Abstract
Objective
To determine the ability of three performance-based measures [Short Physical Performance Battery (SPPB), gait speed, and Grip Strength] and a self-report measure [Vulnerable Elders Survey (VES-13)] to predict functional decline among older women with breast cancer.
Patients and Methods
Longitudinal data from a study of women ≥ 65 years, with newly diagnosed stage I–III breast cancer, recruited from ambulatory oncology clinics between July 2010 and April 2014, was used. The primary outcome was functional decline, Yes or No, defined as a decrease in ≥ 1-point from baseline to 12 months, on Activities of Daily Living Scales. Multivariable logistic regression and Receiver Operator Curve analyses were conducted
Results
Among 123 participants 18 (15%) developed functional decline. The predictive abilities for measures were: SPPB [Adjusted odds ratio (AOR) = 1.65 per unit decrease in scores, 95% confidence interval (CI) =1.33–2.05; area under the receiver operator curve (AUC) = 0.93; sensitivity=94%, specificity=80%]; gait speed (AOR = 1.76 per unit increase in usual walking time, CI=1.29–2.41; AUC=0.93; sensitivity=87%, specificity=79%); VES-13 (AOR = 1.64 per unit increase in scores, CI=1.31–2.05; AUC=0.87; sensitivity=83%, specificity=84%); and grip strength: (AOR = 1.18 per unit decrease in grip strength, CI=1.06–1.30; AUC=0.80; sensitivity=67%, specificity=77%).
Conclusion
SPPB, gait speed, grip strength and VES-13 all demonstrated excellent predictive abilities for functional decline. Larger studies are warranted to confirm the utility of these measures for identifying older adults with cancer at increased risk for functional decline, who may then be targeted for studies to explore the effects of interventions to improve function.
Keywords: Functional Decline physical performance gait speed, Older Women Breast Cancer
INTRODUCTION
Functional decline, defined as increasing difficulty to independently complete Activities of Daily Living (ADLs) and Instrumental Activities of Daily Living (IADLs), is a significant and profound marker of morbidity and mortality.1, 2 Existing literature has shown that mortality progressively increases with increasing limitations in ADLs and IADLS, increasing from 15% for those with one limitation in IADLs, to 21% for those with two limitations in ADLs and to 37% for those with five or six limitations in ADLs.3 For this and many other reasons, the Centers for Medicare and Medicaid Services has declared mobility maintenance and functional independence among at risk older adults a priority for aging research.4
Cancer and related-treatment augment the disablement process, and accelerate the trajectory towards functional decline. Indeed, as many as forty percent of older women with breast cancer have functional disability at initial breast cancer diagnosis5 and about twenty percent develop functional decline within a year of initiating breast cancer treatment.
Given the likely adverse impact of functional decline on the overall health status of older cancer survivors1, it is imperative to develop strategies and interventions to prevent and attenuate functional decline among cancer survivors. The first and critical step to developing any preventive strategies, is the early identification of those at increased risk for developing functional decline.
Although several self-report and performance-based measures for predicting functional decline have been developed and validated among older adults in general, a limited number have been tested among older adults with cancer. In particular, existing data on the ability of performance-based measures to predict functional decline among older adults with cancer is scant. Therefore, using data from a longitudinal study of older women with newly diagnosed breast cancer we sought to examine and compare the ability of three performance-based and one self-report measure to predict functional decline among older women with breast cancer. This information is clinically relevant for cancer treatment-decision making and for informing interventions aimed at preventing functional decline among older breast cancer survivors.
PATIENTS and METHODS
Study Design and Patient Population
A more detailed description of the study design and methods have been published already6 but in brief we used longitudinal data from a prospective cohort study of women with newly diagnosed stage I–III breast cancer who were 65 years of age and older. Patients were excluded from study participation if they had already received chemotherapy, hormonal or targeted therapy or breast irradiation for the current diagnosis of breast cancer prior to enrollment. Receipt of breast surgery before enrollment was allowed. Patient recruitment from ambulatory oncology clinics at an academic center begun in 2008. In September 2010, performance-based measures were included in study assessments. Therefore, for the current analyses we only included participants enrolled from September 2010 onward who had repeated measures of ADL and IADL at 12 months. The study was approved by the Institutional Review Board.
Study Procedures and Data Collection
Using weekly multi-disciplinary breast cancer conference list, potentially eligible patients were identified and approached for informed consent by a trained research assistant during patients’ initial visit with a medical or radiation oncologist. A Comprehensive Geriatric Assessment (CGA) which included a functional assessment was completed by consenting patients at baseline and 12 months from study enrollment.
