Abstract
Rationale
The therapeutic potential of monoamine releasers with prominent dopaminergic effects is hindered by their high abuse liability.
Objectives
The present study examined the effects of several novel ‘norepinephrine (NE)-preferring’ monoamine releasers relative to nonselective monoamine releasers, d-amphetamine and d-methamphetamine, in rhesus monkeys trained to discriminate cocaine. NE-preferring releasers were approximately 13-fold more potent for NE compared to dopamine release and ranged in potency for serotonin release (PAL-329<l-methamphetamine<PAL-169).
Methods
Adult rhesus macaques were trained to discriminate 0.4 mg/kg, IM cocaine on a 30-response fixed ratio schedule of food reinforcement. Substitution studies determined the extent to which test drugs produced cocaine-like discriminative-stimulus effects and their time course. Drug interaction studies determined whether pretreatment with test drugs altered the discriminable effects of cocaine.
Results
Results show that cocaine, d-amphetamine, and d-methamphetamine dose-dependently substituted for cocaine with similar potencies. Among the ‘NE-preferring’ releasers, PAL-329 and l-methamphetamine also dose-dependently substituted for cocaine but differed in potency. PAL-169 failed to substitute for cocaine up to a dose that disrupted responding. When administered prior to cocaine, only d-amphetamine and PAL-329 significantly shifted the cocaine dose-effect function leftward indicating enhancement of cocaine’s discriminative stimulus effects.
Conclusions
These data suggest that greater potency for NE relative to dopamine release (up to 13-fold) does not interfere with the ability of a monoamine releaser to produce cocaine-like discriminative effects but that increased serotonin release may have an inhibitory effect. Further characterization of these and other ‘NE-preferring’ monoamine releasers should provide insight into their potential for the management of cocaine addiction.
Keywords: monoamine releaser, norepinephrine, dopamine, drug discrimination, cocaine use disorder, nonhuman primate
Introduction
The relative success of agonist, or ‘substitution,’ therapies for the treatment of opioid and nicotine abuse (Wood and Henningfield 1995; Mello and Negus 1996; Grabowski et al 2004) has encouraged the evaluation of candidate medications using this approach for the management of cocaine use disorder. Inasmuch as the abuse-related effects of cocaine can be largely attributed to increased dopamine (DA) neurotransmission (Ritz et al 1987; Cline et al 1992), most agonist-based candidate medications act, like cocaine, indirectly to increase extracellular DA in the mesolimbic system. For example, d-amphetamine (d-AMPH) and d-methamphetamine (d-MA), two monoamine releasers with prominent dopaminergic effects (Rothman and Baumann 2003), can decrease cocaine self-administration without greatly altering other motivated behavior in laboratory animals and human subjects (see Czoty et al 2016 for review). However, the therapeutic potential of these monoamine releasers is overshadowed by their own abuse liability (Hart et al 2001; Lile et al 2013; Kirkpatrick et al 2012) and their status as Schedule II agents under the Controlled Substances Act.
In view of the potential utility of monoamine releasers for the management of cocaine use disorder, various strategies have been adopted to design effective drugs with reduced abuse potential. For example, considerable attention has focused on the development of novel ‘dual’ releasers that increase extracellular levels of both DA and serotonin (5-HT). This strategy is predicated on the well-supported idea that increased 5-HT neurotransmission may inhibit DA release and its behavioral sequelae (Rothman and Baumann 2006). Indeed, dual DA/5-HT releasers have been shown to attenuate locomotor responses to indirect DA agonists (d-amphetamine, cocaine; Baumann et al, 2011) as well as corresponding increases in extracellular DA in brain regions implicated in their abuse-related effects (Baumann et al 2011); see also (Howell and Cunningham 2015). Preclinical self-administration studies also support this strategy, showing that monoamine releasers that are non-selective DA/5-HT releasers (e.g., PAL-287) or that release 5-HT selectively (e.g., fenfluramine) are less efficacious reinforcers than drugs that have selective dopaminergic actions (Woods and Tessel 1974; Glowa and Fantegrossi 1997; Wee et al 2005; Wee and Woolverton 2006). Unfortunately, however, such dual DA/5-HT or 5-HT-selective releasers fail to decrease IV cocaine self-administration at doses below those that decrease food-maintained behavior, indicative of a general disruption in motivated behavior (Negus et al 2007; Banks et al 2011). Thus, the ratio of DA to 5-HT activity appears to be an important factor in determining the balance between abuse potential and behavioral selectivity in the effects of monoamine releasers (Negus et al 2009).
In addition to increasing synaptic levels of DA and 5-HT, d-MA and d-AMPH, like cocaine, also can increase synaptic levels of norepinephrine (NE). Although the role of such NE-related actions in the ability of these drugs to reduce cocaine self-administration is unclear (Rothman et al 2000; Rothman and Baumann 2003; Banks et al 2014), the results of previous studies have shown that alterations in NE activity can modulate behavioral effects of cocaine itself (reviewed by Schmidt and Weinshenker 2014). For example, NE reuptake inhibitors can substitute for and/or enhance the discriminative stimulus effects of low doses of cocaine in rats and monkeys (Kleven and Koek 1997; Spealman 1995) and partially reinstate extinguished cocaine self-administration in monkeys (Platt et al 2007). Dopamine β-hydroxylase (DβH) inhibition, which decreases neuronal NE and increases extracellular dopamine levels by blocking the conversion of DA to NE, also has been reported to enhance the discriminative-stimulus effects of cocaine in rats and to reinstate extinguished cocaine self-administration in monkeys (Manvich et al 2013; Schroeder et al 2013) but not rats (Cooper et al 2014). Although further work is needed to clarify species-related differences in the neurochemical and behavioral effects of DβH inhibition, it seems evident that modulating NE neuronal activity can directly influence the behavioral effects of cocaine (see also Kohut et al 2013). Moreover, increased or decreased noradrenergic activity alone is not associated with reinforcement, consistent with the low abuse liability of directly- or indirectly-acting NE-related drugs (Wee and Woolverton 2004; Wee et al 2006; Walsh et al 2013) and supporting the further investigation of candidate medications with prominent noradrenergic actions for cocaine use disorder.
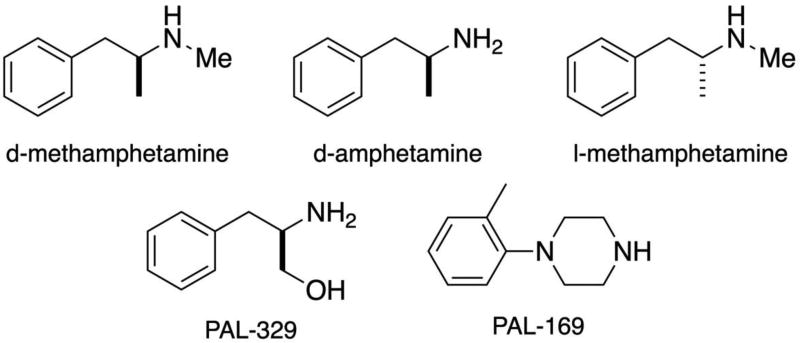
We recently reported that l-MA, like its stereoisomer d-MA, can produce cocaine-like discriminative stimulus effects and selectively reduce the reinforcing effects of cocaine (Kohut et al 2016). L-MA is a “NE-preferring” monoamine releaser that, in vitro, is relatively equipotent to d-MA in releasing NE but approximately 15-fold less potent in releasing DA (Rothman and Baumann 2003). The stereoisomers of MA also release 5-HT with similar potency; however, the relative roles of NE and 5-HT release in the cocaine-like discriminative stimulus effects of l-MA is unclear. The present studies were conducted to examine the contribution of NE and 5-HT release in l-MA’s cocaine-like discriminative stimulus effects by evaluating novel ‘NE preferring’ monoamine releasers with varying potency as 5-HT releasers (PAL-329, l-MA, PAL-169; see Figure 1) in rhesus monkeys trained to discriminate cocaine.
