Abstract
Purpose
The CLEOPATRA (Clinical Evaluation of Trastuzumab and Pertuzumab) study demonstrated superior progression-free survival (PFS) and overall survival when pertuzumab was added to trastuzumab and docetaxel. Paclitaxel given once per week is effective and less toxic than docetaxel. We performed a phase II study to evaluate the efficacy and safety of pertuzumab and trastuzumab with paclitaxel given once per week.
Patients and Methods
Patients with metastatic human epidermal growth factor receptor 2–positive breast cancer with zero to one prior therapy were enrolled. Treatment consisted of paclitaxel 80 mg/m2 once per week plus trastuzumab (8 mg/kg loading dose → 6 mg/kg) once every 3 weeks plus pertuzumab (840 mg loading dose → 420 mg) once every 3 weeks, all given intravenously. The primary end point was 6-month PFS assessed by Kaplan-Meier methods.
Results
From January 2011 to December 2013, we enrolled 69 patients: 51 (74%) and 18 (26%) treated in first- and second-line metastatic settings, respectively. At a median follow-up of 21 months (range, 3 to 38 months), 6-month PFS was 86% (95% CI, 75% to 92%). The median PFS was 19.5 months (95% CI, 14 to 26 months) overall. PFS was 24.2 months (95% CI, 14 months to not reached [NR]) and 16.4 months (95% CI, 8.5 months to NR) for those without and with prior treatment, respectively. At 1 year, Kaplan-Meier PFS was 70% (95% CI, 56% to 79%) overall, 71% (95% CI, 55% to 82%) for those without prior therapy, and 66% (95% CI, 40% to 83%) for those with prior therapy. Treatment was well-tolerated; there was no febrile neutropenia or symptomatic left ventricular systolic dysfunction.
Conclusion
Paclitaxel given once per week with trastuzumab and pertuzumab is highly active and well tolerated and seems to be an effective alternative to docetaxel-based combination therapy.
INTRODUCTION
The human epidermal growth factor receptor (HER) family consists of four members—EGFR, HER2, HER3, and HER4—which are transmembrane receptor tyrosine kinases that regulate cell growth and survival, differentiation, and migration, as well as other cellular responses.1 HER2 protein was shown to be highly expressed in approximately 20% of breast tumors as a result of amplification of the HER2 gene. Its overexpression portends an aggressive natural history compared with other breast tumors.2 Homo- or heterodimerization is required for HER signaling, and the HER2-HER3 dimer is the most potent in inducing cell proliferation.3 With the advent of trastuzumab, a humanized monoclonal antibody that binds to domain IV of HER2, the natural history of this disease has been significantly altered in both the metastatic and early-stage settings, thus leading to the approval of this agent in both clinical settings.4–14
Pertuzumab is a humanized monoclonal antibody that targets HER2. However, unlike trastuzumab, it binds to domain II of the receptor and is thus able to disrupt HER2 dimerization and ligand-activated signaling with other growth factor receptors, including other HER family members.15 Recently, the CLEOPATRA (Clinical Evaluation of Trastuzumab and Pertuzumab) randomized phase III trial for clinical evaluation of pertuzumab and trastuzumab demonstrated that the addition of pertuzumab to standard docetaxel and trastuzumab increases both progression-free survival (PFS) and overall survival (OS).16,17 Pertuzumab is approved for use with docetaxel and trastuzumab in first-line and neoadjuvant settings by regulatory agencies in the United States and other countries.18 Paclitaxel given once per week is highly effective in the treatment of breast cancer in both advanced and early-stage settings.19–22 In direct comparison with docetaxel (administered once every 3 weeks) in the adjuvant setting, paclitaxel given once per week was more effective and less toxic.22 By using the efficacy and safety data from the adjuvant setting, we aimed to determine whether paclitaxel given once per week is effective, safe, and tolerable when added to dual anti-HER2 antibody therapy in the metastatic setting, so we conducted a phase II trial of paclitaxel with pertuzumab and trastuzumab.
