Abstract
Background
Streptococcus pneumoniae, the leading pathogen of bacterial infections in infants and the elderly, is responsible for pneumococcal diseases with severe morbidity and mortality. Emergence of drug-resistant strains presented new challenges for treatment and prevention. Vaccination has proven to be an effective means of preventing pneumococcal infection worldwide. Detailed epidemiological information of antibiotic susceptibilities and serotype distribution will be of great help to the management of pneumococcal infections.
Methods
A total of 881 S. pneumoniae isolates were collected from patients at 23 teaching hospitals in 17 different cities from 2011 to 2016. The main specimen types included sputum, blood, broncho-alveolar lavage fluid, pharyngeal swabs, and cerebrospinal fluid. Minimum inhibitory concentrations (MICs) were determined using the agar dilution method. Capsular serotypes were identified using latex agglutination and quellung reaction test. Molecular epidemiology was investigated using multilocus sequence typing.
Results
S. pneumoniae isolates were highly resistant to macrolides, tetracycline, and trimethoprim/sulfamethoxazole. The rate of resistance to penicillin was 51.6% (oral breakpoint). However, levofloxacin and moxifloxacin maintained excellent antimicrobial activity and all of the isolated strains were susceptible to vancomycin.
Twenty-two serotypes were identified among the 881 isolates. Prevalent serotypes were 19F (25.7%), 19A (14.0%), 15 (6.8%), 6B (3.6%), 6A (3.0%), and 17 (2.8%). The overall vaccine coverage rates for 7- and 13-valent pneumococcal conjugate vaccines were 37.5% and 58.3%, respectively. Vaccine coverage rates in young children and economically underdeveloped regions were higher than those in older adults and developed regions. Vaccine-covered serotypes demonstrated higher resistance compared with uncovered serotypes.
Molecular epidemiological typing demonstrated that S. pneumoniae showed significant clonal dissemination and that ST271 (120, 28.3%), ST320 (73, 17.2%) and ST81 (27, 6.6%) were the major STs.
Conclusions
High resistance to clinical routine antibiotics was observed for all 881 S. pneumoniae strains. Drug resistance varied among different serotypes and age groups. Prevalent serotypes among the isolates were 19F, 19A, 15, 6B, 6A, and 17. Community-acquired strains should also be included in future studies to gain a better understanding of the prevalence and resistance of S. pneumoniae in China.
Electronic supplementary material
The online version of this article (doi: 10.1186/s12879-017-2880-0) contains supplementary material, which is available to authorized users.
Keywords: Streptococcus pneumoniae, Serotyping, Antimicrobial resistance, Multilocus sequence typing
Background
Streptococcus pneumoniae is a major cause of morbidity and mortality in infants, children, and older adults (≥ 65 years), causing pneumonia and invasive pneumococcal diseases (IPDs) worldwide [1–3]. The disease burden is rapidly worsening in many countries due to the increasing number of people affected by chronic diseases and the increasing disease risks in older age groups [4, 5]. Although IPD is the most severe form with the highest case fatality rate, non-bacteremic pneumonia is the most common manifestation of pneumococcal disease in adults [5]. The population of China is the largest in the world and, given its economic development and improved medical conditions in recent decades, the aging Chinese population is growing; therefore the prevention and control of pneumococcal infections has become an important public health challenge.
Antibiotics are the primary choice for treatment of pneumococcal infections. However, increasing resistance of pneumococci to conventional antibiotics has made the role of antibiotics in the treatment of infection more and more limited [6]. Vaccination has proven to be an effective means of preventing pneumococcal infection worldwide. Immunization with 7-valent pneumococcal conjunctive vaccine (PCV7) and 23-valent pneumococcal polysaccharide vaccine (PPV23) has been recommended for children younger than 24 months of age, high-risk groups, and older adults. For children, PCV7 became commercially available in China in 2008 and was replaced by the 13-valent pneumococcal conjugate vaccine (PCV13) in 2017. Currently, PCV7 is not included in the national immunization program and PCV7 immunization is given only on an individual basis.
A previous study showed that serotype distribution varies with geographical location and age [7]. To effectively control pneumococcal disease in China, the serotype combinations included in vaccines must closely match the distribution of pneumococcal serotypes. Therefore, it is necessary to determine the epidemiology of S. pneumoniae after vaccination with the PCV7 available in China. This study analyzed the antimicrobial resistance, serotype distribution, and molecular epidemiology characteristics of 881 pneumococcal isolates from multiple hospitals in China from 2011 to 2016.
Methods
Bacterial isolates
This study was conducted at the Peking University People’s Hospital, a teaching hospital located in Beijing, China. A total of 881 S. pneumoniae isolates were collected from pediatric and adult patients with pneumococcal infections in 17 cities across China during the years 2011, 2012, 2013, and 2016. The study protocols were approved by Ethics Committee of the Pecking University People’s Hospital and all participants provided written informed consent prior to study commencement. For participants younger than 18 years of age, written informed consent was obtained from each participant’s parents or legal guardian. One isolate was collected from each patient. Duplicate isolates and patients colonized by bacteria but without any clinical evidence of infection were excluded. Isolates were obtained from sputum, blood, broncho-alveolar lavage fluid (BALF), cerebrospinal fluid (CSF), pharyngeal swabs, nasal swabs, and middle ear fluid. Sputum samples from children < 2 years old were collected by nasotracheal aspiration. S. pneumoniae isolates from sputums were included if they met the following criteria: less than 10 squamous epithelial cells and more than 25 leukocytes per low power field. Isolates were transported to Peking University People’s Hospital for antibiotic susceptibility testing and serotyping annually. Isolates were identified based on typical colony morphology, Gram staining, optochin sensitivity tests (Oxoid, Hampshire, UK), and Omni serum assays (Statens Serum Institut, Copenhagen, Denmark).
In vitro antimicrobial susceptibility testing
The agar dilution method was used to determine the minimum inhibitory concentrations (MICs) of the 881 S. pneumoniae isolates against 15 antibiotics (penicillin, amoxicillin/clavulanic acid, ceftriaxone, cefuroxime, cefaclor, vancomycin, erythromycin, azithromycin, clarithromycin, tetracycline, levofloxacin, moxifloxacin, trimethoprim/sulfamethoxazole, chloramphenicol and clindamycin) in accordance with the guidelines established by the Clinical and Laboratory Standards Institute (CLSI) [8]. The CLSI 2013 criteria for MICs were applied to classify isolates as susceptible, intermediate, or resistant. The oral penicillin breakpoint was used to classify isolates as penicillin-susceptible (MIC ≤ 0.06 μg/ml), penicillin-intermediate (MIC between 0.12 and 1 μg/ml), or penicillin-resistant (MIC ≥ 2 μg/ml). For ceftriaxone, the non-meningitis breakpoint was used to classify isolates as susceptible (MIC ≤ 1 μg/ml) or resistant (MIC ≥ 4 μg/ml) [9]. S. pneumoniae ATCC 49619 was used as the quality control strain and was included in each set of tests to ensure accurate results. MICs were calculated as the MIC50 and MIC90 (MICs that inhibit 50% and 90% of the isolates, respectively).
