Abstract
Background
Heart failure in women increases around the time of menopause when high-fat diets may result in obesity. The heart produces brain natriuretic peptide (BNP), also known as B-type natriuretic peptide. This aims of this study were to assess cardiac hypertrophy and BNP levels in ovariectomized rats fed a high-fat diet.
Material/Methods
Forty-eight female Wistar rats were divided into four groups: sham-operated rats fed a control diet (SC) (n=12); ovariectomized rats fed a control diet (OC) (n=12); sham-operated rats fed a high-fat diet (SF) (n=12); and ovariectomized rats fed a high-fat diet (OF) (n=12). Body weight and blood pressure were measured weekly for 24 weeks. Rats were then euthanized, and plasma samples and heart tissue were studied for gene expression, hydroxyproline levels, and histological examination.
Results
A high-fat diet and ovariectomy (group OF) increased the weight body and the systolic blood pressure after three months and five months, respectively. Cardiomyocyte hypertrophy was associated with increased expression of ventricular BNP, decreased natriuretic peptide receptor (NPR)-A and increased levels of hydroxyproline and transforming growth factor (TGF)-β. The plasma levels of BNP and estradiol were inversely correlated; expression of estrogen receptor (ER)β and ERα were reduced.
Conclusions
The findings of this study showed that, in the ovariectomized rats fed a high-fat diet, the BNP-NPR-A receptor complex was involved in cardiac remodeling. BNP may be a marker of cardiac hypertrophy in this animal model.
MeSH Keywords: Estradiol; Hypertension; Natriuretic Peptide, Brain; Obesity, Abdominal
Background
Obesity is clinically associated with the development of cardiovascular disease, but the mechanisms involved remain poorly understood. Obesity is a risk factor for the development of hypertension, associated with endothelial dysfunction [1–3]. High blood pressure, associated with expression of inflammatory cytokines and fibrinolytic factors, are associated with obesity [4–6]. High blood pressure and cardiac hypertrophy can be associated with atherogenesis and oxidative stress, which contributes to heart failure [7].
Brain natriuretic peptide (BNP), which is also known as B-type natriuretic peptide, has been reported to be associated with cardiac overload and shows antiproliferative and antifibrinolytic activity [8]. Also, BNP modulates the expression of the transforming growth factor (TGF)-β gene that mainly promotes fibrosis and cardiac hypertrophy [9]. Therefore, BNP has been proposed as a potential marker for heart disease and to evaluate the progression of cardiovascular disease, including cardiac hypertrophy [10,11]. The effects of BNP are by binding to the A-receptor natriuretic peptide (NPR-A), leading to increased synthesis of cyclic guanosine monophosphate (cGMP) [12]. BNP as a mediator of cardiac disease may participate in the regulation of several hypertrophic pathways, including via cytokines and growth factors that can stimulate the production of cardiac natriuretic hormones, and some authors have proposed a link between endocrine function and remodeling in myocardial and smooth muscle cells [13–15].
Ovarian hormones have an important role in the cardiovascular system as premenopausal women have a lower risk of developing cardiovascular disease when compared with men at the same age; this gender difference disappears after menopause [16–19]. Also, the contribution of ovarian hormones on the pattern of cardiac hypertrophy has been demonstrated by studies showing that the physiological replacement with 17β-estradiol (E2) in female mice limits left ventricular hypertrophy induced by pressure overload [20]. A previous study in our laboratory, using a rat model of obesity, has shown that a high-fat diet and ovariectomy increased blood pressure as well as inducing the development of cardiac hypertrophy [21]. This established animal model was used in the present study.
This aims of this study were to assess cardiac hypertrophy, and BNP levels in ovariectomized rats fed a high-fat diet. Estradiol levels, and gene expression for estrogen receptor alpha (ERα) and beta (ERβ), measurement blood pressure, cardiac hypertrophy, and fibrosis were evaluated.
Material and Methods
Animals studied
Forty-eight female Wistar rats were maintained in an environment with controlled light (12 hours) and dark (12 hours), and controlled temperature (23±1ºC), in the Institute of Multidisciplinary Health, University Federal of Bahia, BA, Brazil. The animals received food and water ad libitum. The study was conducted for 24 weeks.
The study was approved by the Ethics Committee in Animal Experimentation of the State University of Feira de Santana and carried out according to the recommendations of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
To simulate the post-menopausal state, female rats were ovariectomized at ten weeks of age. The animals were ovariectomized or underwent sham operation using general anesthesia with a tribromoethanol solution consisting of 2.5% (0.025 g/ml) tribromoethanol diluted in 0.9% normal saline, administered at a dose of 1 ml of solution per 100 gm of animal body weight.
Animal diets
Animals were fed a standardized laboratory rat diet from the American Institute of Nutrition 93 (AIN 93) (Pragsoluções Biosciences, São Paulo, SP, Brazil) for a week of acclimatization. After the procedure of ovariectomy, the rats received the following diets: a diet containing 54.4% of total calories from fat (Pragsoluções Biosciences, São Paulo, SP, Brazil) or a control diet or standard diet AIN 93 (Pragsoluções Biosciences, São Paulo, SP, Brazil). The specific compositions of the diets have been reported in a previous study [21].
The 48 female Wistar rats were divided into four groups: sham-operated rats fed a control diet (SC) (n=12); ovariectomized rats fed a control diet (OC) (n=12); sham-operated rats fed a high-fat diet (SF) (n=12); and ovariectomized rats fed a high-fat diet (OF) (n=12).
Rat cardiac and plasma samples
Measurements of systolic blood pressure were made by the tail plethysmography method using an electronic sphygmomanometer LE5001 (Panlab, Barcelona, Spain). After five minutes of pre-heating, the animal tail was placed in contact with a cuff, and a pulse transducer was used to measure the heart rate, and the mean systolic and diastolic blood pressures, calculated from five consecutive data readings each day.
Body weight and blood pressure were measured weekly for 24 weeks. The rats were then euthanized by decapitation, and the heart was removed, weighed, and used for further analysis of gene expression, histology, immunohistochemistry, and determination of hydroxyproline levels (a total of four hearts per group for each of three techniques).
Blood was collected in heparinized tubes containing protease inhibitors. After centrifugation (10,000×G) for 15 minutes, plasma was separated for measurement of estradiol, transforming growth factor (TGF)-β and brain natriuretic peptide (BNP), which is also known as B-type natriuretic peptide. RNA extraction and polymerase chain reaction (PCR) were performed to determine the gene expression of BNP and the natriuretic peptide receptor (NPR)-A gene, NPR-A, and levels of estrogen receptor (ER) genes in the ventricles.
Plasma measurements using ELISA
The plasma measurement of BNP levels was made by an enzyme-linked immunosorbent assay (ELISA) (RayBio® BNP EIA Kit) (Cat. No. EIA-BNP-1) (RayBiotech, Inc. Norcross, GA, USA).
