Abstract
High-intensity eccentric muscle contraction induces muscle damage. Damaged muscles recover through different processes, including degeneration, inflammation, regeneration, and fibrosis; some of these processes are mediated through the actions of cytokines. The transforming growth factor-beta (TGF-β) is one such cytokine involved in muscle recovery and repair. In this regard, TGF-β regulates the skeletal muscle inflammatory response, inhibits muscle regeneration, regulates extracellular matrix remodeling, and promotes fibrosis. Although some studies have suggested that inhibition of TGF-β after muscle damage promotes muscle regeneration and recovery, other studies have noted that TGF-β inhibition actually reduces muscle strength because it leads to incomplete muscle regeneration. Despite the importance of TGF-β in the repair of damaged muscles, most studies have focused on examining its role in muscle diseases such as chronic inflammatory diseases or Duchenne’s muscular dystrophy. Here, we have reviewed the existing literature for examining the role of TGF-β in muscle damage and regeneration after eccentric muscle contraction.
Keywords: Eccentric muscle contraction, Muscle damage, Muscle regeneration, Transforming growth factor-β
INTRODUCTION
Repeated high-intensity eccentric muscle contraction induces muscle damage (Stauber et al., 1990). Following injury, muscles recover through a number of steps involving degeneration, inflammation, muscle regeneration, and fibrosis (Quintero et al., 2009; Wong et al., 2015). Various cytokines, such as interleukin-1 beta, interleukin-6, tumor necrosis factor-alpha (TNF-α), fibroblast growth factor, and transforming growth factor-beta (TGF-β), are involved in this healing process (Wong et al., 2015). TGF-β is a multifunctional cytokine that acts on multiple different cell types. In particular, in skeletal muscle, it inhibits myogenic responses, regulates extracellular matrix (ECM) remodeling, and stimulates fibrosis (Kim et al., 2005). Muscle levels of TGF-β have been found to be elevated after eccentric muscle contraction in both animals and humans (Barash et al., 2004; Hamada et al., 2005).
Several studies have reported that inhibition of TGF-β action improves muscle regeneration and recovery from injury by reducing the levels of factors related to muscle damage, such as creatine kinase and fibrosis (Chan et al., 2005; Nozaki et al., 2012; Taniguti et al., 2011). On the other hand, Gumucio et al. (2013) reported that inducing muscle damage through eccentric muscle contraction and at the same time inhibiting TGF-β action resulted in rapid recovery of muscle strength in the short-term, but led to incomplete structural regeneration, ultimately reducing muscle strength in the long-term. These findings suggest that TGF-β plays important roles in the regeneration of damaged muscles. This review article aims at describing the role of TGF-β in muscle damage and regeneration based on a review of the literature pertaining to eccentric muscle contraction.
BIOLOGICAL FUNCTION OF TGF-β IN SKELETAL MUSCLE
TGF-β exists as three subtypes namely TGF-β1, TGF-β2, and TGF-β3 (Gumucio et al., 2015). Myostatin is a new type of cytokine referred to as a ‘myokine’ that also acts through a TGF-β-like signaling pathway (McPherron et al., 1997).
TGF-β is involved in cell growth regulation, wound healing, fibrosis, carcinogenesis, angiogenesis, and inflammation (Kim et al., 2005). During the inflammatory process that occurs after muscle injury, TGF-β plays two different roles: initially, acting as a potential chemotactic factor for neutrophils and monocytes, it stimulates the inflammatory response by inducing the synthesis of TNF-α in monocytes; then, it then suppresses the inflammatory response allowing for recovery from injury to occur (Sanjabi et al., 2009; Wahl, 1994). An in vivo study has confirmed the presence of TGF-β1 at sites of inflammation (Yagnik et al., 2004).
TGF-β also functions in muscle cell differentiation and fusion of myofibers, and can inhibit the expression of various muscle-specific proteins (Florini et al., 1991; Liu et al., 2001). In particular, it is known to delay muscle regeneration because it inhibits cell proliferation and delays muscle cell differentiation through a Smad-3 signaling system pathway, thereby preventing activation of positive regulators of muscle cell differentiation, such as MyoD and myogenin (Liu et al., 2001). Other studies have also reported that TGF-β inhibits the activation of cyclin-dependent kinases and reduces expression of MyoD and myogenin (Gumucio et al., 2015; McCroskery et al., 2003), thereby also limiting the process of differentiation and fusion of satellite cells (Mendias et al., 2012). The inhibition of satellite cell activation induces muscle atrophy. Narola et al. (2013) reported that overexpression of TGF-β1 in mouse muscle induced muscle weakness and atrophy, and Mendias et al. (2012) reported that treating mouse muscle with TGF-β significantly reduced the size of muscle fibers as well as their maximal isometric strength.
