Abstract
Most childhood deaths in the United States occur in hospitals and the majority of these in intensive care settings. Pediatric intensive care unit (PICU) clinicians must anticipate, identify and effectively treat the dying child’s pain and suffering, as well as support the psychosocial and spiritual needs of the child and their family. Such timely therapy and support may facilitate comfort and a peaceful death, and also help family members adjust to their loss. Effective communication that is candid and compassionate is paramount to successful end of life (EOL) care, as is creating an environment that encourages parent participation in their child’s care and fosters meaningful family interactions. Despite these supportive efforts, parents whose children die often experience reduced mental and physical health during bereavement. Finally, many ethical issues surround EOL care in the PICU, and as such, several professional societies have published recommendations and policies addressing these issues. PICU clinicians should stay current on these recommendations and policies and maintain a working understanding of their implications.
Keywords: pediatric death, end-of-life, pediatric intensive care unit, family support, parental grief
INTRODUCTION
Pediatric Death in the PICU
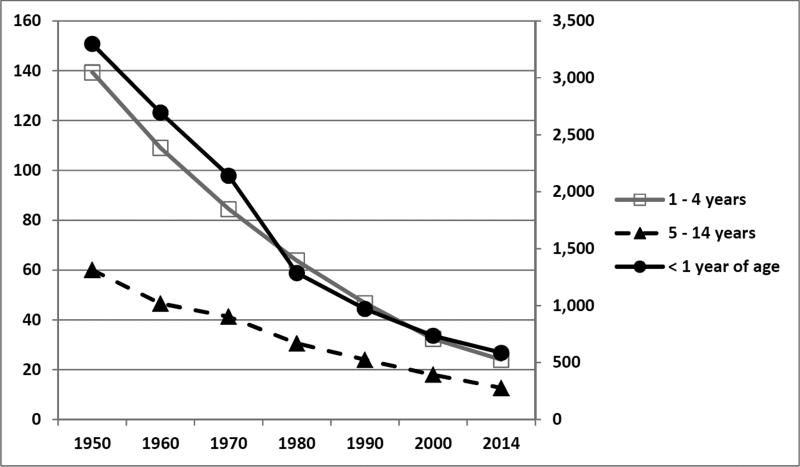
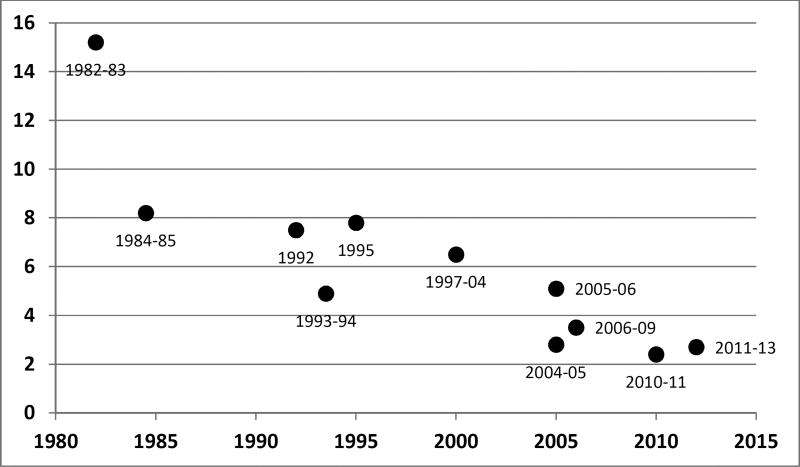
Overall pediatric mortality is decreasing in the United States. In 1980, over 64,000 infants and children less than 15 years of age died in the US. In striking contrast, 2014 data show that number has almost decreased in half with only 32,295 reported deaths among this age group (Figure 1).1 Similarly, mortality rates among pediatric intensive care unit (PICU) admissions have also decreased over time (Figure 2).2–8 Three recent multicenter studies have reported PICU mortality rates less than 3%.9–11
Figure 1. Death rates among three pediatric cohorts for all causes in the United States.
The figure depicts the decreasing mortality rates among three pediatric cohorts from 1950 through 2014. The less than one year of age cohort (solid black circles, solid black line) is plotted on the right-sided secondary axis. The 1 – 4 year old cohort (open squares, gray line) and the 5 – 14 year old cohorts are plotted on the left-sided, primary axis. Mortality rates are expressed in deaths per 100,000 resident population.
Data from National Center for Health Statistics. Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD. 2016. Table 21. Death rates for all causes, by sex, race, Hispanic origin, and age: United States, selected years 1950–2014. Page 113.
Figure 2. Mortality rates among pediatric intensive care unit admissions over time.
This figure depicts the declining rates of mortality in the pediatric intensive care unit over the past three decades. The years next to each data point indicate the years that the data were collected.
- Namachivayam P, Shann F, Shekerdemian L, et al. Three decades of pediatric intensive care: Who was admitted, what happened in intensive care, and what happened afterward. Pediatr Crit Care Med. 2010;11:549–555.
- Typpo KV, Petersen NJ, Hallman DM, Markovitz BP, Mariscalco MM. Day 1 multiple organ dysfunction syndrome is associated with poor functional outcome and mortality in the pediatric intensive care unit. Pediatr Crit Care Med. 2009;10:562–570.
- Pollack MM, Holubkov R, Funai T, et al.; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network. Simultaneous Prediction of New Morbidity, Mortality, and Survival Without New Morbidity From Pediatric Intensive Care: A New Paradigm for Outcomes Assessment. Crit Care Med. 2015;43:1699–1709.
- Burns JP, Sellers DE, Meyer EC, Lewis-Newby M, Truog RD. Epidemiology of death in the PICU at five U.S. teaching hospitals. Crit Care Med. 2014;42:2101–2108.
- Conlon NP, Breatnach C, O’Hare BP, Mannion DW, Lyons BJ. Health-related quality of life after prolonged pediatric intensive care unit stay. Pediatr Crit Care Med. 2009;10:41–44.
- Visser IH, Hazelzet JA, Albers MJ, et al. Mortality prediction models for pediatric intensive care: comparison of overall and subgroup specific performance. Intensive Care Med. 2013;39:942–950.
- Gemke RJ, Bonsel GJ, van Vught AJ. Long-term survival and state of health after paediatric intensive care. Arch Dis Child. 1995;73:196–201.
- Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24:743–752.
- Pollack MM, Ruttimann UE, Getson PR. Pediatric risk of mortality (PRISM) score. Crit Care Med. 1988;16:1110–1116.
Approximately half of the deaths among US children one to nineteen years of age occur in hospitals with an additional 14% occurring in Emergency Departments.12,13 Of hospital deaths, the majority occur in intensive care settings.14,15 Consequently, a sound understanding of the principles and practices of palliative and EOL care should be maintained by all clinicians in the field.