The plasma levels of estradiol were determined using an ELISA kit (Cat. No. KAQ0621) (Biosource, CA, USA). The determination of plasma levels of transforming growth factor (TGF)-β was performed using an ELISA kit for rat TGF-β (Cat. No. E0131Ra) (Uscn Wuhan Life Science Inc.).
Gene expression analysis
Rat cardiac RNA was extracted using Brazol® reagent, 1 mL/100 mg, following the manufacturer’s protocol. Moloney murine leukemia virus reverse transcriptase (M-MLV RT) was used under standard conditions in a 20 μl reaction, in which 1 μg of RNA was reverse transcribed.
The following oligonucleotides were used for amplification:
BNP, 258 bp, 5′-TCTGCTCCTGCTTTTCCTTA and GAACTATGT GCCATCTTGGA-3′ as forward and reverse primers, respectively;
NPR-A, 454 bp, 5′-CTCAACATCACAGTAAATCACC and CCTGAA GGCACCTGTCTCG-3′ as forward and reverse primers, respectively;
ERα, 623 bp, 5′-TAAGAACCGGAGGAAGAGTTG and TCATGCG GAATCGACTTG-3′ as forward and reverse primers, respectively and
ERβ, 159 bp, 5′-GCTCCTCTATGCAGAACCTCAAA and CAGAAGT GAGCATCCCTCTTTG-3′ as forward and reverse primers, respectively.
The conditions of the polymerase chain reaction (PCR) were standardized and defined after testing [22]. Primers were manufactured by Prodimol Biotechnology (Belo Horizonte, Brazil). The PCR amplifications for BNP were performed at 95°C for 5 min, followed by 35 cycles at 94°C for 30 s, 59°C for 30 s, and 72°C for 30 s. The PCR amplifications for NPR-A were performed at 95ºC for 5 min followed by 40 cycles at 94ºC for 15 s, 58ºC for 30 s, and 72ºC for 30 s. The PCR amplifications for ERα were performed at 95 ºC for 5 min followed by 25 cycles at 94ºC for 30 s, 45ºC for 30 s, and at 72ºC for 1 min. The PCR amplifications for ERβ were performed at 95 ºC for 5 min followed by 30 cycles at 95ºC for 30 s, 60ºC for 30 s, and 72ºC for 40 s. The final extension was for 10 min. All reaction products were separated on a 1.0–1.5% agarose gel and stained with ethidium bromide. β-Actin was amplified as an internal control in each PCR test using specific primers to provide a semi-quantitative assessment. All PCR reactions were performed in triplicate.
Rat cardiac morphology and histology
The rat hearts were removed and fixed in 4% formaldehyde.Transmural slices were taken from the middle region of the ventricular myocardium, and longitudinal and transverse sections were taken. The rat heart tissues were embedded in paraffin wax and processed using conventional histological techniques. Tissue sections of 5 mm thickness were cut onto glass slides, and the sections were stained with histochemically with hematoxylin and eosin (H&E). Morphometric measurements were performed using computerized interactive image analysis with Image Pro Plus 4.1 imaging software (Media Cybernetics, Silver Springs, MD, USA), a method previously shown to reduce interobserver variability. For the linear measurements (diameters) of cardiomyocytes, an imaging magnification of ×400 objective was used. Sixty cells were measured by region, in at least ten different fields, with the smallest cardiomyocyte diameter measured as the line a line that intersected the cell nucleus. Photomicrographs were taken of the rat cardiac histological fields with a magnification of ×200.
Immunohistochemistry for rat cardiac α-smooth muscle actin (ASMA)
The unstained 5 mm rat cardiac tissue sections were deparaffinized prior to immunohistochemistry. The tissue sections were incubated with primary monoclonal anti-mouse α-smooth muscle actin (ASMA) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted 1: 500 in PBS solution and 1% bovine serum albumin (BSA) at 8ºC overnight in a humid chamber. The reaction product was detected with an avidin-biotin-peroxidase complex (Leica Biosystems, Newcastle, United Kingdom). Antigen-antibody binding was detected using the chromogen, diaminobenzidine (DAB) (Cat. No. K3468) (DakoCytomation, CA, USA), one drop diluted in 2 ml of buffer and the tissue sections were incubated for 1 minute, then washed in distilled water to remove any remaining DAB. Counterstaining was carried out in Harris hematoxylin for 5 minutes. A negative control was included that omitted the use of the primary antibody.
Positive immunohistochemical staining (brown) was localized by the primary antibody used and was quantified by the histological evaluation of 30 fields per slide with positive immunostaining attributed to the marked ventricular area by assigning a score of 0–4. A score of 0 was equivalent to 0–5% of the set; a score of 1 was equivalent to 5–25%; a score of 2 was equivalent to 25–50%; a score 3 was equivalent to 50–75%; and a score of 4 was equivalent to 75–100% [26]. Immunohistochemical staining scoring analysis was performed with a ×400 objective, and photomicrographs were taken with an objective magnification of ×200.
Rat cardiac hydroxyproline levels as an indirect measure of collagen content
The measurement of rat cardiac hydroxyproline, as an indirect measure of collagen content, was performed using a modified version of the colorimetric assay, as described previously [24]. Briefly, whole hearts from four animals per group were selected at random, and the ventricles were separated from the atria. The samples of rat cardiac ventricles were then lyophilized overnight using the Labconco 3 freeze dryer (Labconco, Kansas City, MI, USA). The samples were weighed and hydrolyzed with HCl solution overnight at 120°C. For the generation of the standard curve, 20–70 mg of hydroxyproline were added to individual wells in a 96-well plate. The hydrolyzed samples were then separately added to the wells and incubated with Ehrlich’s reagent (p-dimethylamino benzaldehyde) for 30 minutes at 80°C. Colorimetric results were read using a Bio-Rad Benchmark Microplate Reader at a wavelength of 557 nm. The calculation of the levels of hydroxyproline was made by linear regression analysis and compared with known standards. The total hydroxyproline levels were calculated in milligram per gram of rat cardiac tissue.
Statistical analysis
The values of two variables, diet and ovariectomy, and the means between groups were determined from the individual variation or variation of error (s2), using two-way analysis of variance (ANOVA) followed by the Bonferroni method of analysis. Correlations were calculated using the two-tailed Pearson’s correlation coefficient (r) (r=0.10–0.30, a weak correlation; r=0.40–0.60, a moderate correlation; r=0.70–1.0, a strong correlation). The results were expressed as mean ± the standard deviation (SD) using the GraphPad Software®. A p-value ≤0.05 was considered to be statistically significant.