TGF-β also regulates ECM remodeling and can stimulate the fibroblasts that produce ECM proteins (Gumucio et al., 2015; Mann et al., 2011). TGF-β is activated by enzymes such as metalloproteinase (MMP)-2 and MMP-9. It inhibits MMP-2 and MMP-9-induced ECM degradation by increasing the secretion of enzymes that inhibit ECM degradation, such as TIMP and plasminogen activator inhibitor-1, while at the same time stimulating the synthesis of ECM proteins (Mann et al., 2011). TGF-β is secreted by damaged muscle fibers (Baoge et al., 2012). This increases the expression of cyclooxygenase-2, thereby escalating the secretion of prostaglandin E2 by fibroblasts (Fang et al., 2014). These elevated levels of prostaglandin E2 may potentially regulate TGF-β-induced fibroblast proliferation and collagen synthesis (Bondesen et al., 2006).
However, overproduction of TGF-β induces progressive deposition of ECM, tissue fibrosis, and scar tissue formation (Border and Noble, 1994). Ultimately, it forms scar tissues, weakening muscle function, inducing pain, and increasing the risk of reinjury (Fig. 1). Chen and Li (2009) stated that scar tissue leads to incomplete functional recovery because it interferes with cell signaling related to differentiation and limits vascular perfusion in the damaged area. Such an action of TGF-β may therefore be a hindering factor in muscle regeneration. Muscle biopsies taken from patients with chronic inflammatory disease or muscular dystrophies show TGF-β1 localization in the ECM or fibrotic connective tissue in the areas of thickening (Bernasconi et al., 1999; Confalonieri et al., 1997).
Fig. 1.
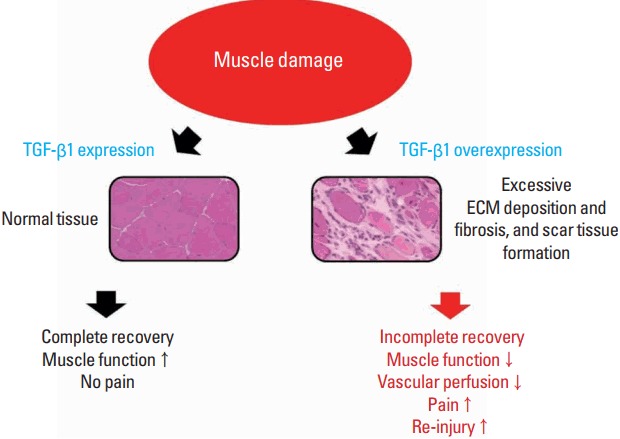
Changes in muscle function caused by TGF-β1 after muscle damage. After muscle damage, TGF-β1 plays a role in muscle recovery by regulating ECM remodeling. However, overexpression of TGF-β1 may induce excessive ECM deposition and fibrosis as well as forming scar tissue, which may lead to incomplete muscle recovery. TGF-β1, transforming growth factor-beta 1; ECM, extracellular matrix.
CHANGE IN MUSCLE TGF-β AFTER DAMAGE CAUSED BY ECCENTRIC MUSCLE CONTRACTION
Most studies have generally explored changes in muscle TGF-β from a pathological perspective, such as examining TGF-β responses in chronic inflammatory myopathy and Duchenne’s muscular dystrophy (Amemiya et al., 2000; Bernasconi et al., 1995), with relatively few studies investigating changes following eccentric muscle contraction.