Recent prospective, multicenter data suggest that approximately 70% of patients dying in a PICU do so in the context of withdrawal or withholding of life-sustaining therapies, with the remaining 30% being fairly equally divided between a diagnosis of brain death and failed cardiopulmonary resuscitation.11,16 One study suggests that PICU deaths can be categorized into one of two groups based on the PICU length of stay at the time of the death.11 Children who died within a week of PICU admission were characterized by the onset of a new illness or injury, and were also more likely to die as a result of brain death or failed resuscitation. In contrast, those who died more than a week into their PICU course were characterized by pre-existing conditions and technology dependence at baseline. Their deaths tended to follow the withdrawal of life-sustaining support.11
In terms of overall causes of death, congenital malformations and disorders related to prematurity accounted for the majority of deaths in children under one year of age in 2014, while unintentional injury was the leading cause of death in children one year and older.1 Within the PICU, multiple organ failure has been reported to be the most common diagnosis at the time of death followed by neurologic and respiratory conditions.11 Despite unintentional injuries being the leading overall cause of death in children over one year, most deaths in the PICU are associated with a pre-existing or chronic condition.11 Given recent data that suggest over half of the children admitted to a PICU have a chronic, complex medical condition17, this finding may merely reflect the population of patients currently being cared for in the PICU. Independent of the reasons, the findings that children with chronic conditions account for a large proportion of both the admissions to and the deaths within a PICU, may have significant implications for the provision of palliative care within that setting. Both the Institute of Medicine18 and the American Academy of Pediatrics19 have long offered that palliative care should be initiated at the time of diagnosis of a life-limiting condition and be implemented concurrently with curative therapies. Consequently, palliative care services should not simply be provided at EOL for these children and their families, but rather, implemented from the time of diagnosis and throughout their often multiple admissions to the PICU. Data suggest that children with a life-limiting condition who are discharged from the PICU with palliative care services in place are more likely to die outside of the hospital than those without such provisions of care.20
DISCUSSION
End of Life Care
Although early implementation of palliative care has the potential to improve outcomes for many children with complex, chronic medical conditions, the provision of quality EOL care in the PICU is an essential pillar of any successful pediatric critical care program.21 To provide such care, it is imperative to meet the physical needs of the patient in terms of pain control and symptom management.22 Survey data suggest that most PICU clinicians express confidence in their ability to treat acute symptoms of the dying patient including pain, agitation, dyspnea, secretions and seizures; however, they appear less comfortable in their ability to treat more chronic issues such as skin breakdown and constipation.23 More than simply addressing physical suffering, effective EOL care must also address the child and their family’s psychosocial and spiritual needs.24 Clearly, critical illness in a child impacts the entire family, and independent of outcome, has the potential for long-standing dysfunction and detriment among family members.25,26,27 The provision of such comprehensive and time-consuming care may be particularly challenging for PICU clinicians,28,29 and current data purport wide variability in identifying and addressing these complex issues associated with EOL care in the PICU.30,31 Clinicians must strive to develop a sound understanding of this field and its potential to impact the quality of life for patients, families, and clinicians alike.
The Needs of Children at the End of Life
The National Quality Forum, Institute of Medicine, and the National Institutes of Health have all identified EOL care as a national priority, including medical care for children with advanced illness. At the EOL, children may have many needs across a vast array of domains, and clinicians must be equipped with the skills necessary to address the needs of each individual in order to provide high quality care. These needs are often divided into physical, psychosocial, spiritual and environmental domains.32 This discussion will focus on the physical and psychosocial needs of the dying child. However, if children do in fact hold certain religious or spiritual beliefs, particularly in the case of adolescents, understanding those beliefs is important, as is ensuring that children are allowed to die comfortably, in the family’s chosen environment, surrounded by the people they love.
Physical Needs
Although little is known about the personal priorities of children nearing death, adult patients have consistently endorsed pain and symptom management as a high priority with regard to their care.33,34 Children at the EOL experience numerous physical symptoms and can suffer if their physical needs are not promptly recognized and addressed. Various pediatric palliative care experts have developed clinical practice guidelines and algorithms to manage EOL symptoms in children with cancer, which can be readily adapted for use in children with other advanced illnesses (Tables 1 and 2).35
Table 1.
Pharmacologic management of pediatric pain at the end of life
| Drug | Initial dose | Route | Interval | Maximum Dosea |
|---|---|---|---|---|
|
| ||||
| Acetaminophen | 10–16 mg/kg | PO/PR/IV | q 4 h | 1 g/dose |
| 4 g/day | ||||
|
| ||||
| Ibuprofen | 5–10 mg/kg | PO | q 6 h | 2.4 g/day |
| 3.4 g/day (adults) | ||||
|
| ||||
| Naproxen | 5–7 mg/kg | PO | q 8–12 h | 1 g/dose |
| 4 g/day | ||||
|
| ||||
| Ketorolac | 0.5 mg/kg | PO, IV | q 6 h | 30 mg/dose (IV) |
| 10 mg/dose (PO) | ||||
|
| ||||
| Tramadol | 1–2 mg/kg | PO | q 6 h | 100 mg/dose |
| 400 mg/day | ||||
|
| ||||
| Morphine | 0.2–0.5 mg/kg | PO, SL, PR | q 3–4 h | Titrate |
| 0.1 mg/kg | IV, SQ | q 2–4 h | ||
| 0.3–0.6 mg/kg (LA) | PO | q 8–12 h | ||
|
| ||||
| Hydromorphone | 0.03–0.08 mg/kg | PO, PR | q 3–4 h | Titrate |
| 0.015 mg/kg | IV, SQ | q 2–4 h | ||
|
| ||||
| Methadone | 0.2 mg/kg | PO | q 8–12 h | Titrate |
| 0.1 mg/kg | IV, SQ | q 8–12 h | ||
|
| ||||
| Fentanyl | 0.5–1 µg/kg/hr | TD | q 48–72 h | Titrate |
| 5–15 µg/kg (sed) | TM | q 4–6 h | ||
| 1–2 µg/kg | IV, SQ | q 1–2 h | ||
|
| ||||
| Oxycodone | 0.05–0.15 mg/kg | PO | q 6 h | Titrate |
| 0.1–0.3 mg/kg (LA) | PO | q 12 h | ||
Abbreviations: PO, by mouth; IV, intravenous; PR, per rectum, SL, sub-lingual; SQ, Subcutaneous; TD, transdermal; TM, transmucosal; sed, sedative; LA, long acting
Common maximum dosage; however, dose escalation may be necessary at EOL
Adapted from Johnson LM, Snaman JM, Cupit MC, Baker JN. End-of-Life Care for Hospitalized Children. Pediatr Clin N Am. 2014;61:835–854.
Table 2.
Pediatric symptom management (non-pain) at the end of life
| Symptom | Medication | Common Pediatric Dosage (<60 kg) |
Maximum Daily Dosagea |
|---|---|---|---|
|
| |||
| Agitation/Delirium | Nonpharmacologic | Familiar objects, low lighting, soothing tones/music | |
| Lorazepam | 0.05 mg/kg/dose PO, SL (preferred for seizure), or PR every 4–6 h | 2 mg per dose | |
| Chloral hydrate | 25–50 mg/kg/day PO/PR divided every 6–8 h | 1 g/day for infants, 2 g/ day for children | |
| Haloperidol | 0.01–0.02 mg/kg per dose PO, SL, or PR every 8–12 h | 0.15 mg/kg/ day | |
|
| |||
| Dyspnea | Nonpharmacologic | Elevate head of bed, fluid restriction, suctioning, bedside fan, flowing air | |
| Morphine | 0.15 mg/kg PO/SL every 2 h PRN (titrate to effect) | ||
| Lorazepam* | 0.05 mg/kg PO/SL every 4–6 h PRN (titrate to effect) | 2 mg per dose | |
|
| |||
| Nausea/Vomiting | Nonpharmacologic | Avoid irritating foods or smells, relaxation, biofeedback, acupuncture, aromatherapy | |
| Ondansetron | 0.15 mg/kg/dose PO/IV every 8 h PRN | 8 mg per dose | |
| Promethazine | >2 y: 0.25 mg/kg/dose PO/IV every 6–8 hr PRN | 1 mg/kg/day | |
| Scopolamine | 8–15 kg: half patch TD every 3 days, >15 kg: 1 patch TD every 3 days | 1 patch every 3 days | |
| Metoclopramide | 0.01–0.02 mg/kg/dose per dose IV every 4 hr | ||
| Lorazepam | 0.05 mg/kg PO/SL every 4–6 h PRN (titrate to effect) | 2 mg per dose | |
|
| |||
| Secretions | Nonpharmacologic | Fluid restriction, gentle suctioning | |
| Glycopyrrolate | 0.04–0.1 mg/kg/d PO every 4–8 h | 1–2 mg per dose or 8 mg/day | |
| 0.01–0.02 mg/kg/d IV every 4–6 h | |||
Abbreviations: IV, intravenous; PR, per rectum, SL, sub-lingual; TD, transdermal
Common maximum dosage; however, dose escalation may be necessary at EOL
Lorazepam used for dyspnea associated with anxiety
Adapted from Johnson L-M, Snaman JM, Cupit MC, Baker JN. End-of-Life Care for Hospitalized Children. Pediatr Clin N Am. 2014;61:835–854.
