced chemiluminescence system (ECL) (Amersham Pharmacia Biotech).
Results
Correlations of RBM3 expression in gastric cancer tissues with clinicopathological parameters
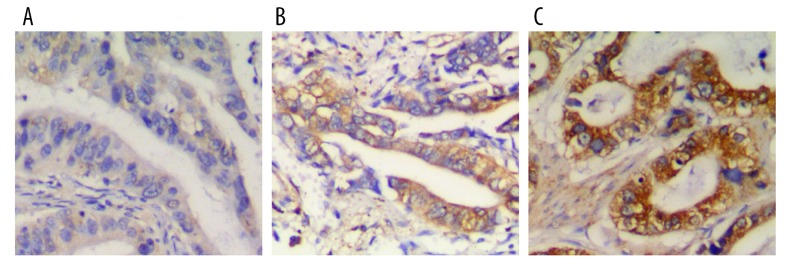
RBM3 expression in gastric cancer tissues was evaluated in 123 patients with gastric cancer. The representative immunohistochemical images for RBM3 are shown in Figure 1 and all data are displayed in Table 1.
Figure 1.
RBM3 expression in gastric cancer: Representative immunohistochemical images of RBM3 expression were evaluated as weak (A), moderate (B), and strong (C) staining.
Table 1.
Associations between RBM3 expression and clinicopathological parameters.
| Clinicopathological parameters | N (positive rate) | RBM3 expression | P-value | |
|---|---|---|---|---|
|
| ||||
| Positive (n=84) | Negative (n=39) | |||
| Age | 0.700 | |||
| < Average | 54 (70.3%) | 38 | 16 | |
| ≥ Average | 69 (66.7%) | 46 | 23 | |
|
| ||||
| Gender | 0.818 | |||
| Female | 28 (71.4%) | 20 | 8 | |
| Male | 95 (67.3%) | 64 | 31 | |
|
| ||||
| Tumor size (cm) | 0.179 | |||
| <5 | 65 (73.8%) | 48 | 17 | |
| ≥5 | 58 (62.1%) | 36 | 22 | |
|
| ||||
| Differentiation grade | 0.161 | |||
| High-moderate | 39 (76.9%) | 30 | 9 | |
| Low | 84 (64.3%) | 54 | 30 | |
|
| ||||
| TNM stage | 0.424 | |||
| I+II | 45 (77.3%) | 33 | 12 | |
| III+IV | 78 (65.4%) | 51 | 27 | |
|
| ||||
| Lymphatic invasion | 0.217 | |||
| No | 38 (76.3%) | 29 | 9 | |
| Yes | 85 (64.7%) | 55 | 30 | |
|
| ||||
| Lauren grade | 0.068 | |||
| Intestinal | 78 (75.6%) | 59 | 19 | |
| Diffuse | 15 (53.3%) | 8 | 7 | |
| Mixed | 30 (56.7%) | 17 | 13 | |
|
| ||||
| Ki-67 expression | 0.436 | |||
| Negative | 51 (72.5%) | 37 | 14 | |
| Positive | 72 (80.7%) | 47 | 25 | |
|
| ||||
| p53 expression | 0.007 | |||
| Negative | 57 (80.7%) | 46 | 11 | |
| Positive | 66 (58.5%) | 38 | 28 | |
As shown in Table 1, among 123 specimens of gastric cancer tissue, 84 (68.3%) samples were stained with positive expression of RBM3 and 39 samples had negative staining of RBM3. The RBM3 expression did not differ by age, sex, or TNM stage. There were no significant differences in RBM3 expression among subgroups by tumor size, differentiation grade, lymphatic invasion, or Lauren grade, whereas RBM3 expression was obviously higher in tumor tissues with sizes less than 5 cm (P=0.179), with high-moderate differentiation grade (P=0.161), with lymphatic invasion (P=0.217), and with intestinal Lauren grade (P=0.068).
Correlations of RBM3 expression with Ki-67 and p53 expression in gastric cancer tissues
There was no obvious correlation between RBM3 and Ki-67 expression, indicating RBM3 expression has no association with proliferation. However, RBM3 expression was significantly and negatively associated with p53 expression. High expression of RBM3 was observed in gastric cancer tissues with negative p53 expression compared with positive p53 expression (P=0.007).
