Abstract
PURPOSE:
To compare endothelial cell density (ECD) loss rates in penetrating keratoplasty (PKP), Descemet's stripping automated endothelial keratoplasty (DSAEK), and deep anterior lamellar keratoplasty (DALK).
DESIGN:
Single-center, multiple-surgeon, retrospective cohort study.
MATERIALS AND METHODS:
Patients who received PKP, DSAEK, or DALK from 2009 to 2014 were analyzed (68 vs. 38 vs. 11 patients, respectively). We excluded patients with therapeutic PKP or regraft, infection, endothelial rejection, or uncontrolled glaucoma. Only clear grafts and initial ECD more than 1000 cell/mm2 were included in the study. The main outcome was ECD loss rate. The follow-up time period was divided into five subgroups: 0–1.5 months, 1.5–6 months, 6–12 months, 12–24 months, and longer than 24 months.
RESULTS:
Average ECD loss rate (cell/mm2/month) declined in all three groups (PKP group: −561.5, −113.2, −36.6, −31.4, and −53.7; DSAEK group: −686.4, −68.3, −21.8, −14.4, and −5.1; DALK group: −576.5, −68, −23.7, 5.9, and 18.3). Although DSAEK group showed faster ECD loss rate in the early postoperative period, it became slower compared to the PKP group within the postoperative 6th month and demonstrated significant difference within 2 years. No ECD loss developed in the DALK group after the 1st postoperative year; this was significantly different from the PKP group.
CONCLUSIONS:
Although ECD loss rate in the DSAEK group was initially larger than that in the PKP group, the DSAEK group possessed better long-term endothelial cell survival rate. The DALK group had a lower ECD loss rate than that of the other groups and maintained a stable ECD at 1 year after surgery.
Keywords: Deep anterior lamellar keratoplasty, Descemet's stripping automated endothelial keratoplasty, endothelial cell density, penetrating keratoplasty
Introduction
Penetrating keratoplasty (PKP) has long been the standard treatment for corneal transplantation and has well-established safety and efficacy rates. Owing to evolving technology, we have treated these diseases with several types of keratoplastic surgeries since the late 20th century. For cases with bullous keratopathy, corneal endothelial transplantation by Descemet's stripping automated endothelial keratoplasty (DSAEK) or Descemet's membrane endothelial keratplasty has the advantages of a more regular corneal contour and faster recovery than that associated with PKP. If the endothelium is not involved, deep anterior lamellar keratoplasty (DALK) is currently considered an alternative surgery that preserves endothelial cells and decreases the risk of endothelial rejection.
Although the techniques are quite different in these three procedures, endothelial cell loss is a common concern after the operation. Endothelial rejection is one type of graft rejection that could damage endothelial cells and reduce corneal transparency. Endothelial cell density (ECD) cannot only represent the condition of the endothelial layer but also predict the prognosis. Even without graft rejection, the ECD of the corneal grafts may still gradually decrease. Therefore, many studies have focused on the ECD after these surgeries. Regarding PKP and DSAEK, some studies[1,2] have demonstrated that DSAEK resulted in lower endothelial cell loss whereas Price et al. revealed a comparable endothelial cell loss between DSEAK and PKP in Fuchs’ dystrophy at 3 postoperative years.[3] As for DALK and PKP, DALK accounted for a more stable ECD in keratoconus[4,5] and macular dystrophy[6] over a long-term period.
Published data on the ECD loss rate among these three procedures are limited, especially in Asian population. Here, we reported the results of the ECD loss rate for these three different types of corneal transplantation among them in National Taiwan University Hospital (NTUH), a tertiary medical center in North Taiwan.
Materials and Methods
This was a retrospective cohort study that enrolled consecutive patients who received PKP, DSAEK, or DALK at NTUH from January 2009 to April 2014. Four surgeons in this single-center performed the procedures. Our study followed the principles of the Declaration of Helsinki, and it was approved by the Institutional Review Board of the NTUH.