Although rare, there are situations in which patients experience intractable physical and/or psychosocial suffering at EOL despite aggressive treatment regimens and escalating doses. These clinical scenarios are most likely to occur in the PICU and may require palliative sedation therapy with infusions such as propofol to achieve continuous deep sedation and relief of pain and distress.35 In these difficult situations, other medications such as ketamine, and more recently dexmedetomidine, may play a vital role in the ease of suffering. Ketamine has been found to alleviate severe pain and decrease the use and escalation of opioids at EOL in both adults and children with cancer.36,37 It has also been found to be effective for neuropathic pain in children at EOL.38 Dexmedetomidine, an α2-adrenoreceptor agonist, is a sedative frequently used in both adult and pediatric ICUs. It was recently evaluated as an adjuvant therapy to treat pain and agitation in children and adolescents with advanced illness at EOL, and a significant decrease in pain scores was observed after the initiation of a dexmedetomidine infusion.39 Although further study is clearly needed, it appears that both ketamine and dexmedetomidine hold promise in treating refractory pain and distress at EOL for children.
In addition to pharmacologic methods of controlling pain at EOL, there are also non-pharmacologic measures. Complementary and alternative medicine (CAM) encompasses a wide range of modalities such as acupuncture, massage, faith healing, and organic supplementation. Parents have reported that CAM benefitted their dying child and did not cause any additional suffering.40 Although a detailed discussion of non-pharmacologic palliative care therapies is beyond the scope of this review, interest appears to be growing in the use of these modalities, and thus, it is important for clinicians to be knowledgeable of their role.
Psychosocial Needs
Children facing EOL often exhibit psychosocial distress linked to various illness-related factors.41 A child’s reaction to illness and their understanding of the concept of death is largely influenced by their cognitive and developmental level.41 Clinical experience suggests that it is three “losses” that cause the most distress for children: 1) loss of control over their bodies and what is happening at any given moment, 2) loss of personal identity, and 3) loss of interpersonal relationships.41 Identifying the extent to which each of these is affecting the dying child can aid clinicians in meeting the emotional needs of their patient. Some pediatric patients at EOL will experience significant emotional distress manifested by signs of anxiety and depression. These children may benefit from consultation with psychology and/or psychiatry teams.
Facilitating communication between parents and their children is a critical aspect of pediatric EOL care.41 A Swedish study found that 100% of participating parents reported satisfaction with their decision to discuss death with their dying child, whereas a small percentage of those who chose not to have this discussion were dissatisfied with their decision.42 The content and approach to these conversations will depend upon the cognitive level of the child; however, it is recommended that these conversations occur in series and can be encouraged through various forums (e.g. talking, writing, drawing).41 Many palliative care experts believe that providing dying children the opportunity to openly discuss death, grief, and illness minimizes their confusion and fears.41 Clinicians must be prepared to patiently and honestly answer the questions of dying children. In addition to the expertise of palliative medicine, ancillary teams such as child life can serve an extremely important role in helping explain concepts of death in developmentally appropriate ways to patients and their siblings.
For children with life-threatening illnesses, efforts to build memories and confirm that they will be remembered are important.43 Legacy building encompasses actions to create items that are remembered including artwork, photographs, and videos.35 Dying children in the PICU are often too sick to participate in legacy building activities, but other forms of memory making do exist for parents and siblings. Regardless of the activity, legacy making may have positive effects for ill children and their families.44
The Needs of Parents at the End of a Child’s Life
The needs of parents in the PICU at the end of a child’s life warrant special attention. Evidence suggests that parents who perceive greater fulfillment of their needs during their child’s PICU stay experience less symptoms of complicated grief during bereavement.45 Communication remains an integral component of addressing bereaved parents’ needs. Parents have reported a desire to receive honest communication that is given in a caring and sensitive tone.46,47 Honest communication for parents includes frequent updates on their child’s condition and prognosis which facilitates decision-making.47 Moreover, honest communication improves parental understanding and reduces conflict.47 Evasive answers and incomplete information may undermine trust and interfere with the parents’ ability to cope with the death of their child.48 Parent-physician interactions with more patient-centered elements, such as increased proportions of empathetic statements, question asking, and emotional talk, positively influence parent satisfaction with care independent of the child’s severity of illness.49 Parents of dying children also desire simple language in lieu of medical jargon.47 High-quality communication at the end of a child’s life fosters trust between families and medical staff and helps to ensure that the dying child receives the best possible care.
Physical and Emotional Health
For parents facing the loss of their child, the relief of their child’s pain and suffering is a critically important need; however, bereaved family members have historically reported poor management of distressful symptoms.50,51,52 PICU clinicians should begin the process of symptom management and preparedness by first educating families on the anticipated physical symptoms associated with EOL and the potential therapies. Parents should be given a platform to express their concerns and to identify their unique preferences35 and should be reassured that relieving their child’s pain and suffering is a top priority of care.53
Although parents of dying children will largely be focused on the needs of their child, they themselves are at risk for physical and mental health issues. Numerous studies have highlighted the detrimental effects of child death on the physical health of bereaved parents,54–58 and even more have identified risks for emotional distress and mental illness.59–67 Although often beyond the scope of the pediatric clinician, psychology or psychiatry services can be utilized to help parents cope more effectively and to assess for signs of psychopathology.
Psychosocial Needs
Numerous psychosocial needs exist for parents at the end of a child’s life and clinicians should strive to provide care that is inclusive of a family’s personal, cultural, religious, or spiritual beliefs.35 Spirituality appears to play a significant role in adult grief reactions,68,69 and as such, facilitating the expression of a family’s religious or spiritual beliefs as a child approaches EOL may provide comfort and a sense of meaning.35 Hospital chaplains may play an important role in helping clinicians identify a family’s specific religious and spiritual needs.
One of the most prominent spiritual needs described by bereaved parents is that of maintaining a connection to their child at the EOL.70 Clinicians may foster this connection by helping parents maintain the parent-child relationship at EOL. As parents struggle with the loss of their traditional roles of protector and provider,53 maintenance of the parent-child relationship may be facilitated by encouraging active parent involvement in patient care, allowing parents to be present during invasive procedures or cardiopulmonary resuscitation,71 providing opportunity to stay with their child at the time of death, and by helping parents create memories that can bring comfort in the future.
Parents also need staff to be kind and compassionate45 and they want to know that the medical team genuinely cares about their child.53 Although clinicians previously unknown to the family may be quickly incorporated into parents’ support network, special effort should be made to include friends and family during the EOL process, as this facilitates continuity of support when the child dies and the parental support network abruptly shifts from the medical team to primarily family members and friends.53
Environmental
Parents experiencing their child’s death have also identified environmental needs in the PICU including the need for easy access to their child, privacy, facilities for self and sibling care, and the ability to accommodate family and friends at the time of death.71 Memories of a welcoming environment can contribute to comfort during bereavement, whereas environmental frustrations may lead to negative interpersonal interactions and greater grief for parents.71 Clinicians with a heightened awareness of a parent’s environmental needs at EOL may enhance the quality of care provided, improve satisfaction with care, and create a supportive atmosphere for families preparing for their child’s imminent death.