Functional Assessment Measures
Short Physical Performance Battery
The Short Physical Performance Battery (SPPB), which consists of three lower extremity physical performance measures had participants complete standing balance, five consecutive chair rises, and a 4-meter gait walk at usual pace. A 4-point scale was used for each task, and summary scores ranged from 0–12 with higher scores denoting higher physical performance. Best performance SPPB scores range from 10–12 and scores ≤ 9 have been found to be associated with increased mortality.7, 8
Usual Gait Speed
We measured usual gait speed (as part of the SPPB) in a flat and unobstructed clinic hallway. The measuring area was demarcated by two strips of brightly colored tape placed 4 meters apart. Each participant performed the gait speed protocol twice with a 20–30 second break between trials. Each walk was performed with a one-meter rolling start, where the participant was already walking upon entering the measuring area. Canes and walkers were allowed if participants regularly used these assistive devices in their daily activities. Participants were instructed to “walk at a comfortable and normal pace” from one tape to the other. The time to walk 4 meters was measured with a manual stopwatch. A research assistant stood in the middle of the 4-meter course and started the stopwatch when the participant’s first foot completely crossed into the timing area and stopped the stopwatch once the participant’s first foot completely came out of the timing area. Pathological gait speed or slow gait speed is defined as walking <0.8m/sec.9
The Vulnerable Elders Survey
The VES-13 is a self-report measure which took participants approximately 4 minutes to complete. The survey asked participants to report their age by three categories (65–74, 75–84, ≥ 85 years); self-rated health (excellent, very good, good, fair, poor); functional limitations (1. stooping, crouching or kneeling; 2. lifting or carrying heavy objects; 3. writing or grasping small objects; 4. walking a quarter of a mile; 5. doing heavy housework); and functional disabilities (1. shopping for personal items; 2. managing money; 3. walking across a room; 4. doing light housework; 5. bathing or showering).10–12 The maximum score is 10 with increasing scores denoting increasing risk of functional decline. Scores of ≥ 3 denote increased vulnerability and has been found to be associated with an increased risk of functional decline.10, 11
Grip Strength
Grip strength was measured with a Jamar dynamometer (Sammons Preston Rolyan, Bolingbrook, Illinois). While sitting and holding the dynamometer in their dominant hand, with the arm extended and the palm of their hand facing their leg, participants was instructed to squeeze the grip as hard as possible. The strongest of 3 attempts (in kilograms) was recorded, with higher values reflecting better performance.13 This was repeated for the other hand and the average of the highest measurement from the two hands was the final measurement. Normative data have been derived for community dwelling older adults and range from a mean of 25.3 kg (s.d =4.8) for women aged 60–69 years to 20.0kg (s.d. = 4.3) for those aged ≥ 80 years in the right hand with slightly lower strength in the left hand.14
Functional Status
The Katz Activities of Daily Living (ADLs), and the Lawton Instrumental Activities of Daily Living (IADLs) were used to evaluate self-reported functional status. ADLs are skills necessary to live independently at home such as bathing, transferring, and dressing15 and IADLs are skills required for living independently in the community such as using the telephone, managing medications, housekeeping, transportation, ability to manage finances, and preparing meals.16 The maximum score for ADLs and IADLs is 6 and 8 respectively, with increasing scores denoting better functional status.
Other Variables
Socio-demographic variables were captured at baseline using a self-administered questionnaire. Data on median household income was obtained from United States Census Bureau website17 using participants’ zip codes from their place of residence at the time of enrollment. Height was measured to the nearest 0.1 cm and weight measured to the nearest 0.01kg to compute Body Mass Index (BMI). Medical records were abstracted to obtain data on tumor characteristics and cancer treatments received, medications and comorbidities. Using the list of comorbidities we derived the Charlson Comorbidity Index (CCI)18 score, based on the presence of eighteen medical conditions.
Analytic Variables
Primary outcome variable
The primary outcome was functional decline within 12 months of study enrollment. Functional decline was defined as a decrease in at least one point on the ADL and/or IADL scales from baseline to 12 months, Yes or No.19, 20
Independent variables
The independent variables were the SPPB scores, usual gait speed, grip strength and VES-13 scores at baseline all analyzed as continuous variables.
Explanatory variables
Explanatory variables included the following baseline variables: age at enrollment (65–74, ≥ 75 years); race (AA, NHW); marital status (married, other); educational status (≤ high school, > high school); median household income (<$35,000, ≥ $35,000); living situation (alone, other); body mass index [BMI] (<25kg/m2, ≥ 25kg/m2); physical activity level (<500-METs/minute/week, ≥ 500-METs/minute/week); stage (I–II, III); hormone receptor status (positive. negative); receipt of surgery (Yes or No); receipt of surgery before or after enrollment(Yes or No); receipt of axillary lymph node assessment (Yes or No); receipt of chemotherapy (Yes or No); receipt of hormone therapy (Yes or No); receipt of radiation therapy (Yes or No); geriatric syndromes [presence of dementia, depression, polypharmacy and/or malnutrition (0–1, ≥ 2)]; functional disability (Yes or No); and comorbidity [CCI score (0–1, ≥ 2)].
Data Analysis
We examined participants’ baseline characteristics using descriptive analysis. Bivariate analysis was used to examine independent variables by primary outcome. We used univariate logistic regression with functional decline (Yes vs. No) as our outcome variable to identify explanatory variables that had a univariate associations with functional decline (p < 0.10), see Table 2.
We developed our multivariable model for each measure by using a stepwise multiple logistic regression approach that included functional decline as the outcome variable, scores of measure as the independent variable and explanatory variables that were associated with functional decline at p-value ≤0.10. We did not include baseline functional status in our models because scores of the four measures were collinear with ADL/IADL scores. We also did not include receipt of surgery (Yes or No) in models because only 5 patients did not undergo surgery. Using this approach a final model was derived for each measure that adjusted for explanatory variables that remained significantly associated with functional decline at p<0.05.
Using this strategy, comorbidity was the only explanatory variable that was found to be independently associated with functional decline in the models for SPPB, grip strength and VES-13 and therefore comorbidity was retained in final models for these measures. Lymph node assessment was independently associated with functional decline in the model for gait speed and therefore retained in the final model for gait speed.
We also conducted sensitivity analysis that adjusted for all variables found to be predictive of functional decline at the univariate level. In these full models, all four measures remained predictive of functional decline, see appendix B for full multivariable model for SPPB.
To compare the discriminatory power of the SPPB, gait speed, grip strength and VES-13 for identifying functional decline at 12 months, logistic regression Receiver Operating Characteristic curves (ROC) were calculated for functional decline, using estimated area under the ROC curve (AUC) with 95% confidence interval as an index of predictive accuracy. Adjustment covariates were the same as those used in multivariable regression models. ROC curve areas of different models were compared using the method of Delong and Delong.21 To reduce the Type 1 error rate associated with pair-wise comparisons p-value <0.01 were considered significant. AUCs were categorized into > 0.9, 0.8 to 0.9, 0.7 to <0.8 and <0.7 to represent outstanding, excellent, acceptable or poor instrument discriminating ability.22 Optimal cut-off scores for the instruments were determined as the cut-offs maximizing the Youden index J=SEc+ SPc-1, where SEc and SPc are sensitivity and specificity, respectively at a chosen cut-point when using only the performance measure alone as a classifier. All analyses were conducted using SAS version 9.4 (SAS institute, Cary, NC).