Animal studies
Several animal studies have observed TGF-β responses to muscle damage through eccentric muscle contraction (Barash et al., 2004; Gumucio et al., 2013; Heinemeier et al., 2007; Smith et al., 2007). The results showed that levels of TGF-β are elevated after eccentric muscle contraction. Smith et al. (2007) reported that 50 repetitions of eccentric contractions with plantar flexor elevated levels of TGF-β1 and TGF-β2 precursors within 48 hr of damage, but such changes did not lead to increased levels of mature TGF-β. Barash et al. (2004) performed 50 repetitions of eccentric contractions in both the tibialis anterior and the extensor digitorum longus in mice and analyzed the gene expression profile 48 hr after exercise using the microarray technique. They found that TGF-β expression was about 2.7 times higher in eccentric contraction legs compared to the unexercised leg or the leg that only underwent isometric contraction. In addition, Heinemeier et al. (2007) reported that electrically inducing muscle contraction in gastrocnemius muscles in mice led to elevated levels of TGF-β1 mRNA regardless of the type of muscle contraction (i.e., isometric, eccentric, or concentric), with the highest level recorded after eccentric muscle contraction. The findings of Barash et al. (2004) and Heinemeier et al. (2007) provide evidence for high levels of TGF-β after eccentric muscle contraction, which may be related to the fact that eccentric muscle contraction induces higher stress and strain in muscles and leads to greater force production and local shear stress during contraction than other types of muscle contraction (Heinemeier et al., 2007). Other studies have reported that TGF-β1 is one of the stress/strain-responsive growth factors (Chiquet et al., 2003), and that TGF-β1 expression is positively correlated with mechanical stimulation of cells (Villarreal and Dillmann, 1992).
Human studies
In a human study, Hamada et al. (2005) instructed the participants to perform eccentric muscle contraction through downhill running and found that, 72 hr after exercise, young male and elderly participants showed increased levels of TGF-β1 mRNA, being 3.7 and 2.0 fold increased respectively. They suggested the underlying cause to be amplified and sustained cellular recruitment related to inflammatory responses at the sites of exercise-induced muscle damage.
The TGF-β response ensuing from eccentric muscle contraction has been reported to not only occur in the immediate period following exercise but is also evident after a longer time, which could be explained by the expression of collagen proteins after eccentric muscle contraction. Hyldahl et al. (2015) reported that expression of collagens I, III, and IV were elevated 27 days after eccentric muscle contraction, and Mackey et al. (2011) also reported that the levels of mRNAs for collagens I and III were considerably increased 30 days after eccentric muscle contraction. Collagen I and III are related to TGF-β signaling. Hyldahl et al. (2015) reported that the levels of the TGF-β receptor type 2 (TGF-βRII) protein, which is one of the components of TGF-β signaling, increased significantly 27 days after eccentric muscle contraction. This is thought to reflect an increase in ECM remodeling after muscle damage because collagen is a key component of the ECM, and TGF-β facilitates remodeling by stimulating the synthesis of ECM proteins (Olczyk et al., 2014).
ROLE OF TGF-β IN MUSCLE DAMAGE AND REGENERATION AFTER ECCENTRIC MUSCLE CONTRACTION
TGF-β and muscle damage
Gumucio et al. (2013) induced muscle damage in the extensor digitorum longus muscle in mice through eccentric muscle contraction and observed the responses during recovery after inhibiting TGF-β. Their findings showed that inhibiting TGF-β in the damaged muscle led to a quick recovery of muscle strength compared to that in the control group at day 3 and day 7 after injury, but after day 21, muscle strength was actually lower than in the control group. Gumucio et al. (2013) suggested that the initial recovery of muscle strength is related to an improvement in tissue morphology, and that the reduction in muscle strength at day 21 may be a result of irregular ECM assembly and atrogin-1 gene expression, both of which promote muscle loss. In fact, Gumucio et al. (2013) observed mottled ECM by immunohistochemistry staining of the damaged muscle 21 days after injury and suggested that the mottled pattern represents an incomplete recovery of the damaged sarcolemma thereby explaining the reduced force transmission. In this latter regard, ECM rich in collagen that envelops muscle fibers can increase force transmission during contraction, so an increase in ECM degradation reduces force transmission (Gao et al., 2008). Incomplete regeneration of ECM may also increase the risk of muscle damage. Mackey et al. (2011) suggested that strengthening the ECM may act as a protective barrier from future injury. In other words, ECM remodeling is essential for strengthening the damaged muscular structure, and through the healthy recovery of muscle, it could induce the “repeated bout effect (Hyldahl et al., 2015; Nosaka and Clarkson, 1995)”.