Parental Bereavement
Studies have demonstrated that bereaved parents suffer more intense grief after the death of a child as compared to the grief associated with the loss of a spouse or a parent.72,73 This intense grief puts these parents at risk for long-term psychological issues such as anxiety,59 depression,59,74,75 post-traumatic stress,63,64,76 substance abuse,65 suicide66 and increased risk for psychiatric hospitalization.67 There is also evidence that bereaved parents may be at higher risk for physical morbidity including certain types of cancer and response to cancer treatments,54,55 multiple sclerosis,77 diabetes56 and myocardial infarction.78 Data not only suggest that this grief results in increased health service utilization and sick leave,64 but also higher rates of mortality for bereaved parents.58
Complicated Grief
It is estimated that 7% of bereaved people will develop a severe and protracted grief response frequently referred to as complicated grief.79,80 This appears to be even more pronounced in parents that experience the death of a child; as many as 60% of parents whose children died in a PICU demonstrated high levels of complicated grief that persisted over time.27 Individuals with complicated grief can experience a wide array of symptoms that interfere with daily function including an intense longing or yearning for the person who died, avoidance of reminders of the deceased, intrusive thoughts, or a feeling of meaninglessness.27,81 Individuals often feel stuck in a chronic state of mourning and are unable to move on with life.27,81 Among parents that experience the death of a child in the PICU, several variables have been associated with an increased risk for complicated grief including being the biological mother or female guardian, trauma as the cause of death, greater attachment-related anxiety and avoidance, and greater grief avoidance.27
Bereavement Interventions
The evidence supporting bereavement prevention and intervention is ever growing, but the general consensus is that the vast majority of cases of grief, though painful, are normative and self-limiting. Thus, only the highest risk individuals and those with complicated grief are likely to show benefit from preventive or therapeutic intervention.82,83 In one study, 60% of parents bereaved by child death in the PICU reported a desire to meet with their child’s intensive care physician in the weeks following the death.84 Parents want to revisit events and management at EOL, gain reassurance about decisions that were made, and provide feedback to the medical team.84 A follow-up meeting between pediatric intensivists and bereaved parents after the death of a child could provide parents with greater clarity regarding EOL events as well as emotional support. These meetings may also enable clinicians to screen for parents and/or siblings exhibiting significant physical or emotional distress so that appropriate referrals can be made. Although further study is needed, these meetings may be a potential platform by which intensive care communication is made more complete, bereaved parental knowledge enhanced and the risk for complicated grief decreased.
Ethical Issues in End-of-Life Care in the PICU
As previously described, the majority of childhood deaths in the PICU occur after withdrawal or withholding of life-sustaining therapies.11,15,85 Decision making in these situations is often complex and may be a source of conflict between families and clinicians. Frequently, the first and most common discussion under such circumstances is regarding withholding of cardiopulmonary resuscitation.
The Do-Not-Resuscitate Order
A Do-Not- Resuscitate (DNR) (also referred to as a Do-Not-Attempt-Resuscitation) order in its purest form, states that cardiopulmonary resuscitation will not be initiated in the event that the child experiences cardiopulmonary arrest. In a study that assessed the meaning, implication, and timing of DNR orders for critically ill children, 67% of clinicians believed that a DNR order only limits care in the event of cardiopulmonary arrest, while one-third (33%) of respondents considered a DNR order to be the impetus to consider or implement limitation of other life-sustaining therapies not related to cardiopulmonary arrest, and 6.2% of respondents believed that a DNR order means a transition to comfort care only.86 Although a majority of clinicians contend that DNR orders guide care only during a cardiopulmonary arrest, many believe that once a DNR order is written for a child, the level and type of care provided changes, frequently through an increased attention to comfort care measures, but also through limitation or withdrawal of diagnostic tests and therapeutic interventions. As written by the President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research, “Any DNR policy should ensure that the order not to resuscitate has no implications for any other treatment decisions. Patients with DNR orders on their charts may still be appropriate candidates for all other vigorous care.”87 The disconnect between clinician understanding and the actual intent of DNR orders can cause significant confusion for family members.
Differences between Withdrawal and Limiting of Life-Sustaining Therapies
Ethical conflict and moral distress may occur when there is confusion regarding what is meant by the withdrawal of life-sustaining therapies versus the withholding, or limiting, of those therapies.88 Both of these actions reflect a shift in patient care goals, typically occurring when there is a decision that the goal of curing the critical illness is no longer possible, or that curing the illness is so improbable that the risk of pain and suffering and decreased quality of life far outweighs the benefits of life-sustaining therapies. Optimally, such decisions are made by consensus after targeted discussions between family and clinicians so that all individuals involved are in agreement.28,89,90
Once there is a shift in care goals, decisions regarding whether to limit the initiation of any new life-sustaining therapies or to withdraw certain therapies can be made. The most common interventions to limit or withdraw include mechanical ventilation, vasoactive agents, blood products or antibiotics, and hydration or nutrition.91 Withholding, or limiting life-sustaining therapies means that no new life-sustaining intervention will be initiated based on the belief that the child is dying and the family and clinicians concur that the intervention will not enhance the probability of meaningful survival and may exacerbate or prolong suffering. Withdrawal of a life-sustaining therapy, on the other hand, refers to the active removal of a therapy that was previously initiated. Despite the physiologic differences in stopping a life-sustaining therapy versus never starting one, it is generally accepted that there is no ethical distinction between withdrawing and withholding life support.92 It is important to educate clinicians and families that when death is the expected outcome for the child, clinicians are simply removing or not starting therapies that would artificially prolong life, and that the underlying disease or condition is the cause of death.92,93
The Doctrine of Double Effect
Management of pain and anxiety is key to providing compassionate EOL care;89 however, clinicians are often concerned that by providing medications such as opioids and anxiolytics to mitigate these symptoms, they may hasten death.
The Doctrine of Double Effect states that an action has two effects: one that is inherently good, and one that is inherently bad, but justifiable. In order to abide by the Doctrine of Double Effect, the following conditions must be met: (1) the nature of the act must be good or at least morally neutral; (2) the clinician’s intention must be to only provide the good effect; (3) there must be distinction between means and effect, such that the bad effect must not be a means to a good effect; and (4) there must be proportionality between the good effect and the bad effect (i.e. the benefits of the good effect must outweigh the risk of the bad effect). Thus, the delivery of anxiolytics and opioid pain medications to a child at EOL, despite the risk of causing respiratory depression or hypotension, is clinically, morally and ethically justifiable if the clinician’s intent is strictly to relieve pain and suffering.89
Conflict over Futile or Potentially Inappropriate Therapies
One of the most ethically troubling events while caring for a critically ill child in the PICU occurs when conflict arises between the family and healthcare providers during EOL decision-making. Typically, conflict occurs when a family requests the initiation of therapies that the clinician believes to be ineffective or inappropriate. Ethical controversy occurs as families believe they are acting in the best interest of their child, and clinicians feel pressured to provide a therapy that they do not believe will help, and may foster pain and discomfort.89,94,95 Accordingly, clinicians are not obligated to provide truly “futile” therapies (defined as those therapies that are unable to meet physiologic goals), nor should they under the ethical principle of beneficence (“do no harm”). However, disagreements about the potential effectiveness of a given therapy often lead to distrust and ineffective communication between the family and clinician.
