Abstract
Objective
The need for effective non-hormonal treatments for hot flash management without unwanted side effects continues. The primary aim of this pilot study was to evaluate the effect of combining a non-hormonal pharmacologic agent with a behavioral treatment for hot flash reduction.
Method
71 postmenopausal women were randomized to one of four groups: venlafaxine 75 mg + hypnosis (VH) versus venlafaxine 75 mg + sham hypnosis (VSH) versus a placebo pill + hypnosis (PH) versus placebo pill + sham hypnosis (PSH). Women recorded hot flash severity and frequency in a daily diary, in real time. The intra-patient difference in hot flash score (frequency × severity) at 8 weeks was analyzed using a General Estimating Equation model, using VSH as the referent arm, controlling for baseline hot flashes.
Results
The active arms including PH or VH were not statistically significantly different than VSH (p=.34, p=.05, respectively). Women in each active arm reported hot flash reductions of about 50%, with the PSH group reporting a 25% reduction. Women receiving the PSH reported statistically significantly smaller reductions in hot flash score than women in the referent VSH arm (p=.001). There were no significant negative side effects during the course of the study.
Conclusion
Hypnosis alone reduced hot flashes equal to venlafaxine alone, but the combination of hypnosis and venlafaxine did not reduce hot flashes more than either treatment alone. More research is needed to clarify whether combining hypnosis with a different antidepressant would provide synergistic benefits.
Keywords: hot flashes/non-pharmacologic treatment, hot flashes/drug therapy, hot flashes/hypnosis, hot flashes/behavioral intervention, menopause, post-menopause, quality of life, breast cancer survivorship
Introduction
Hot flashes are experienced by up to 75% of women in menopause including those with a history of cancer.[1] For many women, hot flashes persist for up to 2 to 4 years and then subside.[2] About 25% of women, however, can experience hot flashes for as many as 15 or more years.[2-4] Although the median age of menopause is 51 years, hot flashes can begin up to 8 years earlier, in the perimenopausal phase.[2, 3]
In cancer survivors, menopause can come prematurely, with hot flashes beginning long before others in their peer group.[5] Endocrine therapies for breast cancer (i.e., tamoxifen and aromatase inhibitors) are associated with hot flashes in a majority of women.[6-9] In general, studies have shown that women with a history of breast cancer experiencing menopause are more likely to experience symptoms than the non-cancer population and experience more severe symptoms.[5, 7]
Overall, for women experiencing hot flashes whether prematurely from cancer treatment or as a result of menopause, they can be a source of bother and distress and can negatively impact quality of life including a loss of productivity.[10-13]
Current Treatment Options
The gold standard for treatment of hot flashes was, until the year 2000, estrogen-based therapy reducing both the frequency and severity of hot flashes by up to 90%.[14, 15] Current research provides insights about the risk/benefit of estrogen therapy for hot flashes with a potential for an increased risk of breast cancer, blood clots and less than expected benefits with respect to cardiovascular and cognitive health making the use of estrogen based therapy less attractive.[16]
Amongst the most effective non-hormonal alternatives for hot flash management are serotonergic antidepressants [17-22] and the anticonvulsant, gabapentin.[23-25] For most of these agents, a 50 to 60% reduction can be achieved in many women.[23] However, unwanted side effects, particularly related to sexual side effects with serotonergic antidepressants and dizziness with gabapentin, inconvenient dosing and negative stigma associated with antidepressants limit the use of these agents. In addition, not all women benefit from these agents and long term efficacy is not known.
Longer term use of hot flash management interventions have been necessitated with the emergence of tamoxifen and aromatase inhibitors; particularly since recent data demonstrate benefit in the treatment of breast cancer with up to 10 years of this endocrine treatment.[26] Behavioral therapies that could potentially be taught to women to do at home represent a viable alternative for women with bothersome hot flashes. In fact, at least one descriptive study attests to the fact that over half of women surveyed state they would be interested in learning about or would prefer behavioral treatments for menopausal symptoms.[27]
One interesting option
Hypnosis is a behavioral intervention whose biologic plausibility to reduce hot flashes lies in the relaxation response and as a trigger to reduce core body temperature based on cooling imagery. Hypnosis is a behavioral mind-body therapy that can be defined as a deep relaxed state involving focused attention, mental imagery, an altered state of consciousness, imagination, and an enhanced capacity for response to suggestion.[28] It is a condition or state where relevant suggestions can produce alterations of perception, memory or mood. A hypnotic induction may for hot flashes is focused on reducing core body temperature and inducing a deep relaxation response. Specific suggestions may include being in a pleasant place where one can feel a cool breeze, or drinking cool water. Hypnosis has been studied in women with a history of breast cancer, being compared to a wait list control group.[29] and in general postmenopausal women compared to an attention control group.[30] In both of these studies, hypnosis was able to reduce the hot flash score about 70%.[29, 30] This reduction is still less than what would be expected from estrogen-based therapy.
Therefore, based on the need for more effective treatments for hot flashes, we developed a pilot proof of concept study to evaluate the combination of two effective interventions, an antidepressant and hypnosis. We conducted a prospective, randomized, single blind trial to evaluate the effect of venlafaxine in combination with a hypnotic relaxation intervention on hot flash frequency and severity in addition to the bother related to hot flashes. Venlafaxine extended release (XL) was chosen as the antidepressant agent as it was the most studied, well established non-hormonal treatment for hot flashes at the time this study was developed and is taken once daily.
The primary aim of the study was to evaluate the effect of venlafaxine XL 75 mg with hypnosis (VH) compared to venlafaxine alone (VSH) for the reduction of hot flashes and our primary hypothesis was that the combination treatment (VH) would reduce hot flashes more than venlafaxine + sham hypnosis (VSH). Secondary aims included an evaluation of the side effects and effects on bother related to hot flashes. In addition, impression of change and satisfaction with assigned treatment were assessed.