RESULTS
Details of patient flow into the parent study have already been described in a previous publication.6 In brief, we screened 614 patients, of whom 206 were enrolled into the parent study. Of these 206 participants, 63 were enrolled before September 2010 and did not complete performance-based measure, leaving 145 participant who completed performance based assessments and were potentially eligible for the current analysis. However, 22 patients (15%) did not complete follow-up assessments at 12 months, (withdrew N=7 and lost to follow-up N=15), and were excluded from these analyses, resulting in 123 evaluable patients. There were no statistically significant differences in baseline characteristics between participants who were excluded versus those who were not, see appendix A.
Participants’ Baseline Characteristics
Participants’ baseline characteristics are displayed in Table 1. The median duration from diagnosis to baseline assessment was 2.0 months, (interquartile range 1.3–2.8 months). The median duration of follow-up was 12.1 months, (interquartile range 11.8–12.6 months). The mean age of the cohort was 74.5 years, (range 65–93 years), 29% were AA, 39% had ≤ high school education and 35% had functional disability at baseline. The cohort had a mean SPPB score of 8.6, SD 3.3; mean usual gait speed of 5.1 seconds, SD 2.4; mean grip strength of 19.9 kg, SD 6.2; and mean VES-13 score of 1.8, SD 2.4 at baseline.
Table 1.
Baseline Characteristics of Participants
| Baseline Characteristics | N=123 (100%) |
|---|---|
| Age Group (Years) | |
| 65 to 74 | 67 (54%) |
| >=75 | 56 (46%) |
| Race | |
| African-American | 36 (29%) |
| Non-Hispanic White | 87 (71%) |
| Educational Status | |
| High school or less | 46 (37%) |
| More than high school | 77 (63%) |
| Marital Status | |
| Married | 50 (41%) |
| Other | 73 (59%) |
| Median Household Income* | |
| ≥ $35,000 | 99 (80%) |
| <$35,000 | 24 (20%) |
| Living Situation | |
| Alone | 51 (42%) |
| Other | 71 (58%) |
| Missing/Unknown | 1 |
| Body Mass Index | |
| < 25 | 29 (24%) |
| 25 to ≤30 | 41 (33%) |
| ≥ 30 | 53 (43%) |
| Physical Activity | |
| <500-METs/minute/week | 63 (52%) |
| ≥500-METs/minute/week | 58 (48%) |
| Missing | 2 |
| Stage | |
| Stage I–II | 109 (90%) |
| Stage III | 12 (10%) |
| Missing | 2 |
| Hormonal Status | |
| Positive | 102 (83%) |
| Negative | 21 (17%) |
| Primary Surgery | |
| Mastectomy | 36 (30%) |
| Lumpectomy | 80 (67%) |
| None | 4 (3%) |
| Missing | 3 |
| Primary Surgery Prior to Enrollment | |
| Yes | 90 (73%) |
| No | 33 (27%) |
| Lymph Node Assessment | |
| Yes | 90 (73%) |
| No | 33 (27%) |
| Radiation Therapy | |
| Yes | 58 (50%) |
| No | 59 (50%) |
| Missing/unknown | 6 |
| Chemotherapy | |
| Yes | 31 (26%) |
| No | 85 (74%) |
| Missing/unknown | 5 |
| Hormonal Therapy | |
| Yes | 95 (81%) |
| No | 23 (19%) |
| Missing/unknown | 5 |
| Functional Disability** | |
| No | 80 (65%) |
| Yes | 43 (35%) |
| Vulnerable Elders Survey Score | |
| Scores 0–2 | 91 (74%) |
| Scores 3–10 | 32 (26%) |
| Short Physical Performance Battery Score | |
| Scores 8–12 | 85 (69%) |
| Scores 0–7 | 38 (31%) |
| Gait Speed in Seconds (4-Meter Walk Time) | |
| ≤6.2 secs | 99 (80%) |
| >6.2 secs | 24 (20%) |
| Grip Strength (Kg) | |
| ≥21 | 53 (43%) |
| <21 | 69 (57%) |
| Charlson Comorbidity Index | |
| 0–1 | 96 (79%) |
| ≥ 2 | 25 (21%) |
| Missing | 2 |
| Geriatric Syndromes*** | |
| 0–1 | 94 (78%) |
| ≥ 2 | 27 (22%) |
| Missing | 2 |
Median Household income is derived from information from the US Census Bureau using Zip codes
Functional disability denotes self-reported ADL/IADL dependence
Geriatric syndromes include dementia, depression, polypharmacy, malnutrition, and falls
Measures According to Functional Decline
Of 123 evaluable participants, fifteen percent (18) developed functional decline. With regards to the trajectory of functional status 15%, 72% and 14% of participants declined, remained stable or improved in functional status, respectively, from baseline to 12 months. The mean baseline scores for the four measures for participants who developed versus those who did not develop functional decline were as follows; SPPB scores 4.1 (SD 2.6) vs. 9.3 (SD 2.8), p<0.0001; gait speed 8.5 seconds (SD 3.7) vs. 4.6 seconds (SD 1.7), p<0.0001; grip strength 15.4 kg (SD 8.3) vs. 20.7 kg (SD 5.4), p=0.0025; and VES-13 scores 4.7 (SD 2.5) vs. 1.3 (SD 2.0), p<0.0001, respectively.