Changes in muscle structure and function caused by inhibition of TGF-β after injury are similar to those resulting from treatment with anti-inflammatory agents. Some studies have suggested that administering anti-inflammatory agents after eccentric muscle contraction is effective for pain relief (Paulsen et al., 2010; Tokmakidis et al., 2003), but may delay recovery, as it hinders muscle regeneration (Mikkelsen et al., 2009; Mishra et al., 1995). According to Hertel (1997), anti-inflammatory agents can facilitate recovery in the short-term, but in the long run, it may have adverse effects on tissue recovery, structure, and functions. TGF-β is associated with inflammatory responses (Kim et al., 2005), which is an important step for muscle regeneration (Laumonier and Menetrey, 2016). In particular, M2 macrophages induce cellular signaling and production of cytokines for muscle regeneration (Novak et al., 2014). One of these cytokines is TGF-β1, which acts as a switch for converting M1 macrophages to M2 macrophages (Garg et al., 2015). Given that TGF-β is dependent on the presence of macrophages during post-damage recovery (Cromack et al., 1990), TGF-β is speculated to be the link connecting inflammatory responses to muscle regeneration in the recovery phase.
However, TGF-β may need to be inhibited in severe muscle damage, such as contusion or laceration, as such damages require long periods for recovery, during which time muscle atrophy or fibrosis may induce excessive scar tissue formation (Chen and Li, 2009; Mann et al., 2011). Agents such as suramin, which inhibits binding of TGF-β to its receptors and thereby prevents fibrosis, have been used in such cases (Nozaki et al., 2008). According to some studies, postdamage TGF-β inhibition by suramin facilitates improvement of muscle strength by reducing scar tissue formation and promoting differentiation of myoblasts and muscle-derived stem cells (Chan et al., 2005; Nozaki et al., 2008). In addition to suramin, decorin, interferon, and losartan are also used as anti-fibrotic agents (Wong et al., 2015). Based on these findings, TGF-β inhibition should be considered in accordance with the type and severity of muscle damage.
TGF-β and muscle regeneration
Some studies have suggested that TGF-β is important for myoblast fusion and myotube formation and is essential for proper muscle development (Han et al., 2012; Kollias and McDermott, 2008). Recently, Gumucio et al. (2015) suggested that TGF-β should not be considered a negative factor for interfering with satellite cell activation. On the contrary, they asserted that TGF-β can actually help optimize satellite cell activation because premature proliferation, differentiation, and fusion of satellite cells interrupt muscle regeneration and may result in the formation of small-sized muscle fibers (Kollias and McDermott, 2008; Murphy et al., 2011). In other words, activating satellite cells, before the debris from damaged muscle fibers are completely removed through inflammatory responses, may lead to incomplete structural regeneration. Hence, timely activation of TGF-β and satellite cells is thought to be critical for normal and maximal muscle recovery.
CONCLUSIONS
TGF-β is a multifunctional cytokine that is involved in a wide array of activities in muscle, from post-muscle damage inflammation to fibrosis. Various biological responses to muscle damage caused by eccentric muscle contraction, such as inflammation, regeneration, and fibrosis, are interdependent, as opposed to being independent, such that interference with one of these responses would hamper recovery. Activation of TGF-β after eccentric muscle contraction is a normal response following injury and should be considered a vital process to optimize muscle recovery. However, most existing TGF-β studies pertaining to eccentric muscle contraction had limitations in their design, as they were animal studies that used electrical stimulation, as opposed to exercise, to induce muscle contraction. Therefore, current research data is inadequate to elucidate the role of TGF-β in muscle recovery following exercise-induced eccentric muscle contraction. Future studies should further explore the roles of TGF-β in the various events that manifest after exercise-induced eccentric muscle contraction.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- Amemiya K, Semino-Mora C, Granger RP, Dalakas MC. Downregulation of TGF-beta1 mRNA and protein in the muscles of patients with inflammatory myopathies after treatment with high-dose intravenous immunoglobulin. Clin Immunol. 2000;94:99–104. doi: 10.1006/clim.1999.4823. [DOI] [PubMed] [Google Scholar]