Methods
Adult postmenopausal women who were eligible for the trial included those with and without a history of breast cancer who either could not or did not wish to take estrogen for hot flash relief. We sought to include women with and without breast cancer since previous studies indicate both groups of women respond to hot flashes similarly and both have a need for more treatment options [6,21-23, 29,30]. Women had to report bothersome hot flashes for at least the past month, at a frequency of at least 4 per day. They could be taking endocrine therapy (tamoxifen or an aromatase inhibitor) but could not be planning to discontinue this therapy during the trial. Women could not have allergies to venlafaxine or related drugs (selective serotonin reuptake inhibitors) and could not be on any other pharmacologic agents for hot flashes during the study. They could not have used either venlafaxine or hypnosis in the past 6 months for any reason, nor could they use other antidepressants during the study for any reason. Women with uncontrolled hypertension were also excluded, defined as 3 consecutive readings over the past year of >160 systolic/100 diastolic.
All potential participants were educated about the study in a neutral way in order to keep the study hypothesis blinded and decrease bias. Women were told that they would be randomized to receive venlafaxine or a placebo pill in addition to one of two types of behavioral treatments, one involving hypnosis and the other involving white noise as a means to focus their attention on their hot flashes in a helpful way. The white noise arm served to control for the non-specific effects of the intervention as it was equivalent in time and attention to the hypnosis arm but did not include the active components of hypnosis: trance and suggestions. Women eligible and consenting to the study were randomized to one of the four following treatment conditions: 1) venlafaxine 75 mg plus hypnosis (VH), 2) venlafaxine 75 mg plus sham hypnosis (VSH), 3) placebo pill plus hypnosis (PH) or 4) placebo pill plus sham hypnosis (PSH).
The study was approved by the Mayo Clinic Foundation Institutional Review Board as well as the Baylor University Institutional Review Board. Written informed consent was obtained by all participants and the trial was registered on the NIH clinical trials website, NCT 01000623. All participants were recruited from the Mayo Clinic, Rochester, MN.
A table of random numbers was created by one of the statisticians without involvement of study staff. These numbers corresponded to one of the four treatment arms and were put into sealed envelopes kept in a locked cabinet. After participants signed consent at the Mayo Clinic and were ready for randomization, the study therapist opened the next sealed envelope for the treatment assignment number. The venlafaxine was over-encapsulated so that both placebo and venlafaxine were identical. The study coordinator who was recruiting participants and collecting data was blinded to the participant's treatment assignment.
Intervention
After randomization, beginning in week 1 and throughout the study, all women kept track of their hot flashes in a daily diary, indicating the number and severity of each hot flash in every 24 hour period. Women were instructed on how to record their hot flashes in real time using a small scratch pad and enter the totals into the diary every day.
Beginning week 2, women began taking their study medication (venlafaxine 37.5 mg or placebo pill) once per day. Women titrated the study medication (venlafaxine 37.5 mg or placebo pill) to two pills per day during week 3 and remained on two pills per day through the end of the study.
Beginning week 2 and continuing through week 5, each woman had weekly visits with the study therapist to learn about and experience either hypnosis or sham hypnosis (white noise) for a total of 4 in-person visits. Weeks 6 through 8, women continued the study medication and their behavioral treatment at least 4 times per week at home, using a CD.
The hypnosis intervention was the same intervention developed and tested previously [29, 30] and consisted of a standard hypnotic induction that provides for the experience of deep relaxation, feelings of safety and comfort. The focus for hot flash reduction involves two key suggestions, one for stress reduction and one for coolness. Participants also received suggestions to dissociate from the anxiety and feelings of lack of control that accompany the hot flash experience. The hypnotic induction took about 20 minutes. The dose of the intervention was also based on previous studies in hypnosis that included 5 in-person visits.[29,30]. We used 4 in-person visits followed by three weeks of home practice in an attempt to decrease the burden of the intervention with clinic visits since these women were healthy and not already going to the clinic. The therapist interaction at each face-to-face session included a discussion of the hot flash experience, the experience the participant was having with the hypnotic intervention and problem solving potential barriers for home practice. Hypnotizability was evaluated at the end of the last face-to-face session. Due to a lack of power, this variable was to be looked at descriptively to evaluate whether it influenced participants' hot flash responses to hypnosis. The results will be reported in a separate paper. Hypnotizability was measured at the end of the last therapist session so as to not bias the therapist toward a perceived outcome. Depth of hypnotic state was not otherwise assessed or measured as the hypnosis was delivered in a standardized way, matching the script to the participant's relaxation but different strategies were not used to try to elicit a certain hypnotic state. This was in order to have a more generalizable intervention.
The sham hypnosis also included a face-to-face interaction with the study therapist for 4 weeks and the use of a CD containing white noise. The white noise was used to assist with the ability to focus attention, by minimizing background noise. The participants were instructed to listen to the white noise CD for 15 minutes, during the face-to-face interactions as well as 4 times per week at home, and to think about their hot flashes in any way they thought might be helpful. During the in-person sessions, before the participant would listen to the white noise CD, the therapist interaction consisted of a discussion very similar to that in the hypnosis intervention: a discussion of the participant's experience with the white noise exercise, problem solving potential barriers to using the CD four times per week and continued discussion about their hot flash experience and coping strategies. This conversation was general and did not include therapist directed information about how to use the white noise, but rather a positive confirmation of what the participant was finding helpful on her own. After the therapist discussion, the participant then listened to the white noise CD and focused her attention on her hot flashes in any way she wished.
Training to deliver the hypnosis and sham hypnosis interventions was provided by a board certified expert in hypnosis who is also a clinical psychologist.[31] The certified hypnotherapist provided an initial three day training session for three nurses at the Mayo Clinic, each with a different degree ranging from a bachelor's to a master's to a PhD in nursing. Education, practice, and demonstrations were completed. The certified hypnotherapist returned to the research site after 6 months to provide a refresher course and to evaluate each interventionists' competency as well as to evaluate the standardized delivery of the both true and sham hypnosis interventions.