Additionally, in comparison with participants who did not develop functional decline, participants who developed functional decline were more likely at baseline to be 75 years or older (21% vs. 9%, p=0.05); to be AA (25% vs. 10%, p=0.04); to have high school level or less education (24% vs. 9%, p=0.02); to have a median household income of <$35,000 (33% vs. 10%, p=0.008); to have functional disability at baseline (26% vs. 9%, p=0.01), to have a comorbidity index ≥ 2 (36% vs. 9%, p =0.0025); to have ≥ 2 geriatric syndromes (30% vs. 11%, p=0.01); to be less physically active (25% vs. 3%, p=0.0007); to have undergone surgery after enrollment (27% vs. 10%, p=0.02); not to have completed axillary lymph node assessment (44% vs. 9%, p<0.0001); and not to have completed radiation therapy (22% vs. 7%, p=0.02). These results were consistent with univariate analysis which identified 12 potential predictors of functional decline, see Table 2
Table 2.
Univariate Analysis Showing Explanatory Variables Associated with Functional Decline at 12 months among Older Women with Breast Cancer
| Odds Ratio | 95% CI | P-value | |
|---|---|---|---|
| Baseline Characteristics | |||
| Age Group (Years) | |||
| 65 to 74 | Referent | ||
| >=75 | 2.77 | 0.97, 7.95 | 0.06 |
| Race | |||
| White | Referent | ||
| African-American | 2.89 | 1.04, 8.03 | 0.04 |
| Educational Status | |||
| More than high school | Referent | ||
| High school or less | 3.14 | 1.12, 8.81 | 0.03 |
| Median Household Income | |||
| ≥$35,000 | Referent | ||
| <$35,000 (Lowest Quartile) | 4.45 | 1.53, 12.99 | 0.006 |
| Marital Status | |||
| Married | Referent | ||
| Other | 1. 95 | 0.65, 5.87 | 0.23 |
| Living Situation | |||
| Other | Referent | ||
| Alone | 1. 48 | 0.54, 4.03 | 0.45 |
| Body Mass Index | |||
| <25 (Normal) | Referent | ||
| ≥ 25 (Overweight/Obese) | 2.77 | 0.60, 12.83 | 0.19 |
| Physical Activity | |||
| ≥500-METs/minute/week | Referent | ||
| <500-METs/minute/week | 9.53 | 2.08, 43.58 | 0.004 |
| Stage | |||
| Stage I–II | Referent | ||
| Stage III | 0.49 | 0.06, 4.06 | 0.51 |
| Primary Surgery | |||
| Yes | Referent | ||
| No | 6.73 | 0.88, 51.45 | 0.07 |
| Primary surgery prior to enrollment | |||
| Yes | Referent | ||
| No | 3.38 | 1.21, 9.46 | 0.02 |
| Lymph Node Assessment | |||
| Yes | Referent | ||
| No | 8.27 | s2.61, 26.22 | 0.0003 |
| Radiation Therapy | |||
| Yes | Referent | ||
| No | 3.82 | 1.16, 12.51 | 0.03 |
| Chemotherapy | |||
| Yes | Referent | ||
| No | 1.94 | 0.52, 7.23 | 0.32 |
| Hormone | |||
| Yes | Referent | ||
| No | 1.25 | 0.33, 4.74 | 0.74 |
| Functional Disability | |||
| Yes | Referent | ||
| No | 3.59 | 1.27, 10.09 | 0.02 |
| Charlson Comorbidity Index | |||
| 0–1 | Referent | ||
| ≥ 2 | 5.44 | 1.87, 15.80 | 0.002 |
| Geriatric Syndromes | |||
| 0–1 | Referent | ||
| ≥ 2 | 3.54 | 1.23, 10.15 | 0.02 |
Discriminatory Abilities of the SPPB, Usual Gait Speed, Grip Strength and the VES-13
SPPB was predictive of functional decline, AUC=0.93 (95% CI: 0.89–0.98). With each one-unit decrease in SPPB scores, the odds of developing functional decline increased by 65% (AOR=1.65, 95% CI: 1.33–2.05), sensitivity=94.4%, specificity=80%, at cut-off ≤ 7, (see Table 3 and Figure 1). Usual gait speed showed similar discriminatory powers, AUC=0.93 (95% CI: 0.88–0.99), sensitivity=86.7%, specificity=78.6%, at cut-off ≥ 4.98 seconds. With each one-second increase in usual walking time, the odds of developing functional decline increased by 76% (AOR=1.76, 95% CI: 1.29–2.41). Results for grip strength were as follows; AUC=0.80 (95% CI: 0.56–0.88), sensitivity of 66.7% and specificity of 76.9%, at cut-off ≤ 17.5kg. With each one kg decrease in grip strength, the odds of developing functional decline increased by 18% (AOR=1.18, 95% CI: 1.06–1.30). Lastly VES-13 scores predicted functional decline; AUC=0.87 (95% CI: 0.76–0.96), sensitivity=83.3%, specificity=83.8%, at cut-off ≥ 3. With each one-unit increase in VES-13 scores, the odds of developing functional decline increased by 64% (AOR=1.64, 95% CI: 1.31–2.05).
Table 3.
Association Between Physical Function Measures and Functional Decline at 12 months: Unadjusted Models
| Physical Function Measures | Odds Ratio (95% CI) | P-Value | Sensitivity | Specificity | Cut-off | Area Under the Curve (95% CI) |
|---|---|---|---|---|---|---|
| Short Physical Performance Battery | ||||||
| 1-unit decrease in scores | 1.63 (1.34, 1.98) | <0.0001 | 94.4 | 80.0 | 7.85 | 0.92 (0.87, 0.97) |
| Gait Speed in Seconds | ||||||
| 1-unit increase in usual walking time | 1.76 (1.33, 2.32) | <0.0001 | 86.7 | 78.6 | 4.98 | 0.91 (0.85, 0.97) |
| Grip Strength (Kg) | ||||||
| 1-unit decrease in grip strength | 1.16 (1.06, 1.26) | 0.002 | 66.7 | 76.9 | 17.5 | 0.72 (0.56, 0.88) |
| Vulnerable Elders Survey Score (VES) | ||||||
| 1-unit increase in scores | 1.61 (1.31, 1.97) | <0.0001 | 83.3 | 83.8 | 2.61 | 0.86 (0.77, 0.96) |
Figure 1. Comparison of Receiver Operator Curves of Four Measures to Predict Functional Decline.