Pneumococcal serotyping
Pneumococcal serotypes/groups were determined for the 881 isolates with Pneumotest-Latex kit (Statens Serum Institut, Copenhagen, Denmark) and type-specific antisera (Statens Serum Institut, Copenhagen, Denmark). The Pneumotest-Latex kit consisted of the 14 latex reagents pools A-I and P-Q. By testing all 14 pools and using the chessboard identification system, it was possible to identify the 23 vaccine serotypes to type/group level. Traditional quellung reaction with type-specific antisera was used for full serotyping of serogroup 19, 6 and 23. Isolates that reacted with the Pneumotest-Latex kit but did not belonged to serotypes or groups included in the PPV23 were classified as Non-vaccine types (NVT). The potential PCV7 and PCV13 vaccine coverage was estimated by calculating the percentage of isolates that expressed the serotypes included in the vaccines and related serotypes (7 for 7F, 9 for 9 V, and 18 for 18C).
Multilocus sequence typing (MLST) procedure
Multilocus sequence typing (MLST) analysis was performed according to the S. pneumoniae MLST protocol [10]. Internal fragments of approximately 550–600 bp from the aroE, gdh, gki, recP, spi, xpt, and ddl genes were amplified by polymerase chain reaction using primers described previously [11]. Alleles and sequence types (STs) were assigned using software available at the Streptococcus pneumoniae MLST Database web page (http://pubmlst.org/spneumoniae). Analysis of STs and assignment to clonal complexes were performed using all STs found in the online database using the eBURST program. STs were grouped into clonal complexes by their similarity to a central allelic profile. Visualization of phylogenetic results was performed using the PHYLOViZ online tool (http://www.phyloviz.net/).
Statistical analysis
Data from the antibiotic susceptibility tests were analyzed using WHONET 5.6 software, Windows-based database software developed by the World Health Organization for the management and analysis of microbiological laboratory data with a special focus on the analysis of antimicrobial susceptibility test results. χ2 and Fisher’s exact probability tests were performed using SPSS for Windows (version 18.0; SPSS, Chicago, IL, USA) to compare proportions. P values < 0.05 were considered statistically significant.
Results
Profile of pneumococcal isolates
During the study period, a total of 881 non-duplicated isolates were collected from 23 teaching hospitals in 17 cities. The number of isolates varied with years and regions and it was detailed in the Additional file 1: Table S1. Of the 881 S. pneumoniae isolates, 131 were obtained from pediatric patients aged 0 to 2 years (≤ 2 years old), 45 were obtained from pediatric patients aged 2 to 5 years (> 2 but ≤ 5 years old), 43 were obtained from patients aged 5 to 18 years (> 5 but ≤ 18 years old), 408 were obtained from adults aged 18 to 65 years (> 18 but ≤ 65 years old), and 254 were obtained from patients > 65 years old. Sputum was the most common specimen source, accounting for 73.1% of samples (644 strains), followed by blood (56 strains), BALF (56 strains), pharyngeal swabs (22 strains) and CSF (13 strains) Table 1. Strains isolated from sterile sites, such as blood, CSF, and pleural fluid, were classified as IPD strains. The 17 cities were divided into three groups based on the per capita gross domestic product (GDP) of the province in which the city is located (China Statistical Yearbook 2016, http://www.stats.gov.cn/tjsj/ndsj/2016/indexeh.htm). Cities in group 1 had a per capita GDP of more than 60,000 Chinese yuan (CNY) and included Tianjin, Beijing, Shanghai, Jiangsu, Zhejiang, Inner Mongolia, Guangdong, and Shandong. Cities in group 2 had a per capita GDP between 50,000 and 60,000 CNY and included Chongqing, Hubei, Jilin, Shaanxi, and Liaoning. Cities in group 3 had a per capita GDP of less than 50,000 CNY and included Ningxia, Hunan, Xinjiang, and Guangxi.
Table 1.
Distribution of Streptococcus pneumoniae specimens by age group and year collected
| Specimen type | Number | % | Age groups | Years | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤ 2 years | > 2 and ≤ 5 years | > 5 and ≤ 18 years | > 18 and ≤ 65 years | > 65 years | 2011 | 2012 | 2013 | 2016 | |||
| Sputum | 644 | 73.1 | 121 | 33 | 26 | 259 | 205 | 34 | 210 | 166 | 234 |
| Blood | 56 | 6.4 | 3 | 3 | 3 | 35 | 12 | 3 | 18 | 17 | 18 |
| Broncho-alveolar lavage | 56 | 6.4 | 1 | 37 | 18 | 13 | 17 | 26 | |||
| Pharyngeal swabs | 22 | 2.5 | 3 | 6 | 4 | 8 | 1 | 3 | 8 | 4 | 7 |
| Cerebrospinal fluid | 13 | 1.5 | 1 | 9 | 3 | 2 | 4 | 7 | |||
| Eye secretion | 10 | 1.1 | 2 | 2 | 5 | 1 | 1 | 1 | 4 | 4 | |
| Secretion | 9 | 1.0 | 1 | 1 | 5 | 2 | 5 | 4 | |||
| Pus | 8 | 0.9 | 1 | 6 | 1 | 4 | 4 | ||||
| Nasal swabs | 6 | 0.7 | 2 | 4 | 3 | 3 | |||||
| Wound swab | 6 | 0.7 | 5 | 1 | 2 | 3 | 1 | ||||
| Pleural fluid | 6 | 0.7 | 5 | 1 | 2 | 4 | |||||
| Tissue | 6 | 0.7 | 2 | 4 | 5 | 1 | |||||
| Sinus swab | 4 | 0.5 | 2 | 2 | 4 | ||||||
| Tracheal | 4 | 0.5 | 3 | 1 | 4 | ||||||
| Middle ear fluid | 3 | 0.3 | 1 | 1 | 1 | 1 | 2 | ||||
| Abdominal fluid | 3 | 0.3 | 3 | 1 | 2 | ||||||