Outcome Measures
The primary endpoint was the hot flash score, which is a measure that takes into account both frequency and severity as recorded in real time by participants in the daily log. This diary has been used in numerous other studies evaluating various pharmacologic agents for hot flashes and has yielded consistent data.[23, 32] To calculate the hot flash score, severity, which included mild, moderate, severe and very severe, was given a value ranging from 1 to 4 respectively. For every 24 hour period, the number of hot flashes in each severity category was multiplied by the value of the severity score and then summed together. For example, if a woman experienced 3 mild and 4 moderate hot flashes in a 24 hour period, her hot flash score would be 3 ×1 + 4 × 2 = 11. The hot flash diary was completed daily for the full 8 weeks of the study.
Participants completed a side effect assessment questionnaire that asks women to rate various potential side effects over the past week. This measure was completed at baseline, week 5 (marking the end of the face-to-face sessions) and week 8 (the end of the study).
An important secondary endpoint included the Menopausal Quality of Life (MENQOL)[33] which provided data on the amount of bother or distress a woman experienced related to hot flashes. This was completed at baseline and at the end of the study (8 weeks) only. Responses on the MENQOL, range from 0 (not bothered) to 6 (extremely bothered). We were interested in the vasomotor subscale which includes three questions (hot flashes, night sweats and sweating). Scores for the subscale were determined by calculating the mean of the three items with a range from 0 to 6. At the end of the study only, women completed a scale that asked about their perception of change in their symptoms since beginning the study using the Subject Global Impression of Change (SGIC) and a single yes/no question about whether they were satisfied with the study treatment.[34]
Women kept a daily log of their home sessions with the behavioral intervention (hypnosis vs. sham hypnosis), indicating the number of days and minutes they used the CD at home. This measure provided the dose and frequency of the behavioral component. Women also marked on the daily log whether they took their medication as prescribed.
Analysis
The primary endpoint was the average intra-patient difference in hot flash (HF) score (frequency × severity) at the end of the study, 8 weeks, controlling for baseline hot flash score. We analyzed data using a General Estimating Equation (GEE) model, using venlafaxine/sham hypnosis as the referent arm. We chose the venlafaxine/sham hypnosis as the referent arm since it can be argued that venlafaxine is standard, first line non-estrogenic treatment for hot flashes having been shown to be effective in large randomized controlled trials.[35] In addition, the effects of venlafaxine on hot flashes were well established so effect size differences could be ascertained with more confidence. The sham hypnosis/placebo arm was included due to the large placebo effects often seen in hot flash research.[32, 36] We wanted to be able to calculate the effect size from the sham arm for future well powered trials.
Compound symmetry covariance structure was considered in the GEE model fitting. Self-reported HF scores from participants were assumed to be equally correlated. Analysis of covariance was used to compare differences and effect sizes were calculated in comparison to the reference arm of venlafaxine and sham hypnosis. As this was a pilot study, not a fully powered randomized, controlled trial, our primary interest was magnitude of effect. Our a priori hypothesis was that the combined arm of venlafaxine and hypnosis would provide an additional 16% reduction in hot flashes over the venlafaxine and sham hypnosis arm. Previous data indicate reductions in hot flashes by venlafaxine to be about 55%. In a population with 4 hot flashes per day, this results in a 2.2 hot flash per day reduction, assuming the lowest possible hot flash score of 4 mild hot flashes per day at baseline. Hence, a further decrease of 16%, to a 71% decrease in hot flashes, would translate to a difference of 0.45 standard deviations, being a moderate effect size. Therefore, participants could expect a further decrease of 1 hot flash per day, or a total of 3 per day decrease.
The vasomotor subscale of the MENQOL was evaluated through the calculation of average intra-patient scores at week 8 controlling for baseline and then analyzed with GEE modeling using compound symmetry covariance structure with the double placebo arm (PSH) as the referent arm. Side effects were analyzed by calculating changes from baseline and evaluating whether that change was statistically significant using paired t-tests. The SGIC was evaluated descriptively by dividing the group into two. Those who responded to the question, “Since beginning the study intervention, my hot flashes are…” by marking a negative 3, negative 2, negative 1 or 0, were classified as having no benefit while those marking a positive 1, 2 or 3 were classified as having perceived benefit. Frequencies were calculated for each arm. Likewise, frequencies for those reporting satisfaction with the study intervention were also calculated for each arm.
All analyses were performed using SPSS 23 and SAS 9.3. Significant p-values were set at <.05.
Results
A total of 71 women were enrolled and randomized in this study, 15 to venlafaxine/hypnosis, 19 to venlafaxine/sham hypnosis, 22 to placebo/hypnosis and 15 to placebo/sham hypnosis, between January, 31, 2010 and December 20, 2011. The CONSORT diagram is shown in Figure 1. Baseline demographics are listed in Table 1. No demographic variables or baseline hot flash characteristics were statistically significantly different between groups at baseline. There were large differences between groups in the number of months since menopause, ranging from 41 to 84, although all women were over 3 years post menopause.
Figure 1. CONSORT diagram.