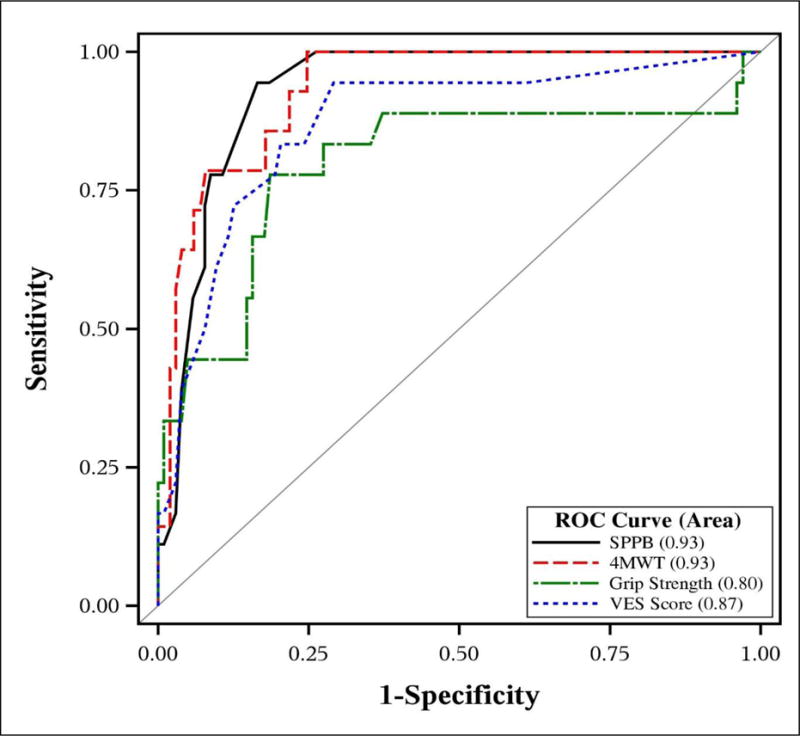
Note: 4MWT denotes 4 minute Walk Test which is Usual Gait Speed
Pair-wise comparisons between SPPB vs. gait speed, SPPB vs.VES-13 and SPPB vs. grip strength showed no statistical significant difference between ROC curves, [difference=−0.009, p-value=0.72, (95% CI: −0.06 to 0.04); difference=−0.07, p-value=0.18, (95% CI: −0.17 to 0.03), and [difference=−0.21, p-value=0.03, (95% CI: −0.39 to −0.04)], respectively.
DISCUSSION
This study, conducted among older women with stage I–III newly diagnosed breast cancer, revealed a high prevalence of functional disability at diagnosis (35%) and subsequent development of functional decline (15%) within 12 months of treatment initiation for breast cancer. The SPPB, usual gait speed, grip strength and VES-13 were all useful instruments for predicting functional decline.
Among older adults in general, performance-based measures such as the SPPB has been found to be a robust and consistent predictor of functional decline, hospitalization, nursing home placement, and death.7, 23 The SPPB has been found to be easy to administer in both epidemiological7 and clinical settings24, has excellent test–retest reliability and has demonstrated sensitivity to change25 making it an ideal measure for use in intervention studies. Gait speed has also been demonstrated among older adults in general to be a well-established, inexpensive and reliable measure of physical performance26, with high inter-rater and test–retest reliability24. Gait speed has well-documented predictive value for major health-related outcomes such as functional decline, hospitalizations, nursing home placements, mortality, poor quality of life and falls27, 28, making it a useful screening measure to identify older persons at risk of such events26. Grip strength, another performance-based measure with excellent reliability(ICC=.92)29 is associated with functional disability.30 The VES-13, the only self-reported measure evaluated in this study, also has a well-established track record as a robust and consistent measure for predicting functional decline and death, irrespective of patient population including older patients with cancer.6, 31
Among older adults with cancer on the other hand, far fewer studies have evaluated the utility of physical performance measures for predicting functional decline, likely due to a concern that such measures may not be easy to administer in busy oncology practices. In a population-based study of 429 older adults with cancer, gait speed was found to be associated with functional decline and death among patients with non-metastatic breast cancer.32 There were no associations between grip strength and functional decline or death.32 In another secondary analysis of a population based study conducted among 413 cancer survivors with a median follow-up of 11.0 years each 1-unit increase in SPPB score and 0.1 m s(−1) increase in fast walk speed predicted a 12% reduction in mortality.33 While these findings suggest that physical performance measures are useful for predicting and quantifying the health status of older cancer survivors, it will be very helpful and even more imperative for such assessments to be tested more proximal to diagnosis where early intervention is more likely to be impactful.
Moreover, and to the best of our knowledge no studies have compared performance-based to self-report measures among patients with cancer to identify the best predictive measures for functional decline34 although one study has compared the utility of different self-report and physical performance measures to predict mortality, the most extreme manifestation of functional decline. Among 200 women 65 years of age and older with gynecological cancers, Cesari M, et al,35 compared six measures which included the VES-13, usual gait speed, SPPB, grip strength, ECOG Performance Status Scale, Karnofsky Performance Status Scale, ADL and IADL on their abilities to predict one-year mortality. The SPPB, usual gait speed and IADL were the only predictive measures.35 Our study results are somewhat consistent with these findings by demonstrating the SPPB and usual gait speed as the most robust predictors of functional decline. Our study therefore informs a gap in evaluating the functional health of older the adults with cancer given that very few to no studies have examined and compared the utility of self-report and performance-based measures to identify the best measures for identifying older patients with cancer at increased risk for functional decline.