Associations of RBM3 expression with overall survival
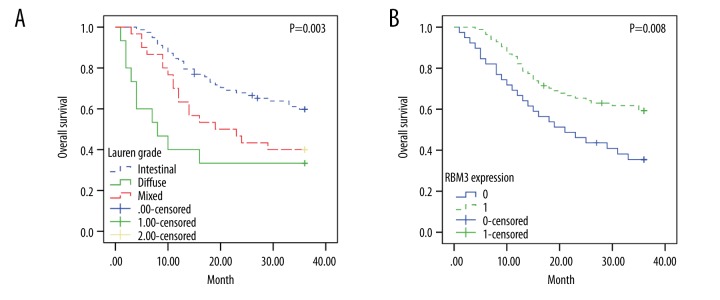
Cox univariate analysis suggested that down-regulated RBM3 expression was associated with reduced overall survival time (HR 0.504, 95% CI: 0.300–0.845, P = 0.009) (Table 2). Figure 2A confirmed that high expression of RBM3 is closely correlated with prolonged overall survival time. However, there was no significant in multivariate analysis with adjusting for age, gender, tumor size, differentiation grade, TNM stage, lymphatic invasion, Lauren grade, Ki-67 and p53 expression. But if Lauren grade was not included in those adjustment factors, the negative association between RBM3 expression with overall survival time remained significant in multivariate analysis (HR 0.541, 95% CI: 0.308–0.952, P=0.033) (data not shown). Lauren grade was a factor of independent prognostic significance both in univariate (Table 2) and multivariate analysis (at level of diffuse) (HR 2.463, 95% CI: 1.172–5.174, P=0.017), which was demonstrated by significant difference in overall survival showed in Figures 2B and 3A.
Table 2.
Cox univariate and multivariate analysis of overall survival according to clinicopathological parameters, ki-67, p53 and RBM3 expression.
| Clinicopathological parameters | Overall survival | |||
|---|---|---|---|---|
|
| ||||
| Unadjusted HR (95%CI) | P-value | Adjusted HR (95%CI) | P-value | |
| Age | 0.456 | 0.511 | ||
| < Average | 1 | 1 | ||
| ≥ Average | 0.823 (0.494–1.372) | 0.834 (0.486–1.432) | ||
|
| ||||
| Gender | 0.214 | 0.120 | ||
| Female | 1 | 1 | ||
| Male | 0.689 (0.383–1.240) | 0.593 (0.307–1.147) | ||
|
| ||||
| Tumor size (cm) | 0.002 | 0.406 | ||
| <5 | 1 | 1 | ||
| ≥5 | 2.292 (1.349–3.896) | 1.294 (0.704–2.379) | ||
|
| ||||
| Differentiation grade | 0.016 | 0.574 | ||
| High-moderate | 1 | 1 | ||
| Low | 2.248 (1.166–4.335) | 1.254 (0.570–2.755) | ||
|
| ||||
| TNM stage | <0.001 | 0.089 | ||
| I+II | 1 | 1 | ||
| III+IV | 4.990 (2.446–10.182) | 2.368 (0.877–6.396) | ||
|
| ||||
| Lymphatic invasion | <0.001 | 0.086 | ||
| No | 1 | 1 | ||
| Yes | 4.943 (2.239–10.912) | 2.326 (0.888) | ||
|
| ||||
| Lauren grade | ||||
| Intestinal | 1 | 0.002 | 1 | 0.017 |
| Diffuse | 3.074 (1.505–6.281) | 0.039 | 2.463 (1.172–5.174) | 0.101 |
| Mixed | 1.847 (1.032–3.304) | 1.728 (0.899–3.321) | ||
|
| ||||
| Ki-67 expression | 0.001 | 0.612 | ||
| Negative | 1 | 1 | ||
| Positive | 2.623 (1.457–4.721) | 1.197 (0.598–2.397) | ||
|
| ||||
| p53 expression | 0.001 | 0.369 | ||
| Negative | 1 | 1 | ||
| Positive | 2.631 (1.520–4.554) | 1.336 (0.710–2.516) | ||
|
| ||||
| RBM3 expression | 0.009 | 0.130 | ||
| Negative | 1 | 1 | ||
| Positive | 0.504 (0.300–0.845) | 0.632 (0.349–1.144) | ||
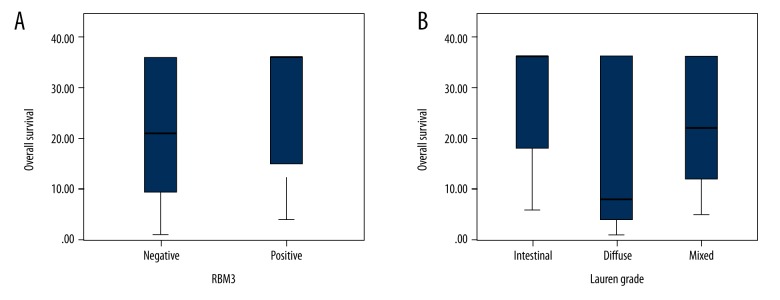
Figure 2.