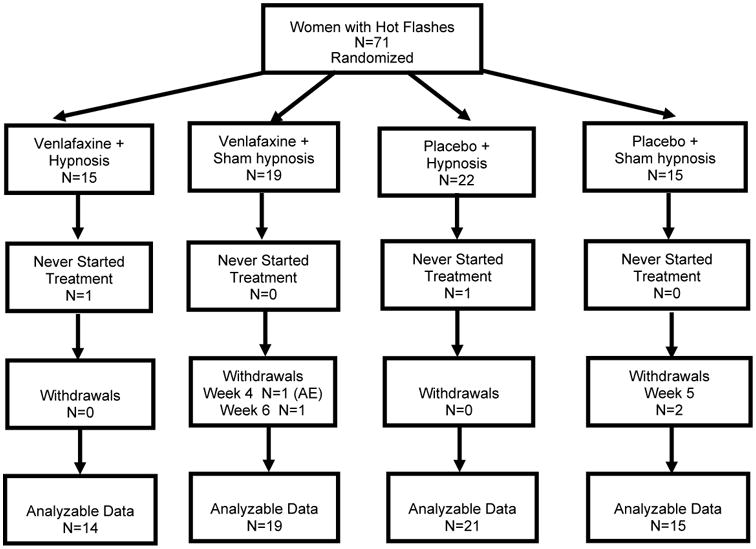
Table 1. Demographics Characteristics (N=71).
| Characteristics | N=15 Venlafaxine + hypnosis | N=19 Venlafaxine + sham hypnosis | N=22 Placebo pill + hypnosis | N=15 Placebo pill + sham hypnosis |
|---|---|---|---|---|
| Age (mean) | 56 | 54 | 54 | 56 |
| Race | ||||
| White | 14 (93%) | 19 (100%) | 20 (90%) | 15 (100%) |
| Black | 1 (7%) | 0 | 1 (5%) | 0 |
| missing | 1 (5%) | |||
| Non-Hispanic | 13 (87%) | 19 (100%) | 19 (86%) | 15 (100%) |
| Not reported/known | 2 (13%) | 0 | 3 (14%) | 0 |
| History of Breast Cancer | ||||
| No | 15 (100%) | 17 (89%) | 20 (95%) | 14 (93%) |
| Menopause status | ||||
| Natural | 11 (73%) | 16 (84%) | 20 (90%) | 11 (73%) |
| Surgical | 4 (27%) | 3 (16%) | 1 (5%) | 4 (27%) |
| missing | 1 (5%) | |||
| Months since menopause (mean) | 84 | 42 | 47 | 84 |
| Average Frequency of Hot Flashes/day | 9 | 9 | 8 | 10 |
| TAM - yes | 0 | 0 | 0 | 1 (7%) |
| AI – yes | 0 | 1 (5%) | 1 (5%) | 0 |
TAM: Tamoxifen; AI: Adjuvant aromatase inhibitor
Primary endpoint
For the GEE modeling, at first, Group × Time were included in the model to investigate the effect of interventions over the time. None of the interaction terms of group*time were significant, so the interaction terms were dropped from the final model (Table 2). Our primary hypothesis, that VH would provide more relief of hot flashes over VSH, was not supported. The women in the combined arm, VH, reported hot flash reductions that were not statistically different than women in the VSH arm, p=.05. Only women in the placebo/sham hypnosis arm reported reductions in hot flashes that were statistically significantly LESS than women in the VSH arm, p=.001. Women receiving the placebo pill + hypnosis (PH) reported hot flash reductions that were not statistically significantly different than women in the VSH arm, p=.34.
Table 2. Average intra-patient difference in hot flash score at 8 weeks (N=65).
| Effects | Estimate | SE | P -values |
|---|---|---|---|
| Group | |||
| Venlafaxine + hypnosis | 26.76 | 13.51 | .05 |
| Placebo pill + sham hypnosis | 63.21 | 19.37 | .001* |
| Placebo pill + hypnosis | 12.26 | 12.97 | .34 |
| Time | -6.62 | 0.86 | <.001* |
Group × Time terms were non-significant. Result from reduced model is given.Referent arm is venlafaxine/sham hypnosis, significant p values were indicated by *
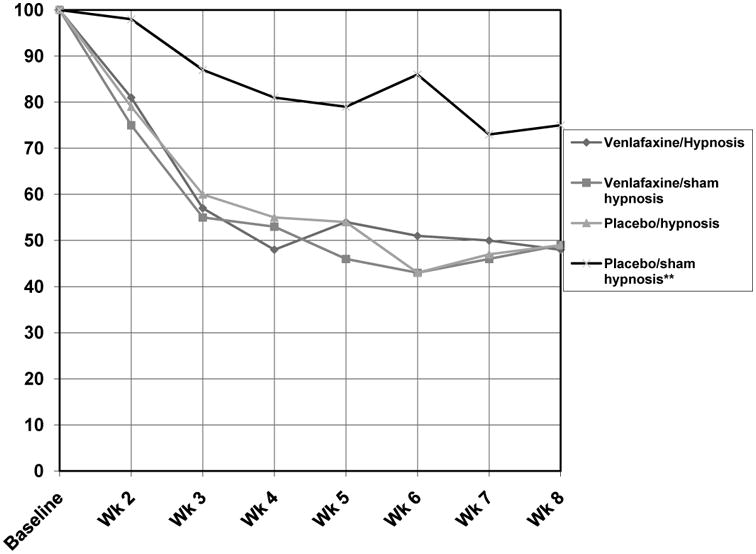
The participants assigned to the placebo/sham hypnosis (PSH) arm experienced a 25% decrease in hot flashes over the study period; while participants in each active arm reported hot flash score reductions of just over 50%. Hot flash score reductions (shown in percent of baseline) over the 8 weeks are shown in Figure 2.
Figure 2. Graph of hot flash scores depicted as percent of baseline for all arms over 8 weeks.
Secondary outcomes
Side effects evaluated included appetite increase/ loss, somnolence, nausea, fatigue, dry mouth, dizziness, diarrhea, blurred vision, sweating and sleep. None of these side effects were significantly worse than baseline during the course of the study. Four side effects significantly improved during the study. These data are shown in Table 3. Sleep and sweating improved significantly in all four groups while somnolence improved in all but the venlafaxine/hypnosis arm. Fatigue significantly improved in venlafaxine/sham hypnosis and placebo/hypnosis groups.