| Bile | 2 | 0.2 | 2 | 1 | 1 | ||||||
| Urine | 2 | 0.2 | 2 | 1 | 1 | ||||||
| Bone marrow | 1 | 0.1 | 1 | 1 | |||||||
| Vaginal swab | 1 | 0.1 | 1 | 1 | |||||||
| Not specified | 19 | 2.2 | 1 | 2 | 15 | 1 | 19 | ||||
Antimicrobial susceptibility of Streptococcus pneumoniae
The in vitro activities of the tested antimicrobial agents against the 881 S. pneumoniae strains are shown in Table 2. Based on the MIC breakpoints of oral penicillin, the percentages of penicillin-resistant S. pneumoniae (PRSP), penicillin-intermediate S. pneumoniae (PISP), and penicillin-susceptible S. pneumoniae (PSSP) isolates were 51.6% (455/881), 12.3% (108/881), and 36.1% (318/881), respectively. The rates of resistance of S. pneumoniae to erythromycin, azithromycin, and clarithromycin were 95.2%, 96.9%, and 92.5%, respectively. Macrolides exhibited weak activity against all of the isolates. Of the 839 strains (95.2%) that were resistant to erythromycin, 776 (92.5% of all strains) were also resistant to clindamycin. S. pneumoniae susceptibilities to amoxicillin/clavulanic acid and ceftriaxone were comparable (using non-meningitis breakpoints), but the rate of resistance to amoxicillin/clavulanic acid was higher than to ceftriaxone (using non-meningitis breakpoints). Overall, the resistance of S. pneumoniae to penicillin (based on breakpoints of oral penicillin) increased from 48.8% in 2011 to 55.9% in 2016 (P = 0.390, Fig. 1). The proportion of PRSP isolates remained stable and there were no statistical differences in rates of PRSP from 2011 to 2016 (Table 3). During the same time period, the proportion of PSSP isolates fell from 39% in 2011 to 33.6% in 2016. The MIC50 of penicillin was 1 μg/ml in 2011 and increased to 2 μg/ml in 2016, indicating a trend towards an increase in resistance to penicillin. Trends towards increases in resistance were also observed for other β-lactams and cephalosporins. However, fluoroquinolones maintained excellent activity against S. pneumoniae; the MIC90 values for levofloxacin and moxifloxacin were 1 μg/ml and 0.25 μg/ml, respectively. There were 19 S. pneumoniae strains resistant to levofloxacin and the MIC values were between 8 and 32 μg/ml. All of the strains were susceptible to vancomycin and no vancomycin-resistant strains were found during the study period. The rates of resistance of penicillin non-susceptible S. pneumoniae (PNSP) to other antimicrobial agents were also significantly higher than those of PSSP. However, no statistical differences in resistance to azithromycin, clarithromycin, levofloxacin, or moxifloxacin were found between PSSP and PNSP strains. The proportion of PSSP isolates varied with patient age; PSSP was more common among older adults compared with children (42.0% vs 18.7%, P < 0.001). There were no significant differences in the proportion of PNSP isolates between IPD and non-IPD strains. The rate of PSSP isolated from patients in emergency units was higher than in other hospital wards (P = 0.041). Similarly, the rate of PNSP isolated from patients in intensive care units (ICUs) was higher than in other wards (P = 0.017, Table 3).
Table 2.
In vitro activity of 14 antimicrobial agents against 881 isolates of Streptococcus pneumoniae
| Antimicrobial agent | PSSP (n = 318) | PISP (n = 108) | PRSP (n = 455) | P | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R% | MIC50 | MIC90 | MIC range | R% | MIC50 | MIC90 | MIC range | R% | MIC50 | MIC90 | MIC range | ||
| Penicillin (oral) | 0 | 0.016 | 0.032 | .004–.064 | 0 | 1 | 1 | .125–1 | 100 | 4 | 4 | 2–64- | – |
| Amoxicillin/clavulanic acid | 0.3 | 0.016 | 0.032 | .008–32 | 0 | 1 | 2 | .008–2 | 60.7 | 8 | 16 | .016–128 | < 0.00001 |
| Ceftriaxone (non meningitis) | 0 | 0.016 | 0.064 | .016–1 | 0.9 | 0.5 | 1 | .016–4 | 40.7 | 2 | 8 | .25–512 | – |
| Cefuroxime oral | 0.6 | 0.032 | 0.125 | .016–8 | 45.4 | 2 | 4 | .016–32 | 97.8 | 16 | 32 | .008–512 | < 0.00001 |
| Cefaclor | 2.2 | 1 | 2 | .016–32 | 88.9 | 128 | 256 | 1–512- | 99.8 | >256 | >256 | 2–512- | < 0.00001 |
| Vancomycin | 0 | 0.25 | 0.25 | .008–.5 | 0 | 0.25 | 0.5 | .064–.5 | 0 | 0.25 | 0.25 | .064–1 | – |
| Erythromycin | 89 | 16 | >256 | .016–512 | 96.3 | 32 | >256 | .032–512 | 99.3 | >256 | >256 | .032–512 | < 0.00001 |
| Azithromycin | 94.6 | 32 | >256 | .016–512 | 94.4 | 32 | >256 | .032–512 | 99.3 | >256 | >256 | .032–512 | 0.135 |
| Clarithromycin | 88.3 | 32 | >256 | .016–512 | 80.6 | 32 | >256 | .016–512 | 98.6 | >256 | >256 | .032–512 | 0.063 |
| Tetracycline | 84.6 | 32 | 64 | .064–128 | 92.6 | 16 | 64 | .25–64 | 97.1 | 32 | 64 | .25–128 | < 0.00001 |
| Levofloxacin | 1.3 | 1 | 1 | .025–32 | 5.6 | 1 | 1 | .25–32 | 2 | 1 | 1 | .5–64 | 0.163 |
| Moxifloxacin | 0.6 | 0.125 | 0.25 | .016–16 | 1.9 | 0.125 | 0.25 | .016–8 | 0.7 | 0.125 | 0.25 | .016–16 | 0.666 |
| Trimethoprim/sulfamethoxazole | 34.9 | 0.5 | 8 | .032–64 | 60.2 | 4 | 8 | .064–32 | 89.5 | 8 | 16 | .125–128 | < 0.00001 |
| Clindamycin | 74.2 | 128 | 256 | .016–512 | 88 | 128 | 256 | .016–256 | 97.8 | 256 | 256 | .016–512 | < 0.00001 |
Fig. 1.
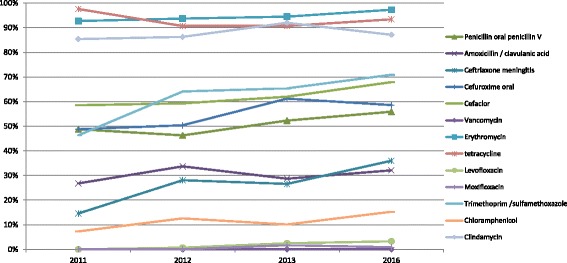
Changes in resistance of S. pneumoniae to different antimicrobial agents from 2011 to 2016
Table 3.