Overall survival distributions according to RBM3 expression and Lauren grade: Box diagram visualizing the distribution of overall survival in different levels of RBM3 expression (A) and Lauren grade (B).
Figure 3.
Prognostic value of Lauren grade and RBM3 expression in gastric cancer: Kaplan-Meier estimates of overall survival according to Lauren grade (A) and RBM3 expression (B).
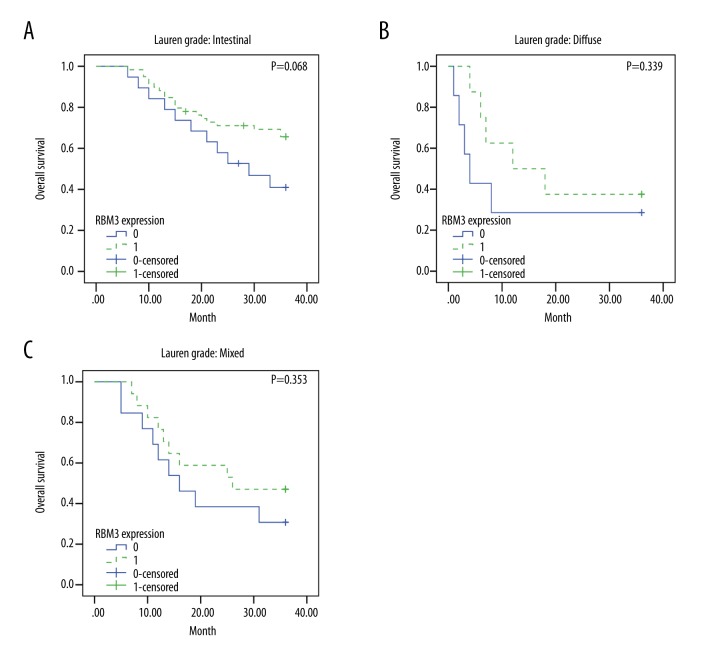
The association between RBM3 expression and overall survival time were confirmed by Kaplan-Meier analysis (Figure 3B). The overall survival time was longer in the group with positive RBM3 expression compared to that with negative RBM3 expression (P=0.008). Figure 4A–4C shows the associations of RBM3 expression with overall survival at different levels (intestinal, diffuse, and mixed) of Lauren grade. The overall survival was higher in the group with positive RBM3 expression compared to negative RBM3 expression at all levels of Lauren grade. However, this association was more significant in intestinal cancer (intestinal: P=0.068 vs. diffuse: P=0.339 or mixed: P=0.353).
Figure 4.
Prognostic value of RBM3 expression at different levels of Lauren grade: Overall survival is estimated by Kaplan-Meier according to RBM3 expression at intestinal (A), diffuse (B), and mixed (C) levels of Lauren grade.