- Baoge L, Van Den Steen E, Rimbaut S, Philips N, Witvrouw E, Almqvist KF, Vanderstraeten G, Vanden Bossche LC. Treatment of skeletal muscle injury: a review. ISRN Orthop. 2012;2012:689012. doi: 10.5402/2012/689012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barash IA, Mathew L, Ryan AF, Chen J, Lieber RL. Rapid muscle-specific gene expression changes after a single bout of eccentric contractions in the mouse. Am J Physiol Cell Physiol. 2004;286:C355–364. doi: 10.1152/ajpcell.00211.2003. [DOI] [PubMed] [Google Scholar]
- Bernasconi P, Di Blasi C, Mora M, Morandi L, Galbiati S, Confalonieri P, Cornelio F, Mantegazza R. Transforming growth factor-beta1 and fibrosis in congenital muscular dystrophies. Neuromuscul Disord. 1999;9:28–33. doi: 10.1016/s0960-8966(98)00093-5. [DOI] [PubMed] [Google Scholar]
- Bernasconi P, Torchiana E, Confalonieri P, Brugnoni R, Barresi R, Mora M, Cornelio F, Morandi L, Mantegazza R. Expression of transforming growth factor-beta 1 in dystrophic patient muscles correlates with fibrosis. Pathogenetic role of a fibrogenic cytokine. J Clin Invest. 1995;96:1137–1144. doi: 10.1172/JCI118101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondesen BA, Mills ST, Pavlath GK. The COX-2 pathway regulates growth of atrophied muscle via multiple mechanisms. Am J Physiol Cell Physiol. 2006;290:C1651–1659. doi: 10.1152/ajpcell.00518.2005. [DOI] [PubMed] [Google Scholar]
- Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- Chan YS, Li Y, Foster W, Fu FH, Huard J. The use of suramin, an antifibrotic agent, to improve muscle recovery after strain injury. Am J Sports Med. 2005;33:43–51. doi: 10.1177/0363546504265190. [DOI] [PubMed] [Google Scholar]
- Chen X, Li Y. Role of matrix metalloproteinases in skeletal muscle: migration, differentiation, regeneration and fibrosis. Cell Adh Migr. 2009;3:337–341. doi: 10.4161/cam.3.4.9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquet M, Renedo AS, Huber F, Flück M. How do fibroblasts translate mechanical signals into changes in extracellular matrix production? Matrix Biol. 2003;22:73–80. doi: 10.1016/s0945-053x(03)00004-0. [DOI] [PubMed] [Google Scholar]
- Confalonieri P, Bernasconi P, Cornelio F, Mantegazza R. Transforming growth factor-beta 1 in polymyositis and dermatomyositis correlates with fibrosis but not with mononuclear cell infiltrate. J Neuropathol Exp Neurol. 1997;56:479–484. doi: 10.1097/00005072-199705000-00003. [DOI] [PubMed] [Google Scholar]
- Cromack DT, Porras-Reyes B, Mustoe TA. Current concepts in wound healing: growth factor and macrophage interaction. J Trauma. 1990;30(12 Suppl):S129–133. doi: 10.1097/00005373-199012001-00026. [DOI] [PubMed] [Google Scholar]
- Fang L, Chang HM, Cheng JC, Leung PC, Sun YP. TGF-β1 induces COX-2 expression and PGE2 production in human granulosa cells through Smad signaling pathways. J Clin Endocrinol Metab. 2014;99:E1217–1226. doi: 10.1210/jc.2013-4100. [DOI] [PubMed] [Google Scholar]
- Florini JR, Ewton DZ, Magri KA. Hormones, growth factors, and myogenic differentiation. Annu Rev Physiol. 1991;53:201–216. doi: 10.1146/annurev.ph.53.030191.001221. [DOI] [PubMed] [Google Scholar]