Results
Ovariectomized rats fed a high-fat diet and hypertension associated with cardiac hypertrophy
Forty-eight female Wistar rats were divided into four groups: sham-operated rats fed a control diet (SC) (n=12); ovariectomized rats fed a control diet (OC) (n=12); sham-operated rats fed a high-fat diet (SF) (n=12); and ovariectomized rats fed a high-fat diet (OF) (n=12).
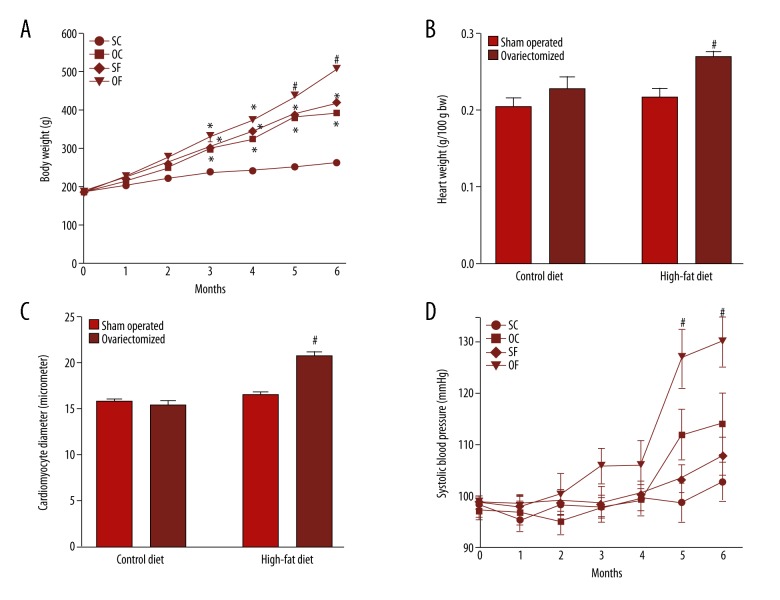
In this study, ovariectomy associated with a high-fat diet (OF group) induced a significant increase (p<0.05) in body weight (Figure 1A). The high-fat diet (SF group) or ovariectomy (OC group) increased the body weight of animals when compared with the control group (SC group). This difference was noted from the third month of the study and remained until the end of the sixth month. Also, the OF group showed an increase in weight gain that started at the third month, with a greater weight gain when compared with all other groups by the fifth and sixth month of the study (Figure 1A).
Figure 1.
Ovariectomy associated with high-fat diet (the OF group) induces weight gain, increase in cardiac weight and increase in systolic blood pressure (SBP). (A) Body weight gain. The body weight gain was measured weekly. The results show an average of each animal group per month. N=12 for each group. (B) This figure shows the results of the heart weight of the animals. After euthanasia, the whole hearts were removed and weighed immediately. The results were normalized by body weight and expressed as gram per 100 grams. N=12 for each group. (C) Cardiomyocyte diameter. For the linear measurements (diameters) of cardiomyocytes, ×400 objective was used. Sixty cells were measured by region in at least ten different fields. As a parameter, the smallest diameter measured in cardiomyocytes is in a line that intersects the nuclei. N=4 for each group. (D) Systolic blood pressure (SBP). The SBP was measured weekly by tail plethysmography, and the results indicate an average of each animal group per month. Measurements were made by tail plethysmography using the electro-sphygmomanometer LE5001. After five minutes of pre-heating at 37°C, the rat tail was placed in contact with a cuff, and a pulse transducer to register data from mean cardiac rate, and mean systolic and diastolic blood pressure. The mean from five consecutive data each day was used as an individual result. N=8 for each group. SC – sham-operated fed a control diet; OC – ovariectomized fed a control diet; SF – sham-operated fed a high-fat diet; OF – ovariectomized fed a high-fat diet. p<0.05 vs. SC; # p<0.05 vs. SC, OC and SF. The results were analyzed by two-way ANOVA followed by Bonferroni’s test.
The heart weight was measured after the sixth month of a high-fat diet and ovariectomy. Ovariectomy or isolated fat diet alone did not alter the heart weight. However, the association of these variables in the ovariectomized rats fed a high-fat diet (OF group) resulted in a significant increase (p<0.05) in heart weight (Figure 1B). The same result was observed regarding the diameter of cardiomyocytes. Ovariectomized rats fed a high-fat diet (OF group) showed a significant (p<0.05) increase in the diameter of cardiomyocytes when compared with the other groups (Figure 1C).
Previous studies on the morphological development of cardiac hypertrophy in this model have been shown previously by our group [21]. With the onset of cardiac remodeling, both cells and the macroscopic structure of the myocardium undergo functional and morphological changes. There was thickening of the walls of the ventricles of in ovariectomized rats fed a high-fat diet (OF group). Also, a reduction in lumen diameter of the cardiac chambers was found in these animals, shown by concentric hypertrophy.
Morphometric analysis showed that the increase in myocardial wall thickness occurred as a result of an increase in the diameter of the cardiomyocytes in ovariectomized rats fed a high-fat diet (OF group) when compared with the other groups (Figure 1C). The systolic blood pressure measurements were made by tail plethysmography, and only the ovariectomized rats fed a high-fat diet (OF group) showed a significant increase (p<0.05) in the systolic blood pressure, and this difference appeared at the fifth month until the end of the study protocol (Figure 1D).
Ovariectomy associated with a high-fat diet increased the parameters involved in cardiac fibrosis
The detection of α-smooth muscle actin (ASMA) in this rat model indicated that the influence of both diet and ovariectomy in the activation of myofibroblasts, and demonstrated the presence of myofibroblasts in the myocardial tissue. The cardiac tissue sections from the SC group were almost negative for ASMA (Figure 2A). However, ASMA-positive cells were observed in rats subjected to ovariectomy or fat diet alone (Figure 2B, 2C). A negative control was tested where the primary antibody was omitted, and no unspecific labeling was found (Figure 2A – upper right corner).
Figure 2.
Ovariectomy associated with high-fat diet (the OF group) increases the parameters involved in rat cardiac fibrosis. A–D are photomicrographs showing the immunohistochemistry for α-smooth muscle actin (ASMA) in the ventricles of sham-operated rats fed control diet (SC); ovariectomized rats fed control diet (OC); sham-operated rats fed a high-fat diet (SF) and ovariectomized rats fed a high-fat diet (OF), respectively. Analysis of the images shows a gradual increase in ASMA expression. The arrows illustrate the presence of activated myofibroblasts in the interstitium of the myocardium of animals under study. Objective magnification ×200. Ovariectomy accounts for 19.66% of the variation (p=0.0077), diet accounts for 45.72% of the variation (p=0.0003). The negative control (omission of the primary antibody) is presented on the upper right corner in A. (E) Positive immunohistochemical staining (brown) for α-smooth muscle actin (ASMA) was localized by the primary antibody used and was quantified by the histological evaluation of 30 fields per slide with positive immunostaining attributed to the marked ventricular area by assigning a score of 0–4. A score of 0 was equivalent to 0–5% of the set; a score of 1 was equivalent to 5–25%; a score of 2 was equivalent to 25–50%; a score 3 was equivalent to 50–75%; and a score of 4 was equivalent to 75–100% [26]. Immunohistochemical staining scoring analysis was performed with ×400 objective, and photomicrographs were taken at an objective magnification of ×200. (F) Hydroxyproline concentrations. Data analyzed by two-way ANOVA followed by Bonferroni’s test. * p<0.05 vs. SC group; # p<0.05 vs. SC, OC and SF; and p<0.05 vs. SC and OC; (n=4, for each group).