Figure 1.
Structures of d-methamphetamine, d-amphetamine, l-methamphetamine, PAL-329, and PAL-169.
Methods
In vitro monoamine release assay
The potency of d-MA, d-AMPH, PAL-329, l-MA, and PAL-169 to evoke neurotransmitter release via rat monoamine transporters (rSERT, rNET, and rDAT) was determined in rat brain synaptosomes as described previously (Rothman et al., 2000). Briefly, rat caudate (for [3H]DA release) or whole brain minus cerebellum and caudate (for [3H]NE and 3H]5HT release) was homogenized in ice-cold 10% sucrose. Following 12 strokes with a Potter-Elvehjem homogenizer, homogenates were centrifuged at 1,000g for 10 min at 0 – 4°C and supernatant retained on ice. Synaptosomal preparations were then incubated to steady-state with 5 nM [3H]DA (30 min), 7 nM [3H]NE (60 min), or 5 nM [3H]5HT (60 min) in uptake buffer (without BSA) plus 1 µM reserpine. Unlabeled compounds (nomifensine (100nM) and GBR12935 (100nM) for SERT; GBR12935 (100nM) and citalopram (100nM) for NET; citalopram (100nM) and desipramine (100nM) for DAT) were added to the sucrose solution to optimize the selectivity of assays for a single transporter by preventing uptake by competing transporters. After incubation to steady-state, 850 µl of synaptosomes were added to 12 × 75 mm polystyrene test tubes which contained 150 µl test drug in uptake buffer. After 5 min ([3H]DA and [3H]5-HT) or 30 min ([3H]NE) the release reaction was terminated by dilution with 4 ml wash buffer (10 mM Tris-HCl pH 7.4 containing 0.9% NaCl at 25°C) followed by rapid vacuum filtration over Whatman GF/B filters and rinsed twice with 4 ml wash buffer using a Brandel Harvester. The retained tritium was counted by a Taurus liquid scintillation counter at 40% efficiency after an overnight extraction in 3 ml Cytoscint (ICN). Nonlinear least-squares curve fitting was used to determine EC50 values (GraphPad Prism, San Diego, CA).
Drug Discrimination Procedures
Subjects
Four cocaine-experienced adult male rhesus monkeys (Macaca mulatta) that weighed between 6 and 10 kg served as subjects. High-protein chow (Purina Monkey Chow, St. Louis, MO) was provided twice daily at least 1-hr after experimental sessions supplemented daily with fresh fruit and vegetables. Banana-flavored food pellets (Purina Mills Test Diet, Richmond, IN) were earned during operant sessions. Water was continuously available from an automatic watering system. A 12-hr light-dark cycle was in effect (lights on 8:00AM – 8:00PM) except where noted below. Animal maintenance and research were conducted in accordance with the guidelines provided by the Institute of Laboratory Animal Resources (National Research Council 2010) and the NIH Office of Laboratory Animal Welfare (OLAW). The facility is licensed by the U.S. Department of Agriculture and protocols were approved by the McLean Hospital Institutional Animal Care and Use Committee.
Apparatus
All monkeys were housed in stainless-steel chambers (56 × 71 × 69 cm), each equipped with a custom operant response panel (28 × 28 cm) mounted on the front wall. The operant panel included three square translucent response keys (5.1 × 5.1 cm) arranged 3.5 cm apart in a horizontal row 9 cm from the top of the panel. Each key could be transilluminated with red or green stimulus lights (SuperBright LEDs; Fairchild Semiconductor, San Jose, CA). An externally mounted pellet dispenser (model G5210; Gerbrands, Arlington, MA) was attached to the back of the response panel and delivered 1-g banana-flavored food pellets to a receptacle mounted on the front of the cage beneath the response panel. All experimental events were controlled through custom programmed software (Med-PC) on a desktop PC located in a separate room that connected to the chambers via a Med Associates (Georgia, VT) Interface.
Discrimination Training
Monkeys discriminated cocaine (0.4 mg/kg, intramuscularly [IM]) from saline under a fixed ratio (FR) 30: time-out (TO) 10-sec schedule of reinforcement during daily sessions. Training sessions consisted of 1 to 5 cycles, and each cycle included a 15-min time-out period followed by a 5-min response period. Cocaine, or saline, was administered 10-min prior to the onset of each 5-min response period. During the response period, the right and left response keys were illuminated and monkeys could earn up to 10 food pellets. Delivery of each pellet was followed by a 10-sec time-out during which response keys were dark and key presses had no programmed consequences. One response key was designated as correct after cocaine injections while the other key was designated as correct following saline injections. The assignment of cocaine and saline keys was counterbalanced across subjects but saline was always associated with green light illumination and drug associated with red lights. A consecutive response contingency was in effect such that pressing the condition-inappropriate key prior to completing the FR30 reset the count toward completion of the response requirement to zero. During training, cocaine was administered only during the final cycle of a session.
The principle dependent variables were (a) percent injection-appropriate responses for the entire cycle, and (b) percent injection-appropriate responses prior to the first completed FR30, and (c) response rate in responses/sec. Criteria for successful discrimination were: (1) at least 80% of total responses before the first completed FR30 on the condition-appropriate key, (2) at least 90% of total responses in each cycle on the stimulus-appropriate key, and (3) rate of responding greater than or equal to 0.2 responses/sec.
Discrimination Testing
Once monkeys met criteria for discrimination in each cycle for four consecutive sessions, testing began. Each test session always followed a training session of criterion-level discrimination. Otherwise, training sessions, which consisted of both saline and cocaine cycles, were continued until criterion levels were obtained for at least two consecutive sessions. Test sessions were identical to training sessions except that responding on either key produced food. Two types of studies were conducted: first, substitution studies to determine the extent to which test drugs produced cocaine-like discriminative-stimulus effects and their time course and, second, drug interaction studies to determine how pretreatment with test drugs altered the discriminable effects of cocaine. In substitution studies, a single dose of test drug was administered 10-min prior to the first cycle, exactly as in training sessions. Subsequent cycles then began at 30-, 60-, 100-, and 300-min after injection of the test compound or until responding was predominantly on the vehicle-appropriate key (i.e., <20% cocaine-lever responding). In interaction studies, saline and selected doses of test compounds were administered prior to re-determination of the cumulative cocaine dose-effect function (0.032-0.32 mg/kg). Pretreatment times for test compounds were based on data from substitution studies and were the earliest time points at which either full substitution or, in the absence of substitution, decreases in response rate to less than 0.2 responses/sec were observed.