Early identification of persons at increased risk for functional decline among older adults with cancer is critically important and clinically relevant. Our previous work demonstrated that among older women with stage I–III newly diagnosed breast cancer one in five developed functional decline and/or death within 12 months of initiating treatment for breast cancer and that women of lower socioeconomic status were the most at risk.6 Hoppe, et al,36 in their study of patients 70 years of age and older receiving first line chemotherapy, demonstrated that 17% of patients developed functional decline after just one cycle of chemotherapy. These two studies speak to the high likelihood of functional decline developing soon after a new diagnosis of cancer and initiation of treatment. Because functional status is a key component for consideration in cancer treatment decision-making and predicts treatment tolerance37, and because of the adverse impact of functional decline on the overall health status of older cancer survivors including nursing home placement and increased mortality2, it is imperative to identify those at increased risk for functional decline and implement early intervention to attenuate the disablement process. Feasible and robust measures such as the SPPB, usual gait speed, grip strength and VES-13 offer different options for early identification of those at increased risk of functional decline, particularly older AA and lower SES breast cancer survivors who are disproportionately affected by poor functional outcomes5, 6.
Our study has some caveats. The sample size is small and the study population was recruited from a single academic institution raising a concern that results may not be generalizable. Patients grip strength may have been affected by the laterality of breast cancer surgery and/or lymph node assessment. We did not collect data on the dominant hand or on the laterality of breast surgery and therefore the grip strength model was not adjusted for these variable. This could have led to an underestimation of relation between grip strength and functional decline. On the other hand, study strengths include prospective data collection by staff trained to administer these functional measures and the availability data on four measures in one population.
In conclusion, this study examined the ability of three performance-based measures and one self-report measure to predict functional decline. All measures demonstrated excellent predictive abilities for predicting functional decline with the SPPB and gait speed showing the strongest predictive abilities. Larger studies are warranted to confirm these results. Oncologists should consider using performance-based measures such as the SPPB and usual gait speed for assessing for the risk of functional decline as these measures can be administered in busy ambulatory oncology settings and may enable oncologists better identify older patients with cancer at increased risk for functional decline who may then be targeted for studies to explore the effects of interventions to improve function.
Table 4.
Association between Physical Function Measures and Functional Decline at 12 months: Adjusted Models
| Physical Function Measures | Odds Ratio (95% CI)* | P-Value | Area Under the Curve (95% CI) |
|---|---|---|---|
| Short Physical Performance Battery | |||
| 1-unit decrease in scores | 1.65 (1.33, 2.04) | <0.0001 | 0.93 (0.89, 0.98) |
| Gait Speed in Seconds | |||
| 1-unit increase in usual walking time | 1.76 (1.29, 2.41)† | 0.0004 | 0.93 (0.88, 0.99) |
| Grip Strength (Kg) | |||
| 1-unit decrease in grip strength | 1.18 (1.06, 1.30) | 0.001 | 0.80 (0.66, 0.94) |
| Vulnerable Elders Survey Score (VES) | |||
| 1-unit increase in scores | 1.64 (1.31, 2.05) | <0.0001 | 0.87 (0.76, 0.96) |
Adjusted for Charlson Comorbidity Index
Adjusted for lymph node assessment
Acknowledgments
This study was supported in part by a Susan Komen Breast Cancer Foundation Career Catalyst in Disparities Research Grant (KG100319) and 1R01MD009699-01 to Cynthia Owusu, M.D. None of the authors have any financial disclosures or conflicts of interests to report.
Appendix A: Baseline Characteristics of Participants who Completed Follow-up at 12 months versus Participants Who Did Not
| Completed Follow-up 123 (85%) |
Did Not Complete Follow-up 22 (15%) |
P-value | |
|---|---|---|---|
| Age Group (Years) | |||
| 65 to 74 | 67 (54) | 13 (59) | |
| >=75 | 56 (46) | 9 (41) | 0.6882 |
| Race | |||
| African-American | 36 (29) | 9 (41) | |
| Non-Hispanic Whites | 87 (71) | 13 (59) | 0.2770 |
| Educational Status | |||
| High school or less | 46 (37) | 11 (52) | |
| More than high school | 77 (63) | 10 (48) | 0.1944 |
| Median Household Income* | |||
| ≥ $35,000 | 99 (80) | 16 (73) | |
| <$35,000 | 24 (20) | 6 (27) | 0.4079 |
| Marital Status | |||
| Married | 50 (41) | 5 (24) | |
| Other | 73 (59) | 16 (76) | 0.2235 |
| Living Situation | |||
| Alone | 51 (42) | 9 (38) | |
| Other | 71 (58) | 13 (62) | 0.7499 |
| Body Mass Index | |||
| <25 (Normal) | 23 (24) | 3 (14) | |
| ≥ 25 (Overweight/Obese) | 94 (76) | 19 (86) | 0.4077 |
| Physical Activity | |||
| <500-METs/minute/week | 63 (52) | 11 (50) | |
| ≥500-METs/minute/week | 58 (48) | 11 (50) | 0.8584 |
| Stage | |||
| Stage I–II | 109 (90) | 19 (86) | |
| Stage III | 12 (10) | 3 (14) | 0.7034 |
| Hormonal Status | |||
| Positive | 102 (83) | 18 (82) | |