There was an increase in expression of ASMA in the ovariectomized rats fed a high-fat diet (OF group, Figure 2D), although there was no difference when compared with the SF group (Figure 2E). Compared with the control group (Figure 2A) only rare myofibroblasts and ASMA was expressed by smooth muscle cells in blood vessels.
Figure 2F shows the presence of hydroxyproline, an indirect measure of collagen. Only the combined effect of ovariectomized rats fed a high-fat diet (OF group) showed an increase in the levels of hydroxyproline in the myocardial tissue in ovariectomized rats fed a high-fat diet (OF group).
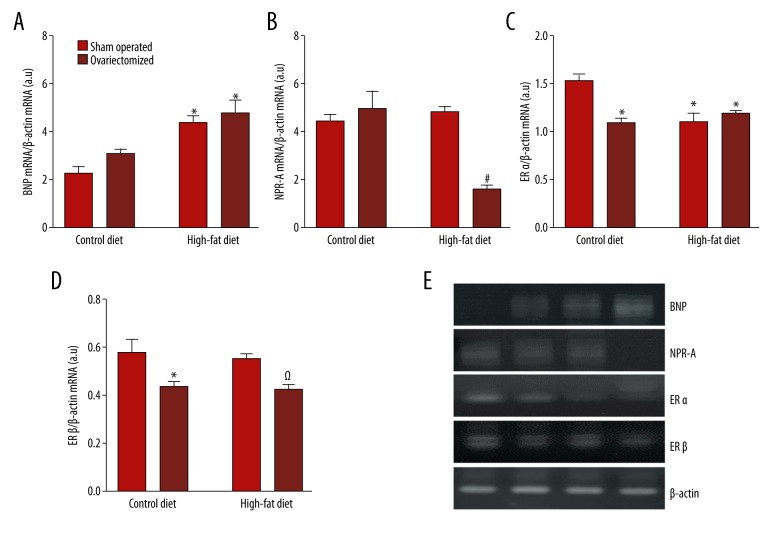
Gene expression for BNP, NPR-A, ERα, and ERβ
The gene expression analysis performed in the ventricle of the animals showed that mRNA levels of expression of brain natriuretic peptide (BNP), which is also known as B-type natriuretic peptide, was increased in ovariectomized rats fed a high-fat diet (OF group) when compared with the SC group (Figure 3A).
Figure 3.
Expression of estrogen receptor genes are associated with cardiac hypertrophy and expression of BNP in ovariectomized rats fed a high-fat diet (the OF group). Gene expression in rat ventricle showing the mRNA detection of BNP, NPR-A, Erα, and ERβ. The mRNA determination was made by polymerase chain reaction (PCR) using the whole hearts (n=4 for each group) from sham-operated rats fed control diet (SC); ovariectomized rats fed control diet (OC); sham-operated rats fed a high-fat diet (SF); and ovariectomized rats fed a high-fat diet (OF). mRNA values have been normalized with mRNA β-actin values. A–D are the ratio of BNP, NPR-A, ERα and ERβ expressed in arbitrary units respectively. (E) Representative ethidium bromide visualization of polymerase chain reaction (PCR) products. N=4 each group. The results were analyzed by two-way ANOVA followed by Bonferroni’s test. * p <0.05 vs. SC group; # p<0.05 vs. SC, OC and SF; Ω p<0.05 vs. SC and SF; (n=4, for each group).
Also, the ovariectomized rats fed a high-fat diet (OF group) showed a significant reduction (p<0.05) in expression of the NPR-A gene in the rat myocardium (Figure 3B). It is possible that downregulation of NPR-A can explain how low estradiol levels interact to promote cardiac hypertrophy.
The expression of ERα and ERβ differed between the experimental groups. Figure 3C shows the pattern of expression of mRNA for ERα. All ovariectomized rats (the OC and OF groups) or rats fed a high-fat diet (the OF group) showed a significant reduction (p<0.05) in expression of ERα compared with the control group. However, analysis of the mRNA for ERβ (Figure 3D) showed a reduction in expression only in the ovariectomized groups (the OC and OF groups) when compared with the sham-operated groups.
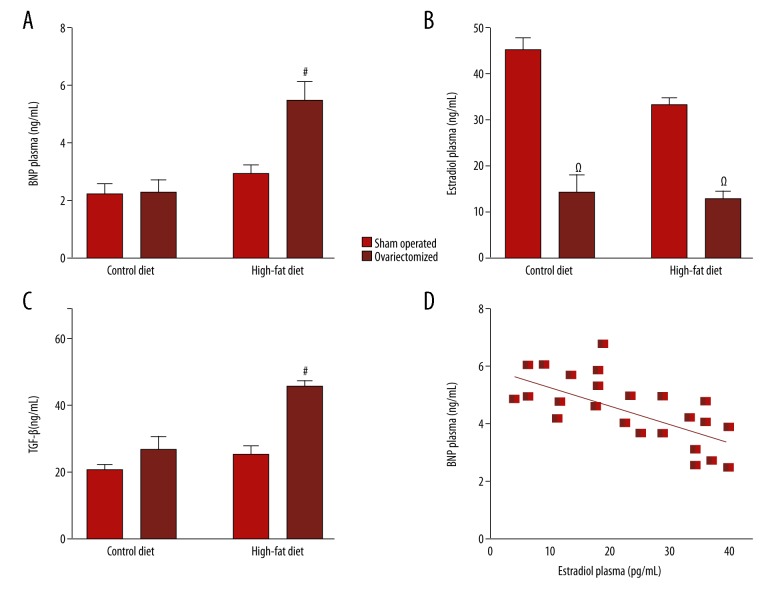
Plasma levels of BNP, estradiol, and transforming growth factor (TGF)-β
The plasma levels of BNP, estradiol and transforming growth factor (TGF)-β were measured by enzyme-linked immunosorbent assay (ELISA). Plasma levels of BNP in the sham-operated animals were very low. However, BNP levels were significantly increased (p<0.05) in ovariectomized rats fed a high-fat diet (OF group) (Figure 4A). This result indicates that the production and release of BNP were associated with increased weight gain and cardiac hypertrophy in the rat model. Also, estradiol levels were reduced in ovariectomized animals (Figure 4B), demonstrating that ovariectomy was effective in reducing plasma levels of estradiol.