Data Analysis
Cocaine-discrimination in test sessions was defined as responding on the cocaine-associated key as a percentage of total responding on both keys, excluding responses during timeouts. The percentage of responding on the cocaine-associated key was not calculated in cases when response rate fell below 0.2 responses/sec. Response rates were calculated by dividing the total responses on both keys when the cue lights were illuminated by the total session time and are expressed as responses per second. Data are presented as mean (± SEM) values for the group of monkeys. Substitution test data were interpreted as follows: (1) Responding on the cocaine-associated lever below 20% was not considered to deviate significantly from responding engendered by saline, whereas (2) doses of a drug that led to 20% – 80% cocaine-like responding were considered to partially substitute for the cocaine training dose and (3) doses of a drug that produced >80% responding on the cocaine-associated lever were considered to fully substitute for cocaine. Given the 90% accuracy criterion for training session performance, these thresholds can be considered significantly different from chance for a conditional discrimination (Sidman, 1980). ED80 values, defined as the dose of each test compound that engendered 80% responding on the cocaine-associated lever, were determined using log-linear interpolation with individual subject dose-effect curve data. Log ED80 values were converted to linear values and corresponding confidence limits (CL) for statistical tests and data presentation. ED80 was used to determine doses or dose combinations that produced levels of responding indicative of full substitution for the cocaine discriminative stimulus. Shifts in the cocaine dose-effect curve during interaction studies were assessed by comparing relative potency calculated as ED80Cocaine/ED80Test Compound. Statistical significance was determined by non-overlapping 95% confidence limits for ED80 values. One-way repeated measures (RM) ANOVA also was used to assess rate-altering effects of each drug or drug combination; further comparisons utilized Dunnett tests, with a criterion significance level of p <.05. All statistical tests were conducted with GraphPad Prism software.
Drugs
Cocaine hydrochloride was supplied by the National Institute of Drug Abuse, NIH. d-methamphetamine and d-amphetamine were obtained from Sigma-Aldrich (St. Louis, MO). PAL-329, l-methamphetamine, and PAL-169 were synthesized by Dr. Bruce E. Blough at Research Triangle Institute (Research Triangle Park, N.C.). All drugs were dissolved in sterile saline (0.9% NaCl) and administered intramuscularly (I.M.). Drug doses (mg/kg) are expressed as the salt.
Results
In vitro monoamine release
Table 1 shows EC50 values (nM +/− SD) for the norepinephrine-preferring releasers, PAL-329, l-MA, and PAL-169 to release dopamine (DA), norepinephrine (NE), and serotonin (5-HT) in relation to reference compounds, d-AMPH and d-MA. In contrast to d-AMPH and d-MA, which are relatively non-selective dopamine/norepinephrine releasers, each of the NE-preferring releasers was between 13–15 –fold selective for releasing norepinephrine over dopamine but differed in their selectivity to promote release of 5-HT with a rank order of PAL-329<l-MA<PAL-169.
Table 1.
EC50 values (nM +/− SD) for monoamine releasers to release norepinephrine (NE), dopamine (DA), and serotonin (5-HT) in in vitro rat synaptosomes. PAL-329 selectivity was approximated using 10,000 nM as the potency for 5-HT release. a Rothman and Baumann, 2003
| Drug | EC50 values | Ratio | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| DA | NE | 5-HT | DA/NE | 5-HT/NE | 5-HT/DA | |
| d-amphetaminea | 24.8 ± 3.5 | 7.07 ± 0.95 | 1765 ± 94 | 3.4 | 245 | 71 |
| d-methamphetaminea | 24.5 ± 2.1 | 12.3 ± 0.7 | 736 ± 45 | 1.9 | 59.8 | 30 |
|
| ||||||
| PAL-329 | 1355 ± 74 | 106 ± 37 | >10000 | 12.8 | -- | -- |
| l-methamphetaminea | 416 ± 20 | 28.5 ± 2.5 | 4640 ± 243 | 14.6 | 162 | 11 |
| PAL-169 | 542 ± 43 | 39.1 ± 5 | 175 ± 13 | 13.9 | 4.5 | 0.3 |
Drug Discrimination
The training dose of cocaine maintained reliable discriminative control throughout the study. During training sessions that immediately preceded control tests, all subjects responded nearly exclusively on the saline-associated key following saline administration (99% ± 0.66) and on the cocaine-associated key after cocaine administration (99% ± 0.67). Mean response rates did not differ significantly after treatment with saline or the training dose of cocaine (2.79 ± 0.46 vs. 3.06 ± 0.10 r/sec, respectively).
Substitution and Time Course
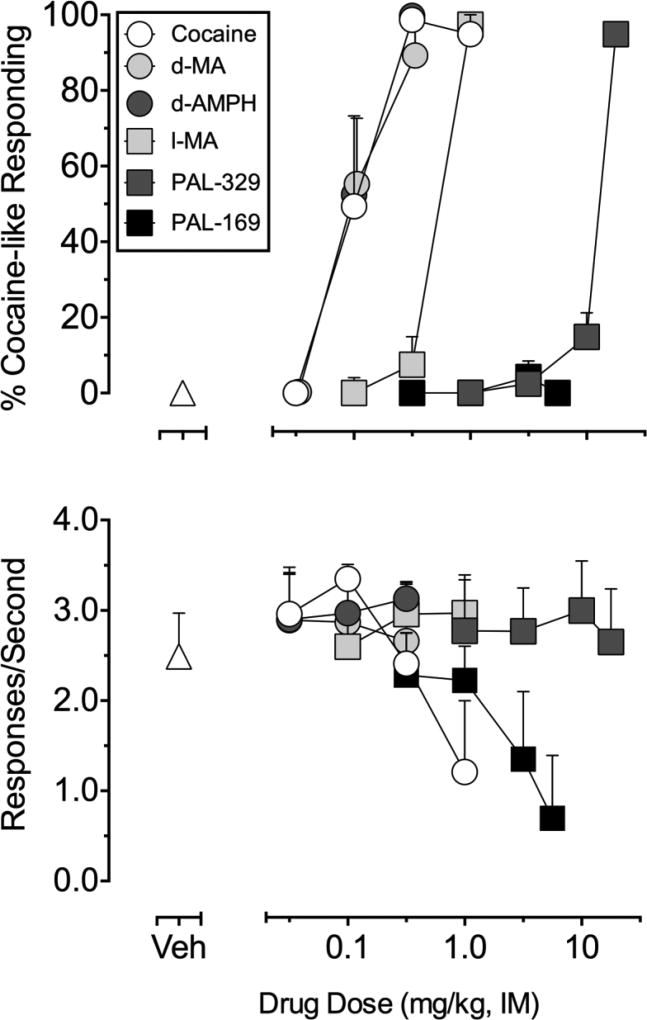
Cocaine and the non-selective monoamine releasers, d-AMPH and d-MA, dose-dependently substituted for the training dose of cocaine with similar potencies (full substitution at 0.32 mg/kg; see Table 2; Figure 2). Among the ‘NE preferring’ monoamine releasers, PAL-329 and l-MA also dose-dependently substituted for the training dose of cocaine but varied in potency: PAL-329 was approximately 100-fold less potent than cocaine whereas l-MA was approximately 5-fold less potent (Table 2; Figure 2). In contrast to the other drugs, PAL-169 failed to substitute for the training dose of cocaine and produced only vehicle lever responding up to a dose (5.6 mg/kg) that decreased rate of responding to below 0.2 responses/sec in 3 of 4 subjects. PAL-169 was the only monoamine releaser studied that produced decreases in rate of responding within the dose ranges tested (see Figure 2; bottom panel).
Table 2.