We included the patients who met the following criteria: (1) received the procedure for the first time, (2) had a clear graft during OPD follow-up, (3) had an initial postoperative ECD more than 1000 cell/mm2 and (4) had a minimum postoperative follow-up of 1 year. The exclusion criteria were as follows: (1) therapeutic indication for infection control such as corneal ulcer or keratouveitis, (2) repeated keratoplastic surgical history, (3) documented rejection episode during outpatient clinic follow-up, and (4) postoperative infection or uncontrolled glaucoma.
One hundred and seventeen patients undergoing PKP, DSAEK, or DALK (68, 38, and 11 patients, respectively) were enrolled in the study. The ECD of the central corneal graft was measured by two experienced technicians using corneal confocal microscopy (Nidek-ConfoScan 3, Tokyo, Japan). The follow-up time period was divided into five subgroups: 0–1.5 months, 1.5–6 months, 6–12 months, 12–24 months, and longer than 24 months. The main outcome measured was ECD loss velocity among the groups. The ECD loss velocity was defined as the difference in ECD divided by the duration of the two consecutive time points. It can be presented as the following equation:
ECD loss velocity (t1 + t2)/2= (ECDt2 − ECDt1)/(t2 − t1)
Where t1 and t2 represent two consecutive time points. Demographic data, surgical indications, and postoperative steroid medications were also examined to compare the groups.
Statistical analysis
Baseline characteristics, such as age and ECD, were compared among the three groups with an ANOVA test; sex was compared with Chi-square test. The results for ECD loss rates were compared between two keratoplastic procedures (PKP vs. DSAEK and PKP vs. DALK) using a Student's t-test first and subsequently by multiple regression analysis with adjustment for age and sex. P < 0.05 was considered statistically significant. For all statistical analyses, SAS 9.4 (SAS Institute Inc., Cary, NC, USA) was used.
Results
The summary of baseline characteristic data is shown in Table 1. Although most of the patients underwent PKP, the number of patients receiving DSAEK and DALK has continued to increase. The average age was oldest in the DSAEK group and youngest in the DALK group. The baseline ECD in the PKP and DSAEK groups represented the preoperative ECD of the donor corneas, whereas it represented the preoperative ECD of the recipient corneas in the DALK group. In view of these baseline ECD values, the ECD between the PKP and DALK groups was comparable, and the DSAEK group had a higher ECD than that of the other two groups.
Table 1.
Demographic data of penetrating keratoplasty, Descemet's stripping automated endothelial keratoplasty, and deep anterior lamellar keratoplasty
| PKP (n=68) | DSAEK (n=38) | DALK (n=11) | P | |
|---|---|---|---|---|
| Mean±SD (age in years) | 53.5±21.1 | 64.36±12.5 | 38.93±19.7 | 0.0003 |
| Gender (%) | ||||
| Male | 31 (46) | 15 (39) | 5 (45) | 0.8237 |
| Female | 37 (54) | 23 (61) | 6 (55) | |
| Baseline ECD, mean±SD (cells/mm2) | 2600±289 | 2806±355 | 2651±503 | 0.0157 |
+Baseline ECD: In PKP and DSAEK group, the baseline ECD represents the donor ECD. In DALK, it means the recipient ECD. SD = Standard deviation, ECD = Endothelial cell density, DALK = Deep anterior lamellar keratoplasty, PKP = Penetrating keratoplasty, DSAEK = Descemet's stripping automated endothelial keratoplasty
In our hospital, PKP had the widest range of indications, varying from stromal surgical opacity to corneal edema. Corneal opacity was the most common indication for PKP. Bullous keratopathy was the main reason in the DSAEK group, and the DALK group was composed of keratoconus and corneal opacity with ECD within the normal range [Table 2]. We examined the postoperative steroid medication at the latest follow-up and found that 0.1% betamethasone was predominant in the PKP and DSAEK groups (86.8% in both groups). Only a few patients were maintained with 0.1% fluorometholone or no steroid over a long-term period. The DALK group had almost equal proportions of 0.1% betamethasone and 0.1% fluorometholone, which was quite different from the other two groups.