Figure 4.
Expression of brain natriuretic peptide (BNP) correlates with plasma estradiol levels in the ovariectomized rats fed a high-fat diet (the OF group), and with transforming growth factor (TGF)-β levels. Plasma levels of brain natriuretic peptide (BNP), which is also known as B-type natriuretic peptide, estradiol and TGF-β represented in A, B and D, respectively were measured by the enzyme-linked immunosorbent assay (ELISA) using plasma samples (n=12 for each group) from sham-operated rats fed control diet (SC); ovariectomized rats fed control diet (OC); sham-operated rats fed a high-fat diet (SF); and ovariectomized rats fed a high-fat diet (OF). C represents the correlation between BNP and estradiol in ovariectomized rats (n=12) fed a control diet (OC) or a high-fat diet (OF) for six months. The results were analyzed by two-way ANOVA followed by Bonferroni’s test, and correlations were analyzed by Pearson’s coefficient. # p<0.05 vs. SC, OC and SF; Ω p<0.05 vs. SC and SF.
There was an inverse relationship between BNP and estradiol in the rat model (Figure 4D). This finding showed that a reduction in estrogen levels was correlated with increased plasma levels of BNP, which is not common in models that do not use diet associated with ovariectomy. Also, TGF-β levels were increased in ovariectomized rats fed a high-fat diet (OF group) (Figure 4C), which may be a possible factor involved in the activation of cardiac remodeling.
Plasma BNP and body weight, blood pressure and diameter of the cardiomyocytes
The relationship between plasma BNP levels and cardiac hypertrophy were analyzed with other variables involved in the development of cardiac remodeling, as shown in Table 1. Plasma BNP levels were significantly correlated (91%) (p=0.004) with the diameter of cardiomyocytes in the groups with a high-fat diet. There was also a significant correlation (89%) (p=0.007) between plasma BNP and body weight but again only in the ovariectomized rats fed a high-fat diet (OF group). However, there was no significant correlation between plasma BNP and systolic blood pressure in the groups fed a control diet and a high-fat diet (p=0.223 and p=0.065, respectively).
Table 1.
Brain natriuretic peptide (BNP) (also known as B-type natriuretic peptide) expression correlates with the diameter of cardiomyocytes and body weight but not with blood pressure in the ovariectomized rats fed a high-fat diet (the OF group).
| Variable | Control diet | High-fat diet | ||
|---|---|---|---|---|
| Pearson (r) | Statistical significance | Pearson (r) | Statistical significance | |
| Plasma levels of BNP vs. diameter of cardiomyocytes | 0.461 | p=0.357 (ns) | 0.914 | p=0.004 |
| Plasma levels of BNP vs. body weight | 0.71 | p=0.746 (ns) | 0.890 | p=0.007 |
| Plasma levels of BNP vs. systolic blood pressure | −0.84 | p=0.223 (ns) | 0.726 | p=0.065 (ns) |
The correlations were made using Pearson’s correlation coefficient (two-tailed) among the entire population of Wistar rats fed control diet, and the whole population of Wistar rats fed a high-fat diet (n=24 for each variable). This type of correlation indicates trends or associations between variables. Statistical significance was determined to be a p-value ≤0.05. ns – not significant. r=0.10–0.30 (weak correlation), 0.40–0.60 (moderate correlation), 0.70–1.0 (strong correlation) [50].
Discussion
The findings of this study, using ovariectomized rats fed a high-fat diet, showed a new aspect of the biology of brain natriuretic peptide (BNP), which is also known as B-type natriuretic peptide, in relation to obesity, reduced estradiol, and cardiac hypertrophy. Forty-eight female Wistar rats were divided into four groups, which included sham-operated rats fed a control diet (SC), ovariectomized rats fed a control diet (OC), sham-operated rats fed a high-fat diet (SF), and ovariectomized rats fed a high-fat diet (OF).
The findings of this study were that in ovariectomized rats fed a high-fat diet (the OF group) there was an increase in body weight, an increase in blood pressure, and cardiac hypertrophy, characterized by an increase in heart weight, increase in diameter of cardiomyocytes and cardiac fibrosis. In ovariectomized rats fed a high-fat diet (the OF group), there was an increase in plasma BNP levels and cardiac BNP expression, a decrease in ventricular synthesis of NPR-A, ERα and ERβ and an increase in expression of transforming growth factor (TGF)-β. The study also showed that the increase in plasma BNP levels was associated with increased body weight and increased the diameter of cardiomyocytes in the ovariectomized rats fed a high-fat diet.
Ventricular hypertrophy is reported to be more common in obese subjects [25]. The probable mechanisms involved include an increase in total blood volume and cardiac output and the increased cardiac burden imposed on the myocardium due to cardiac compression [26,27]. Obesity also favors a state of imbalance between pro-inflammatory and anti-inflammatory factors that affect cardiac remodeling, which may contribute to inflammation and fibrosis. The chronic cardiac overload eventually leads to contractile dysfunction and heart failure [28], since weight loss by either bariatric surgery or diet has been found to promote a significant improvement in cardiac function [29].
In this study, the OC and SF rats showed increased body weight beginning on the third month of the high-fat diet. However, the ovariectomized rats fed a high-fat diet (the OF group) had a greater gain in body weight in the last two months of the study. Systolic blood pressure was associated with body weight, which was increased in the last two months of the study for the ovariectomized rats fed a high-fat diet (the OF group). The weight gain could be a cause of the changes found in the myocardium of the experimental animals.
The ovariectomized rats fed a high-fat diet (the OF group) demonstrated increased systolic blood pressure and increased heart weight and diameter of cardiomyocytes. These results could indicate a direct relationship between the development of cardiac overload and cardiac hypertrophy. This pattern of hypertrophy was probably due to increased pressure on the cardiac structures together with overproduction of inflammatory cytokines by adipose tissue. Also, an unfavorable hormonal state due to estrogen deficiency could be responsible for reducing the production of mediators of vasodilation [30] which can lead to an increase in peripheral resistance. Reduced estrogen levels may result in an inflammatory profile in the ovariectomized rat groups [31], especially in the ovariectomized rats fed a high-fat diet (the OF group) that showed an increase in TGF-β expression. Also, the findings of the increase in blood pressure in the ovariectomized rats fed a high-fat diet (the OF group) suggest that estrogen deficiency and a high-fat diet may create the environment for the development of hypertension. Previously published studies have shown that CYP1B1 (cytochrome P450) protects against Ang II-induced hypertension and associated cardiovascular changes in female mice [32]. The findings of this study also indicate that cardiac remodeling and an increase in peripheral vascular resistance may begin at an early stage when obesity is associated with a lack of estrogen.