ED80 estimates (95% C.L.) and relative potency for substitution with peak pretreatment time for cocaine and monoaminergic releasers. n=4.
| Drug (peak time) | ED80(mg/kg) | Relative Potency |
|---|---|---|
| Cocaine (10 min) | 0.16 (0.10–0.27) | 1 |
| d-AMPH (10 min) | 0.14 (0.07–0.27) | 1.14 |
| d-MA (30 min) | 0.15 (0.08–0.29) # | 1.06 |
| PAL-329 (30 min) | 16.22 (15.6–16.8) * | 0.01 |
| l-MA (10 min) | 0.79 (0.73–0.86) * | 0.20 |
| PAL-169 (30 min) | ND | ND |
ND - not determined
significantly less potent than cocaine
n=3
Figure 2.
Discriminative stimulus effects of cocaine, d-methamphetamine (d-MA), d-amphetamine (d-AMPH), l-methamphetamine (l-MA), PAL-329, and PAL-169 in rhesus monkeys (n=4) trained to discriminate cocaine (0.4 mg/kg i.m.). Abscissae: Drug dose in mg/kg (log-scale). Top ordinates: percentage cocaine-like responding for the entire component. Bottom ordinates: rate of responding expressed as responses/second. Data are Mean +/− SEM for the group of monkeys from the pretreatment time that elicited peak cocaine-like responding for each drug and are presented as mean +/− SEM for the group. Cocaine 10-min pretreatment, d-MA 30-min pretreatment, d-AMPH 10-min pretreatment, l-MA 10-min pretreatment, PAL-329 30-min pretreatment, and PAL-169 30-min pretreatment. Substitution data was excluded for two subjects following 1.0 mg/kg cocaine and 5.6 mg/kg PAL-169, and one subject for 3.2 mg/kg PAL-169 because rate of responding was significantly suppressed (<0.2 responses/sec).
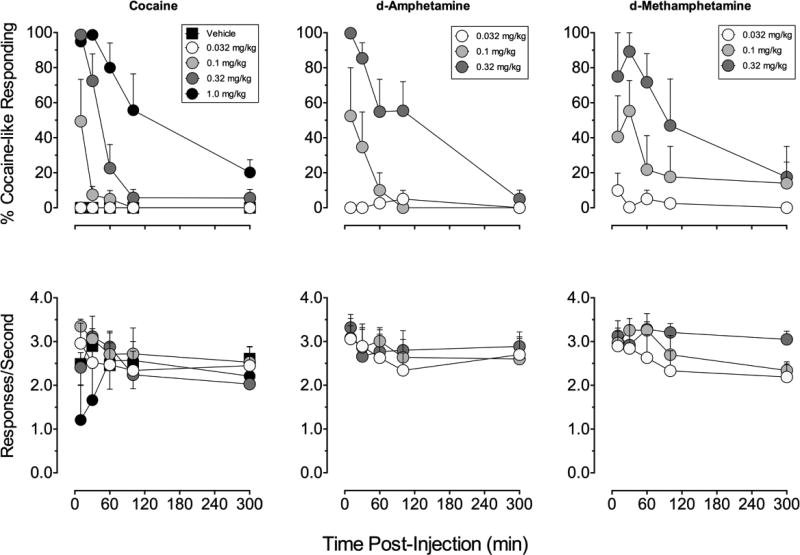
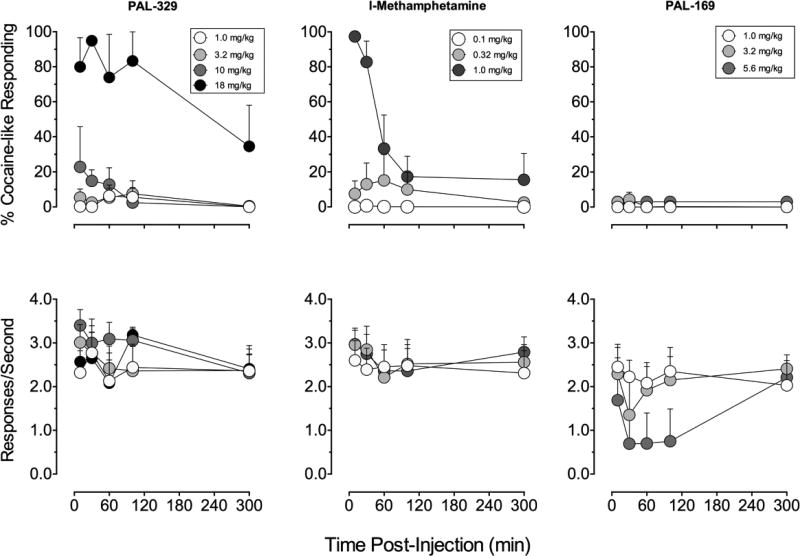
The time course of cocaine-like discriminative-stimulus effects varied among drugs. Inspection of group data (Figure 3, top panels) shows that cocaine and d-AMPH produced maximal cocaine-like responding 10-min after administration of 0.32 mg/kg whereas, consistent with a reportedly slower onset to action in human studies (Newton et al, 2005), d-MA produced partial substitution (~75%) at 10-min and full substitution at 30-min after IM administration of 0.32 mg/kg. The ‘NE preferring’ monoamine releasers PAL-329 and l-MA also engendered full substitution for cocaine at the 10-min time point but differed in their duration of action (Figure 4; top panels). A high dose of 18 mg/kg PAL-329 maintained near full cocaine-like responding >100-min post-injection, with partial substitution still evident at 300-min. The cocaine-like discriminative stimulus effects of 1.0 mg/kg l-MA dropped to below 80% after the 30-min time point with predominantly vehicle lever responding 100-min after administration. Doses of PAL-169 produced only vehicle lever responding at all time points tested and the rate altering effects were maintained for >120-min but returned to baseline by 300-min
Figure 3.
Time-course of cocaine-like discriminative stimulus effects of cocaine, d-amphetamine, and d-methamphetamine in rhesus monkeys (n=4) trained to discriminate cocaine (0.4 mg/kg i.m.). Abscissae: Time after drug administration (min). Top ordinates: percentage cocaine-like responding for the entire response period. Bottom ordinates: rate of responding expressed as responses per second. Data are from the entire component after each pretreatment time for each drug dose and are presented as mean +/− SEM for the group. Substitution data from two subjects were excluded from 1.0 mg/kg cocaine at 10- and 30-min because rate of responding was significantly suppressed (<0.2 responses/sec).
Figure 4.
Time-course of cocaine-like discriminative stimulus effects of PAL-329, l-methamphetamine, and PAL-169 in rhesus monkeys (n=4) trained to discriminate cocaine (0.4 mg/kg i.m.). Abscissae: Time after drug administration (min). Top ordinates: percentage cocaine-like responding for the entire session. Bottom ordinates: rate of responding expressed as responses/second. Other details as in Figure 2. Substitution data from two subjects were excluded from 5.6 mg/kg PAL-329 at 10–100 min and one subject for 3.2 mg/kg PAL-169 (30-min) and 18 mg/kg PAL-329 (100-min) because rate of responding was significantly suppressed (<0.2 responses/sec).