Table 3. Mean change baseline to week 5, Negative number=worsening.
| Side effects | Venlafaxine/hypnosis N=14 | Venlafaxine/ sham hypnosis N=19 | Placebo pill/hypnosis N=21 | Placebo pill/sham hypnosis N=15 |
|---|---|---|---|---|
| Appetite loss | -.15 | -.65 | .10 | -.25 |
| Somnolence | .33 | 3.53* | 1.65* | 1.92* |
| Nausea | -.08 | .53 | -.25 | .50 |
| Dizzy | .38 | .29 | .05 | .50 |
| Appetite increase | -.08 | 1.35 | .30 | .58 |
| Fatigue | .54 | 2.71* | 1.45* | 1.58 |
| Dry mouth | -.54 | .29 | .35 | .75 |
| Sweating | 3.15* | 2.88* | 3.37* | 2.25* |
| Diarrhea | .15 | .12 | .60 | 1.00 |
| Blurred vision | 0 | .29 | -.20 | .42 |
| Sleep | 2.54* | 3.35* | 2.70* | 2.17* |
significant changes evaluated through paired t-tests
Menopausal quality of life was evaluated by the MENQOL and results are shown in Table 4 for the vasomotor subscale, again using the double placebo arm as the referent arm. In this model, there was a significant time by group interaction for the combination of venlafaxine and hypnosis (p=.02), a trend toward significance (p=.05) for the hypnosis alone arm and an insignificant effect for venlafaxine alone (p=.09) compared to the control arm. At baseline, means for the vasomotor subscale were 4.10 (SD 1.6) for the venlafaxine/hypnosis arm, 3.25 (1.4) for venlafaxine/sham hypnosis, 3.16 (1.6) for placebo/hypnosis and 3.76 (2.0) for the placebo/sham hypnosis arm. Means at week 8 were 2.24 (1.4) for venlafaxine/hypnosis, 1.88 (1.5) for venlafaxine/sham hypnosis, 1.84 (1.3) for placebo/hypnosis and 3 (1.8) for placebo/sham hypnosis.
Table 4. Average intra-patient change in MENQOL vasomotor subscale at 8 weeks (N=65).
| Factor | Estimate | SE | P values |
|---|---|---|---|
| Venlafaxine + sham hypnosis | 6.43 | 10.44 | .54 |
| Venlafaxine + Hypnosis | -9.03 | 11.51 | .43 |
| Placebo pill + Hypnosis | 7.84 | 10.85 | .47 |
| Time | 0.98 | 0.85 | .25 |
| Venlafaxine × Time | 1.99 | 1.17 | .09 |
| Venlafaxine + Hypnosis × Time | 3.22 | 1.33 | .02* |
| Hypnosis only × Time | 2.14 | 1.11 | .05 |
Referent arm is placebo/focused attention, significant p values are marked as *
The mean number of practice days and days participants took medication are shown in Table 5. Total practice days and medication days were not statistically significantly different between arms and were poorly correlated with hot flash scores at 8 weeks and the vasomotor scale of the MENQOL with absolute Pearson correlation coefficients ranging from .03 to .19 (data not shown). The venlafaxine/hypnosis arm reported the most minutes with the hypnosis CD and the least number of days taking the study medication.
Table 5. Mean number of minutes, days of practice, and taking medication over the study period.
| Treatment assignment | Mean total number of days listened to CD* (SD) | Mean total number of minutes listened to CD (SD) | Mean total number of days took study med** (SD) |
|---|---|---|---|
| Venlafaxine & Hypnosis | 35 (11) | 1069 (110) | 46 (6) |
| Venlafaxine & Sham Hypnosis | 37 (11) | 763 (468) | 48 (2) |
| Placebo & Hypnosis | 29 (11) | 805 (455) | 47 (3) |
| Placebo & Sham Hypnosis | 39 (8) | 898(170) | 48 (1) |
target dose was at least four times per week × 7 weeks = 28 days
target was daily medication use, 7 days × 7 weeks = 49 days
Impression of benefit and satisfaction
More women who received venlafaxine/sham hypnosis or placebo/hypnosis perceived benefit related to hot flash improvement with 100% and 90% of women feeling their hot flashes were better, respectively. Only 67% of those receiving placebo/sham hypnosis perceived benefit. These frequencies are shown in Table 6. When asked about satisfaction with the intervention received, 83% said they were satisfied with venlafaxine/sham hypnosis and 79% were satisfied with placebo/hypnosis (Table 6).
Table 6.
Subjective Global Impression of Change and Satisfaction.
| Venlafaxine & Hypnosis (N=12) | Placebo/sham hypnosis (N=12) | Placebo/Hypnosis (N=19) | Venlafaxine/Sham hypnosis (N=15) | |
|---|---|---|---|---|
| Impression of benefit | ||||
| No Benefit (-3, 02, -1, 0) | 2 (17%) | 4 (33%) | 2 (10%) | 0 |
| Benefit (+1, +2, +3) | 10 (83%) | 8 (67%) | 17 (90%) | 15 (100%) |
| Satisfaction | ||||
| No | 4 (33%) | 6 (50%) | 4 (21%) | 2 (17%) |
| Yes | 8 (67%) | 6 (50%) | 15 (79%) | 10 (83%) |
Discussion
In this pilot study, the combination of venlafaxine and hypnosis did not yield greater reductions in hot flashes. While this was a surprise, the dose of 75 mg of venlafaxine may have been too strong eclipsing the effect of hypnosis and resulting in a mechanistic ceiling effect. However, based on the practice and medication log, since the venlafaxine/hypnosis arm listened to the CD the longest and took less medication, it could be that this was not as good of an evaluation of the combination of the two treatments as it could have been.
Although this was a pilot study designed to compare the combination of a pharmacologic and behavioral intervention versus a pharmacologic agent alone without expectations of adequate power, effects were strong enough that statistically significant reductions in hot flashes were demonstrated with both venlafaxine alone (with sham hypnosis) and hypnosis alone (with a placebo pill) being better than the double placebo arm (placebo/sham hypnosis) but not significantly different from each other. Therefore, this study provides supportive data demonstrating significant benefit from hypnosis alone, equal to that of venlafaxine, for the amelioration of hot flashes, without unwanted side effects.