Table 2.
Surgical indication of penetrating keratoplasty, Descemet's stripping automated endothelial keratoplasty, and deep anterior lamellar keratoplasty
| Surgical indication | Number of cases | ||
|---|---|---|---|
| PKP (%) | DSAEK (%) | DALK (%) | |
| Bullous keratopathy | 17 (25) | 24 (63) | |
| Fuchs’ dystrophy | 5 (7) | 10 (26) | |
| ICE syndrome | 4 (11) | ||
| Corneal opacity | 30 (44) | 5 (45) | |
| Keratoconus | 9 (13) | 6 (55) | |
| Corneal dystrophy | 3 (5) | ||
| Corneal melting | 3 (5) | ||
| Peter's anomaly with sclera and cornea | 1 (1) | ||
| Total number of cases | 68 | 38 | 11 |
DALK = Deep anterior lamellar keratoplasty, PKP = Penetrating keratoplasty, DSAEK = Descemet's stripping automated endothelial keratoplasty, ICE = Iridocorneal endothelial
The trends of ECD loss rate in the three groups showed a maximum in the early postoperative 1½ months (DALK, PKP, and DSAEK: −576.5 ± 522.9, −561.5 ± 425.7, and −686.4 ± 447.7 cells/mm2/month, respectively). Thereafter, it declined gradually and became stationary after 1 year [Figure 1]. After we adjusted the age and sex, we found the ECD loss rate was highest in the DSAEK group initially but became lower than that of the PKP group at the end of 6th postoperative month and reached a statistically significant difference in the 2nd postoperative year [Table 3]. Similarly, the ECD loss rate in the DALK group gradually became slower than that of the PKP group and reached significance in the first and second postoperative years [Table 4]. In addition, we found that the value of the ECD was not correlated with the loss rate of the ECD throughout the study period.
Figure 1.
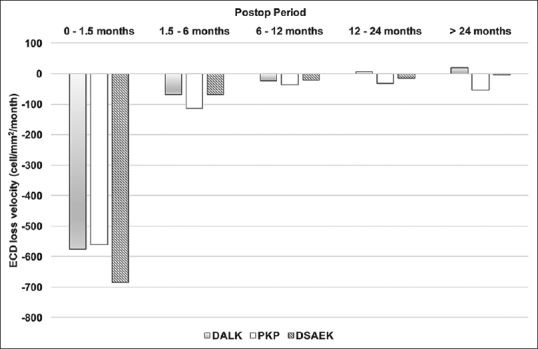
The postoperative ECD loss rate in the three groups. The ECD loss rate between the DALK and PKP groups was comparable initially, but the DALK became stationary at 1 year postoperatively. The ECD loss rate in the DSAEK group was the highest in the beginning but declined within 6 months postoperatively. DALK = Deep anterior lamellar keratoplasty, PKP = Penetrating keratoplasty, DSAEK = Descemet's stripping automated endothelial keratoplasty, ECD = Endothelial cell density
Table 3.
Comparison of endothelial cell density loss rate between penetrating keratoplasty and Descemet's stripping automated endothelial keratoplasty
| Follow-up period (months) | ECD loss rate, mean±SD (cells/mm2/month) | P | ||
|---|---|---|---|---|
| PKP | DSAEK | t-test | Adjusted for age and sex | |
| 0-1.5 | −561.5±425.7 | −686.4±447.7 | 0.36 | 0.78 |
| 1.5-6 | −113.2±121.4 | −68.3±152.4 | 0.15 | 0.053 |
| 6-12 | −36.6±63.5 | −21.8±77.2 | 0.46 | 0.54 |
| 12-24 | −31.4±42.0 | −14.4±45.0 | 0.27 | 0.29 |
| >24 | −53.7±14.0 | −5.1±13.0 | 0.001* | 0.012* |
*P<0.05. PKP = Penetrating keratoplasty, DSAEK = Descemet's stripping automated endothelial keratoplasty, SD = Standard deviation, ECD = Endothelial cell density
Table 4.