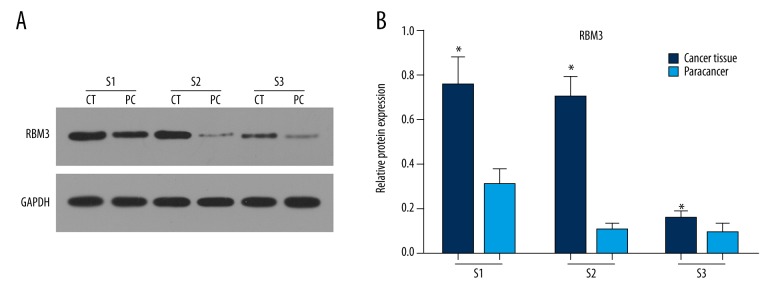
The expression level of RBM3 was higher in gastric cancer tissues than in paracancerous normal tissues.
To further confirm the prognostic significance of RBM3, Western blot assay was conducted to determine RBM3 expression in gastric cancer tissue and corresponding paracancerous normal tissue. As showed in Figure 5A, 5B, RBM3 expression was obviously higher in sample 1 (S 1) and sample 2 (S 2) than in sample 3 (S 3). However, in all samples, upregulated RBM3 expression was observed in gastric cancer tissues compared to that in paracancerous normal tissues.
Figure 5.
The expression levels of RBM3 protein in 3 gastric cancer tissues and corresponding paracancerous normal tissues: Western blot assay showed that RBM3 expression was higher in cancer tissues (CT) than in paracancerous normal tissues (PT) (A, B).
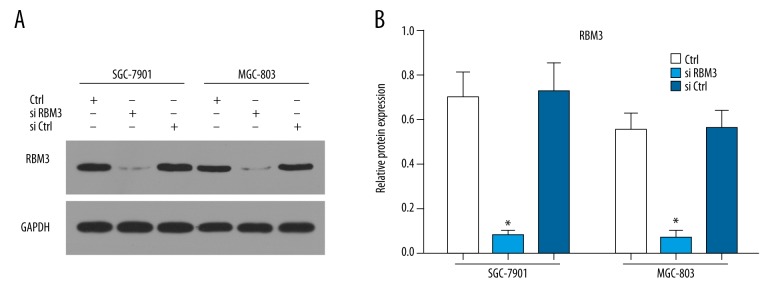
Specificity of the RBM3 antibody was confirmed by silencing RBM3 with siRNA
To test the specificity of the RBM3 antibody, we silenced the expression of RBM 3 by the use of RBM3 siRNA in 2 gastric cancer cell lines: SGC-7901 and MGC-803. Figure 6A, 6B shows that the expression levels of RBM3 protein determined by Western blot were significantly reduced both in SGC-7901 and MGC-803 in the group treated with RBM3 siRNA, indicating the good specificity of RBM3 antibody used in this study.
Figure 6.
Silencing RBM3 with siRNA showed reduced expression of RBM3: RBM3 expression was significantly reduced after transfection with siRNA (siRBM3) compared to control and negative transfection control (siCtrl) (A, B).
Discussion
Our results demonstrated that patents with high RBM3 expression in gastric cancer tissues had a prolonged overall survival time. There was lower RBM3 expression in normal tissues compared with the tumor tissues. The strong correlation between RBM3 and overall survival was closely associated with growth patterns. Consistent with studies of other forms of cancer, our results suggest that RBM3 is a useful biomarker for good prognosis [27–30]. However, the underlying mechanisms that support these findings remain unknown. Furthermore, the prognostic significance of RBM3 has not yet been reported.
Thus, we investigated a consecutive cohort in which none of the patients had received chemotherapy or other treatment before surgery. We found that RBM3 expression was higher in gastric cancer tissues than in normal tissues. In accordance with our results, increasing evidence shows that the expression of RBM3 protein is associated with the development of breast cancer, ovarian cancer, and prostate cancer, and malignant and pigmentary tumors [16–20[. The expression of RBM3 protein and mRNA, as evaluated by immunohistochemical method, cell culture, and PCR, confirmed that RBM3 protein is not expressed or rarely expressed in normal tissues, and the expression of RBM3 protein was higher in tumors than in normal tissue [16–20]. However, many studies have shown that up-regulation of RBM3 protein expression can improve the survival rate of certain tumors, such as breast cancer, ovarian cancer, melanoma, and colon cancer [16–20,27–30], which is related to the fact that its expression is involved in DNA repair, protecting DNA integrity [22,25], although the mechanism is not entirely clear. The lack of high RBM3 expression and DNA integrity increases the sensitivity of tumor cells to cisplatin and improves the prognosis [27].