The results in this study are consistent with other hot flash studies that have found significant reductions with hypnosis alone when compared with an attention control group,[29, 30] and a recent study demonstrating reductions with hypnosis that were greater than those seen with the use of gabapentin.[37] As illustrated by the data in this study and the aforementioned publications, there are preliminary data that hypnosis can be effective against hot flashes in women with natural, surgical and chemotherapy-induced menopause.
We had hoped to recruit more women with a history of breast cancer but were unable to do so. Many of the women with breast cancer were interested in trying hypnosis, but did not want to take a pharmacologic agent. Therefore, while we only had 5 women with a history of breast cancer, we were not able to further test any differences in response based on that characteristic. However, as mentioned, in previous studies, women with and without breast cancer have responded similarly to hot flash interventions [6,21-23, 29,30].
Recently, the North American Menopause Society listed both hypnosis and cognitive behavioral therapy as evidence based treatments for hot flashes.[38] It is important to note that studies evaluating hypnosis have demonstrated reductions in both frequency and severity as well as interference or bother[34, 35], as in this study, while studies with cognitive behavioral therapy have demonstrated improvements in perceptions of distress or bother but not actual decreases in frequency or severity.[39-41]
Therefore, it may be that hypnosis is an intervention that can impact the actual physiology of hot flashes and not just cognitive perception. Recently, hot flash physiology has moved beyond the knowledge that estrogen deprivation causes a narrowed temperature regulatory zone. Current hypotheses include that hot flashes are centrally mediated, involving neurotransmitters such as serotonin, since estrogen concentrations can impact neurotransmitters.[42, 43] In fact, one small study (N=39) found statistically significantly lower serotonin concentrations in women with more severe, compared to less severe, hot flashes.[44] Therefore, there may be an imbalance in the sympathetic/parasympathetic balance that contributes to hot flash persistence and by eliciting a deep relaxation response with hypnosis, the shift from sympathetic (norepinephrine) to parasympathetic (serotonin) activity is facilitated.[43, 45, 46]
Vasomotor symptom bother was evaluated in this study in addition to decreases in the number and severity of hot flashes. The data demonstrated consistent and significant improvements in vasomotor symptom bother for the combination arm of VH and trends toward significant reductions in the other two active arms with VSH and PH. The lack of significance is likely a result of low power in this pilot study.
There is a concern about the ability to broadly disseminate this intervention. When developing the intervention, there were only a very small group of professionals who had expertise in using hypnosis for hot flashes and in general, many areas may not have providers certified or trained to deliver hypnosis. In addition, this intervention currently requires coming in to see the practitioner and this is often viewed as inconvenient and does limit access to resources that are not widely available. It is important to note that for this study, three nurses were trained by a certified hypnotherapist and clinical psychologist, to provide this intervention. The nurses each had different levels of education (bachelor's degree, master's degree and PhD), but all were well experienced licensed professionals. The intervention was taught to each of these health care professionals and clearly, results of this trial indicate that the hypnosis was being delivered effectively. This suggests that this intervention can be taught effectively to licensed health care providers of several educational levels. Work is ongoing to evaluate methods for women to self-administer the hypnosis in order to improve the dissemination of this helpful therapy.
The placebo/sham hypnosis group was the intended true control group and did experience a reduction in hot flashes consistent with placebo arms in other pharmacologic hot flash intervention studies, about a 25% reduction.[23, 32] As depicted in the CONSORT diagram, women in this arm did not disproportionately withdraw or have missing data, leading to the conclusion that a sham hypnosis arm using white noise may be an appropriate control group adequately controlling for non-specific effects of general relaxation, provider attention and home practice with an audio file. As has been articulated previously, research in hot flashes does evoke a placebo response [32, 36, 47] and therefore, appropriately controlled trials are needed to build the evidence base.
The strengths of the study included a rigorous 4 arm randomized design that involved an adequate control group for both venlafaxine and hypnosis. In addition, since the treatments could not be blinded, we utilized a blinded hypothesis. This means that when educating women about the study, for the non-pharmacologic component, the study was described as evaluating two different behavioral approaches. We did not “label” the study as being about hypnosis. This strategy reduces bias toward an experimental arm. Further, data collection was completed by a study coordinator who was blinded to the participant's treatment assignment.
Limitations of this study include the fact that it was a small pilot study with a very homogeneous population of white women without a history of breast cancer. A larger study would be necessary to more definitively evaluate the effects of a combined intervention on hot flashes. Although a mechanism was proposed for how hypnosis may impact hot flashes, we did not test that hypothesis in this study. Finally, this pilot study, as a proof of concept, was only 8 weeks in length. Longer studies are needed to determine whether an intervention might meet ongoing needs for hot flash control.
Conclusion
Hypnosis is an effective treatment for hot flashes. Future research is needed to clarify whether hypnosis could be combined with a different low dose pharmacologic intervention to provide optimal relief of hot flashes synergistically without ceiling effects and without side effects, or to identify a population who could benefit more from such a combination. Second, the hypnosis intervention requires additional development and testing in order to increase the potential for broader dissemination, as mentioned above. Studies that include longer term follow up are needed to also evaluate what sort of maintenance doses might be needed to keep hot flashes at bay. Finally, research is needed to investigate how hypnotic relaxation impacts hot flashes and also how the induction can be modified to focus on the menopausal symptom cluster of hot flashes, sleep and mood to provide significant improvement and benefit in all areas related to menopausal quality of life.
Acknowledgments
Support for this study was received from the National Cancer Institute, grant number R21 CA 131795. The views expressed in this article are those of the authors and do not necessarily represent the views of the National Cancer Institute, or National Institutes of Health.
Footnotes
None of the authors report any conflict of interest, financial or otherwise.