Comparison of endothelial cell density loss rate between penetrating keratoplasty and deep anterior lamellar keratoplasty
| Follow-up period (months) | ECD loss rate, mean±SD (cells/mm2/month) | P | ||
|---|---|---|---|---|
| PKP | DALK | t-test | Adjusted for age and sex | |
| 0-1.5 | −561.5±425.7 | −576.5±522.9 | 0.38 | 0.33 |
| 1.5-6 | −113.2±121.4 | −68±142.4 | 0.32 | 0.59 |
| 6-12 | −36.6±63.5 | −23.7±37.5 | 0.59 | 0.42 |
| 12-24 | −31.4±42.0 | 5.9±24.8 | 0.008* | 0.032* |
| >24 | −53.7±14.0 | 18.3±33.1 | 0.007* | 0.049* |
*P<0.05. PKP = Penetrating keratoplasty, DALK = Deep anterior lamellar keratoplasty, SD = Standard deviation, ECD = Endothelial cell density
Discussion
Our main interest in the study was the natural course of ECD loss in the above-mentioned three keratoplastic procedures. Acute endothelial rejection may occur in patients undergoing PKP or DSAEK but not in patients with DALK. Furthermore, the onset period of acute corneal rejection and subsequent treatments varies greatly between cases, which could deeply influence the ECD. To minimize variation in the three groups, we excluded the acute endothelial rejection cases during enrollment for our study. Other complicated cases, such as active or chronic infections, repeated keratoplastic surgeries and postoperative uncontrolled glaucoma, were also excluded to elucidate the natural course of these three procedures with less bias. Compared to PKP, the DSAEK and DALK surgeries are relatively new and difficult. The beginners need deep learning curves. Then, we excluded all complication cases to reduce the bias of different keratoplastic procedures. To minimize the effect of the different disease entities and different surgeons, we chose the cases with initial postoperative ECD more than 1000 cells/mm2 to make the three groups have similar initial conditions before analyzing their natural courses.
It is believed that corneal endothelial cells are responsible for graft survival in any kind of keratoplastic surgery. The normal corneal ECD declines with age and the physiologic loss is approximately 0.6% per year.[7] PKP has been shown to have accelerated endothelial cell loss with a rate of 28.8% after 6 months, 39.8% after 12 months, 49% after 24 months,[8] and between 69% and 75% after 5 years.[9] The contributing factors include the initial surgical trauma, cellular interactions between the donor and recipient,[10] immune reactions,[11,12,13] accelerated cellular aging, secondary glaucoma as well as donor status and donor preservation conditions.[4,14] In contrast to PKP, DSAEK seems to be associated with less chronic immune reactions and a substantially smaller surgical wound. Low immune reaction in DSAEK had been postulated to relate to reduction in suture numbers, reduced immunogenicity of endothelium graft, and no exposure to surface which made the antigen presenting cells more difficult to access the graft.[15] However, due to vigorous manipulation of the endothelial graft during surgery, the endothelial cell loss might be tremendous in the early postoperative period and result in primary graft failure. Several articles have focused on the difference in the ECD loss rate between PKP and DSAEK. The average ECD loss percentage increased with time in both PKP and DSAEK groups (PKP vs. DSAEK: 6 months: 34% vs. 11%, 1 year: 28%–32% vs. 20%–35.8%, 2 years: 45% vs. 36%, 3 years: 47%–48% vs. 39%–53%, 5 years: 60.9 vs. 48.7%, and 10 years: 70%–82% vs. 53%–80%).[1,16,17,18,19,20] In addition, cell loss increased by only 6%–7% between 6 months and 2 years postoperatively for endothelial keratoplasty, but the loss was 25% for PKP.[9,21]
Regarding ECD loss in the DSAEK and PKP groups, there have been different points of view among ophthalmologists. Price et al. suggested that DSAEK had a substantially high ECD loss rate within the 1st year but less cell loss in subsequent years. At 3 years, however, the median endothelial cell loss was comparable for the DSAEK and PKP groups, and the results were persistent in the subjects with Fuchs’ dystrophy and non-Fuchs’ dystrophy.[3] On the contrary, Ang et al. showed that DSAEK resulted in lower endothelial cell loss for up to 3 years in patients with Fuchs’ dystrophy and bullous keratopathy.[1]