Our results show that, although there were no significant associations between RBM3 expression and clinicopathological parameters, high RBM3 expression was clearly associated with larger size, higher differentiation grades, and less lymphatic invasion of tumors. In particular, there was a strong correlation between RBM3 expression and Lauren grade. A recent study showed that RBM3 expression was upregulated in upper gastrointestinal cancers, especially in cases with a background of intestinal metaplasia [31]. Thus, we hypothesize that RBM3 level is higher in cases with intestinal Lauren grade. Our results demonstrate that more positive expression numbers of RBM3 were observed in intestinal cases compared to diffuse or mixed cases.
The association of RBM3 expression and proliferation assessed by Ki-67 expression was weak, while a significant negative correlation between RBM3 and tumor biomarker p53 was observed, indicating that high RBM3 expression is a favorable prognostic factor. We also found that Ki-67 and p53 were prognostic factors, but were not independent from other clinicopathological parameters in this study, which is consistent with other reports [13,31–33]. However, our study revealed that RBM3 is independent of prognostic factors, excluding Lauren grade. In line with our results, RBM3 is a favorable and independent prognostic factor in several cancer forms, including breast cancer, ovarian cancer, prostate cancer, and colorectal cancer [16–20,27–30,34]. Thus, these results suggest that RBM3 is a better and independent prognostic factor compared to Ki-67 and p53.
Further Cox analysis revealed that upregulated RBM3 expression was closely associated with prolonged overall survival time in univariate analysis, and in multivariate analysis if adjustment factors did not include Lauren grade. Interestingly, Lauren grade was the only factor with independent prognostic significance. These results were confirmed by Kaplan-Meier analysis. Thus, considering the high RBM3 expression in intestinal cases, we presume that upregulated RBM3 expression partially contributed to the favorable prognosis in cases with intestinal Lauren grade. The box diagram and Kaplan-Meier analysis demonstrate these suggestions.
Conclusions
Our study demonstrates that upregulated RBM3 expression is an independent factor of favorable prognosis in patients with gastric cancer. In addition, high RBM3 expression was more frequently observed in intestinal-type carcinoma compared to diffuse-type and mixed-type. High RBM3 expression has a significant association with overall survival, suggesting that RBM3 has important prognostic significance. Since RBM3 is a potential prognostic biomarker, these findings should be pursued in future studies in various cancer forms.
Footnotes
Conflict of interest
We declare that we have no financial or personal relationships with other people or organization that could inappropriately influence our work. There is no professional or other personal interest of any nature or kind in any product, service, and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled, “High RBM3 expression is independently associated with a prolonged overall survival in intestinal-type gastric cancer”.
Source of support: This work was supported by Wenzhou Science and Technology Bureau (Grant No. Y20150165)
References
- 1.Katoh M. Epithelial-mesenchymal transition in gastric cancer (Review) Int J Oncol. 2005;27(6):1677–83. [PubMed] [Google Scholar]
- 2.Takahashi T, Saikawa Y, Kitagawa Y. Gastric cancer: Current status of diagnosis and treatment. Cancers. 2013;5(1):48–63. doi: 10.3390/cancers5010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seto Y, Shimoyama S, Kitayama J, et al. Lymph node metastasis and preoperative diagnosis of depth of invasion in early gastric cancer. Gastric Cancer. 2001;4(1):34. doi: 10.1007/s101200100014. [DOI] [PubMed] [Google Scholar]