A recent consensus policy statement from the American Thoracic Society, the American Association for Critical Care Nurses, the American College of Chest Physicians, the European Society for Intensive Care Medicine, and the Society of Critical Care Medicine provides recommendations addressing the management of requests from patients and families for treatment that clinicians deem to be inappropriate and believe should not be administered.96 These clinical recommendations include: (1) creating institutional strategies, such as proactive communication and expert consultants, to minimize or prevent care disputes, (2) using the term “potentially inappropriate” rather than “medically futile” or “futility” when discussing treatments that may have some chance of meeting a patient care goal, no matter how small, but that the clinicians believe are clinically not indicated and their non-use ethically justified; (3) saving the term “futile” to use only in those specific occasions in which the family requests care that cannot achieve the intended goal in any way. Family meetings early in the PICU admission, in which clinicians listen to family perceptions and concerns and explain clinical situations in clear language, are instrumental in laying a foundation of trust and good communication.90
Brain Death and Organ Donation
As more children are kept alive through life-saving technologies, the need for donated pediatric organs for transplantation has dramatically increased.93,97 The Uniform Determination of Death Act (UDDA), released in 1981, helped establish what is known as the “Dead Donor Rule,” which states that “vital organs should only be taken from dead subjects and, correlatively, living subjects must not be killed by organ retrieval.” However, the UDDA did not adequately discuss the unique aspects of declaring brain death in infants and children.98 Subsequently, the guidelines for the determination of brain death in children were published in 1987, and updated in 2011.99,100 The majority of transplanted organs in children have traditionally been procured from brain dead donors.93
As the demand for healthy organs grows, alternative methods for procurement and transplantation have been developed. One controversial method is organ donation after cardiac death (DCD), previously referred to as “non-heart-beating organ donation.”93 Donation after cardiac death involves procurement of organs from donors who have cessation of circulation, and who have been declared dead by circulatory standards, usually related to withdrawal of life-sustaining therapies. Although DCD practices vary, the following conditions generally must be present prior to DCD donation: (1) informed written consent for DCD must be obtained from the parents or legal guardians; (2) there must be irreversible, end-stage illness where a decision to withdraw life-sustaining therapies was made prior to the decision to donate; (3) withdrawal of support must occur in the ICU or operating suite to assure adequate treatment of pain and anxiety; (4) a specified observation period must be provided (generally 60 to 120 minutes); and (5) if cardiac function and circulation stop within the observation period, the patient may be declared dead and the organs procured after a waiting period of approximately 2 to 5 minutes.93,101 However, if those conditions are not met during the observation period, EOL care is continued and the patient is no longer considered a potential DCD donor.93,101
While DCD has been endorsed by several professional organizations, controversy exists regarding the ethics of DCD.101–104 Concerns include violation of the “Dead Donor Rule,” including the concern that donors may endure pain and suffering if death is declared prematurely; irreversible damage to donated organs from ischemia if death is not pronounced within the necessary timeframe; and conflict of interest between the needs of the donor and the needs of the transplant recipient.93,101 Additional research is warranted to address these ethical concerns.
Key points.
Most childhood deaths in the United States occur in hospitals and the majority of these in intensive care settings. Thus, the ability to provide high quality end of life (EOL) care is an essential component of successful pediatric critical care programs.
The ability to anticipate, identify and treat pain and suffering at EOL while concurrently attending to the psychosocial needs of the dying child and their family may facilitate a peaceful death and help families adjust during bereavement.
Parents often experience reduced mental and physical health following the loss of their child.
EOL care in the pediatric intensive care unit is often associated with challenging ethical issues. Clinicians must maintain a sound and working understanding of these matters.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No financial support was obtained for this review.
References
- 1.National Center for Health Statistics. Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD: 2016. Table 20. Leading causes of death and numbers of deaths, by age: United States, 1980 and 2014; p. 111. [PubMed] [Google Scholar]
- 2.Conlon NP, Breatnach C, O’Hare BP, Mannion DW, Lyons BJ. Health-related quality of life after prolonged pediatric intensive care unit stay. Pediatr Crit Care Med. 2009;10:41–44. doi: 10.1097/PCC.0b013e31819371f6. [DOI] [PubMed] [Google Scholar]
- 3.Visser IH, Hazelzet JA, Albers MJ, et al. Mortality prediction models for pediatric intensive care: comparison of overall and subgroup specific performance. Intensive Care Med. 2013;39:942–950. doi: 10.1007/s00134-013-2857-4. [DOI] [PubMed] [Google Scholar]
- 4.Gemke RJ, Bonsel GJ, van Vught AJ. Long-term survival and state of health after paediatric intensive care. Arch Dis Child. 1995;73:196–201. doi: 10.1136/adc.73.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24:743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Pollack MM, Ruttimann UE, Getson PR. Pediatric risk of mortality (PRISM) score. Crit Care Med. 1988;16:1110–1116. doi: 10.1097/00003246-198811000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Namachivayam P, Shann F, Shekerdemian L, et al. Three decades of pediatric intensive care: Who was admitted, what happened in intensive care, and what happened afterward. Pediatr Crit Care Med. 2010;11:549–555. doi: 10.1097/PCC.0b013e3181ce7427. [DOI] [PubMed] [Google Scholar]
- 8.Aspesberro F, Mangione-Smith R, Zimmerman JJ. Health-related quality of life following pediatric critical illness. Intensive Care Med. 2015;41:1235–1246. doi: 10.1007/s00134-015-3780-7. [DOI] [PubMed] [Google Scholar]
- 9.Typpo KV, Petersen NJ, Hallman DM, Markovitz BP, Mariscalco MM. Day 1 multiple organ dysfunction syndrome is associated with poor functional outcome and mortality in the pediatric intensive care unit. Pediatr Crit Care Med. 2009;10:562–570. doi: 10.1097/PCC.0b013e3181a64be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pollack MM, Holubkov R, Funai T, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network. Simultaneous Prediction of New Morbidity, Mortality, and Survival Without New Morbidity From Pediatric Intensive Care: A New Paradigm for Outcomes Assessment. Crit Care Med. 2015;43:1699–1709. doi: 10.1097/CCM.0000000000001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burns JP, Sellers DE, Meyer EC, Lewis-Newby M, Truog RD. Epidemiology of death in the PICU at five U. S. teaching hospitals. Crit Care Med. 2014;42:2101–2108. doi: 10.1097/CCM.0000000000000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feudtner C, Silveira MJ, Shabbout M, Hoskins RE. Distance from home when death occurs: a population-based study of Washington State, 1989–2002. Pediatrics. 2006;117:e932–939. doi: 10.1542/peds.2005-2078. [DOI] [PubMed] [Google Scholar]