Interaction Studies
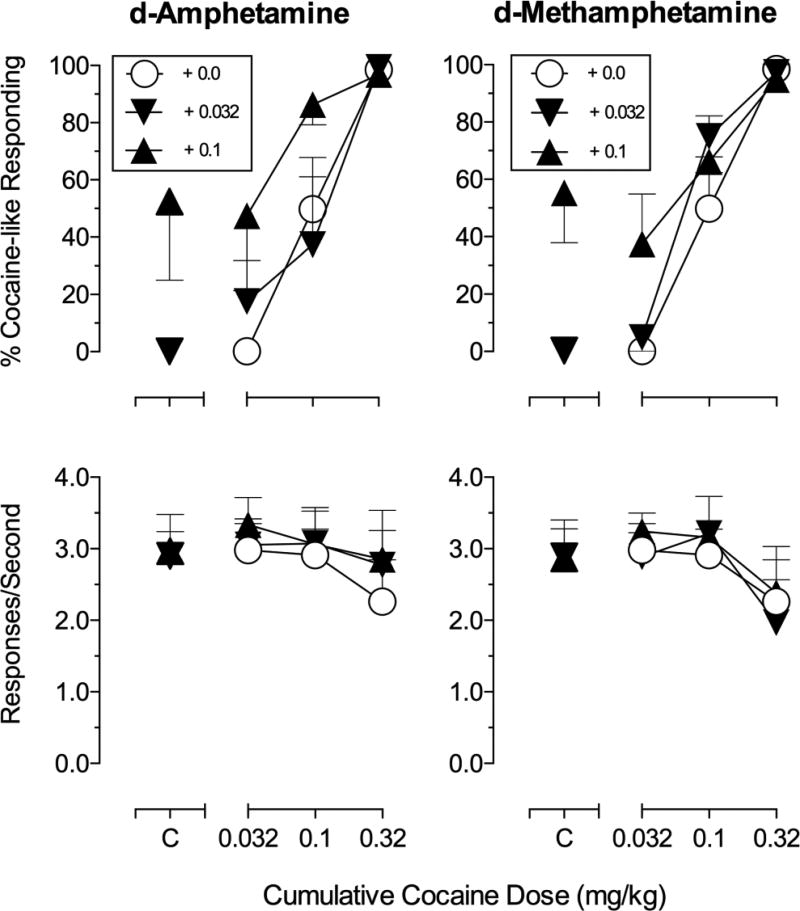
Control cocaine dose-effect curves (Figure 5 and 6) and ED80 analysis reflect averaged data from the first and last control dose-effect curve for all subjects (see above). As in time-course studies, cumulative cocaine doses produced dose-dependent increases in cocaine-like responding, with full substitution following administration of 0.32 mg/kg cocaine. When administered alone, both d-AMPH and d-MA produced vehicle-like responding following a dose of 0.032 mg/kg and approximately 50% cocaine-like responding after doses of 0.1 mg/kg. When administered prior to cumulative dosing with cocaine, only 0.1 mg/kg d-AMPH significantly shifted the cocaine dose-effect curve leftward; pretreament with the lower dose of d-AMPH or either dose of d-MA did not appreciably alter cocaine’s discriminative stimulus effects or overall rates of responding (see Table 3; Figure 5).
Figure 5.
Effects of pretreatment with d-amphetamine and d-methamphetamine on the discriminative stimulus effects of cocaine (0.032-0.32 mg/kg) in rhesus monkeys (n=4). Abscissae: Cumulative cocaine dose in mg/kg (log-scale), C = control injections of each drug dose from corresponding pretreatment times determined from time-course data. Top ordinates: percentage cocaine-like responding for the entire component. Bottom ordinates: rate of responding expressed as responses per second.
Figure 6.
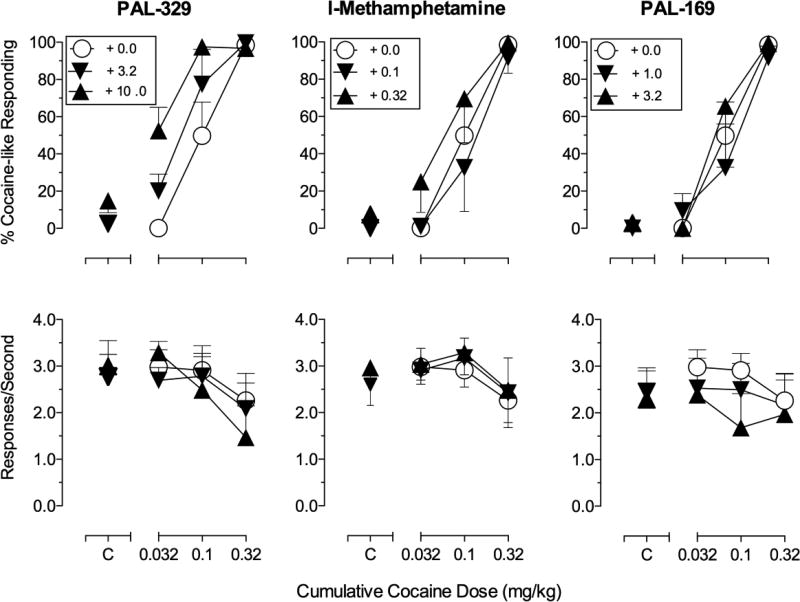
Effects of pretreatment with PAL-329, l-methamphetamine, and PAL-169 on the discriminative stimulus effects of cocaine (0.032-0.32 mg/kg) in rhesus monkeys (n=4). Abscissae: Cumulative cocaine dose in mg/kg (log-scale), C = control injections of each drug dose from corresponding pretreatment times from time-course data. Top ordinates: percentage cocaine-like responding for the entire component. Bottom ordinates: rate of responding expressed as responses/second. Substitution data from one subject was excluded from 3.2 mg/kg PAL-169 (cocaine doses 0.1–0.32 mg/kg) and from 10.0 mg/kg PAL-329 (cocaine dose 0.32 mg/kg) because rate of responding was significantly suppressed (<0.2 responses/sec).
Table 3.
ED80 estimates (95% C.L.) and relative potency of cocaine after pretreatment with nonselective monoamine releasers. n=4.
| Drug | ED80(mg/kg) | Relative Potency |
|---|---|---|
| Cocaine | 0.17 (0.11–0.27) | 1 |
| + 0.032 mg/kg d-Amph | 0.17 (0.10–0.28) | 1.00 |
| + 0.1 mg/kg d-Amph | 0.05 (0.03–0.10)* | 3.40 |
| + 0.032 mg/kg d-MA | 0.13 (0.08–0.20) | 1.31 |
| + 0.1 mg/kg d-MA | 0.08 (0.04–0.18) | 2.13 |
|
| ||
| + 0.1 mg/kg l-MA | 0.21 (0.11–0.41) | 0.81 |
| + 0.32 mg/kg l-MA | 0.11 (0.05–0.23) | 1.54 |
| + 3.2 mg/kg PAL-329 | 0.10 (0.06–0.17) | 1.70 |
| + 10.0 mg/kg PAL-329 | 0.05 (0.04–0.08)* | 3.40 |
| + 1.0 mg/kg PAL-169 | 0.20 (0.11–0.38) | 0.85 |
| + 3.2 mg/kg PAL-169# | 0.12 (0.06–0.25) | 1.42 |
n=3
significantly different from cocaine alone
The ‘NE preferring’ drugs varied in their interactive effects with cocaine (Figure 6). The highest dose of PAL-329 (10 mg/kg) which, when administered alone produced primarily vehicle-like responding (<20% cocaine-like responding), significantly shifted the cocaine dose-effect function approximately 3-fold to the left. However, like d-MA, no tested dose of l-MA or PAL-169 significantly altered the position of the cocaine dose-effect function (see Table 3). Response rates were not significantly altered by pretreatment with PAL-329, l-MA, or PAL-169 over the range of tested doses, though a modest decrease was evident after 3.2 mg/kg PAL-169 (Figure 6, bottom).