In our study, we did not measure the ECD of donors after preparation of DSAEK grafts, which could cause more ECD loss and explained the DSAEK group had a substantially high ECD loss rate in the early postoperative period. However, the ECD loss rate decreased to less than that of the PKP group within the 6th postoperative month. Although it was not significant initially, the trend in the difference between the two groups persisted and reached significance in the 1st postoperative year. Similar results in another study showed significantly lower ECD in the DSAEK group until 6 months, which increased to higher than that of the PKP group at postoperative 2 years.[17] The initial large ECD loss might indicate that surgical trauma and manipulation were greater in the DSAEK group than in the PKP group. PKP is a well-established procedure that is familiar to experienced corneal specialists, whereas DSAEK was not performed frequently at the NTUH until 2009. Therefore, it was reasonable that the DSAEK group had more ECD loss than that in the other two groups in the early surgical period. However, the ECD loss rate in the DSAEK group became less than that of the PKP group within 6 months, and the loss velocity of the ECD was not correlated to the value of the postoperative ECD. More immunological loads, stronger inflammation reactions, and longer wound healing in PKP as compared to DSAEK could explain this phenomenon. Our results of the ECD loss rate were also consistent with other studies[1,9,21] that focused on the loss percentage of ECD. Therefore, we were in agreement in that DSAEK had less endothelial cell loss than PKP over a long period.
With evolving surgical techniques, DALK has become the alternative procedure for patients with corneal stromal diseases such as keratoconus, stromal dystrophy, or corneal opacities. Theoretically, the ECD would be more stable in DALK than in PKP because Descemet's membrane and the endothelial layer of the recipients are preserved in DALK. However, the postoperative ECD loss in DALK was still found and could be related to surgical injury and postoperative inflammation. In general, the reported long-term endothelial cell loss was lower in DALK than in PKP.[4,6,22,23] One randomized multicenter clinical trial revealed that the ECD loss was significantly higher after PKP compared with that after DALK procedures performed without perforation of Descemet's membrane in patients with corneal stromal pathology but was not different from the cases with perforation of Descemet's membrane while performing DALK.[24] The ECD loss rate in the DALK group in our study demonstrated nearly no ECD loss after the 1st postoperative year; even the initial loss rate was comparable with the PKP group.
The postoperative steroid usage somehow reflected the difference in ECD stability in these three groups. In the DALK group, the clinicians tended to use less potent topical corticosteroid (e.g., 0.1% fluorometholone), whereas the majority of the PKP and DSAEK groups were treated with 0.1% betamethasone. In our opinion, long-term steroids may not be necessary in the DALK group if there is no obvious stromal rejection.
The retrospective design was the main limitation of this study. In addition, the variation in the time for the ECD measurement, different surgeons and surgical techniques, and variant postoperative management are all issues to be considered. Different technicians and possible different measurement locations on the cornea may lead to some variability in the ECD data. We included diverse etiologies of corneal diseases that were different from other studies, which focused on specific disease etiology, and this might have caused potential variations in this study as well. To minimize this, we excluded the cases with complications. We selected less complicated cases and chose a follow-up period of at least 1 year to determine the natural course of the ECD loss rate in these three groups of patients.
Our study in eyes of Asian patients showed that the ECD loss rate in the DSAEK group was more in the early postoperative period but became slower after the 2nd postoperative year than that of the PKP. As for DALK, it resulted in a stable ECD after the 1st postoperative year and may not need long-term steroid therapy.
Conclusions
Initially postoperative ECD loss rate in the DSAEK group was larger than that in the PKP group, but the DSAEK group possessed better long-term endothelial cell survival rate in two years later. The DALK group had a lower ECD loss rate than that of the other two groups and maintained a stable ECD at one year after surgery. DALK patients may not need long-term use of steroid.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors declare that there are no conflicts of interests of this paper.