- 13.Chang E, MacLeod R, Drake R. Characteristics influencing location of death for children with life-limiting illness. Arch Dis Child. 2013;98:419–424. doi: 10.1136/archdischild-2012-301893. [DOI] [PubMed] [Google Scholar]
- 14.Angus DC, Barnato AE, Linde-Zwirble WT, et al. Robert Wood Johnson Foundation ICU End-Of-Life Peer Group. Use of intensive care at the end of life in the United States: an epidemiologic study. Crit Care Med. 2004;32:638–643. doi: 10.1097/01.ccm.0000114816.62331.08. [DOI] [PubMed] [Google Scholar]
- 15.Carter BS, Howenstein M, Gilmer MJ, Throop P, France D, Whitlock JA. Circumstances surrounding the deaths of hospitalized children: opportunities for pediatric palliative care. Pediatrics. 2004;114:e361–366. doi: 10.1542/peds.2003-0654-F. [DOI] [PubMed] [Google Scholar]
- 16.Meert KL, Keele L, Morrison W, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network. End-of-Life Practices Among Tertiary Care PICUs in the United States: A Multicenter Study. Pediatr Crit Care Med. 2015;16:e231–238. doi: 10.1097/PCC.0000000000000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan T, Rodean J, Richardson T, et al. Pediatric Critical Care Resource Use by Children with Medical Complexity. J Pediatr. 2016;177:197–203.e1. doi: 10.1016/j.jpeds.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 18.Committee on Palliative and End-of-Life Care for Children and their Families: When Children Die: Improving Palliative and End-of-Life Care for Children and their Families. Washington, DC: National Academy Press; 2003. [Google Scholar]
- 19.American Academy of Pediatrics. Committee on Bioethics and Committee on Hospital Care: Palliative care for children. Pediatrics. 2000;106:351–357. [PubMed] [Google Scholar]
- 20.Fraser LK, Miller M, Draper ES, McKinney PA, Parslow RC. Paediatric Intensive Care Audit Network. Place of death and palliative care following discharge from paediatric intensive care units. Arch Dis Child. 2011;96:1195–1198. doi: 10.1136/adc.2009.178269. [DOI] [PubMed] [Google Scholar]
- 21.Boss R, Nelson J, Weissman D, et al. Integrating palliative care into the PICU: a report from the Improving Palliative Care in the ICU Advisory Board. Pediatr Crit Care Med. 2014;15:762–767. doi: 10.1097/PCC.0000000000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosenthal AC, Weissman DE, Curtis JR, et al. Integrating palliative care in the surgical and trauma intensive care unit: a report from the Improving Palliative Care in the Intensive Care Unit (IPAL-ICU) Project Advisory Board and the Center to Advance Palliative Care. Crit Care Med. 2012;40:1199–1206. doi: 10.1097/CCM.0b013e31823bc8e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones PM, Carter BS. Pediatric palliative care: feedback from the pediatric intensivist community. Am J Hosp Palliat Care. 2010;27:450–455. doi: 10.1177/1049909109360410. [DOI] [PubMed] [Google Scholar]
- 24.WHO Definition of Palliative Care. [Accessed February 27, 2017]; http://www.who.int/cancer/palliative/definition/en/
- 25.Balluffi A, Kassam-Adams N, Kazak A, Tucker M, Dominguez T, Helfaer M. Traumatic stress in parents of children admitted to the pediatric intensive care unit. Pediatr Crit Care Med. 2004;5:547–553. doi: 10.1097/01.PCC.0000137354.19807.44. [DOI] [PubMed] [Google Scholar]
- 26.Meert KL, Slomine BS, Christensen JR, et al. Therapeutic Hypothermia after Pediatric Cardiac Arrest Trial Investigators. Family Burden After Out-of-Hospital Cardiac Arrest in Children. Pediatr Crit Care Med. 2016;17:498–507. doi: 10.1097/PCC.0000000000000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meert KL, Donaldson AE, Newth CJ, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network. Complicated grief and associated risk factors among parents following a child's death in the pediatric intensive care unit. Arch Pediatr Adolesc Med. 2010;164:1045–1051. doi: 10.1001/archpediatrics.2010.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee KJ, Dupree CY. Staff experiences with end-of-life care in the pediatric intensive care unit. J Palliat Med. 2008;11:986–990. doi: 10.1089/jpm.2007.0283. [DOI] [PubMed] [Google Scholar]
- 29.Stayer D, Lockhart JS. Living with Dying in the Pediatric Intensive Care Unit: A Nursing Perspective. Am J Crit Care. 2016;25:350–356. doi: 10.4037/ajcc2016251. [DOI] [PubMed] [Google Scholar]
- 30.Keele L, Meert KL, Berg RA, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network. Limiting and Withdrawing Life Support in the PICU: For Whom Are These Options Discussed? Pediatr Crit Care Med. 2016;17:110–120. doi: 10.1097/PCC.0000000000000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lago PM, Piva J, Garcia PC, et al. Brazilian Pediatric Center of Studies on Ethics. End-of-life practices in seven Brazilian pediatric intensive care units. Pediatr Crit Care Med. 2008;9:26–31. doi: 10.1097/01.PCC.0000298654.92048.BD. [DOI] [PubMed] [Google Scholar]
- 32.Donnelly JP, Huff SM, Lindsey ML, McMahon KA, Schumacher JD. The needs of children with life-limiting conditions: a healthcare-provider-based model. Am Hosp Palliat Care. 2005;22:259–267. doi: 10.1177/104990910502200406. [DOI] [PubMed] [Google Scholar]
- 33.Steinhauser KE, Christakis NA, Clipp EC, McNeilly M, McIntyre L, Tulsky JA. Factors considered important at the end of life by patients, family, physicians, and other care providers. JAMA. 2000;284:2476–2482. doi: 10.1001/jama.284.19.2476. [DOI] [PubMed] [Google Scholar]
- 34.Patrick DL, Engelberg RA, Curtis JR. Evaluating the quality of dying and death. J Pain Symptom Manage. 2001;22:717–726. doi: 10.1016/s0885-3924(01)00333-5. [DOI] [PubMed] [Google Scholar]
- 35.Johnson L-M, Snaman JM, Cupit MC, Baker JN. End-of-Life Care for Hospitalized Children. Pediatr Clin N Am. 2014;61:835–854. doi: 10.1016/j.pcl.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 36.Conway M, White N, St Jean C, Zempsky WT, Steven K. Use of Continuous Intravenous Ketamine for End-Stage Cancer Pain in Children. J Pediatr Oncol Nurs. 2009;26:100–106. doi: 10.1177/1043454208328768. [DOI] [PubMed] [Google Scholar]
- 37.Campbell-Fleming JM, Williams A. The Use of Ketamine as Adjuvant Therapy to Control Severe Pain. Clin J Oncol Nurs. 2008;12:102–107. doi: 10.1188/08.CJON.102-107. [DOI] [PubMed] [Google Scholar]
- 38.Taylor M, Jakaci R, May C, Howrie D, Maurer S. Ketamine PCA for Treatment of End-of-Life Neuropathic Pain in Pediatrics. American Journal of Hospice and Palliative Medicine. 2015;32:841–848. doi: 10.1177/1049909114543640. [DOI] [PubMed] [Google Scholar]
- 39.Burns J, Jackson K, Sheehy K, Finkel JC, Quezado ZM. The Use of Dexmedetomidine in Pediatric Palliative Care: A Preliminary Study. J Palliat Med. 2017:1–5. doi: 10.1089/jpm.2016.0419. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 40.Heath JA, Oh LJ, Clark NE. Complementary and Alternative Medicine Use in Children with Cancer at the End of Life. J Palliat Med. 2012;15:1218–1221. doi: 10.1089/jpm.2012.0150. [DOI] [PubMed] [Google Scholar]
- 41.McSherry M, Kehoe K, Carroll JM, et al. Psychosocial and Spirtual Needs of Children Living with a Life-Limiting Illness. Pediatr Clin N Am. 2007;54:609–629. doi: 10.1016/j.pcl.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Kreicbergs U, Valdimarsdottir U, Onelov E, Henter JI, Steineck G. Talking about death with children who have severe malignant disease. N Engl J Med. 2004;351:1175–1186. doi: 10.1056/NEJMoa040366. [DOI] [PubMed] [Google Scholar]