Proportion of PSSP and PNSP isolates by age, year, and vaccination status
| Groups | Number of isolates | PSSP % | PNSP % | P value | |
|---|---|---|---|---|---|
| Age | ≤ 2 years | 131 | 19.1 | 80.9 | < 0.00001 |
| > 2 and ≤ 5 years | 45 | 15.6 | 84.4 | 0.003083 | |
| > 5 and ≤ 18 years | 43 | 20.9 | 79.1 | 0.032561 | |
| > 18 and ≤ 65 years | 408 | 42.2 | 57.8 | 0.000646 | |
| > 65 | 254 | 41.7 | 58.3 | 0.029919 | |
| Years | 2011 | 41 | 39 | 61 | 0.700859 |
| 2012 | 270 | 39.3 | 60.7 | 0.210449 | |
| 2013 | 237 | 35.9 | 64.1 | 0.897489 | |
| 2016 | 333 | 33.6 | 66.4 | 0.215055 | |
| PCV7 | < 0.00001 | ||||
| PCV7 covered | 330 | 12.1 | 87.9 | ||
| PCV13 | < 0.00001 | ||||
| PCV13 covered | 514 | 14.8 | 85.2 | ||
| IPD | 0.223755 | ||||
| IPD | 75 | 42.7 | 57.3 | ||
| Ward types | unknown | 25 | 36 | 64 | 0.982413 |
| emergency | 55 | 49.1 | 50.9 | 0.040078 | |
| out patient | 103 | 34 | 66 | 0.616566 | |
| in patient | 635 | 36.9 | 63.1 | 0.524408 | |
| ICU | 63 | 22.2 | 77.8 | 0.016520 | |
| Major serotypes | 19F | 226 | 3.5 | 96.5 | < 0.00001 |
| 19A | 123 | 5.7 | 94.3 | < 0.00001 | |
| 15 | 58 | 46.6 | 53.4 | 0.089938 | |
| 6B | 31 | 19.4 | 80.6 | 0.046833 | |
| 6A | 26 | 3.8 | 96.2 | 0.000491 | |
| 17 | 25 | 84 | 16 | < 0.00001 | |
| 14 | 21 | 0 | 100 | – | |
| 23F | 19 | 10.5 | 89.5 | 0.018531 | |
| NVT | 234 | 69.2 | 30.8 | < 0.00001 | |
Serotyping and vaccine coverage
Of the 881 isolates, 234 isolates were non-typable and 22 different kinds of serotypes were identified. The serotype distribution of these S. pneumoniae isolates is shown in Fig. 2. Among the typable isolates, 19F (25.7%), 19A (14.0%), 15 (6.8%), 6B (3.6%), 6A (3.0%), and 17 (2.8%) were the most common serotypes. The most common serotypes in all age groups were 19F and 19A.
Fig. 2.
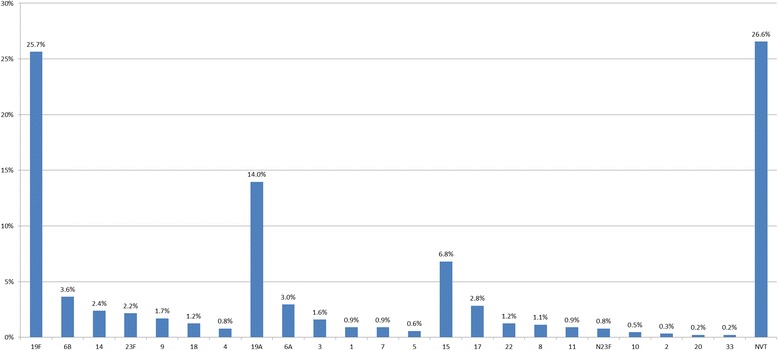
Distribution and proportion of 881 S. pneumoniae strains collected between 2011 and 2016. N23F strains belong to serogroup 23 but not serotype 23F. NVT: serotypes not included in the PPV23. Serotypes 4, 6B, 9, 14, 18, 19F, and 23F are included in PCV7. Serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9, 14, 18C, 19A, 19F, and 23F are included in PCV13
The overall vaccine coverage rates for PCV7 and PCV13, which include isolates 1, 3, 5, 6A, 7, and 19A, were 37.5% and 58.3%, respectively. Importantly, vaccine coverage rates varied by patient age; vaccine coverage rates in young children were significantly higher than in older adults (Table 4).
Table 4.
Coverage (%) of PCV7 and PCV13 in different age groups from 2011 to 2016
| Age groups | PCV7 covered | PCV13 covered | ||||||
|---|---|---|---|---|---|---|---|---|
| 2011 | 2012 | 2013 | 2016 | 2011 | 2012 | 2013 | 2016 | |
| ≤ 2 years | 42.9 | 48.1 | 37.2 | 55.6 | 71.4 | 74.1 | 62.8 | 59.3 |
| > 2 and ≤ 18 years | 50 | 52.6 | 41.7 | 37.8 | 62.5 | 84.2 | 70.8 | 64.9 |
| > 18 and ≤ 65 years | 43.8 | 31.5 | 36.7 | 31.8 | 62.5 | 56.5 | 52 | 51.2 |
| > 65 years | 30 | 41.1 | 38.9 | 35.4 | 40 | 58.9 | 63.9 | 53.5 |
Immunization coverage in this study varied considerably by geographic area. The highest coverage of PCV7 and PCV13 was in Jiangsu with rates of 63.2% and 78.9%, respectively. In contrast, the lowest coverage was in Jilin with rates of 12.9% and 32.3%, respectively. We also found that vaccine coverage was associated with economic development. There was no difference in PCV7 or PCV13 coverage between groups 1 and 2, whereas PCV7 and PCV13 coverage was significantly higher in group 3 compared with groups 1 and 2 (Table 5).
Table 5.