In this study, using a rat model, the influence of the biological peptide, BNP, on cardiac hypertrophy was studied. The clinical importance of BNP-guided treatment for heart failure may lie in its ability to indicate an imbalance in cardiac overload [33]. Hemodynamic changes and the activity of the neurohormonal system may activate the synthesis and release of BNP, as small changes in the levels of renin, aldosterone, cortisol, epinephrine, norepinephrine, as well as cytokines such as IL-6, can promote the wide variation in BNP levels [34]. There may be a relationship between the production and release of BNP with the hemodynamic and neurohormonal state [34]. Research on the hormones produced by the adrenal cortex and components of the renin-angiotensin system may explain the increase in BNP and may be considered in future studies using this animal model. From the findings of this study, it may be possible to propose that TGF-β associated with inflammation secondary to obesity is a key factor in cardiac remodeling and cardiac hypertrophy.
In this study, ovariectomized animals fed a high-fat diet showed higher levels of expression of BNP mRNA in the ventricles as well as higher circulating levels of BNP. In previously published studies, the relationship between body mass index (BMI) and plasma BNP levels has been controversial [35–37]. The controversial relationship between natriuretic peptides and obesity may indicate the existence of different mechanisms for clearance or synthesis of BNP in obesity. Some studies have shown that an increase in expression of NPR-C in adipose tissue and reduced expression of BNP by the ventricle could be the main mechanism to explain the low BNP levels found in obese individuals [38,39]. However, in this study, increased expression of BNP mRNA and protein, in the ventricle and plasma of the rat model was associated with the greatest gain in body weight. These findings are supported by previous studies that have found an increase in the expression of the BNP precursor, NT-proBNP, in patients with severe obesity [40]. However, there was no correlation between BNP expression levels and obesity in these patients [40]. It is possible that, in the present study, mechanisms that stimulate increased production of BNP, such as local stimulators on the rat heart, combined to increase the clearance of this peptide, resulting in increased plasma concentrations.
Increased BNP levels found in women may be explained by the stimulating effect on BNP production by female steroid hormones [41]. However, with cardiac overload or cardiovascular disease, levels of BNP rise regardless of gender but is associated with an increased cardiac clinical risk in women [42]. In this study, increased plasma levels of BNP in ovariectomized animals fed a high-fat diet (the OF group), were inversely correlated with plasma estradiol levels, indicating that even in the absence of hormonal stimulation, the production and release of BNP was increased due to the state of cardiac remodeling [43]. Therefore, BNP may also be a marker of cardiac hypertrophy. Resistance to the biological effects of BNP has been reported and can be explained by changes in the synthesis or function of the NPR-A receptor, as shown in this study by the reduction in expression of the NPR-A gene in the ventricles of the ovariectomized rats fed a high-fat diet (the OF group). This form of resistance to BNP has been reported in patients with heart failure [44].
The major structural changes that occur during the progression of cardiac remodeling are the conversion of fibroblasts to myofibroblasts and excessive deposition of extracellular matrix. Myofibroblasts may then express markers such as α-smooth muscle actin (ASMA). In this study, an increase in ASMA in myocardial tissue was found in ovariectomized rats fed a high-fat diet (the OF group), indicating the presence of cardiac remodeling in this group; this was supported by the increase in hydroxyproline expression and increased levels of TGF-β in this group. TGF-β is a cytokine that may work by activating the transition of myofibroblasts to fibroblasts as part of the fibrotic process involving ASMA and collagen synthesis [45].
The effects on estrogen receptors (ER) mediated by estradiol have been associated with modulation of cardiac hypertrophy in animal models, and in humans as estradiol stimulates the release of cardioprotective factors and contribute to the regulation of metabolic diseases such as obesity [20,46]. The findings of this study demonstrated that the ERα gene underwent downregulation in ovariectomized rats fed a high-fat diet (the OF group), while the ERβ gene was also downregulated. The marked reduction in ER gene expression and reduced plasma levels of this hormone in the OF group may partly explain the development of cardiac hypertrophy in these animals. However, in this rat model, a high-fat diet without ovariectomy was also associated with reduced expression of ERα. As yet, this finding cannot be explained, but a hypothesis is that obesity may lead to a modification of the constitution of the cardiomyocyte cell membrane, including the type of phospholipids, and also the density of receptors expressed in the membrane may be altered [47]. An excess of adipose tissue may, by itself, explain the modification of gene expression observed in our study.
Additionally, previous studies have shown that estradiol can modulate the process of fibrosis in cardiac tissue as a selective agonist for ERβ preventing the differentiation of myofibroblasts and collagen deposition in cardiac tissue of mice treated with Ang II [20,46,48]. However, this finding has not been reported in ERβ knockout mice, and the anti-hypertrophic and antifibrotic effect has not been shown to occur with the use of a selective agonist for ERα [48,49]. Therefore, this study may demonstrate the involvement of ERβ receptor in the anti-hypertrophic effect of estradiol which may regulate the metabolism of fat distribution and also through the ERβ gene [49]. A summary of the possible pathways involved in ovariectomized rats fed a high-fat diet (the OF group) and the role of BNP are summarized in Figure 5.
Figure 5.
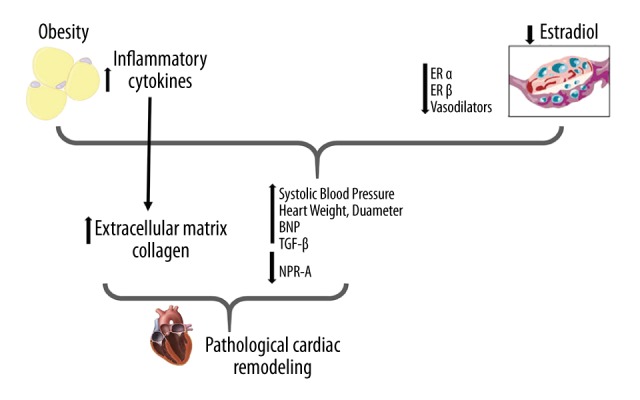
Summary of pathways involved in ovariectomized rats fed a high-fat diet (the OF group): the role of brain natriuretic peptide (BNP). Obesity, characterized by an increase in adipocytes, leads to a high level of inflammatory cytokines, which, together with the effects of estradiol reduction contribute to the elevation of blood pressure in these animals. Such events initiate the stimulus for cardiac remodeling, characterized by cardiomyocyte hypertrophy, and increased brain natriuretic peptide (BNP) production. Obesity may still represent a risk factor for cardiac remodeling because it stimulates the production of extracellular matrix (ECM) and inflammatory factors such as TGF-β. These factors, associated with the reduction of the biological activity of BNP (lower expression of NPR-A) may intensify cardiac hypertrophy and reduce the anti-hypertrophic activity of BNP.