PATIENTS AND METHODS
Patients
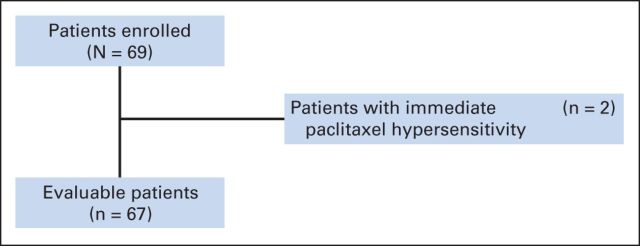
All patients were enrolled from Memorial Sloan Kettering Cancer Center. Eligible patients had HER2-positive breast cancer with metastatic disease. HER2 positivity was defined as 3+ by immunohistochemistry or amplified by fluorescent in situ hybridization with a ratio of ≥ 2.0. Other eligibility criteria included age of 18 years or older, an Eastern Cooperative Oncology Group performance status of 0 to 1, measurable or nonmeasurable disease, zero to one prior treatment, adequate organ function, and baseline left ventricular ejection fraction (LVEF) of ≥ 50% by echocardiogram within 4 weeks before enrollment. Patients may have had adjuvant trastuzumab and stable treated brain metastasis ≥ 2 months before enrollment. Exclusion criteria included a history of prior cardiac morbidities within 12 months (unstable angina, myocardial infarction, congestive heart failure, uncontrolled ventricular arrhythmias), prior pertuzumab, and grade ≥ 2 neuropathy (Fig 1).
Fig 1.
CONSORT diagram.
Treatment
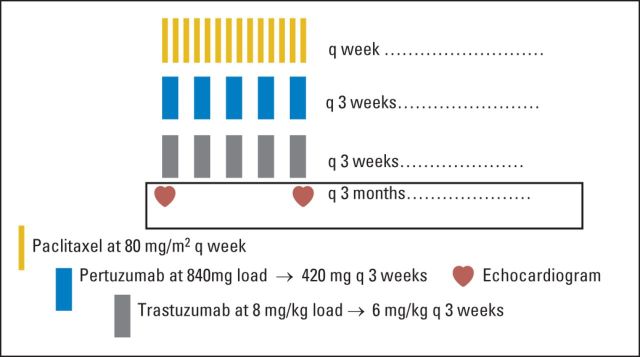
Patients received paclitaxel 80 mg/m2 once per week plus trastuzumab (8 mg/kg loading dose → 6 mg/kg) once every 3 weeks plus pertuzumab (840 mg loading dose → 420 mg) once every 3 weeks, all given intravenously (Fig 2). One cycle consisted of three doses of paclitaxel given once per week with dual anti-HER2 antibody therapy. There was no mandate on the order of administration of chemotherapy and the antibodies. After 6 months of therapy, if patients were deemed to be progression-free, paclitaxel could be held at the discretion of the treating physician, and patients were maintained on antibodies alone. Patients remained on treatment until progression of disease.
Fig 2.
Treatment schema.
Criteria for Treatment Modifications
For paclitaxel, in general, if the patient experienced grade 3 or greater toxicity, treatment was held until the adverse event (AE) improved to grade ≤ 2 (except for neuropathy). In terms of neuropathy, if the patient experienced grade ≥ 2 toxicity, treatment was held until the AE improved to grade ≤ 1. The patient had 3 weeks to improve. If she improved, then paclitaxel dose was reduced. Only two dose reductions were permitted (ie, 80 to 60 mg/m2 and then 60 to 45 mg/m2). For the two antibodies, treatment was held if the patient had a significant asymptomatic LVEF decline of 10% to 15% to less than 50% or ≥ 16% (from baseline) or experienced clinical heart failure, as defined by the study. The patient would then have to have a recovery with a repeat echocardiogram or multigated angiogram within 3 weeks to have anti-HER2 therapies restarted. Dose reductions were not permitted.