Coverage (%) of PCV7 and PCV13 in different regions in China
| Group by GDP | Province | Number | non-PCV7 covered (%) | PCV7 covered (%) | P value | non-PCV13 covered (%) | PCV13 covered (%) | P value |
|---|---|---|---|---|---|---|---|---|
| Group 1 | Beijing | 175 | 120 (68.6) | 55 (31.4) | 84 (48.0) | 91 (52.0) | ||
| Group 1 | Guangzhou | 91 | 50 (54.9) | 41 (45.1) | 25 (27.5) | 66 (72.5) | ||
| Group 1 | Zhejiang | 82 | 61 (74.4) | 21 (25.6) | 44 (53.7) | 38 (46.3) | ||
| Group 1 | Jiangsu | 38 | 14 (36.8) | 24 (63.2) | 8 (21.1) | 30 (78.9) | ||
| Group 1 | Inner Mongolia | 19 | 11 (57.9) | 8 (42.1) | 11 (57.9) | 8 (42.1) | ||
| Group 1 | Shandong | 29 | 20 (69.0) | 9 (31.0) | 13 (44.8) | 16 (55.2) | ||
| Group 1 | Shanghai | 59 | 34 (57.6) | 25 (42.4) | 24 (40.7) | 35 (59.3) | ||
| Group 1 | Tianjin | 50 | 33 (66.0) | 17 (34.0) | 29 (58.0) | 21 (42.0) | ||
| Group 1 | 543 | 343 (63.2) | 200 (36.8) | 0.627101 | 238 (43.8) | 305 (56.2) | 0.097211 | |
| Group 2 | Chongqing | 79 | 44 (55.7) | 35 (44.3) | 30 (38.0) | 49 (62.0) | ||
| Group 2 | Jilin | 31 | 27 (87.1) | 4 (12.9) | 21 (67.7) | 10 (32.3) | ||
| Group 2 | Liaoning | 44 | 37 (84.1) | 7 (15.9) | 20 (45.5) | 24 (54.5) | ||
| Group 2 | Hubei | 52 | 31 (59.6) | 21 (40.4) | 22 (42.3) | 30 (57.7) | ||
| Group 2 | Shaanxi | 31 | 15 (48.4) | 16 (51.6) | 7 (22.6) | 24 (77.4) | ||
| Group 2 | 237 | 154 (65.0) | 83 (35.0) | 0.364746 | 100 (42.2) | 137 (57.8) | 0.844537 | |
| Group 3 | Hunan | 11 | 5 (45.5) | 6 (54.5) | 3 (27.3) | 8 (72.7) | ||
| Group 3 | Guangxi | 7 | 5 (71.4) | 2 (28.6) | 3 (42.9) | 4 (57.1) | ||
| Group 3 | Ningxia | 39 | 19 (48.7) | 20 (51.3) | 10 (25.6) | 29 (74.4) | ||
| Group 3 | Xinjiang | 44 | 25 (56.8) | 19 (43.2) | 13 (29.5) | 31 (70.5) | ||
| Group 3 | 101 | 54 (53.5) | 47 (46.5) | 0.045169 | 29 (28.7) | 72 (71.3) | 0.005041 |
The sensitivities of the S. pneumoniae serotypes to antimicrobial agents varied significantly. In serogroups 19 (including 19F and 19A), 6 (including 6A and 6B), 14, and 23F, the proportion of PNSP isolates was significantly higher compared with the other common serotypes (Table 3), whereas the proportion of PSSP isolates was higher in serotypes 15 and 17 and in the non-typable strains. The proportion of PNSP isolates was higher among vaccine-covered strains (87.9% for PCV7 and 85.2% for PCV13) than non-covered strains (49.4% for PCV7 and 33.8% for PCV13).
Multilocus sequence typing
Of the 881 strains, 424 (strains isolated in 2016 were not tested by MLST) were identified as 82 STs. Among the tested strains, ST271 (120, 28.3%), ST320 (73, 17.2%), and ST81 (27, 6.6%) were the most common STs. Of the 19F isolates that were identified with specific STs, 98 (77.8%) were ST271 and 15 (11.9%) were ST320. Of the 19A isolates, 55 (75.3%) were ST320 and 15 (20.6%) were ST271. We found that 19F–ST271 and 19A–ST320 isolates were more resistant to several of the tested antibiotics, especially β-lactams (Table 6). The phylogenetic tree generated using the PHYLOViZ online tool showed that all of the tested strains exhibited obvious clonal aggregation, with the vast majority of the population made up of resistant clones and serotypes, whereas susceptible clones and serotypes showed a decentralized pattern (Fig. 3).
Table 6.
Sequence types, serotypes, antibiotic resistance rates (%), and age distributions for 424 Streptococcus pneumoniae isolates analyzed by MLST
| Clonal Complex | ST | Number | Serotypes (Number) | Resistance rates of different antibiotics | Number of strains in different ages | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Penicillin | Levofloxacin | Erythromycin | <= 2 | (2,5] | (5,18] | (18,65] | > 65 | ||||
| CC271 | 271 | 120 | 19F(98), 19A(15), NVT(5), 15(1), 1(1) | 97.5 | 0 | 100 | 35 | 7 | 9 | 41 | 28 |
| 320 | 73 | 19A(55), 19F(15), 18(1), 15(1), NVT(1) | 98.6 | 2.7 | 100 | 12 | 10 | 0 | 29 | 22 | |
| 236 | 6 | 19F(5), NVT(1) | 83.3 | 16.7 | 100 | 1 | 0 | 0 | 3 | 2 | |
| 1937 | 2 | 19F(2) | 100 | 0 | 100 | 0 | 0 | 0 | 1 | 1 | |
| CC81 | 81 | 28 | 23F(8), 15(6), NVT(6), N23F(3), 6B(2), 3(1), 17(1), 18(1) | 92.9 | 3.6 | 100 | 5 | 3 | 0 | 15 | 5 |
| 83 | 1 | 19F(1) | 100 | 0 | 100 | 1 | 0 | 0 | 0 | 0 | |
| CC876 | 876 | 17 | 14(11), NVT(3), 18(2), 4(1) | 47.1 | 5.9 | 100 | 2 | 3 | 0 | 10 | 2 |
| 5749 | 1 | 14(1) | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 1 | |
| CC505 | 505 | 14 | NVT(9), 3(3), 17(1), 19F(1) | 0 | 0 | 92.9 | 0 | 0 | 2 | 6 | 6 |
| 12,449 | 2 | NVT(2) | 0 | 0 | 100 | 0 | 0 | 0 | 2 | 0 | |
| CC180 | 180 | 13 | NVT(12), 8(1) | 7.7 | 0 | 92.3 | 1 | 0 | 0 | 8 | 4 |
| 297 | 1 | NVT(1) | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 1 | |
| CC3397 | 3397 | 9 | 15(7), 8(2) | 0 | 0 | 88.9 | 3 | 1 | 0 | 3 | 2 |
| 7768 | 3 | 15(3) | 33.3 | 0 | 100 | 2 | 1 | 0 | 0 | 0 | |
| 10,098 | 1 | 15(1) | 0 | 0 | 100 | 1 | 0 | 0 | 0 | 0 | |
| CC1263 | 6011 | 4 | 15(2), 4(1), NVT(1) | 0 | 0 | 100 | 0 | 0 | 0 | 2 | 2 |
| 4660 | 2 | 9(1), NVT(1) | 0 | 0 | 100 | 0 | 0 | 0 | 2 | 0 | |
| 1263 | 2 | 9(1), NVT(1) | 0 | 0 | 100 | 0 | 0 | 0 | 1 | 1 | |
| 280 | 1 | 1(1) | 0 | 0 | 0 | 1 | 0 | ||||
| CC5972 | 6946 | 4 | 17(3), 8(1) | 0 | 25 | 100 | 0 | 0 | 0 | 2 | 2 |
| 5972 | 3 | NVT(2), 5(1) | 0 | 0 | 100 | 0 | 0 | 0 | 2 | 1 | |
| 7759 | 2 | 3(1), NVT(1) | 50 | 0 | 100 | 0 | 0 | 0 | 2 | 0 | |
| CC2754 | 2754 | 6 | NVT(3), 15(2), 8(1) | 0 | 0 | 100 | 0 | 0 | 0 | 5 | 1 |
| 7752 | 1 | NVT(1) | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 1 | |