Conclusions
The findings of this study have shown that measurement of plasma brain natriuretic peptide (BNP), which is also known as B-type natriuretic peptide, was associated with cardiac hypertrophy in ovariectomized rats fed a high-fat diet. The BNP-NPR-A receptor complex may be involved in cardiac remodeling and BNP may be a marker of cardiac hypertrophy in this animal model. Future clinical studies on the role of BNP in the heart in obesity and the menopause are indicated.
Footnotes
Conflict of interest
None.
Source of support: The study was supported by the National Council for Scientific and Technological Development (CNPq) and Coordination of Improvement of Higher Level Personnel (CAPES)
References
- 1.Ouchi N, Ohishi M, Kihara S, et al. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension. 2003;42:231–34. doi: 10.1161/01.HYP.0000083488.67550.B8. [DOI] [PubMed] [Google Scholar]
- 2.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–80. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 3.Van Haare J, Kooi ME, Vink H, et al. Early impairment of coronary microvascular perfusion capacity in rats on a high-fat diet. Cardiovasc Diabetol. 2015;14:150. doi: 10.1186/s12933-015-0312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rocha VZ, Folco EJ. Inflammatory concepts of obesity. Int J Inflamm. 2011;2011:529061. doi: 10.4061/2011/529061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torrisi JS, Hespe GE, Cuzzone DA, et al. Inhibition of inflammation and iNOS improves lymphatic function in obesity. Sci Rep. 2016;6:19817. doi: 10.1038/srep19817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobsen MJ, Mentzel CMJ, Olesen AS, et al. Altered methylation profile of lymphocytes is concordant with perturbation of lipids metabolism and inflammatory response in obesity. J Diabetes Res. 2016;2016:8539057. doi: 10.1155/2016/8539057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L, Liu M, Jiang H, et al. Extracellular high-mobility group box 1 mediates pressure overload-induced cardiac hypertrophy and heart failure. J Cell Mol Med. 20:459–70. doi: 10.1111/jcmm.12743. 20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamura N, Ogawa Y, Chusho H, et al. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc Natl Acad Sci USA. 2000;97:4239–44. doi: 10.1073/pnas.070371497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kapoun AM, Liang F, O’Young G, et al. B-type natriuretic peptide exerts broad functional opposition to transforming growth factor-beta in primary human cardiac fibroblasts: Fibrosis, myofibroblast conversion, proliferation, and inflammation. Circ Res. 2004;94:453–61. doi: 10.1161/01.RES.0000117070.86556.9F. [DOI] [PubMed] [Google Scholar]
- 10.Namdari M, Eatemadi A, Negahdari B. Natriuretic peptides and their therapeutic potential in heart failure treatment: An updated review. Cell Mol Biol (Noisy-le-grand) 2016;62:1–7. [PubMed] [Google Scholar]
- 11.Lin Y-K, Chen Y-C, Chen Y-A, et al. B-type natriuretic peptide modulates pulmonary vein arrhythmogenesis: A novel potential contributor to the genesis of atrial tachyarrhythmia in heart failure. J Cardiovasc Electrophysiol. 2016;27:1462–71. doi: 10.1111/jce.13093. [DOI] [PubMed] [Google Scholar]
- 12.Pandey KN. Guanylyl cyclase/atrial natriuretic peptide receptor-A: Role in the pathophysiology of cardiovascular regulation. Can J Physiol Pharmacol. 2011;89:557–73. doi: 10.1139/y11-054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harada E, Nakagawa O, Yoshimura M, et al. Effect of interleukin-1 beta on cardiac hypertrophy and production of natriuretic peptides in rat cardiocyte culture. J Mol Cell Cardiol. 1999;31:1997–2006. doi: 10.1006/jmcc.1999.1030. [DOI] [PubMed] [Google Scholar]
- 14.Sakata Y, Yamamoto K, Masuyama T, et al. Ventricular production of natriuretic peptides and ventricular structural remodeling in hypertensive heart failure. J Hypertens. 2001;19:1905–12. doi: 10.1097/00004872-200110000-00027. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka T, Kanda T, Takahashi T, et al. Interleukin-6-induced reciprocal expression of SERCA and natriuretic peptides mRNA in cultured rat ventricular myocytes. J Int Med Res. 2004;32:57–61. doi: 10.1177/147323000403200109. [DOI] [PubMed] [Google Scholar]
- 16.Cavasin MA, Sankey SS, Yu A-L, et al. Estrogen and testosterone have opposing effects on chronic cardiac remodeling and function in mice with myocardial infarction. Am J Physiol Heart Circ Physiol. 2003;284:H1560–69. doi: 10.1152/ajpheart.01087.2002. [DOI] [PubMed] [Google Scholar]
- 17.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–71. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neal-Perry G, Nejat E, Dicken C. The neuroendocrine physiology of female reproductive aging: An update. Maturitas. 2010;67:34–38. doi: 10.1016/j.maturitas.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perheentupa A, Huhtaniemi I. Aging of the human ovary and testis. Mol Cell Endocrinol. 2009;299:2–13. doi: 10.1016/j.mce.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Pedram A, Razandi M, Lubahn D, et al. Estrogen inhibits cardiac hypertrophy: Role of estrogen receptor-beta to inhibit calcineurin. Endocrinology. 2008;149:3361–69. doi: 10.1210/en.2008-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Andrade EN, Gonçalves GKN, de Oliveira THC, et al. Natriuretic peptide system: A link between fat mass and cardiac hypertrophy and hypertension in fat-fed female rats. Regul Pept. 2011;167:149–55. doi: 10.1016/j.regpep.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Belo NO, Sairam MR, Dos Reis AM. Impairment of the natriuretic peptide system in follitropin receptor knockout mice and reversal by estradiol: Implications for obesity-associated hypertension in menopause. Endocrinology. 2008;149:1399–406. doi: 10.1210/en.2007-0572. [DOI] [PubMed] [Google Scholar]
- 23.Kliem V, Johnson RJ, Alpers CE, et al. Mechanisms involved in the pathogenesis of tubulointerstitial fibrosis in 5/6-nephrectomized rats. Kidney Int. 1996;49:666–78. doi: 10.1038/ki.1996.95. [DOI] [PubMed] [Google Scholar]
- 24.Edwards CA, O’Brien WD. Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin Chim Acta. 1980;104:161–67. doi: 10.1016/0009-8981(80)90192-8. [DOI] [PubMed] [Google Scholar]