| Negative | 21 (17) | 4 (18) | 0.8991 |
| Primary Surgery | |||
| Mastectomy | 36 (30) | 4 (19) | |
| Lumpectomy | 80 (67) | 16 (76) | |
| None | 4 (3) | 1 (5) | 0.4670 |
| Primary surgery prior to enrollment | |||
| Yes | 90 (73) | 16 (73) | |
| No | 33 (27) | 6 (27) | 0.9655 |
| Sentinel Node Biopsy/Axillary Node Dissection | |||
| Yes | 102 (85) | 18 (86) | |
| No | 18 (15) | 3 (14) | 0.9999 |
| Radiation Therapy | |||
| Yes | 58 (50) | 13 (62) | |
| No | 59 (50) | 8 (30) | 0.2978 |
| Chemotherapy | |||
| Yes | 31 (26) | 4 (18) | |
| No | 87 (74) | 18 (82) | 0.5931 |
| Hormonal Therapy | |||
| Yes | 95 (81) | 18 (82) | |
| No | 23 (19) | 4 (18) | 0.9999 |
| Functional Disability** | |||
| Yes | 43 (35) | 10 (45) | |
| No | 80 (65) | 32 (55) | 0.3465 |
| Vulnerable Elders Survey Score | |||
| Scores 0–2 | 91 (74) | 12 (55) | |
| Scores 3–10 | 32 (26) | 10 (45) | 0.0641 |
| Short Physical Performance Battery Score | |||
| Scores 8–12 | 85 (69) | 12 (55) | |
| Scores 0–7 | 38 (30) | 10 (45) | 0.1813 |
| 4 Meters Walk Time (seconds) | |||
| ≤6.2 secs | 99 (80) | 15 (68) | |
| >6.2 secs | 24 (20) | 7 (32) | 0.1947 |
| Grip Strength (Kg) | |||
| ≥21 | 53 (43) | 7 (32) | |
| <21 | 69 (57) | 15 (68) | 0.3087 |
| Charlson Comorbidity Index | |||
| 0–1 | 96 (79) | 14 (63) | |
| ≥ 2 | 25 (21) | 8 (37) | 0.1078 |
| Geriatric Syndromes*** | |||
| 0–1 | 94 (78) | 18 (86) | |
| ≥ 2 | 27 (22) | 3 (14) | 0.5660 |
Median Household income is derived from information from the US Census Bureau using Zip codes
Functional disability denotes self-reported ADL/IADL dependence
Geriatric syndromes include dementia, depression, polypharmacy, malnutrition, and falls
Appendix B: Multivariate Logistic Model of SPPB Scores Adjusted for All Explanatory Variables Predictive of Functional Decline on Univariate Analysis
| Odds Ratio | 95% CI | P-value | |
|---|---|---|---|
| Baseline Characteristics | |||
| SPPB Score | |||
| 1-unit decrease in scores | 1.78 | 1.21, 2.63 | 0.004 |
| Age Group (Years) | |||
| 65 to 74 | Referent | ||
| >=75 | 1.56 | 0.25, 9.99 | 0.62 |
| Race | |||
| White | Referent | ||
| African-American | 5.00 | 0.62, 40.65 | 0.13 |
| Educational Status | |||
| More than high school | Referent | ||
| High school or less | 1.82 | 0.33, 10.03 | 049 |
| Median Household Income* | |||
| ≥$35,000 | Referent | ||
| <$35,000 (Lowest Quartile) | 1.42 | 0.20, 9.97 | 0.73 |
| Physical Activity | |||
| ≥500-METs/minute/week | Referent | ||
| <500-METs/minute/week | 4.35 | 0.43, 44.22 | 0.21 |
| Primary surgery prior to enrollment | |||
| Yes | Referent | ||
| No | 2.43 | 0.35, 17.10 | 0.37 |
| Sentinel Node Biopsy/Axillary Node Dissection | |||
| Yes | Referent | ||
| No | 2.50 | 0.36, 17.45 | 0.35 |
| Radiation Therapy | |||
| Yes | Referent | ||
| No | 4.86 | 0.57, 2.78 | 0.15 |
| Charlson Comorbidity Index | |||
| 0–1 | Referent | ||
| ≥ 2 | 10.61 | 1.58, 71.25 | 0.02 |
| Geriatric Syndromes** | |||
| 0–1 | Referent | ||
| ≥ 2 | 2.06 | 0.24, 7.45 | 0.51 |
Median Household income is derived from information from the US Census Bureau using Zip codes
Geriatric syndromes include dementia, depression, polypharmacy, malnutrition, and falls
Footnotes
Disclosures and Conflict of Interest Statements
The authors have no conflicts of interest to disclose.
Author Contributions
Study Concept: C Owusu
Study Design: C Owusu
Data Acquisition: C Owusu
Quality Control of Data and Algorithms: C Owusu
Data Analysis and Interpretation: C Owusu, S Margevicius, SM Koroukian, M Schluchter, NA Berger
Statistical Analysis: C Owusu, S Margevicius, M Schluchter
Manuscript Preparation: C Owusu, S Margevicius, M Schluchter
Manuscript Editing: C Owusu, S Margevicius, SM Koroukian, M Schluchter, NA Berger
Manuscript Review: C Owusu, S Margevicius, SM Koroukian, M Schluchter, NA Berger
References
- 1.Inouye SK, Peduzzi PN, Robison JT, Hughes JS, Horwitz RI, Concato J. Importance of functional measures in predicting mortality among older hospitalized patients. Jama. 1998;279:1187–1193. doi: 10.1001/jama.279.15.1187. [DOI] [PubMed] [Google Scholar]
- 2.Wolinsky FD, Callahan CM, Fitzgerald JF, Johnson RJ. Changes in functional status and the risks of subsequent nursing home placement and death. J Gerontol. 1993;48:S94–101. [PubMed] [Google Scholar]
- 3.Manton KG. A longitudinal study of functional change and mortality in the United States. J Gerontol. 1988;43:S153–161. doi: 10.1093/geronj/43.5.s153. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Medicare and Medicaid Services. Medicare Evidentiary Priorities. 2008 http://www.cms.hhs.gov/coverageGenInfo/07_EvidentiaryPriorities.asp (Accessed October 20, 2009)
- 5.Owusu C, Schluchter M, Koroukian SM, Mazhuvanchery S, Berger NA. Racial disparities in functional disability among older women with newly diagnosed nonmetastatic breast cancer. Cancer. 2013;119:3839–3846. doi: 10.1002/cncr.28232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owusu C, Margevicius S, Schluchter M, Koroukian SM, Schmitz KH, Berger NA. Vulnerable elders survey and socioeconomic status predict functional decline and death among older women with newly diagnosed nonmetastatic breast cancer. Cancer. 2016;122:2579–2586. doi: 10.1002/cncr.30046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 8.Pavasini R, Guralnik J, Brown JC, et al. Short Physical Performance Battery and all-cause mortality: systematic review and meta-analysis. BMC Med. 2016;14:215. doi: 10.1186/s12916-016-0763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. Jama. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Min LC, Elliott MN, Wenger NS, Saliba D. Higher vulnerable elders survey scores predict death and functional decline in vulnerable older people. J Am Geriatr Soc. 2006;54:507–511. doi: 10.1111/j.1532-5415.2005.00615.x. [DOI] [PubMed] [Google Scholar]