- Garg K, Corona BT, Walters TJ. Therapeutic strategies for preventing skeletal muscle fibrosis after injury. Front Pharmacol. 2015;6:87. doi: 10.3389/fphar.2015.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Kostrominova TY, Faulkner JA, Wineman AS. Age-related changes in the mechanical properties of the epimysium in skeletal muscles of rats. J Biomech. 2008;41:465–469. doi: 10.1016/j.jbiomech.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumucio JP, Flood MD, Phan AC, Brooks SV, Mendias CL. Targeted inhibition of TGF-β results in an initial improvement but long-term deficit in force production after contraction-induced skeletal muscle injury. J Appl Physiol (1985) 2013;115:539–545. doi: 10.1152/japplphysiol.00374.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumucio JP, Sugg KB, Mendias CL. TGF-β superfamily signaling in muscle and tendon adaptation to resistance exercise. Exerc Sport Sci Rev. 2015;43:93–99. doi: 10.1249/JES.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada K, Vannier E, Sacheck JM, Witsell AL, Roubenoff R. Senescence of human skeletal muscle impairs the local inflammatory cytokine response to acute eccentric exercise. FASEB J. 2005;19:264–266. doi: 10.1096/fj.03-1286fje. [DOI] [PubMed] [Google Scholar]
- Han D, Zhao H, Parada C, Hacia JG, Bringas P, Jr, Chai Y. A TGFβ-Smad4-Fgf6 signaling cascade controls myogenic differentiation and myoblast fusion during tongue development. Development. 2012;139:1640–1650. doi: 10.1242/dev.076653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemeier KM, Olesen JL, Haddad F, Langberg H, Kjaer M, Baldwin KM, Schjerling P. Expression of collagen and related growth factors in rat tendon and skeletal muscle in response to specific contraction types. J Physiol. 2007;582(Pt 3):1303–1316. doi: 10.1113/jphysiol.2007.127639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertel J. The role of nonsteroidal anti-inflammatory drugs in the treatment of acute soft tissue injuries. J Athl Train. 1997;32:350–358. [PMC free article] [PubMed] [Google Scholar]
- Hyldahl RD, Nelson B, Xin L, Welling T, Groscost L, Hubal MJ, Chipkin S, Clarkson PM, Parcell AC. Extracellular matrix remodeling and its contribution to protective adaptation following lengthening contractions in human muscle. FASEB J. 2015;29:2894–2904. doi: 10.1096/fj.14-266668. [DOI] [PubMed] [Google Scholar]
- Kim HS, Luo L, Pflugfelder SC, Li DQ. Doxycycline inhibits TGF-beta1-induced MMP-9 via Smad and MAPK pathways in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:840–848. doi: 10.1167/iovs.04-0929. [DOI] [PubMed] [Google Scholar]
- Kollias HD, McDermott JC. Transforming growth factor-beta and myostatin signaling in skeletal muscle. J Appl Physiol (1985) 2008;104:579–587. doi: 10.1152/japplphysiol.01091.2007. [DOI] [PubMed] [Google Scholar]
- Laumonier T, Menetrey J. Muscle injuries and strategies for improving their repair. J Exp Orthop. 2016;3:15. doi: 10.1186/s40634-016-0051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Black BL, Derynck R. TGF-beta inhibits muscle differentiation through functional repression of myogenic transcription factors by Smad3. Genes Dev. 2001;15:2950–2966. doi: 10.1101/gad.925901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey AL, Brandstetter S, Schjerling P, Bojsen-Moller J, Qvortrup K, Pedersen MM, Doessing S, Kjaer M, Magnusson SP, Langberg H. Sequenced response of extracellular matrix deadhesion and fibrotic regulators after muscle damage is involved in protection against future injury in human skeletal muscle. FASEB J. 2011;25:1943–1959. doi: 10.1096/fj.10-176487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann CJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL, et al. Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle. 2011;1:21. doi: 10.1186/2044-5040-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol. 2003;162:1135–1147. doi: 10.1083/jcb.200207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- Mendias CL, Gumucio JP, Davis ME, Bromley CW, Davis CS, Brooks SV. Transforming growth factor-beta induces skeletal muscle atrophy and fibrosis through the induction of atrogin-1 and scleraxis. Muscle Nerve. 2012;45:55–59. doi: 10.1002/mus.22232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen UR, Langberg H, Helmark IC, Skovgaard D, Andersen LL, Kjaer M, Mackey AL. Local NSAID infusion inhibits satellite cell proliferation in human skeletal muscle after eccentric exercise. J Appl Physiol (1985) 2009;107:1600–1611. doi: 10.1152/japplphysiol.00707.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra DK, Fridén J, Schmitz MC, Lieber RL. Anti-inflammatory medication after muscle injury. A treatment resulting in short-term improvement but subsequent loss of muscle function. J Bone Joint Surg Am. 1995;77:1510–1519. doi: 10.2106/00004623-199510000-00005. [DOI] [PubMed] [Google Scholar]
- Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narola J, Pandey SN, Glick A, Chen YW. Conditional expression of TGF-β1 in skeletal muscles causes endomysial fibrosis and myofibers atrophy. PLoS One. 2013;8:e79356. doi: 10.1371/journal.pone.0079356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosaka K, Clarkson PM. Muscle damage following repeated bouts of high force eccentric exercise. Med Sci Sports Exerc. 1995;27:1263–1269. [PubMed] [Google Scholar]
- Novak ML, Weinheimer-Haus EM, Koh TJ. Macrophage activation and skeletal muscle healing following traumatic injury. J Pathol. 2014;232:344–355. doi: 10.1002/path.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki M, Li Y, Zhu J, Ambrosio F, Uehara K, Fu FH, Huard J. Improved muscle healing after contusion injury by the inhibitory effect of suramin on myostatin, a negative regulator of muscle growth. Am J Sports Med. 2008;36:2354–2362. doi: 10.1177/0363546508322886. [DOI] [PubMed] [Google Scholar]
- Nozaki M, Ota S, Terada S, Li Y, Uehara K, Gharaibeh B, Fu FH, Huard J. Timing of the administration of suramin treatment after muscle injury. Muscle Nerve. 2012;46:70–79. doi: 10.1002/mus.23280. [DOI] [PubMed] [Google Scholar]
- Olczyk P, Mencner Ł, Komosinska-Vassev K. The role of the extracellular matrix components in cutaneous wound healing. Biomed Res Int. 2014;2014:747584. doi: 10.1155/2014/747584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen G, Egner IM, Drange M, Langberg H, Benestad HB, Fjeld JG, Hallén J, Raastad T. A COX-2 inhibitor reduces muscle soreness, but does not influence recovery and adaptation after eccentric exercise. Scand J Med Sci Sports. 2010;20:e195–207. doi: 10.1111/j.1600-0838.2009.00947.x. [DOI] [PubMed] [Google Scholar]
- Quintero AJ, Wright VJ, Fu FH, Huard J. Stem cells for the treatment of skeletal muscle injury. Clin Sports Med. 2009;28:1–11. doi: 10.1016/j.csm.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA. Anti-inflammatory and pro-inflammatory roles of TGF-beta, IL-10, and IL-22 in immunity and autoimmunity. Curr Opin Pharmacol. 2009;9:447–453. doi: 10.1016/j.coph.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, Stauber F, Waters C, Alway SE, Stauber WT. Transforming growth factor-beta following skeletal muscle strain injury in rats. J Appl Physiol (1985) 2007;102:755–761. doi: 10.1152/japplphysiol.01503.2005. [DOI] [PubMed] [Google Scholar]
- Stauber WT, Clarkson PM, Fritz VK, Evans WJ. Extracellular matrix disruption and pain after eccentric muscle action. J Appl Physiol (1985) 1990;69:868–874. doi: 10.1152/jappl.1990.69.3.868. [DOI] [PubMed] [Google Scholar]
- Taniguti AP, Pertille A, Matsumura CY, Santo Neto H, Marques MJ. Prevention of muscle fibrosis and myonecrosis in mdx mice by suramin, a TGF-β1 blocker. Muscle Nerve. 2011;43:82–87. doi: 10.1002/mus.21869. [DOI] [PubMed] [Google Scholar]
- Tokmakidis SP, Kokkinidis EA, Smilios I, Douda H. The effects of ibuprofen on delayed muscle soreness and muscular performance after eccentric exercise. J Strength Cond Res. 2003;17:53–59. doi: 10.1519/1533-4287(2003)017<0053:teoiod>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Villarreal FJ, Dillmann WH. Cardiac hypertrophy-induced changes in mRNA levels for TGF-beta 1, fibronectin, and collagen. Am J Physiol. 1992;262(6 Pt 2):H1861–1866. doi: 10.1152/ajpheart.1992.262.6.H1861. [DOI] [PubMed] [Google Scholar]
- Wahl SM. Transforming growth factor beta: the good, the bad, and the ugly. J Exp Med. 1994;180:1587–1590. doi: 10.1084/jem.180.5.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S, Ning A, Lee C, Feeley BT. Return to sport after muscle injury. Curr Rev Musculoskelet Med. 2015;8:168–175. doi: 10.1007/s12178-015-9262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagnik DR, Evans BJ, Florey O, Mason JC, Landis RC, Haskard DO. Macrophage release of transforming growth factor beta1 during resolution of monosodium urate monohydrate crystal-induced inflammation. Arthritis Rheum. 2004;50:2273–2280. doi: 10.1002/art.20317. [DOI] [PubMed] [Google Scholar]