- 25.Alexescu TG, Cozma A, Sitar-Tăut A, et al. Cardiac changes in overweight and obese patients. Rom J Intern Med. 2016;54:161–72. doi: 10.1515/rjim-2016-0022. [DOI] [PubMed] [Google Scholar]
- 26.Alpert MA. Obesity cardiomyopathy: Pathophysiology and evolution of the clinical syndrome. Am J Med Sci. 2001;321:225–36. doi: 10.1097/00000441-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: Experimental findings and therapeutic strategies. Pharmacol Ther. 2010;128:191–227. doi: 10.1016/j.pharmthera.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Manabe I. Chronic inflammation links cardiovascular, metabolic and renal diseases. Circ J. 2011;75:2739–48. doi: 10.1253/circj.cj-11-1184. [DOI] [PubMed] [Google Scholar]
- 29.Rider OJ, Francis JM, Ali MK, et al. Beneficial cardiovascular effects of bariatric surgical and dietary weight loss in obesity. J Am Coll Cardiol. 2009;54:718–26. doi: 10.1016/j.jacc.2009.02.086. [DOI] [PubMed] [Google Scholar]
- 30.Schillaci G, Verdecchia P, Borgioni C, et al. Early cardiac changes after menopause. Hypertension. 1998;32:764–69. doi: 10.1161/01.hyp.32.4.764. [DOI] [PubMed] [Google Scholar]
- 31.de Medeiros ARS, Lamas AZ, Caliman IF, et al. Tibolone has anti-inflammatory effects in estrogen-deficient female rats on the natriuretic peptide system and TNF-alpha. Regul Pept. 2012;179:55–60. doi: 10.1016/j.regpep.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 32.Jennings BL, George LW, Pingili AK, et al. Estrogen metabolism by cytochrome P450 1B1 modulates the hypertensive effect of angiotensin II in female mice. Hypertension. 2014;64:134–40. doi: 10.1161/HYPERTENSIONAHA.114.03275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLellan J, Heneghan CJ, Perera R, et al. B-type natriuretic peptide-guided treatment for heart failure. Cochrane Database Syst Rev. 2016;12:CD008966. doi: 10.1002/14651858.CD008966.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emdin M, Passino C, Prontera C, et al. Cardiac natriuretic hormones, neuro-hormones, thyroid hormones and cytokines in normal subjects and patients with heart failure. Clin Chem Lab Med. 2004;42:627–36. doi: 10.1515/CCLM.2004.108. [DOI] [PubMed] [Google Scholar]
- 35.Daniels LB, Clopton P, Bhalla V, et al. How obesity affects the cut-points for B-type natriuretic peptide in the diagnosis of acute heart failure. Results from the Breathing Not Properly Multinational Study. Am Heart J. 2006;151:999–1005. doi: 10.1016/j.ahj.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 36.Beleigoli A, Diniz M, Nunes M, et al. Reduced brain natriuretic peptide levels in class III obesity: The role of metabolic and cardiovascular factors. Obes Facts. 2011;4:427–32. doi: 10.1159/000335174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foster MC, Hwang S-J, Porter SA, Massaro JM, et al. Fatty kidney, hypertension, and chronic kidney disease: The Framingham Heart Study. Hypertension. 2011;58:784–90. doi: 10.1161/HYPERTENSIONAHA.111.175315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang TJ, Larson MG, Levy D, et al. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109:594–600. doi: 10.1161/01.CIR.0000112582.16683.EA. [DOI] [PubMed] [Google Scholar]
- 39.Das SR, Drazner MH, Dries DL, et al. Impact of body mass and body composition on circulating levels of natriuretic peptides: Results from the Dallas Heart Study. Circulation. 2005;112:2163–68. doi: 10.1161/CIRCULATIONAHA.105.555573. [DOI] [PubMed] [Google Scholar]
- 40.Fernandes F, Ramires FJA, Buck PC, et al. N-terminal-pro-brain natriuretic peptide, but not brain natriuretic peptide, is increased in patients with severe obesity. Braz J Med Biol Res. 2007;40:153–58. [PubMed] [Google Scholar]
- 41.Maffei S, Del Ry S, Prontera C, Clerico A. Increase in circulating levels of cardiac natriuretic peptides after hormone replacement therapy in postmenopausal women. Clin Sci (Lond) 2001;101:447–53. [PubMed] [Google Scholar]
- 42.Ballo P, Betti I, Barchielli A, et al. Prognostic role of N-terminal pro-brain natriuretic peptide in asymptomatic hypertensive and diabetic patients in primary care: Impact of age and gender: Results from the PROBE-HF study. Clin Res Cardiol. 2016;105:421–31. doi: 10.1007/s00392-015-0937-x. [DOI] [PubMed] [Google Scholar]
- 43.Gjesdal O, Bluemke DA, Lima JA. Cardiac remodeling at the population level – risk factors, screening, and outcomes. Nat Rev Cardiol. 2011;8:673–85. doi: 10.1038/nrcardio.2011.154. [DOI] [PubMed] [Google Scholar]
- 44.Tsutamoto T, Kanamori T, Morigami N, et al. Possibility of downregulation of atrial natriuretic peptide receptor coupled to guanylate cyclase in peripheral vascular beds of patients with chronic severe heart failure. Circulation. 1993;87:70–75. doi: 10.1161/01.cir.87.1.70. [DOI] [PubMed] [Google Scholar]
- 45.Mewhort HEM, Lipon BD, Svystonyuk D, et al. Monocytes increase human cardiac myofibroblast-mediated extracellular matrix remodeling through TGF-β1. Am J Physiol Heart Circ Physiol. 2016;310:H716–24. doi: 10.1152/ajpheart.00309.2015. [DOI] [PubMed] [Google Scholar]
- 46.Pedram A, Razandi M, O’Mahony F, et al. Estrogen receptor-beta prevents cardiac fibrosis. Mol Endocrinol. 2010;24:2152–65. doi: 10.1210/me.2010-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kris-Etherton P, Daniels SR, Eckel RH, et al. AHA scientific statement: Summary of the Scientific Conference on Dietary Fatty Acids and Cardiovascular Health. Conference summary from the Nutrition Committee of the American Heart Association. J Nutr. 2001;131:1322–26. doi: 10.1093/jn/131.4.1322. [DOI] [PubMed] [Google Scholar]
- 48.Pedram A, Razandi M, Aitkenhead M, Levin ER. Estrogen inhibits cardiomyocyte hypertrophy in vitro. Antagonism of calcineurin-related hypertrophy through induction of MCIP1. J Biol Chem. 2005;280:26339–48. doi: 10.1074/jbc.M414409200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yepuru M, Eswaraka J, Kearbey JD, et al. Estrogen receptor-{beta}-selective ligands alleviate high-fat diet- and ovariectomy-induced obesity in mice. J Biol Chem. 2010;285:31292–303. doi: 10.1074/jbc.M110.147850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dancey CP, Reidy J, Viali L. 3nd ed. [Estatística sem matemática para psicologia: Usando SPSS para Windows]. Porto Alegre: Artmed; 2008. [in Portuguese] [Google Scholar]