Discussion
The present study was conducted to compare the cocaine-like discriminative stimulus effects of ‘NE-preferring’ monoamine releasers PAL-329, l-MA, and PAL-169 and non-selective monoamine releasers, d-MA and d-AMPH in rhesus monkeys. Though the three NE-preferring drugs differ in the potency with which they release NE, each exhibit approximately 13-fold greater in vitro potency in releasing NE than DA (Kuczenski et al 1995; Melega et al 1999; see Table 1). Two of the drugs, PAL-329 and l-MA, fully substituted for the training dose of cocaine in the present studies, indicating that their greater potency to release NE over DA (NE/DA ratio) did not interfere with the expression of cocaine-like discriminative stimulus effects, which are thought to involve predominantly dopaminergic actions. The third compound, PAL-169, up to doses that markedly reduced response rates, did not produce any cocaine-like discriminative-stimulus effects. In this regard, PAL-169—unlike PAL-329 and l-MA—also exhibits approximately 3-fold greater potency in releasing 5-HT than DA (5-HT/DA ratio). Previous studies have shown that increased 5-HT neurotransmission has an inhibitory effect on extracellular DA-mediated effects (Rothman and Baumann 2006; Baumann et al 2011) and behavioral studies have described an inverse relationship between the ability of monoamine releasers to produce cocaine-like reinforcing effects and their 5-HT/DA ratio (Glowa and Fantegrossi 1997; Wee et al 2005). Thus, it is tempting to suggest that the 5-HT-related actions of PAL-169 may have attenuated its ability to produce cocaine-like discriminative-stimulus effects in the present study. However, this suggestion is not supported by previous findings with the related drug PAL-287, which has a 5-HT/DA ratio like that of PAL-169 (PAL-287 EC50 for DA=12.6, NE=11.1, 5-HT=3.4). In those studies, PAL-287 produced dose-related and full substitution in rhesus monkeys trained to discriminate cocaine from saline (Negus et al 2007; Banks et al 2014). It is noteworthy that, unlike PAL-169 but like d-MA and d-AMPH, PAL-287 releases NE and DA with similar potency. Possibly, behaviorally active doses of PAL-169 produce a combination of prominent NE-mediated and 5-HT-mediated, rather than DA-mediated, actions that preclude the expression of cocaine-like discriminative-stimulus effects. It is also possible that some unknown, off-target (i.e., non-monoaminergic) mechanism may be responsible for this difference.
Both PAL-329 and l-MA were less potent in producing cocaine-like discriminative stimulus effects than d-MA and d-AMPH, (relative potencies approximately 100:5:1:1 for PAL-329:l-MA:d-MA:d-AMPH). Although the rank order of potency is similar to the rank order of their in vitro potencies for releasing DA and NE, there is limited correspondence between relative potencies for producing cocaine-like discriminative stimulus effects and, based on EC50 values, relative potencies for releasing DA (55:16:1:1) or NE (approximately 15:4:1.7:1), or relative DA/NE ratios (3.8:4.3:0.6:1; cf. Tables 1 and 2). It also is interesting to note that NE-preferring and nonselective monoamine releasers appeared to differ in their ability to produce partial (20–80%) substitution for cocaine when administered alone. For example, doses of PAL-329 and l-MA below those that produced full substitution failed to engender >20% responding on the cocaine-associated key whereas 0.01 mg/kg of d-MA or d-AMPH produced partial (approximately 50%) substitution for the training dose of cocaine. It may be that the NE-related actions of PAL-329 and l-MA predominated until the dose was sufficiently high to reach a “threshold” level of DA release (Desai and Bergman 2010; Kohut et al 2014). Interestingly, previous findings in squirrel monkeys have shown that norepinephrine reuptake inhibitors substitute for the discriminative stimulus effects of a low, but not high, cocaine training dose (Spealman 1995) suggesting that the substitution profile of these compounds may change with a higher or lower training dose than that used in the present study. Additional research is necessary to determine whether differences in time-course or maximal increase in extracullar neurotransmitter levels may account for the differences in substitution profile.
The present studies indicate that d-AMPH accentuates the discriminative stimulus effects of cocaine. Previous studies in rodents, on the other hand, have found that both d-MA and d-AMPH produced leftward shifts in the dose-effect function for cocaine discrimination that were greater than predicted by simple addition (Li et al 2006). Such differences in drug interactions may be the result of species-related differences in monoamine systems that have been reported previously. Alternatively, these findings may reflect differences in the relative potency of the drugs in the two sets of studies. In contrast to their similar potencies in monkeys shown in the present study, d-MA and d-AMPH were more potent than cocaine in rodents—a difference that, in turn, may be attributable to dissimilar training doses of cocaine (3 or 10 mg/kg, IP rat, Terry et al 1994; 0.4 mg/kg, IM monkey, present study).
The NE-preferring monoamine releaser PAL-329 produced a significant leftward shift in the cocaine dose-effect function. Doses of PAL-329 that engendered <20% cocaine-like responding when administered alone produced a 50% or greater increase in cocaine-like responding when combined with low and intermediate doses of cocaine which had little effect alone. On the other hand, PAL-169 or l-MA did not appreciably modify cocaine’s discriminative stimulus effects. As discussed above, PAL-169 failed to produce cocaine-like discriminative-stimulus effects, and it is not surprising that it also failed to accentuate those of cocaine itself. However, the difference in the modification of cocaine’s effects by PAL-329 and l-MA is somewhat surprising. It is possible that the difference in their potencies for releasing 5-HT was a contributing factor, i.e., cocaine-like effects produced by the release of NE and DA were more effectively dampened by the serotonergic activity of l-MA than PAL-329. In this regard, it is noteworthy that the only other leftward shifts in the cocaine dose-effect function in the interaction studies occurred after d-AMPH which, like PAL-329, has negligible activity as a 5-HT releaser, and may help to explain the absence of a shift after d-MA which has an 5-HT/DA EC50 ratio more similar to l-MA. However, the role of 5-HT in such drug interactions remains speculative in the absence of additional comparison data.
We have previously reported that l-MA is about 3–5-fold less potent than d-MA in rats or monkeys trained to discriminate either cocaine or d-MA (Yasar and Bergman 1994; Desai and Bergman 2010; Kohut et al 2016). The present studies add to these results showing that l-MA also has a shorter duration of cocaine-like effects than d-MA. These observations are consistent with previous studies indicating that the subjective effects of l-MA disappear more quickly than those of d-MA (Mendelson et al 2006) and the shorter duration of l-MA’s cocaine-like effects do not appear to be related to large differences in pharmacokinetics of the two isomers. PET studies in baboons have shown a similar time-course of distribution in various brain regions following their intravenous administration (Fowler et al 2007). On the other hand, a pharmacokinetic comparison of d- and l-MA in human subjects found that metabolism of MA is stereoselective, particularly in regard to AMPH (Li et al 2010). Differences in the bioactivity of d-MA or l-MA metabolites, in turn, may contribute to differences in the time-course of the behavioral effects resulting from administration of the two isomers of methamphetamine.