References
- 1.Ang M, Mehta JS, Lim F, Bose S, Htoon HM, Tan D. Endothelial cell loss and graft survival after Descemet's stripping automated endothelial keratoplasty and penetrating keratoplasty. Ophthalmology. 2012;119:2239–44. doi: 10.1016/j.ophtha.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 2.Hjortdal J, Ehlers N. Descemet's stripping automated endothelial keratoplasty and penetrating keratoplasty for Fuchs’ endothelial dystrophy. Acta Ophthalmol. 2009;87:310–4. doi: 10.1111/j.1755-3768.2008.01492.x. [DOI] [PubMed] [Google Scholar]
- 3.Price MO, Gorovoy M, Price FW, Jr, Benetz BA, Menegay HJ, Lass JH. Descemet's stripping automated endothelial keratoplasty: Three-year graft and endothelial cell survival compared with penetrating keratoplasty. Ophthalmology. 2013;120:246–51. doi: 10.1016/j.ophtha.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kubaloglu A, Koytak A, Sari ES, Akyol S, Kurnaz E, Ozerturk Y. Corneal endothelium after deep anterior lamellar keratoplasty and penetrating keratoplasty for keratoconus: A four-year comparative study. Indian J Ophthalmol. 2012;60:35–40. doi: 10.4103/0301-4738.90490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu H, Chen Y, Wang P, Li B, Wang W, Su Y, et al. Efficacy and safety of deep anterior lamellar keratoplasty vs. penetrating keratoplasty for keratoconus: A meta-analysis. PLoS One. 2015;10:e0113332. doi: 10.1371/journal.pone.0113332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sogutlu Sari E, Kubaloglu A, Unal M, Pinero D, Bulut N, Erol MK, et al. Deep anterior lamellar keratoplasty versus penetrating keratoplasty for macular corneal dystrophy: A randomized trial. Am J Ophthalmol. 2013;156:267–74.e1. doi: 10.1016/j.ajo.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Bourne WM, Hodge DO, Nelson LR. Corneal endothelium five years after transplantation. Am J Ophthalmol. 1994;118:185–96. doi: 10.1016/s0002-9394(14)72898-3. [DOI] [PubMed] [Google Scholar]
- 8.Bertelmann E, Pleyer U, Rieck P. Risk factors for endothelial cell loss post-keratoplasty. Acta Ophthalmol Scand. 2006;84:766–70. doi: 10.1111/j.1600-0420.2006.00726.x. [DOI] [PubMed] [Google Scholar]
- 9.Cornea Donor Study Investigator Group. Lass JH, Gal RL, Dontchev M, Beck RW, Kollman C, et al. Donor age and corneal endothelial cell loss 5 years after successful corneal transplantation. Specular microscopy ancillary study results. Ophthalmology. 2008;115:627–32.e8. doi: 10.1016/j.ophtha.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wollensak G, Green WR. Analysis of sex-mismatched human corneal transplants by fluorescence in situ hybridization of the sex-chromosomes. Exp Eye Res. 1999;68:341–6. doi: 10.1006/exer.1998.0611. [DOI] [PubMed] [Google Scholar]
- 11.Musch DC, Schwartz AE, Fitzgerald-Shelton K, Sugar A, Meyer RF. The effect of allograft rejection after penetrating keratoplasty on central endothelial cell density. Am J Ophthalmol. 1991;111:739–42. doi: 10.1016/s0002-9394(14)76782-0. [DOI] [PubMed] [Google Scholar]
- 12.Bertelmann E, Hartmann C, Scherer M, Rieck P. Outcome of rotational keratoplasty: Comparison of endothelial cell loss in autografts vs. allografts. Arch Ophthalmol. 2004;122:1437–40. doi: 10.1001/archopht.122.10.1437. [DOI] [PubMed] [Google Scholar]