- 4.Kong Y, Zheng Y, Jia Y, et al. Decreased LIPF expression is correlated with DGKA and predicts poor outcome of gastric cancer. Oncol Rep. 2016;36(4):1852–60. doi: 10.3892/or.2016.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lv J, Lv CQ, Wang BL, et al. Membrane glycolipids content variety in gastrointestinal tumors and transplantable hepatomas in mice. Med Sci Monit Basic Res. 2016;22:87–90. doi: 10.12659/MSMBR.899635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmid A, Thybusch A, Kremer B, Hennebruns D. Differential effects of radical D2-lymphadenectomy and splenectomy in surgically treated gastric cancer patients. Hepato-gastroenterology. 2000;47(32):579–85. [PubMed] [Google Scholar]
- 7.Pan D, Chen H, Li L, Li Z. Effect of splenectomy combined with resection for gastric carcinoma on patient prognosis. Med Sci Monit. 2016;22:4205–9. doi: 10.12659/MSM.897842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Ma L, Zhang XM, Zhou ZX. Long-term outcomes after D2 gastrectomy for early gastric cancer: Survival analysis of a single-center experience in China. Asian Pac J Cancer Prev. 2014;15(17):7219–22. doi: 10.7314/apjcp.2014.15.17.7219. [DOI] [PubMed] [Google Scholar]
- 9.Cui X, Huang Q, Li X, et al. Relationship between interleukin-10 gene C-819T polymorphism and gastric cancer risk: Insights from a meta-analysis. Med Sci Monit. 2016;22:2839–45. doi: 10.12659/MSM.895333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng J, Liang H, Sun D, et al. Prognosis of gastric cancer patients with node-negative metastasis following curative resection: Outcomes of the survival and recurrence. Can J Gastroenterol. 2008;22(10):835–39. doi: 10.1155/2008/761821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X, Cai H, Sheng W, Wang Y. Long-term results and prognostic factors of gastric cancer patients with microscopic peritoneal carcinomatosis. PloS One. 2012;7(5):e37284. doi: 10.1371/journal.pone.0037284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto S, Tomita Y, Hoshida Y, et al. Expression of hepatoma-derived growth factor is correlated with lymph node metastasis and prognosis of gastric carcinoma. Clin Cancer Res. 2006;12(1):117–22. doi: 10.1158/1078-0432.CCR-05-1347. [DOI] [PubMed] [Google Scholar]
- 13.Joo YE, Chung IJ, Park YK, et al. Expression of cyclooxygenase-2, p53 and Ki-67 in gastric cancer. J Korean Med Sci. 2006;21(5):871–76. doi: 10.3346/jkms.2006.21.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kishore S, Luber S, Zavolan M. Deciphering the role of RNA-binding proteins in the post-transcriptional control of gene expression. Brief Funct Genomics. 2010;9(5–6):391–404. doi: 10.1093/bfgp/elq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang H, Rao JN, Wang JY. Posttranscriptional regulation of intestinal epithelial tight junction barrier by RNA-binding proteins and microRNAs. Tissue Barriers. 2014;2(1):e28320. doi: 10.4161/tisb.28320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jang HH, Lee HN, Kim SY, et al. Expression of RNA-binding motif protein 3 (RBM3) and cold-inducible RNA-binding protein (CIRP) is associated with improved clinical outcome in patients with colon cancer. Anticancer Res. 2017;37(4):1779–85. doi: 10.21873/anticanres.11511. [DOI] [PubMed] [Google Scholar]
- 17.Grupp K, Wilking J, Prien K, et al. High RNA-binding motif protein 3 expression is an independent prognostic marker in operated prostate cancer and tightly linked to ERG activation and PTEN deletions. Eur J Cancer. 2014;50(4):852–61. doi: 10.1016/j.ejca.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Melling N, Simon R, Mirlacher M, et al. Loss of RNA-binding motif protein 3 expression is associated with right-sided localization and poor prognosis in colorectal cancer. Histopathology. 2016;68(2):191–98. doi: 10.1111/his.12726. [DOI] [PubMed] [Google Scholar]
- 19.Boman K. Decreased expression of RNA-binding motif protein 3 correlates with tumour progression and poor prognosis in urothelial bladder cancer. BMC Urol. 2013;13(1):17. doi: 10.1186/1471-2490-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jögi A, Brennan DJ, Rydén L, et al. Nuclear expression of the RNA-binding protein RBM3 is associated with an improved clinical outcome in breast cancer. Mod Pathol. 2009;22(12):1564–74. doi: 10.1038/modpathol.2009.124. [DOI] [PubMed] [Google Scholar]