References
- 1.Stearns V, Ullmer L, Lopez JF, Smith Y, Isaacs C, Hayes D. Hot flushes. Lancet (London, England) 2002;360(9348):1851–1861. doi: 10.1016/s0140-6736(02)11774-0. [DOI] [PubMed] [Google Scholar]
- 2.Gracia CR, Freeman EW. Acute consequences of the menopausal transition: the rise of common menopausal symptoms. Endocrinology and metabolism clinics of North America. 2004;33(4):675–689. doi: 10.1016/j.ecl.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Politi MC, Schleinitz MD, Col NF. Revisiting the duration of vasomotor symptoms of menopause: a meta-analysis. Journal of general internal medicine. 2008;23(9):1507–1513. doi: 10.1007/s11606-008-0655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Col NF, Guthrie JR, Politi M, Dennerstein L. Duration of vasomotor symptoms in middle-aged women: a longitudinal study. Menopause (New York, NY) 2009;16(3):453–457. doi: 10.1097/gme.0b013e31818d414e. [DOI] [PubMed] [Google Scholar]
- 5.Carpenter JS, Johnson D, Wagner L, Andrykowski M. Hot flashes and related outcomes in breast cancer survivors and matched comparison women. Oncology nursing forum. 2002;29(3):E16–25. doi: 10.1188/02.ONF.E16-E25. [DOI] [PubMed] [Google Scholar]
- 6.Bardia A, Novotny P, Sloan J, Barton D, Loprinzi C. Efficacy of nonestrogenic hot flash therapies among women stratified by breast cancer history and tamoxifen use: a pooled analysis. Menopause (New York, NY) 2009;16(3):477–483. doi: 10.1097/gme.0b013e31818c91ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marino JL, Saunders CM, Emery LI, Green H, Doherty DA, Hickey M. Nature and severity of menopausal symptoms and their impact on quality of life and sexual function in cancer survivors compared with women without a cancer history. Menopause (New York, NY) 2014;21(3):267–274. doi: 10.1097/GME.0b013e3182976f46. [DOI] [PubMed] [Google Scholar]
- 8.Mao JJ, Chung A, Benton A, et al. Online discussion of drug side effects and discontinuation among breast cancer survivors. Pharmacoepidemiology and drug safety. 2013;22(3):256–262. doi: 10.1002/pds.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morales L, Neven P, Timmerman D, et al. Acute effects of tamoxifen and third-generation aromatase inhibitors on menopausal symptoms of breast cancer patients. Anti-cancer drugs. 2004;15(8):753–760. doi: 10.1097/00001813-200409000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Hunter M, Rendall M. Bio-psycho-socio-cultural perspectives on menopause. Best practice & research Clinical obstetrics & gynaecology. 2007;21(2):261–274. doi: 10.1016/j.bpobgyn.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Carpenter JS. The Hot Flash Related Daily Interference Scale: a tool for assessing the impact of hot flashes on quality of life following breast cancer. Journal of pain and symptom management. 2001;22(6):979–989. doi: 10.1016/s0885-3924(01)00353-0. [DOI] [PubMed] [Google Scholar]
- 12.Brown L, Bryant C, Judd FK. Positive well-being during the menopausal transition: a systematic review. Climacteric : the journal of the International Menopause Society. 2015;18(4):456–469. doi: 10.3109/13697137.2014.989827. [DOI] [PubMed] [Google Scholar]
- 13.Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: a comprehensive review. Health and quality of life outcomes. 2005;347 doi: 10.1186/1477-7525-3-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greendale GA, Reboussin BA, Hogan P, et al. Symptom relief and side effects of postmenopausal hormones: results from the Postmenopausal Estrogen/Progestin Interventions Trial. Obstetrics and gynecology. 1998;92(6):982–988. doi: 10.1016/s0029-7844(98)00305-6. [DOI] [PubMed] [Google Scholar]
- 15.Manson JE, Kaunitz AM. Menopause Management--Getting Clinical Care Back on Track. The New England journal of medicine. 2016;374(9):803–806. doi: 10.1056/NEJMp1514242. [DOI] [PubMed] [Google Scholar]
- 16.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. Jama. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 17.Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet (London, England) 2000;356(9247):2059–2063. doi: 10.1016/S0140-6736(00)03403-6. [DOI] [PubMed] [Google Scholar]
- 18.Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20(6):1578–1583. doi: 10.1200/JCO.2002.20.6.1578. [DOI] [PubMed] [Google Scholar]
- 19.Stearns V, Slack R, Greep N, et al. Paroxetine is an effective treatment for hot flashes: results from a prospective randomized clinical trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(28):6919–6930. doi: 10.1200/JCO.2005.10.081. [DOI] [PubMed] [Google Scholar]
- 20.Stearns V, Beebe KL, Iyengar M, Dube E. Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. Jama. 2003;289(21):2827–2834. doi: 10.1001/jama.289.21.2827. [DOI] [PubMed] [Google Scholar]
- 21.Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. Jama. 2011;305(3):267–274. doi: 10.1001/jama.2010.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barton DL, Lavasseur BI, Sloan JA, et al. Phase III, placebo-controlled trial of three doses of citalopram for the treatment of hot flashes: NCCTG trial N05C9. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(20):3278–3283. doi: 10.1200/JCO.2009.26.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loprinzi CL, Sloan J, Stearns V, et al. Newer antidepressants and gabapentin for hot flashes: an individual patient pooled analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(17):2831–2837. doi: 10.1200/JCO.2008.19.6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guttuso T, Jr, Kurlan R, Mcdermott MP, Kieburtz K. Gabapentin's effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstetrics and gynecology. 2003;101(2):337–345. doi: 10.1016/s0029-7844(02)02712-6. [DOI] [PubMed] [Google Scholar]