- 43.Levetown M, Liben S, Audet M. Palliative care in the pediatric intensive care unit. In: Carter BS, Levetown M, editors. Palliative care for infants, children, and adolescents: a practical handbook. Baltimore (MD): John Hopkins University Press; 2004. pp. 273–291. [Google Scholar]
- 44.Allen RS, Hilgeman MM, Ege MA, Shuster JL, Jr, Burgio LD. Legacy activities as interventions approaching the end of life. J Palliat Med. 2008;11:1029–1038. doi: 10.1089/jpm.2007.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meert KL, Templin TN, Michelson KN, et al. The Bereaved Parent Needs Assessment: A new instrument to assess the needs of parents whose children died in the pediatric intensive care unit. Crit Care Med. 2012;40:3050–3057. doi: 10.1097/CCM.0b013e31825fe164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mack JW, Hilden JM, Watterson J, et al. Parent and physician perspective on quality of life at the end of life in children with cancer. J Clin Oncol. 2005;23:9155–9161. doi: 10.1200/JCO.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 47.Meert KL, Eggly S, Pollack M, et al. National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network. Parents’ perspectives on physician-parent communication near the time of a child’s death in the pediatric intensive care unit. Pediatr Crit Care Med. 2008;9:2–7. doi: 10.1097/01.PCC.0000298644.13882.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bright KL, Huff MB, Hollon K. A Broken Heart – The Physician’s Role: Bereaved Parents’ Perceptions of Interactions with Physicians. Clin Pediatr. 2009;48:376–382. doi: 10.1177/0009922808327055. [DOI] [PubMed] [Google Scholar]
- 49.October TW, Hinds PS, Wang J, Dizon ZB, Cheng YI, Roter DL. Parent Satisfaction With Communication Is Associated With Physician’s Patient-Centered Communication Patterns During Family Conferences. Pediatr Crit Care Med. 2016;17:490–497. doi: 10.1097/PCC.0000000000000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Homer CJ, Marino B, Cleary PD, et al. Quality of care at a children’s hospital: the parents’ perspective. Arch Pediatr Adolesc Med. 1999;153:1123–1129. doi: 10.1001/archpedi.153.11.1123. [DOI] [PubMed] [Google Scholar]
- 51.Wolfe J, Grier HE, Klar N, et al. Symptoms and suffering at the end of life in children with cancer. N Engl J Med. 2000;342:326–333. doi: 10.1056/NEJM200002033420506. [DOI] [PubMed] [Google Scholar]
- 52.Teno JM, Clarridge BR, Casey V, et al. Family perspectives on end-of-life care at the last place of care. JAMA. 2004;291:88–93. doi: 10.1001/jama.291.1.88. [DOI] [PubMed] [Google Scholar]
- 53.Meyer EC, Burns JP, Griffith JL, Truog RD. Parental perspectives on end-of-life care in the pediatric intensive care unit. Crit Care Med. 2002;30:226–231. doi: 10.1097/00003246-200201000-00032. [DOI] [PubMed] [Google Scholar]
- 54.Li J, Johansen C, Hansen D, Olsen J. Cancer incidence in parents who lost a child. a nationwide study in Denmark. Cancer. 2002;95:2237–2242. doi: 10.1002/cncr.10943. [DOI] [PubMed] [Google Scholar]
- 55.Levav I, Kohn R, Iscovich J, Abramson JH, Tsai WY, Vigdorovich D. Cancer incidence and survival following bereavement. Am J Public Health. 2000;90:1601–1607. doi: 10.2105/ajph.90.10.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olsen J, Li J, Precht DH. Hospitalization because of diabetes and bereavement: a national cohort study of parents who lost a child. Diabet Med. 2005;22:1338–1342. doi: 10.1111/j.1464-5491.2005.01642.x. [DOI] [PubMed] [Google Scholar]
- 57.Lannen PK, Wolfe J, Prigerson HG, Kreicbergs UC. Unresolved grief in a national sample of bereaved parents: impaired mental and physical health 4 to 9 years later. J Clinical Oncol. 2008;26:5870–5876. doi: 10.1200/JCO.2007.14.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rostila M, Saarela J, Kawachi I. Mortality in parents following the death of a child: a nationwide follow-up study from Sweden. J Epidemiol Community Health. 2012;66:927–933. doi: 10.1136/jech-2011-200339. [DOI] [PubMed] [Google Scholar]
- 59.Kreicbergs U, Valdimarsdottir U, Onelov E, Henter J, Steineck G. Anxiety and depression in parents 4–9 years after the loss of a child owing to a malignancy: a population-based follow-up. Psychol Med. 2004;34:1431–1441. doi: 10.1017/s0033291704002740. [DOI] [PubMed] [Google Scholar]
- 60.McCarthy MC, Clark NE, Ting CL, Conroy R, Anderson VA, Heath JA. Prevalence and predictors of parental grief and depression after the death of a child from cancer. J Palliat Med. 2010;13:1321–1326. doi: 10.1089/jpm.2010.0037. [DOI] [PubMed] [Google Scholar]
- 61.Rogers CH, Floyd FJ, Seltzer MM, Greenberg J, Hong J. Long-term effects of the death of a child on parents’ adjustment in midlife. J Fam Psych. 2008;22:203–211. doi: 10.1037/0893-3200.22.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murphy SA, Braun T, Tillery L, Cain K, Johnson LC, Beaton R. PTSD among bereaved parents following the violent deaths of their 12- to 28-year-old children: a longitudinal prospective analysis. J Trauma Stress. 1999;12:273–291. doi: 10.1023/A:1024724425597. [DOI] [PubMed] [Google Scholar]
- 63.Murphy SA, Johnson LC, Chung IJ, Beaton RD. The prevalence of PTSD following the violent death of a child and predictors of change 5 years later. J Trauma Stress. 2003;16:17–25. doi: 10.1023/A:1022003126168. [DOI] [PubMed] [Google Scholar]
- 64.Dyregrov A, Dyregrov K. Long-term impact of sudden infant death: A 12- to 15-year follow-up. Death Stud. 1999;23:635–661. doi: 10.1080/074811899200812. [DOI] [PubMed] [Google Scholar]
- 65.Vance JC, Najman JM, Boyle FM, Embelton G, Foster WJ, Thearle MJ. Alcohol and drug usage in parents soon after stillbirth, neonatal death or SIDS. J Paediatr Child Health. 1994;30:269–272. doi: 10.1111/j.1440-1754.1994.tb00632.x. [DOI] [PubMed] [Google Scholar]
- 66.Qin P, Mortensen PB. The impact of parental status on the risk of completed suicide. Arch Gen Psychiatry. 2003;60:797–802. doi: 10.1001/archpsyc.60.8.797. [DOI] [PubMed] [Google Scholar]
- 67.Li J, Laursen TM, Precht DH, Olsen J, Mortensen PB. Hospitalization for mental illness among parents after the death of a child. N Engl J Med. 2005;352:1190–1196. doi: 10.1056/NEJMoa033160. [DOI] [PubMed] [Google Scholar]
- 68.Walsh K, King M, Jones L, Tookman A, Blizard R. Spiritual beliefs may affect outcome of bereavement: prospective study. BMJ. 2002;324:1551–1555. doi: 10.1136/bmj.324.7353.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hawthorne DM, Youngblut JM, Brooten D. Parent Spirituality, Grief, and Mental Health at 1 and 3 Months After Their Infant’s/Child’s Death in the Intensive Care Unit. J Pediatr Nurs. 2016;31:73–80. doi: 10.1016/j.pedn.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meert KL, Thurston CS, Briller SH. The spiritual needs of parents at the time of their child’s death in the pediatric intensive care until and during bereavement: a qualitative study. Pediatr Crit Care Med. 2005;6:420–427. doi: 10.1097/01.PCC.0000163679.87749.CA. [DOI] [PubMed] [Google Scholar]
- 71.Meert KL, Briller SH, Meyers S, Thurston CS. Exploring parents’ environmental needs at the time of a child’s death in the pediatric intensive care unit. Pediatr Crit Care Med. 2008;9:623–628. doi: 10.1097/PCC.0b013e31818d30d5. [DOI] [PubMed] [Google Scholar]
- 72.Sanders CM. A Comparison of Adult Bereavement in the Death of a Spouse, Child, and Parent. Omega. 1979;10:303–322. [Google Scholar]
- 73.Middleton W, Raphael B, Burnett P, Martinek N. A longitudinal study comparing bereaved spouses, adult children, and parents. Aust N Z J Psychiatry. 1998;32:235–241. doi: 10.3109/00048679809062734. [DOI] [PubMed] [Google Scholar]