| CC3173 | 3173 | 5 | 6A(4), 6B(1) | 80 | 0 | 100 | 4 | 0 | 0 | 1 | 0 |
| 6340 | 1 | 19F(1) | 100 | 0 | 100 | 0 | 0 | 0 | 0 | 1 | |
| CC2758 | 2758 | 1 | 7(1) | 0 | 0 | 100 | 1 | 0 | 0 | 0 | 0 |
| 7402 | 1 | 11(1) | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 1 | |
| 11,967 | 1 | NVT(1) | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 1 | |
| CC3387 | 3387 | 2 | NVT(2) | 0 | 0 | 100 | 2 | 0 | 0 | 0 | 0 |
| 90 | 1 | 6B(1) | 0 | 0 | 100 | 1 | 0 | 0 | 0 | 0 | |
| CC2912 | 2912 | 1 | 6B(1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 8738 | 1 | NVT(1) | 100 | 0 | 100 | 1 | 0 | 0 | 0 | 0 | |
| CC342 | 342 | 1 | 17(1) | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 1 |
| 6325 | 1 | 1(1) | 0 | 0 | 100 | 0 | 0 | 0 | 1 | 0 | |
| Singletons | 9789 | 7 | 6B(3), 6A(2), NVT(2) | 100 | 0 | 100 | 2 | 0 | 0 | 4 | 1 |
| 6202 | 7 | 15(6), 10(1) | 0 | 0 | 100 | 0 | 0 | 0 | 7 | 0 | |
| 99 | 5 | 1(1),3(1),4(1),5(1),19F(1) | 0 | 0 | 100 | 0 | 0 | 0 | 2 | 3 | |
| 4389 | 4 | NVT(4) | 0 | 0 | 50 | 0 | 0 | 0 | 1 | 3 | |
| OTHER | 69 | NVT(22), 17(7), 18(6), 15(4), 19A(3), 1(2), 14(2), 19F(2), 2(2), 20(2), 22(2), 23F(2), 3(2), 7(2), 8(2), 9(2), N23F(2), 4(1), 6A(1), 6B(1) | 14.5 | 0 | 89.9 | 16 | 3 | 3 | 30 | 17 | |
Fig. 3.
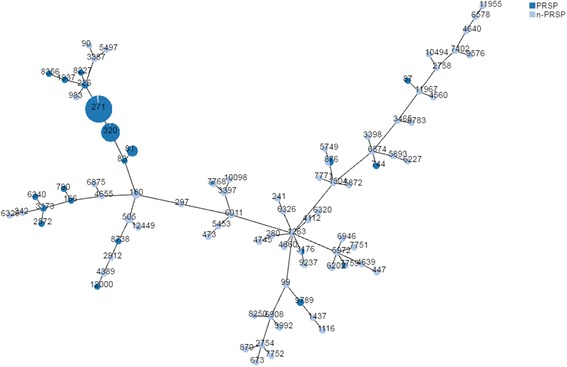
Phylogenetic tree of 424 S. pneumoniae strains generated by the PHYLOViZ online tool
Discussion
Infections caused by S. pneumoniae have traditionally been treated with β-lactams, to which this species was extremely sensitive when penicillin was first introduced in the 1940s [12]. However, resistance was first observed in the 1960s and has continued to increase throughout the world in recent decades [12, 13]. The emergence of resistance to penicillin and other β-lactam antibiotics in pneumococci has led to the increased adoption of macrolides, fluoroquinolones, and other non-β-lactam antibiotics to treat pneumococcal infections [14]. However, resistance to antimicrobials continues to increase, complicating efforts to treat pneumococcal disease in both adults and children.
The data from this study showed that S. pneumoniae isolated from adults and children during the investigation period were highly resistant to β-lactams, macrolides, and trimethoprim/sulfamethoxazole, which is consistent with a previous study [15]. Based on the MIC breakpoints of oral penicillin, the proportion of PRSP increased from 48.8% in 2011 to 55.9% in 2016. MICs of most of the antimicrobial agents tested against PRSP were higher than against PSSP, indicating co-resistance between these antimicrobials. Consequently, the increasing prevalence of multidrug resistant (MDR) in S. pneumoniae in China is becoming a serious health threat.
Macrolides, including erythromycin, azithromycin, and clarithromycin, had the lowest antibacterial activity against both PNSP and PSSP strains, with MIC90 values greater than 256 μg/ml. Since the early 1990s, the American Thoracic Society treatment guidelines have listed macrolide antibiotics as the first-line empiric therapy for outpatients with community-acquired pneumonia [16], resulting in the widespread use of these agents. In Europe, resistance of S. pneumoniae to erythromycin has been reported to be between 14.7 and 17.1% [17]. However, more than 90% of clinical S. pneumoniae strains in this study were found to be resistant to macrolides; therefore macrolides should be used cautiously as empiric therapy against pneumococcal infection in China. Abuse of macrolides in outpatient practices and the clonal spread of MDR strains is likely responsible for the high prevalence of macrolide resistance in China [18]. Additionally, macrolide-resistant S. pneumoniae strains identified in this study were found to be co-resistant to other antibacterials, such as tetracycline (94.2%), clindamycin (92.5%), and penicillin (53.9%; oral penicillin V). Other researchers have shown that the majority of MDR S. pneumoniae in China are macrolide-lincosamide-streptogramin B resistant and that they carry the erm(B) gene [19]. Multiple antibiotic resistance is widespread in China and an increase in MDR S. pneumoniae strains has also been observed in other parts of Asia such as Japan, Korea, and Taiwan [20]. In contrast, fluoroquinolones showed exceptional activity against S. pneumoniae in this study, which is in accordance with other reports [18, 21, 22], indicating that fluoroquinolones could be a better option for the treatment of pneumococcal infections in adult patients.
Interestingly, in this study, strains isolated from patients in emergency units were more susceptible to penicillin, whereas strains isolated from patients in ICUs were more resistant to penicillin. This could indicate that S. pneumoniae strains within the community were more susceptible to penicillin, whereas more resistant strains, for which treatments were more likely to fail, developed within medical institutions.
In this study, the proportion of PNSP isolates in serotypes 19F, 19A, and 6B was higher than in other serotypes. Previous studies have indicated that recombination efficiency varies with S. pneumoniae serotype, with certain strains having been identified as particularly efficient at recombination. For example, serotypes 3 and 18C were found to be much less transformable with a selective marker compared with 19F, 19A, 23F, 6B, and 14 [23]. Interspecies and intraspecies genetic transformations likely play an important role in most multi-antimicrobial resistance mechanisms in pneumococci [24].