- 11.Saliba D, Orlando M, Wenger NS, Hays RD, Rubenstein LZ. Identifying a short functional disability screen for older persons. J Gerontol A Biol Sci Med Sci. 2000;55:M750–756. doi: 10.1093/gerona/55.12.m750. [DOI] [PubMed] [Google Scholar]
- 12.Higashi T, Shekelle PG, Adams JL, et al. Quality of care is associated with survival in vulnerable older patients. Ann Intern Med. 2005;143:274–281. doi: 10.7326/0003-4819-143-4-200508160-00008. [DOI] [PubMed] [Google Scholar]
- 13.Rantanen T, Guralnik JM, Sakari-Rantala R, et al. Disability, physical activity, and muscle strength in older women: the Women’s Health and Aging Study. Arch Phys Med Rehabil. 1999;80:130–135. doi: 10.1016/s0003-9993(99)90109-0. [DOI] [PubMed] [Google Scholar]
- 14.Desrosiers J, Bravo G, Hebert R, Dutil E. Normative data for grip strength of elderly men and women. Am J Occup Ther. 1995;49:637–644. doi: 10.5014/ajot.49.7.637. [DOI] [PubMed] [Google Scholar]
- 15.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies Of Illness In The Aged. The Index Of Adl: A Standardized Measure Of Biological And Psychosocial Function. Jama. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 16.Lawton MP. Scales to measure competence in everyday activities. Psychopharmacol Bull. 1988;24:609–614. [PubMed] [Google Scholar]
- 17.U.S. Census Bureau. State and national population projections. [Accessed February 2013]. http://www.census.gov/population/projections/data/national/2012.html.
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Min LC, Wenger NS, Reuben DB, Saliba D. A short functional survey is responsive to changes in functional status in vulnerable older people. J Am Geriatr Soc. 2008;56:1932–1936. doi: 10.1111/j.1532-5415.2008.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suijker JJ, Buurman BM, van Rijn M, et al. A simple validated questionnaire predicted functional decline in community-dwelling older persons: prospective cohort studies. J Clin Epidemiol. 2014;67:1121–1130. doi: 10.1016/j.jclinepi.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 21.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 22.Hosmer D, Lemeshow S. Applied Logistic Regression. 2nd. New York, NY: Wiley-Interscience; 2000. [Google Scholar]
- 23.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 25.Ostir GV, Volpato S, Fried LP, Chaves P, Guralnik JM. Reliability and sensitivity to change assessed for a summary measure of lower body function: results from the Women’s Health and Aging Study. J Clin Epidemiol. 2002;55:916–921. doi: 10.1016/s0895-4356(02)00436-5. [DOI] [PubMed] [Google Scholar]
- 26.Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people–results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- 27.Graham JE, Ostir GV, Fisher SR, Ottenbacher KJ. Assessing walking speed in clinical research: a systematic review. J Eval Clin Pract. 2008;14:552–562. doi: 10.1111/j.1365-2753.2007.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13:881–889. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- 29.Bohannon RW, Schaubert KL. Test-retest reliability of grip-strength measures obtained over a 12-week interval from community-dwelling elders. J Hand Ther. 2005;18:426–427. doi: 10.1197/j.jht.2005.07.003. quiz 428. [DOI] [PubMed] [Google Scholar]
- 30.Rantanen T, Guralnik JM, Foley D, et al. Midlife hand grip strength as a predictor of old age disability. Jama. 1999;281:558–560. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- 31.Min L, Yoon W, Mariano J, et al. The Vulnerable Elders-13 Survey Predicts 5-Year Functional Decline and Mortality Outcomes in Older Ambulatory Care Patients. J Am Geriatr Soc. 2009 doi: 10.1111/j.1532-5415.2009.02497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klepin HD, Geiger AM, Tooze JA, et al. Physical performance and subsequent disability and survival in older adults with malignancy: results from the health, aging and body composition study. J Am Geriatr Soc. 2010;58:76–82. doi: 10.1111/j.1532-5415.2009.02620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown JC, Harhay MO, Harhay MN. Physical function as a prognostic biomarker among cancer survivors. Br J Cancer. 2015;112:194–198. doi: 10.1038/bjc.2014.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verweij NM, Schiphorst AH, Pronk A, van den Bos F, Hamaker ME. Physical performance measures for predicting outcome in cancer patients: a systematic review. Acta Oncol. 2016:1–6. doi: 10.1080/0284186X.2016.1219047. [DOI] [PubMed] [Google Scholar]
- 35.Cesari M, Cerullo F, Zamboni V, et al. Functional status and mortality in older women with gynecological cancer. J Gerontol A Biol Sci Med Sci. 2013;68:1129–1133. doi: 10.1093/gerona/glt073. [DOI] [PubMed] [Google Scholar]
- 36.Hoppe S, Rainfray M, Fonck M, et al. Functional decline in older patients with cancer receiving first-line chemotherapy. J Clin Oncol. 2013;31:3877–3882. doi: 10.1200/JCO.2012.47.7430. [DOI] [PubMed] [Google Scholar]
- 37.Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012;118:3377–3386. doi: 10.1002/cncr.26646. [DOI] [PubMed] [Google Scholar]