- 74.McCarthy MC, Clark NE, Ting CL, Conroy R, Anderson VA, Heath JA. Prevalence and predictors of parental grief and depression after the death of a child from cancer. J Palliat Med. 2010;13:1321–1326. doi: 10.1089/jpm.2010.0037. [DOI] [PubMed] [Google Scholar]
- 75.Rogers CH, Floyd FJ, Seltzer MM, Greenberg J, Hong J. Long-term effects of the death of a child on parents’ adjustment in midlife. J Fam Psych. 2008;22:203–211. doi: 10.1037/0893-3200.22.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Murphy SA, Braun T, Tillery L, Cain KC, Johnson LC, Beaton RD. PTSD among bereaved parents following the violent deaths of their 12- to 28-year-old children: a longitudinal prospective analysis. J Trauma Stress. 1999;12:273–291. doi: 10.1023/A:1024724425597. [DOI] [PubMed] [Google Scholar]
- 77.Li J, Johansen C, Brønnum-Hansen H, Stenager E, Koch-Henriksen N, Olsen J. The risk of multiple sclerosis in bereaved parents: a nationwide cohort study in Denmark. Neurology. 2004;62:726–729. doi: 10.1212/01.wnl.0000113766.21896.b1. [DOI] [PubMed] [Google Scholar]
- 78.Li J, Hansen D, Mortensen PB, Olsen J. Myocardial infarction in parents who lost a child: a nationwide prospective cohort study in Denmark. Circulation. 2002;106:1634–1639. doi: 10.1161/01.cir.0000031569.45667.58. [DOI] [PubMed] [Google Scholar]
- 79.Kersting A, Brähler E, Glaesmer H, Wagner B. Prevalence of complicated grief in a representative population-based sample. J Affect Disord. 2011;131:339–343. doi: 10.1016/j.jad.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 80.He L, Tang S, Yu W, Xu W, Xie Q, Wang J. The prevalence, comorbidity and risks of prolonged grief disorder among bereaved Chinese adults. Psychiatry Res. 2014;219:347–352. doi: 10.1016/j.psychres.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 81.Kacel E, Gao X, Prigerson HG. Understanding bereavement: what every oncology practitioner should know. J Support Oncol. 2011;9:172–180. doi: 10.1016/j.suponc.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jordan J, Neimeyer R. Does grief counseling work? Death Stud. 2003;27:765–786. doi: 10.1080/713842360. [DOI] [PubMed] [Google Scholar]
- 83.Schut H, Stroebe MS, van den Bout J, Terheggen M. The efficacy of bereavement interventions: Determining who benefits. In: Stroebe MS, Hansson RO, Stroebe W, editors. Handbook of Bereavement Research. 1. Washington DC: American Psychological Association; 1993. pp. 705–738. [Google Scholar]
- 84.Meert KL, Eggly S, Pollack M, et al. National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network. Parents’ Perspectives Regarding a Physician-Parent Conference after Their Child’s Death in the Pediatric Intensive Care Unit. J Pediatr. 2007;151:50–55. doi: 10.1016/j.jpeds.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zawistowski CA, DeVita MA. A descriptive study of children dying in the pediatric intensive care unit after withdrawal of life-sustaining treatment. Pediatr Crit Care Med. 2004;5:216–223. doi: 10.1097/01.pcc.0000123547.28099.44. [DOI] [PubMed] [Google Scholar]
- 86.Sanderson A, Zurakowski D, Wolfe J. Clinician perspectives regarding the do-not-resuscitate order. JAMA Pediatr. 2013;167:954–958. doi: 10.1001/jamapediatrics.2013.2204. [DOI] [PubMed] [Google Scholar]
- 87.Deciding to Forgo Life-Sustaining Treatment. Washington, DC: US Government Printing Office; 1983. President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research. [Google Scholar]
- 88.Garros D, Austin W, Carnevale FA. Moral distress in pediatric intensive care. JAMA Pediatr. 2015;169:885–886. doi: 10.1001/jamapediatrics.2015.1663. [DOI] [PubMed] [Google Scholar]
- 89.Truog RD, Cist AF, Brackett SE, et al. Recommendations for end-of-life care in the intensive care unit: The Ethics Committee of the Society of Critical Care Medicine. Crit Care Med. 2001;29:2332–2348. doi: 10.1097/00003246-200112000-00017. [DOI] [PubMed] [Google Scholar]
- 90.Michelson KN, Patel R, Haber-Barker N, Emanuel L, Frader J. End-of-life care decisions in the pediatric intensive care unit: roles professionals play. Pediatr Crit Care Med. 2013;14:e34–44. doi: 10.1097/PCC.0b013e31826e7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Prendergast TJ, Puntillo KA. Withdrawal of life support: intensive caring at the end of life. JAMA. 2002;288:2732–2740. doi: 10.1001/jama.288.21.2732. [DOI] [PubMed] [Google Scholar]
- 92.Solomon MZ, Sellers DE, Heller KS, et al. New and lingering controversies in pediatric end-of-life care. Pediatrics. 2005;116:872–883. doi: 10.1542/peds.2004-0905. [DOI] [PubMed] [Google Scholar]
- 93.Sarnaik AA, Clark JA, Meert KL, Sarnaik AP. Views of pediatric intensive care physicians on the ethics of organ donation after cardiac death. Crit Care Med. 2013;41:1733–1744. doi: 10.1097/CCM.0b013e31828a219e. [DOI] [PubMed] [Google Scholar]
- 94.De Vos MA, Bos AP, Plötz FB, et al. Talking with parents about end-of-life decisions for their children. Pediatr. 2015;135:e465–e476. doi: 10.1542/peds.2014-1903. [DOI] [PubMed] [Google Scholar]
- 95.Morparia K, Dickerman M, Hoehn KS. Futility: unilateral decision making is not the default for pediatric intensivists. Pediatr Crit Care Med. 2012;13:e311–e315. doi: 10.1097/PCC.0b013e31824ea12c. [DOI] [PubMed] [Google Scholar]
- 96.Bosslet GT, Pope TM, Rubenfeld GD, et al. on behalf of The American Thoracic Society ad hoc Committee on Futile and Potentially Inappropriate Treatment. An Official ATS/AACN/ACCP/ESICM/SCCM Policy Statement: Responding to Requests for Potentially Inappropriate Treatments in Intensive Care Units. Amer J Resp Crit Care Med. 2015;191:1318–1330. doi: 10.1164/rccm.201505-0924ST. [DOI] [PubMed] [Google Scholar]
- 97.Webster PA, Markham L. Pediatric organ donation: A national survey examining consent rates and characteristics of donor hospitals. Pediatr Crit Care Med. 2009;10:500–504. doi: 10.1097/PCC.0b013e318198b06b. [DOI] [PubMed] [Google Scholar]
- 98.Robertson JA. The dead donor rule. Hastings Cent Rep. 1999;29:6–14. [PubMed] [Google Scholar]
- 99.American Academy of Pediatrics, Task Force on Brain Death in Children. Report of Special Task Force: guidelines for determination of brain death in children. Pediatrics. 1987;80:298–300. [PubMed] [Google Scholar]
- 100.Nakagawa TA, Ashwal S, Mathur M, Mysore M, the Society of Critical Care Medicine, Section on Critical Care and Section on Neurology of the American Academy of Pediatrics, and the Child Neurology Society Guidelines for the determination of brain death in infants and children: an update of the 1987 task force recommendations. Pediatrics. 2011;128:e720–e740. doi: 10.1542/peds.2011-1511. [DOI] [PubMed] [Google Scholar]
- 101.American Academy of Pediatrics Committee on Bioethics. Ethical controversies in organ donation after circulatory death. Pediatrics. 2013;131:1021–1026. doi: 10.1542/peds.2013-0672. [DOI] [PubMed] [Google Scholar]
- 102.Potts JT. Non-heart-beating organ transplantation: medical and ethical issues in procurement. Washington, DC: National Academy Press; 1997. [PubMed] [Google Scholar]
- 103.American Academy of Pediatrics Committee on Hospital Care, Section on Surgery, and Section on Critical Care. Policy Statement - pediatric organ donation and transplantation. Pediatrics. 2010;125:822–828. doi: 10.1542/peds.2010-0081. [DOI] [PubMed] [Google Scholar]
- 104.Recommendations for nonheartbeating organ donation. A position paper by the Ethics Committee, American College of Critical Care Medicine, Society of Critical Care Medicine. Crit Care Med. 2001;29:1826–1831. doi: 10.1097/00003246-200109000-00029. [DOI] [PubMed] [Google Scholar]