Vaccination is an alternative method of preventing pneumococcal infection. It has been reported that the prevalence of vaccine-covered serotypes decreased significantly after large-scale application of PCV7. Studies have shown that the introduction of pneumococcal conjugate vaccines has not only reduced the burden of pneumococcal disease in children [25], but has also greatly impacted the burden of disease in adults by preventing the spread of vaccine-related resistant strains to adults [26, 27]. PCV7, which was replaced by PCV13 in 2016, has been used in China since 2008 on an individual basis. In this study, the average vaccine coverage of PCV7 (37.5%) and PCV13 (58.3%) in the population was similar to the vaccine coverage before they were introduced into China [28], suggesting that the effects of PCV7 and PCV13 in China were limited. Exclusion from the national immunization program and high prices could be responsible for low vaccination rates and poor herd immunity in the target population [29]. We also found that in areas with better economic development, vaccine coverage was lower than in less economically developed regions. This is likely due to historically higher rates of vaccine use in economically developed regions, resulting in greater herd protection and a subsequent decline in the rate of vaccine coverage. Considering the protective effect of vaccines reported in other countries and the high coverage of PCV13 in both children and adults in China, inclusion of PCV13 in the national immunization program could result in significant changes in the serotype distribution of S. pneumoniae in both children and adults.
There were some limitations to this study. Most importantly, all strains were obtained from sophisticated medical institutions, whereas no strains were obtained from community-based clinics. Patients are typically admitted to large medical institutions when empirical treatment has failed or when existing diseases worsen. Patients infected with strains that have lower levels of resistance and virulence are more likely to be cured by the empirical treatment regimen and would not need to go to a large hospital for further medication. Because of this screening mechanism, the data from this study only explain resistance and serotype distribution status of S. pneumoniae isolated from hospitals. Additionally, while the effect of PCV13 has been evaluated mainly in IPD strains [30], the number of IPD strains in this study was very limited. This may be due to less blood cultures were prescribed in China. The proportion of blood cultures in all microbiology specimens varied with hospitals in this study, ranging from 20% to 50%. The percentages of blood-cultures were higher in large cities and teaching hospitals and were lower in small cities and primary hospitals. In China, doctors diagnose respiratory infections relied a lot on X-ray imaging findings and white blood cell count, both of them returned results on the same day. Although doctors also prescribed microbiology cultures (usually sputum culture) pathogen detection, doctors may empirically use antibiotics if X-ray imaging and white blood cell count indicating a bacterial infection. Further study on IPD strains is required to assess the effectiveness of the PCV13 vaccine in China. The number of strains varied with years and regions, and the number of strains in some years and regions was relatively small. A very small sample size would inevitably introduce random errors, especially in the comparison of vaccine coverage rates between different age groups and regions. Therefore, in order to clarify the resistance and serotype distribution of S. pneumoniae in the population, our future studies will assess strains from primary health care institutions in the community.
Conclusions
High resistance to β-lactams and macrolides was observed of all 881 S. pneumoniae strains. However, fluoroquinolones maintained excellent activity against S. pneumoniae. Drug resistance varied among different serotypes and age groups. Serotypes 19F, 19A, 15, 6B, 6A and 17 were the most common serotypes. The serotype 19F, 19A, 6A, 6B, 14 and 23F demonstrated higher resistance compared with other serotypes. Vaccine coverage in this study varied considerably associated geographic area and economic development. Through molecular biology analysis, obvious clonal aggregation was observed. Community-acquired and more invasive strains should also be included in future studies to gain a better understanding of the prevalence and resistance of S. pneumoniae in China.
Acknowledgments
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81401695 and No. 81625014).
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ATCC
American Type Culture Collection
- BALF
Broncho-alveolar lavage fluid
- CLSI
Clinical and Laboratory Standards Institute
- CNY
Chinese yuan
- CSF
Cerebrospinal fluid
- GDP
Gross domestic product
- ICU
Intensive care unit
- IPD
Invasive pneumococcal disease
- MDR
Multidrug resistant
- MIC
Minimum inhibitory concentration
- MLST
Multilocus sequence typing
- PCV13
13-valent pneumococcal conjugate vaccine
- PCV7
7-valent pneumococcal conjunctive vaccine
- PISP
Penicillin-intermediate S. pneumoniae
- PNSP
Penicillin non-susceptible S. pneumoniae
- PPV23
23-valent pneumococcal polysaccharide vaccine
- PRSP
Penicillin-resistant S. pneumoniae
- PSSP
Penicillin-susceptible S. pneumoniae
- ST
Sequence type
- SXT
Trimethoprim/sulfamethoxazole
Additional file
Number of Streptococcus pneumoniae strains in different cities and years. This table describes the numbers of strains in different cities and years. The cities in this table were divided into 3 group based on the annual per capita GDP. (DOCX 16 kb)
Authors’ contributions
HW, CZ1 and ZL conceived and designed experiments. CZ1 ZL and FZ performed antibiotic susceptibility testing and serotyping. CZ1 and ZL performed MLST. XZ, PJ, JZ, BH, ZH, KL, HS, RZ, BC, CZ2, WJ, YM, YC, XX1, QY, YJ, QF, XX2, HL, LW, YN and HL contributed strains and case data collection. HW, CZ1 and ZL wrote the manuscript. CZ1 refers to Chunjiang Zhao. CZ2 refers to Chao Zhuo. XX1 refers to Xuesong Xu. XX2 refers to Xiuli Xu. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Study protocols were reviewed and approved by the Ethical Committee of Peking University People’s Hospital (No. 2016PHB135). All participants provided written informed consent prior to study commencement. For participants younger than 18 years of age, written informed consent was obtained from each participant’s parents or legal guardian.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (doi: 10.1186/s12879-017-2880-0) contains supplementary material, which is available to authorized users.
Contributor Information
Chunjiang Zhao, Email: chunkiang@163.com.
Zongbo Li, Email: lizongbo@bjmu.edu.cn.
Feifei Zhang, Email: feifei_517@aliyun.com.
Xiaobing Zhang, Email: xiaobing_zhang@aliyun.com.
Ping Ji, Email: ping_ji881@163.com.
Ji Zeng, Email: ji_zeng@outlook.com.
Bijie Hu, Email: bijie_hu@aliyun.com.
Zhidong Hu, Email: zhidong_hu881@outlook.com.
Kang Liao, Email: nimei.kan@gmail.com.
Hongli Sun, Email: hongli_sun@aliyun.com.
Rong Zhang, Email: zhangrong735@yahoo.com.
Bin Cao, Email: caobin1999@gmail.com.
Chao Zhuo, Email: chao_zhuo@outlook.com.
Wei Jia, Email: wei.jiachina@outlook.com.
Yaning Mei, Email: yaning_mei@outlook.com.
Yunzhuo Chu, Email: yunzhuo_chu@aliyun.com.
Xuesong Xu, Email: xuesong_xu@yahoo.com.
Qing Yang, Email: qing_yang@aliyun.com.