Assessments
Serial tumor assessments, based on RECIST version 1.1, were performed every 3 months with a computed tomography scan of the chest and abdomen with or without a scan of the pelvis. Monitoring for LVEF occurred at baseline and every 3 months with an echocardiogram and a strain imaging analysis within 3 months after completion of treatment. Patients were seen once per cycle, a CBC was performed before each chemotherapy dose, and chemistry laboratory assessments were performed every 3 weeks. Blood samples for exploratory cardiac biomarkers (troponin, brain natriuretic peptide, and neuregulin-β) were collected every other cycle at six time points. AEs were monitored continuously and were graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events, version 4.0.
Statistical Analysis
The primary end point of this study was the proportion of patients who were progression free at 6 months or later. Events for the PFS end point were defined as progression or death, whichever came first. We powered our study on the basis of a binary end point of the proportion of patients progression-free at 6 months. For the primary analysis, evaluable patients included all patients who received at least one full dose of therapy. A single-stage design was used to evaluate the efficacy of this regimen. We elected a target progression-free rate below 50% as the unpromising 6-month progression-free rate and 65% or higher as promising. These target rates were based on data from Slamon et al,4 which showed that the combination of paclitaxel and trastuzumab led to a median time to progression of 7.4 months in patients with untreated HER2-positive metastatic breast cancer. We chose 6 months as opposed to 7 months because patients with one prior treatment were included. On the basis of these proportions, we needed 69 evaluable patients at a one-sided type I error of 5% and 80% power for a single-stage design. At the end of the study, if 42 or more patients were alive and progression free 6 months after therapy, the regimen would be considered a success and deemed worthy of further study. PFS estimates were calculated by using Kaplan-Meier methods. Secondary end points for this study included estimating 6-month and median OS by using Kaplan-Meier methods, describing safety (including cardiac safety) and tolerability, and assessing biomarkers (not presented in this article). AEs with frequencies of 25% or greater were described by using percentages. For cardiac safety based on prior data, we considered a cardiac event rate of ≤ 4% and an asymptomatic LVEF decline (of ≥ 10% from baseline to < 50%) rate of ≤ 20% as acceptable. For this study, to be consistent with data from the CLEOPATRA trial, a cardiac event was defined as symptomatic left ventricular systolic dysfunction (LVSD; deaths and nondeaths), non-LVSD cardiac death, or probable cardiac death. All analyses were conducted by using SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
Study Population
From January 2011 to December of 2013, we enrolled 69 patients whose baseline characteristics are provided in Table 1. The median age was 53 years (range, 26 to 84 years). In all, 49 patients (71%) had visceral disease and 20 patients (29%) had nonvisceral disease. Fifty-seven patients (83%) had measurable disease and 12 patients (17%) had nonmeasurable disease. Overall, 44 patients (64%) had estrogen receptor–positive and/or progesterone receptor–positive disease. In this group, 51 patients (74%) were being treated in the first-line setting, and 18 patients (26%) were being treated in the second-line setting. In terms of prior trastuzumab exposure, 22 patients (33%) received trastuzumab for neoadjuvant or adjuvant therapy, 14 (20%) received trastuzumab as part of one line of therapy for metastatic breast cancer, and six (9%) received trastuzumab in both settings.
Table 1.
Baseline Characteristics for All Patients (N = 69)
| Characteristic | No. | % |
|---|---|---|
| Age, years | ||
| Median | 53 | |
| Range | 26-84 | |
| Race | ||
| Asian | 10 | 14 |
| Black | 15 | 22 |
| White | 44 | 64 |
| ECOG PS | ||
| 0 | 41 | 59 |
| 1 | 27 | 39 |
| ≥ 2 | 1 | 1 |
| Disease type at screening | ||
| Nonvisceral | 20 | 29 |
| Visceral | 49 | 71 |
| Measurable disease | 57 | 83 |
| Nonmeasurable disease | 12 | 17 |
| Hormone receptor status | ||
| ER positive and/or PgR positive | 44 | 64 |
| ER negative and PgR negative | 25 | 36 |
| HER2 status assessed by IHC | ||
| 0 or 1+ | 3 | 4 |
| 2+ | 8 | 12 |
| 3+ | 56 | 81 |
| Unknown | 2 | 3 |
| HER2 status assessed by FISH | ||
| Positive | 29 | 42 |
| Negative | 1 | 1 |
| Unknown | 39 | 57 |
| Prior adjuvant or neoadjuvant therapy | ||
| No | 39 | 57 |
| Yes* | 30 | 43 |
| Anthracycline | 19 | 28 |
| Taxane | 25 | 32 |
| Hormone | 22 | 32 |
| Trastuzumab | 22 | 32 |
| Other | 1 | 1 |
| Prior treatment of metastatic disease | ||
| None | 51 | 74 |
| One prior therapy | 18 | 26 |
| Chemotherapy + trastuzumab | 12 | 17 |
| Endocrine therapy + trastuzumab | 1 | 1 |
| Chemotherapy + trastuzumab + lapatinib | 1 | 1 |
| Endocrine therapy alone | 4 | 6 |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; ER, estrogen receptor; FISH, fluorescent in situ hybridization; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; PgR, progesterone receptor.