Finally, it may be useful to consider the present findings within the framework of agonist-type medications for the management of cocaine use disorder (Kohut et al 2016). The agonist replacement approach to medication development is intended to identify compounds with biochemical and behavioral effects that overlap those of cocaine, presumably easing abnormalities in brain chemistry and function that have been characterized in long-term drug abusers (Rothman et al 2006). Overlapping behavioral effects also may encourage compliance with the medication regimen, a key factor in treatment outcomes. In the present experiments, two monoamine releasers, l-MA and PAL-329, were shown to produce cocaine-like discriminative-stimulus effects in monkeys, suggesting that they meet the above criteria. One of these compounds, l-MA, also has been shown to serve as a positive reinforcer in rodents (Yokel and Pickens 1973) and monkeys (Winger et al 1994), further confirming the overlap with behavioral effects of cocaine. Both compounds also exhibit an approximately 15-fold greater potency in releasing NE than DA, which may be therapeutically advantageous. For example, the subjective effects of l-MA in human studies are similar in some respects to those of d-MA. However, the subjective effects of the two isomers also differ in potentially important ways. While both l-MA and d-MA produce subjective ratings of “drug liking” and “good effects” in experienced stimulant users, only l-MA produces concomitant ratings of bad or aversive drug effects (Mendelson et al 2006), a factor which may limit its abuse liability. It is important to note that both Mendelson et al (2006) and the present study only included male subjects and it will be important for future studies to extend these findings to female subjects to determine whether sex as a biological variable influences responses to monoamine releasers. Regardless, it is tempting to suggest that the differences in the behavioral effects of the two isomers can be attributed to differences in their relative potencies for releasing NE and DA. However, it also is possible that the dissimilar behavioral profiles reflect differences in potency of drugs like d-MA and d-AMPH (high) and l-MA and PAL-329 (low) for releasing DA. Further characterization of these and additional compounds with similar pharmacological profiles as “NE-preferring” monoamine releasers varying in potency for releasing DA should provide insight into the mechanism of their behavioral effects and, as well, their value for the management of cocaine use disorder.
Acknowledgments
This work was funded by National Institutes of Health grants DA002519 (to JB), DA039306 (to SJK), and DA12970 (to BEB). The authors thank Olga Smirnova and Kevin Costa for assistance with conducting these studies, and Dr. Roger D. Spealman for comments on an earlier version of this manuscript.
Footnotes
The authors have no conflicts of interest to declare.
References
- Banks ML, Bauer CT, Blough BE, Rothman RB, Partilla JS, Baumann MH, Negus SS. Abuse-related effects of dual dopamine/serotonin releasers with varying potency to release norepinephrine in male rats and rhesus monkeys. Exp Clin Psychopharmacology. 2014;22:274–284. doi: 10.1037/a0036595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Negus SS. Effects of monoamine releasers with varying selectivity for releasing dopamine/norepinephrine versus serotonin on choice between cocaine and food in rhesus monkeys. Behav Pharmacol. 2011;22:824–836. doi: 10.1097/FBP.0b013e32834d63ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Woolverton WL, Wee S, Blough BE, Rothman RB. In Vivo Effects of Amphetamine Analogs Reveal Evidence for Serotonergic Inhibition of Mesolimbic Dopamine Transmission in the Rat. J Pharmacol Exp Ther. 2011;337:218–225. doi: 10.1124/jpet.110.176271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline EJ, Scheffel U, Boja JW, Carroll FI, Katz JL, Kuhar MJ. Behavioral effects of novel cocaine analogs: a comparison with in vivo receptor binding potency. J Pharmacol Exp Ther. 1992;260:1174–1179. [PubMed] [Google Scholar]
- Cooper DA, Kimmel HL, Manvich DF, Schmidt KT, Weinshenker D, Howell LL. Effects of pharmacologic dopamine β-hydroxylase inhibition on cocaine-induced reinstatement and dopamine neurochemistry in squirrel monkeys. J Pharmacol Exp Ther. 2014;350:144–152. doi: 10.1124/jpet.113.212357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Stoops WW, Rush CR. Evaluation of the “Pipeline” for Development of Medications for Cocaine Use Disorder: A Review of Translational Preclinical, Human Laboratory, and Clinical Trial Research. Pharmacol Rev. 2016;68:533–562. doi: 10.1124/pr.115.011668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai RI, Bergman J. Drug Discrimination in Methamphetamine-Trained Rats: Effects of Cholinergic Nicotinic Compounds. J Pharmacol Exp Ther. 2010;335:807–816. doi: 10.1124/jpet.110.173773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JS, Kroll C, Ferrieri R, Alexoff D, Logan J, Dewey SL, Schiffer W, Schlyer D, Carter P, King P, Shea C, Xu Y, Muench L, Benveniste H, Vaska P, Volkow ND. PET Studies of d-Methamphetamine Pharmacokinetics in Primates: Comparison with l-Methamphetamine and ()-Cocaine. J Nuclear Med. 2007;48:1724–1732. doi: 10.2967/jnumed.107.040279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowa JR, Fantegrossi WE. Effects of dopaminergic drugs on food- and cocaine-maintained responding. IV: continuous cocaine infusions. Drug Alcohol Depend. 1997;45:71–79. doi: 10.1016/s0376-8716(97)01350-1. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addictive Behaviors. 2004;29:1439–1464. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Hart C, Ward A, Haney M, Foltin R, Fischman M. Methamphetamine self-administration by humans. Psychopharmacology. 2001;157:75–81. doi: 10.1007/s002130100738. [DOI] [PubMed] [Google Scholar]
- Howell LL, Cunningham KA. Serotonin 5-HT2 receptor interactions with dopamine function: implications for therapeutics in cocaine use disorder. Pharmacol Rev. 2015;67:176–197. doi: 10.1124/pr.114.009514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ILAR-NRC. Guide for the care and use of laboratory animals, 8th edition (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council) Vol. 125 The National Academies press; 2011. [Google Scholar]
- Kirkpatrick MG, Gunderson EW, Johanson CE, Levin FR, Foltin RW, Hart CL. Comparison of intranasal methamphetamine and d-amphetamine self-administration by humans. Addiction. 2012;107:783–791. doi: 10.1111/j.1360-0443.2011.03706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleven MS, Koek W. Discriminative stimulus properties of cocaine: enhancement by beta-adrenergic receptor antagonists. Psychopharmacology. 1997;131:307–312. doi: 10.1007/s002130050297. [DOI] [PubMed] [Google Scholar]