- 21.Jonsson L, Gaber A, Ulmert D, et al. High RBM3 expression in prostate cancer independently predicts a reduced risk of biochemical recurrence and disease progression. Diagn Pathol. 2011;6:91. doi: 10.1186/1746-1596-6-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehlén Å, Nodin B, Rexhepaj E, et al. RBM3-regulated genes promote DNA integrity and affect clinical outcome in epithelial ovarian cancer. Transl Oncol. 2011;4(4):212–21. doi: 10.1593/tlo.11106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartkova J, Rajpert-De ME, Skakkebaek NE, et al. DNA damage response in human testes and testicular germ cell tumours: biology and implications for therapy. Int J Androl. 2007;30(4):282–91. doi: 10.1111/j.1365-2605.2007.00772.x. [DOI] [PubMed] [Google Scholar]
- 24.Sureban SM, Ramalingam S, Natarajan G, et al. Translation regulatory factor RBM3 is a proto-oncogene that prevents mitotic catastrophe. Oncogene. 2008;27(33):4544–56. doi: 10.1038/onc.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wellmann S, Bührer C, Moderegger E, et al. Oxygen-regulated expression of the RNA-binding proteins RBM3 and CIRP by a HIF-1-independent mechanism. J Cell Sci. 2004;117(Pt 9):1785–94. doi: 10.1242/jcs.01026. [DOI] [PubMed] [Google Scholar]
- 26.Chappell SA, Mauro VP. The internal ribosome entry site (IRES) contained within the RNA-binding motif protein 3 (Rbm3) mRNA is composed of functionally distinct elements. J Biol Chem. 2003;278(36):33793–800. doi: 10.1074/jbc.M303495200. [DOI] [PubMed] [Google Scholar]
- 27.Ehlén Å, Brennan DJ, Nodin B, et al. Expression of the RNA-binding protein RBM3 is associated with a favourable prognosis and cisplatin sensitivity in epithelial ovarian cancer. J Transl Med. 2010;8(1):78. doi: 10.1186/1479-5876-8-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jonsson L, Bergman J, Nodin B, et al. Low RBM3 protein expression correlates with tumour progression and poor prognosis in malignant melanoma: An analysis of 215 cases from the Malmö Diet and Cancer Study. J Transl Med. 2011;9(1):114. doi: 10.1186/1479-5876-9-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hjelm B, Brennan DJ, Zendehrokh N, et al. High nuclear RBM3 expression is associated with an improved prognosis in colorectal cancer. Proteomics Clin Appl. 2011;5(11–12):624–35. doi: 10.1002/prca.201100020. [DOI] [PubMed] [Google Scholar]
- 30.Olofsson S, Nodin B, Gaber A, et al. 1587P low RBM3 protein expression correlates with clinical stage, prognostic index and increased risk of treatment failure in testicular non-seminomatous germ cell cancer. Plos One. 2015;10(3):e0121300. doi: 10.1371/journal.pone.0121300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jonsson L, Hedner C, Gaber A, et al. High expression of RNA-binding motif protein 3 in esophageal and gastric adenocarcinoma correlates with intestinal metaplasia-associated tumours and independently predicts a reduced risk of recurrence and death. Biomark Res. 2014;2:11. doi: 10.1186/2050-7771-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oshima CT, Iriya K, Forones NM. Ki-67 as a prognostic marker in colorectal cancer but not in gastric cancer. Neoplasma. 2005;52(5):420–24. [PubMed] [Google Scholar]
- 33.Sayar I, Akbas EM, Isik A, et al. Relationship among mismatch repair deficiency, CDX2 loss, p53 and E-cadherin in colon carcinoma and suitability of using a double panel of mismatch repair proteins by immunohistochemistry. Pol J Pathol. 2015;66(3):246–53. doi: 10.5114/pjp.2015.54958. [DOI] [PubMed] [Google Scholar]
- 34.Isik A, Soyturk M, Süleyman S, et al. Correlation of bowel wall thickening seen using computerized tomography with colonoscopies: A preliminary study. Surg Laparosc Endosc Percutan Tech. 2017;27(3):154–57. doi: 10.1097/SLE.0000000000000389. [DOI] [PubMed] [Google Scholar]