Patients may have received either adjuvant or neoadjuvant therapy.
Efficacy
Of the 69 enrolled patients, two were not evaluable because they experienced immediate hypersensitivity reactions to paclitaxel and came off study on the same day they enrolled (before receiving antibody therapy). Thus, 67 patients had follow-up data available for efficacy analysis (Fig 1) and all 67 were included for analysis. At a median follow-up period of 21 months (range, 3 to 38 months), the 6-month PFS was 86% (95% CI, 75% to 92%) for these 67 patients; it was 89% (95% CI, 76% to 95%) for those without previous treatment and 78% (95% CI, 51% to 91%) for those with previous treatment (Fig 3A). Similarly, when the PFS curves were analyzed on the basis of previous treatment exposure for metastatic breast cancer, the PFS curve was lower for the 18 (26%) with one prior therapy compared with the 51 patients (74%) who had no prior therapy (Fig 3B). The median PFS was 19.5 months (95% CI, 14 to 26 months; Fig 3A); it was 24.2 months (95% CI, 14 months to not reached [NR]) for those without prior treatment and 16.4 months (95% CI, 8.5 months to NR) for those with prior treatment (Fig 3B). The 1-year Kaplan-Meier estimate for the percentage of patients alive and progression-free was 70% (95% CI, 56% to 79%); the 1-year estimate was 71% (95% CI, 55% to 82%) for those without prior therapy and 66% (95% CI, 40% to 83%) for those with prior therapy. In this study, 64 of 67 patients were assessable for the 6-month PFS status for the primary analysis. The remaining three patients were not assessed because one came off study and two were lost to follow-up before the 6-month time point, so they did not have disease assessment. Of these 64 patients, 54 had clinical benefit as follows: seven (11%) had a complete response (CR), 31 (48%) had a partial response (PR), and 16 (25%) had stable disease (SD; Table 2).
Fig 3.
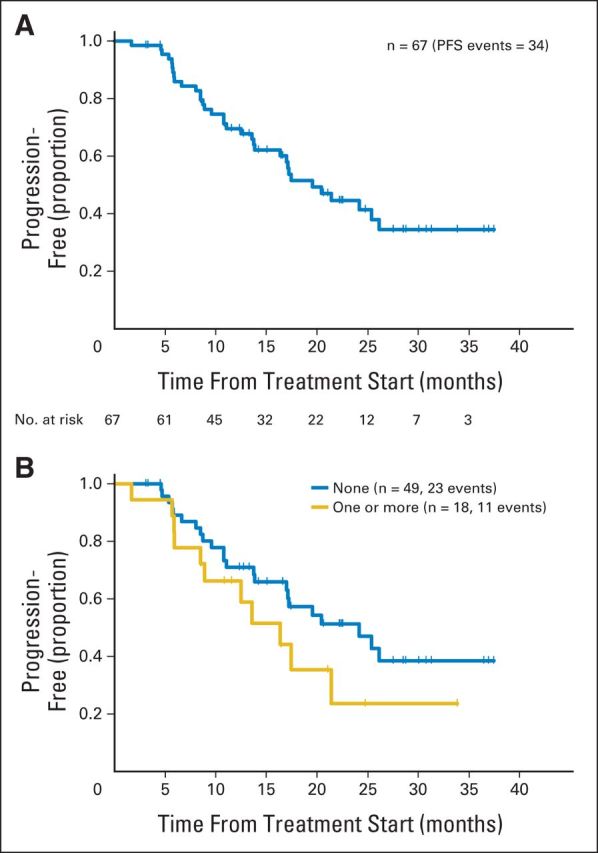
(A) Progression-free survival (PFS) and (B) PFS based on line of therapy.