- 13.Birnbaum F, Reinhard T, Böhringer D, Sundmacher R. Endothelial cell loss after autologous rotational keratoplasty. Graefes Arch Clin Exp Ophthalmol. 2005;243:57–9. doi: 10.1007/s00417-004-0902-2. [DOI] [PubMed] [Google Scholar]
- 14.Böhringer D, Reinhard T, Spelsberg H, Sundmacher R. Influencing factors on chronic endothelial cell loss characterised in a homogeneous group of patients. Br J Ophthalmol. 2002;86:35–8. doi: 10.1136/bjo.86.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prakash G, Jhanji V, Titiyal JS. Will Descemet's stripping with automated endothelial keratoplasty (DSAEK) lower the rates of allograft rejection in corneal transplants for endothelial failure? Med Hypotheses. 2007;69:1117–9. doi: 10.1016/j.mehy.2007.01.083. [DOI] [PubMed] [Google Scholar]
- 16.Price MO, Gorovoy M, Benetz BA, Price FW, Jr, Menegay HJ, Debanne SM, et al. Descemet's stripping automated endothelial keratoplasty outcomes compared with penetrating keratoplasty from the Cornea Donor Study. Ophthalmology. 2010;117:438–44. doi: 10.1016/j.ophtha.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishiyama S, Mori Y, Nejima R, Tokudome T, Shimmura S, Miyata K, et al. Comparison of long-term outcomes of visual function and endothelial cell survival after Descemet stripping automated endothelial keratoplasty and penetrating keratoplasty using mixed-effects models. Cornea. 2016;35:1526–32. doi: 10.1097/ICO.0000000000001003. [DOI] [PubMed] [Google Scholar]
- 18.Ang M, Mehta JS, Anshu A, Wong HK, Htoon HM, Tan D. Endothelial cell counts after Descemet's stripping automated endothelial keratoplasty versus penetrating keratoplasty in Asian eyes. Clin Ophthalmol. 2012;6:537–44. doi: 10.2147/OPTH.S26343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ang M, Soh Y, Htoon HM, Mehta JS, Tan D. Five-year graft survival comparing descemet stripping automated endothelial keratoplasty and penetrating keratoplasty. Ophthalmology. 2016;123:1646–52. doi: 10.1016/j.ophtha.2016.04.049. [DOI] [PubMed] [Google Scholar]
- 20.Price MO, Calhoun P, Kollman C, Price FW, Jr, Lass JH. Descemet stripping endothelial keratoplasty: Ten-year endothelial cell loss compared with penetrating keratoplasty. Ophthalmology. 2016;123:1421–7. doi: 10.1016/j.ophtha.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Busin M, Bhatt PR, Scorcia V. A modified technique for descemet membrane stripping automated endothelial keratoplasty to minimize endothelial cell loss. Arch Ophthalmol. 2008;126:1133–7. doi: 10.1001/archopht.126.8.1133. [DOI] [PubMed] [Google Scholar]
- 22.Borderie VM, Sandali O, Bullet J, Gaujoux T, Touzeau O, Laroche L. Long-term results of deep anterior lamellar versus penetrating keratoplasty. Ophthalmology. 2012;119:249–55. doi: 10.1016/j.ophtha.2011.07.057. [DOI] [PubMed] [Google Scholar]
- 23.Zhang YM, Wu SQ, Yao YF. Long-term comparison of full-bed deep anterior lamellar keratoplasty and penetrating keratoplasty in treating keratoconus. J Zhejiang Univ Sci B. 2013;14:438–50. doi: 10.1631/jzus.B1200272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng YY, Visser N, Schouten JS, Wijdh RJ, Pels E, van Cleynenbreugel H, et al. Endothelial cell loss and visual outcome of deep anterior lamellar keratoplasty versus penetrating keratoplasty: A randomized multicenter clinical trial. Ophthalmology. 2011;118:302–9. doi: 10.1016/j.ophtha.2010.06.005. [DOI] [PubMed] [Google Scholar]