- Kohut SJ, Bergman J, Blough BE. Effects of L-methamphetamine treatment on cocaine- and food-maintained behavior in rhesus monkeys. Psychopharmacology. 2016;233:1067–1075. doi: 10.1007/s00213-015-4186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohut SJ, Fivel PA, Mello NK, Mello NK. Differential effects of acute and chronic treatment with the α2-adrenergic agonist, lofexidine, on cocaine self-administration in rhesus monkeys. Drug Alcohol Depend. 2013;133:593–599. doi: 10.1016/j.drugalcdep.2013.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohut SJ, Hiranita T, Hong S-K, Ebbs AL, Tronci V, Green J, Garcés-Ramírez L, Chun LE, Mereu M, Newman AH, Katz JL, Tanda G. Preference for distinct functional conformations of the dopamine transporter alters the relationship between subjective effects of cocaine and stimulation of mesolimbic dopamine. Biological Psychiatry. 2014;76:802–809. doi: 10.1016/j.biopsych.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS, Cho AK, Melega W. Hippocampus norepinephrine, caudate dopamine and serotonin, and behavioral responses to the stereoisomers of amphetamine and methamphetamine. J Neurosci. 1995;15:1308–1317. doi: 10.1523/JNEUROSCI.15-02-01308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Everhart T, Jacob P, III, Jones R, Mendelson J. Stereoselectivity in the human metabolism of methamphetamine. British J Clin Pharmacol. 2010;69:187–192. doi: 10.1111/j.1365-2125.2009.03576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S-M, Campbell BL, Katz JL. Interactions of Cocaine with Dopamine Uptake Inhibitors or Dopamine Releasers in Rats Discriminating Cocaine. J Pharmacol Exp Ther. 2006;317:1088–1096. doi: 10.1124/jpet.105.100594. [DOI] [PubMed] [Google Scholar]
- Lile JA, Charnigo RJ, Nader MA. The relative reinforcing strength of methamphetamine and D-amphetamine in monkeys self-administering cocaine. Behav Pharmacol. 2013;24:482–485. doi: 10.1097/FBP.0b013e3283644d44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manvich DF, Manvich DF, DePoy LM, DePoy LM, Weinshenker D, Weinshenker D. Dopamine β-hydroxylase inhibitors enhance the discriminative stimulus effects of cocaine in rats. J Pharmacol Exp Ther. 2013;347:564–573. doi: 10.1124/jpet.113.207746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melega WP, Cho AK, Schmitz D, Kuczenski R, Segal DS. l-methamphetamine pharmacokinetics and pharmacodynamics for assessment of in vivo deprenyl-derived l-methamphetamine. J Pharmacol Exp Ther. 1999;288:752–758. [PubMed] [Google Scholar]
- Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14:375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- Mendelson J, Uemura N, Harris D, Nath R, Fernandez E, Jacobelli P, Everhart E, Jones R. Human pharmacology of the methamphetamine stereoisomers. Clin Pharmacol Ther. 2006;80:403–420. doi: 10.1016/j.clpt.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Negus SS, Baumann MH, Rothman RB, Mello NK, Blough BE. Selective Suppression of Cocaine- versus Food-Maintained Responding by Monoamine Releasers in Rhesus Monkeys: Benzylpiperazine, (+)Phenmetrazine, and 4-Benzylpiperidine. J Pharmacol Exp Ther. 2009;329:272–281. doi: 10.1124/jpet.108.143701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Blough BE, Baumann MH, Rothman RB. Monoamine releasers with varying selectivity for dopamine/norepinephrine versus serotonin release as candidate “agonist” medications for cocaine dependence: studies in assays of cocaine discrimination and cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 2007;320:627–636. doi: 10.1124/jpet.106.107383. [DOI] [PubMed] [Google Scholar]
- Newton TF, De La Garza R, Kalechstein AD, Nestor L. Cocaine and methamphetamine produce different patterns of subjective and cardiovascular effects. Pharmacol Biochem Behav. 2005;82:90–97. doi: 10.1016/j.pbb.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. Noradrenergic mechanisms in cocaine-induced reinstatement of drug seeking in squirrel monkeys. J Pharmacol Exp Ther. 2007;322:894–902. doi: 10.1124/jpet.107.121806. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Balance between Dopamine and Serotonin Release Modulates Behavioral Effects of Amphetamine-Type Drugs. Annals of the New York Academy of Sciences. 2006;1074:245–260. doi: 10.1196/annals.1369.064. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur J Pharmacol. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2000;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Baumann MH. Appetite Suppressants as Agonist Substitution Therapies for Stimulant Dependence. Annals of the New York Academy of Sciences. 2006;965:109–126. doi: 10.1111/j.1749-6632.2002.tb04155.x. [DOI] [PubMed] [Google Scholar]
- Schmidt KT, Weinshenker D. Adrenaline rush: the role of adrenergic receptors in stimulant-induced behaviors. Mol Pharmacol. 2014;85:640–650. doi: 10.1124/mol.113.090118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JP, Epps SA, Grice TW, Weinshenker D. The selective dopamine β-hydroxylase inhibitor nepicastat attenuates multiple aspects of cocaine-seeking behavior. Neuropsychopharmacology. 2013;38:1032–1038. doi: 10.1038/npp.2012.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M. A note on the measurement of a conditional discrimination. J Exp Anal Behav. 1980;33:285–289. doi: 10.1901/jeab.1980.33-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spealman RD. Noradrenergic involvement in the discriminative stimulus effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 1995;275:53–62. [PubMed] [Google Scholar]
- Terry P, Witkin JM, Katz JL. Pharmacological Characterization of the novel discriminative stimulus effects of a low dose of cocaine. J Pharmacol Exp Ther. 1994;270:1041–1048. [PubMed] [Google Scholar]
- Walsh SL, Middleton LS, Wong CJ, Nuzzo PA, Campbell CL, Rush CR, Lofwall MR. Atomoxetine does not alter cocaine use in cocaine dependent individuals: A double blind randomized trial. Drug Alcohol Depend. 2013;130:150–157. doi: 10.1016/j.drugalcdep.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Woolverton WL. Evaluation of the reinforcing effects of atomoxetine in monkeys: comparison to methylphenidate and desipramine. Drug Alcohol Depend. 2004;75:271–276. doi: 10.1016/j.drugalcdep.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Wee S, Woolverton WL. Self-administration of mixtures of fenfluramine and amphetamine by rhesus monkeys. Pharmacol Biochem Beh. 2006;84:337–343. doi: 10.1016/j.pbb.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL. Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. J Pharmacol Exp Ther. 2005;313:848–854. doi: 10.1124/jpet.104.080101. [DOI] [PubMed] [Google Scholar]
- Wee S, Wang Z, He R, Zhou J, Kozikowski AP, Woolverton WL. Role of the increased noradrenergic neurotransmission in drug self-administration. Drug Alcohol Depend. 2006;82:151–157. doi: 10.1016/j.drugalcdep.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Winger GD, Yasar S, Negus SS, Goldberg SR. Intravenous self-administration studies with l-deprenyl (selegiline) in monkeys*. Clin Pharmacol Ther. 1994;56:774–780. doi: 10.1038/clpt.1994.208. [DOI] [PubMed] [Google Scholar]
- Wood AJJ, Henningfield JE. Nicotine Medications for Smoking Cessation. N Engl J Med. 1995;333:1196–1203. doi: 10.1056/NEJM199511023331807. [DOI] [PubMed] [Google Scholar]
- Woods JH, Tessel RE. Fenfluramine: amphetamine congener that fails to maintain drug-taking behavior in the rhesus monkey. Science. 1974;185:1067–1069. doi: 10.1126/science.185.4156.1067. [DOI] [PubMed] [Google Scholar]
- Yasar S, Bergman J. Amphetamine-like effect of l-deprenyl (selegiline) in drug discrimination studies*. Clin Pharmacol Ther. 1994;56:768–773. doi: 10.1038/clpt.1994.207. [DOI] [PubMed] [Google Scholar]
- Yokel RA, Pickens R. Self-administration of optical isomers of amphetamine and methylamphetamine by rats. J Pharmacol Exp Ther. 1973;187:27–33. [PubMed] [Google Scholar]