Yan Jin, Email: yan_jin33@yahoo.com.
Quan Fu, Email: quan_fu41@yahoo.com.
Xiuli Xu, Email: xiuli_xu1967@126.com.
Hongling Li, Email: pumch.micro@gmail.com.
Lijun Wang, Email: lijunwang456@sina.com.
Yuxing Ni, Email: yuxing_ni@163.com.
Hongjie Liang, Email: hongjie_liang@21cn.com.
Hui Wang, Phone: +86-10-88326300, Email: whuibj@163.com.
References
- 1.Peto L, et al. The bacterial aetiology of adult community-acquired pneumonia in Asia: a systematic review. Trans R Soc Trop Med Hyg. 2014;108(6):326–337. doi: 10.1093/trstmh/tru058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jain S, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 2012;67(1):71–79. doi: 10.1136/thx.2009.129502. [DOI] [PubMed] [Google Scholar]
- 4.Morimoto K, et al. The burden and etiology of community-onset pneumonia in the aging Japanese population: a multicenter prospective study. PLoS One. 2015;10(3):e0122247. doi: 10.1371/journal.pone.0122247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Said MA, et al. Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. PLoS One. 2013;8(4):e60273. doi: 10.1371/journal.pone.0060273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao C, et al. Antimicrobial resistance trends among 5608 clinical gram-positive isolates in China: results from the gram-positive Cocci resistance surveillance program (2005-2010) Diagn Microbiol Infect Dis. 2012;73(2):174–181. doi: 10.1016/j.diagmicrobio.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Hausdorff WP, Feikin DR, Klugman KP. Epidemiological differences among pneumococcal serotypes. Lancet Infect Dis. 2005;5(2):83–93. doi: 10.1016/S1473-3099(05)70083-9. [DOI] [PubMed] [Google Scholar]
- 8.Institute., C.a.L.S . Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard-ninth edition. Document M7-A9. Wayne: CLSI; 2012. [Google Scholar]
- 9.Institute., C.a.L.S . Performance standards for antimicrobial susceptibility testing; twenty-third informational supplement. Document M100-S23. Wayne: CLSI; 2013. [Google Scholar]
- 10.Enright MC, Spratt BG. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology. 1998;144(Pt 11):3049–3060. doi: 10.1099/00221287-144-11-3049. [DOI] [PubMed] [Google Scholar]
- 11.Moore MR, et al. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J Infect Dis. 2008;197(7):1016–1027. doi: 10.1086/528996. [DOI] [PubMed] [Google Scholar]
- 12.Linares J, et al. Changes in antimicrobial resistance, serotypes and genotypes in Streptococcus pneumoniae over a 30-year period. Clin Microbiol Infect. 2010;16(5):402–410. doi: 10.1111/j.1469-0691.2010.03182.x. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs MR, et al. Emergence of multiply resistant pneumococci. N Engl J Med. 1978;299(14):735–740. doi: 10.1056/NEJM197810052991402. [DOI] [PubMed] [Google Scholar]
- 14.Lin H, et al. Trends and patterns of antibiotic consumption in shanghai municipality, China: a 6 year surveillance with sales records, 2009-14. J Antimicrob Chemother. 2016;71(6):1723–1729. doi: 10.1093/jac/dkw013. [DOI] [PubMed] [Google Scholar]
- 15.Zhao C, et al. Phenotypic and genotypic characteristic of invasive pneumococcal isolates from both children and adult patients from a multicenter surveillance in China 2005-2011. PLoS One. 2013;8(12):e82361. doi: 10.1371/journal.pone.0082361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niederman MS, et al. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163(7):1730–1754. doi: 10.1164/ajrccm.163.7.at1010. [DOI] [PubMed] [Google Scholar]
- 17.Torres A, et al. The aetiology and antibiotic management of community-acquired pneumonia in adults in Europe: a literature review. Eur J Clin Microbiol Infect Dis. 2014;33(7):1065–1079. doi: 10.1007/s10096-014-2067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao Y, Wei Z, Shen P, Ji J, Sun Z, Yu H, et al. Bacterial-resistance among outpatients of county hospitals in China: significant geographic distinctions and minor differences between central cities. Microbes Infect. Elsevier Masson. 2015;17:417–25. doi: 10.1016/j.micinf.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, et al. Molecular characterization of erm(B)- and mef(E)-mediated erythromycin-resistant Streptococcus pneumoniae in China and complete DNA sequence of Tn2010. J Appl Microbiol. 2011;110(1):254–265. doi: 10.1111/j.1365-2672.2010.04875.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim SH, et al. Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: an Asian network for surveillance of resistant pathogens (ANSORP) study. Antimicrob Agents Chemother. 2012;56(3):1418–1426. doi: 10.1128/AAC.05658-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel SN, et al. Susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. Antimicrob Agents Chemother. 2011;55(8):3703–8. doi: 10.1128/AAC.00237-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yanagihara K, et al. Nationwide surveillance of bacterial respiratory pathogens conducted by the surveillance committee of Japanese Society of Chemotherapy, the Japanese Association for Infectious Diseases, and the Japanese Society for Clinical Microbiology in 2010: general view of the pathogens’ antibacterial susceptibility. J Infect Chemother. 2015;21(6):410–420. doi: 10.1016/j.jiac.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Kim L, et al. Biological and epidemiological features of antibiotic-resistant Streptococcus pneumoniae in pre- and post-conjugate vaccine eras: a United States perspective. Clin Microbiol Rev. 2016;29(3):525–552. doi: 10.1128/CMR.00058-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andam CP, Hanage WP. Mechanisms of genome evolution of streptococcus. Infect Genet Evol. 2015;33:334–342. doi: 10.1016/j.meegid.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitney CG, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348(18):1737–1746. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 26.Whitney CG, Klugman KP. Vaccines as tools against resistance: the example of pneumococcal conjugate vaccine. Semin Pediatr Infect Dis. 2004;15(2):86–93. doi: 10.1053/j.spid.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention (CDC). Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease--United States, 1998-2003. MMWR Morb Mortal Wkly Rep. 2005;54(36):893–7. [PubMed]
- 28.Xiao SK, et al. Resistance and serotype distribution of Streptococcus pneumoniae among adults and children in China. Zhonghua Jie He He Hu Xi Za Zhi. 2010;33(8):601–607. [PubMed] [Google Scholar]
- 29.Boulton ML, et al. Trends in childhood pneumococcal vaccine coverage in shanghai, China, 2005-2011: a retrospective cohort study. BMC Public Health. 2016;16:109. doi: 10.1186/s12889-016-2785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruce MG, et al. Impact of the 13-valent pneumococcal conjugate vaccine (pcv13) on invasive pneumococcal disease and carriage in Alaska. Vaccine. 2015;33(38):4813–4819. doi: 10.1016/j.vaccine.2015.07.080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.