- 25.Pandya KJ, Morrow GR, Roscoe JA, et al. Gabapentin for hot flashes in 420 women with breast cancer: a randomised double-blind placebo-controlled trial. Lancet (London, England) 2005;366(9488):818–824. doi: 10.1016/S0140-6736(05)67215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goss PE, Ingle JN, Pritchard KI, et al. Extending Aromatase-Inhibitor Adjuvant Therapy to 10 Years. The New England journal of medicine. 2016;375(3):209–219. doi: 10.1056/NEJMoa1604700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunter MS, Grunfeld EA, Mittal S, et al. Menopausal symptoms in women with breast cancer: prevalence and treatment preferences. Psycho-oncology. 2004;13(11):769–778. doi: 10.1002/pon.793. [DOI] [PubMed] [Google Scholar]
- 28.Elkins GR, Barabasz AF, Council JR, Spiegel D. Advancing research and practice: the revised APA Division 30 definition of hypnosis. The International journal of clinical and experimental hypnosis. 2015;63(1):1–9. doi: 10.1080/00207144.2014.961870. [DOI] [PubMed] [Google Scholar]
- 29.Elkins G, Marcus J, Stearns V, et al. Randomized trial of a hypnosis intervention for treatment of hot flashes among breast cancer survivors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(31):5022–5026. doi: 10.1200/JCO.2008.16.6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elkins GR, Fisher WI, Johnson AK, Carpenter JS, Keith TZ. Clinical hypnosis in the treatment of postmenopausal hot flashes: a randomized controlled trial. Menopause (New York, NY) 2013;20(3):291–298. doi: 10.1097/GME.0b013e31826ce3ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elkins GR. Handbook of Medical and Psychological Hypnosis: Foundations, Applications, and Professional Issues. Springer Publishing Inc; New York: 2016. [Google Scholar]
- 32.Sloan JA, Loprinzi CL, Novotny PJ, Barton DL, Lavasseur BI, Windschitl H. Methodologic lessons learned from hot flash studies. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2001;19(23):4280–4290. doi: 10.1200/JCO.2001.19.23.4280. [DOI] [PubMed] [Google Scholar]
- 33.Hilditch JR, Lewis J, Peter A, et al. A menopause-specific quality of life questionnaire: development and psychometric properties. Maturitas. 1996;24(3):161–175. doi: 10.1016/s0378-5122(96)82006-8. [DOI] [PubMed] [Google Scholar]
- 34.Guy W, editor. ECDEU Assessment Manual for Psychopharmacology. US Department of Health, Education, and Welfare; Washington DC: 1976. Revised Publication Adm 76-338. [Google Scholar]
- 35.Ramaswami R, Villarreal MD, Pitta DM, Carpenter JS, Stebbing J, Kalesan B. Venlafaxine in management of hot flashes in women with breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2015 Jul;152(2):231–237. doi: 10.1007/s10549-015-3465-5. [DOI] [PubMed] [Google Scholar]
- 36.Mahon SM, Kaplan M. Placebo effect in hot flush research. Lancet Oncol. 2012 May;13(5):e188. doi: 10.1016/S1470-2045(12)70197-3. author reply e190. [DOI] [PubMed] [Google Scholar]
- 37.Maclaughlan David S, Salzillo S, Bowe P, et al. Randomised controlled trial comparing hypnotherapy versus gabapentin for the treatment of hot flashes in breast cancer survivors: a pilot study. BMJ open. 2013;3(9):e003138. doi: 10.1136/bmjopen-2013-003138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause (New York, NY) 2015;22(11):1155–1174. doi: 10.1097/GME.0000000000000546. [DOI] [PubMed] [Google Scholar]
- 39.Mann E, Smith MJ, Hellier J, et al. Cognitive behavioural treatment for women who have menopausal symptoms after breast cancer treatment (MENOS 1): a randomised controlled trial. The Lancet Oncology. 2012;13(3):309–318. doi: 10.1016/S1470-2045(11)70364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ayers B, Smith M, Hellier J, Mann E, Hunter MS. Effectiveness of group and self-help cognitive behavior therapy in reducing problematic menopausal hot flushes and night sweats (MENOS 2): a randomized controlled trial. Menopause (New York, NY) 2012;19(7):749–759. doi: 10.1097/gme.0b013e31823fe835. [DOI] [PubMed] [Google Scholar]
- 41.Duijts SF, Van Beurden M, Oldenburg HS, et al. Efficacy of cognitive behavioral therapy and physical exercise in alleviating treatment-induced menopausal symptoms in patients with breast cancer: results of a randomized, controlled, multicenter trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(33):4124–4133. doi: 10.1200/JCO.2012.41.8525. [DOI] [PubMed] [Google Scholar]
- 42.Berendsen H. Hot flushes and serotonin. Menopause Int. 2002;8:30–34. [Google Scholar]
- 43.Deecher DC, Dorries K. Understanding the pathophysiology of vasomotor symptoms (hot flushes and night sweats) that occur in perimenopause, menopause, and postmenopause life stages. Archives of women's mental health. 2007;10(6):247–257. doi: 10.1007/s00737-007-0209-5. [DOI] [PubMed] [Google Scholar]
- 44.Slopien R, Meczekalski B, Warenik-Szymankiewicz A. Relationship between climacteric symptoms and serum serotonin levels in postmenopausal women. Climacteric : the journal of the International Menopause Society. 2003;6(1):53–57. [PubMed] [Google Scholar]
- 45.Carpenter JS, Gilchrist JM, Chen K, Gautam S, Freedman RR. Hot flashes, core body temperature, and metabolic parameters in breast cancer survivors. Menopause (New York, NY) 2004;11(4):375–381. doi: 10.1097/01.gme.0000113848.74835.1a. [DOI] [PubMed] [Google Scholar]
- 46.Thurston RC, Christie IC, Matthews KA. Hot flashes and cardiac vagal control: a link to cardiovascular risk? Menopause (New York, NY) 2010;17(3):456–461. doi: 10.1097/gme.0b013e3181c7dea7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.National Institutes of Health State-of-the-Science Conference statement: management of menopause-related symptoms. Annals of internal medicine. 2005;142(12 Pt 1):1003–1013. [PubMed] [Google Scholar]