Table 2.
Efficacy at 6 Months (N = 69)
| Status | No. | % |
|---|---|---|
| Progression free | 54 | 84 |
| Complete response | 7 | 11 |
| Partial response | 31 | 48 |
| Stable disease | 16 | 25 |
| Progression of disease | 10 | 16 |
NOTE. In all, 64 patients were eligible for 6-month progression-free survival assessment. Five patients did not have scans at the 6-month time point: two came onto and off the study on the same day because of paclitaxel hypersensitivity; one came off the study and two were lost to follow-up before the 6-month time point and did not have imaging studies.
Treatment Exposure
The median duration of study treatment was 13.8 months (range, 0 to 38.7 months) for all enrolled patients. The per-study median duration of therapy in all patients who received at least one cycle of treatment was 14.5 months (range, 1.2 to 38.7 months). The median number of study treatment cycles per patient was 20 (range, two to 55). The median number of cycles of paclitaxel exposure was 10 (range, two to 29). The median paclitaxel dose intensity was 80 mg/m2 per week, and it was identical from months 1 to 3 and from months 4 to 6. The median number of cycles of dual antibody exposure was 20 (range, two to 55).
Toxicity Profile and Cardiac Safety
Overall, the study regimen was well tolerated with no unexpected toxicities (Table 3). Grade 3 or higher adverse events included fatigue (four patients [6%]), diarrhea (two [3%]), sensory neuropathy (two [3%]), palmar-plantar erythrodysesthesia (two [3%]), AST elevation (two [3%]), ALT elevation (two [3%]), nausea (one [1.5%]), and dry skin (one [1.5%]). Overall, two patients were hospitalized, one for sepsis (non-neutropenic) as a result of cellulitis and one for grade 4 hypomagnesemia. Of note, the incidence of febrile neutropenia was 0%.
Table 3.
Adverse Events (safety population)
| Adverse Event | Grade 1 to 2 |
Grade 3 to 4 |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Diarrhea | 54 | 81 | 2 | 3 |
| Fatigue | 53 | 79 | 4 | 6 |
| Peripheral neuropathy | 53 | 79 | 2 | 3 |
| Alopecia | 48 | 72 | 0 | |
| AST elevation | 42 | 63 | 2 | 3 |
| ALT elevation | 37 | 55 | 2 | 3 |
| Dry skin | 35 | 52 | 1 | 1.5 |
| Mucositis | 37 | 55 | 0 | |
| Acneiform rash | 37 | 55 | 0 | |
| Nausea | 33 | 49 | 1 | 1.5 |
| Arthralgia | 36 | 54 | 0 | |
| Hot flashes | 33 | 49 | 0 | |
| Dyspepsia | 26 | 43 | 0 | |
| Xerophthalmia | 28 | 42 | 0 | |
| Palmar-plantar erythrodysesthesia syndrome | 25 | 37 | 2 | 3 |
| Anorexia | 23 | 34 | 0 | |
| Cough | 35 | 52 | 0 | |
| Peripheral edema | 26 | 39 | 0 | |
| Epistaxis | 25 | 37 | 0 | |
| Epiphora | 24 | 36 | 0 | |
| Dyspnea | 22 | 33 | 0 | |
NOTE. Safety population includes all patients who received at least one dose of study drug. Adverse events of all grades shown have a frequency of 25% or higher.
In this study, the median LVEF was preserved throughout. No patients experienced a cardiac event as defined by the protocol (symptomatic LVSD). The median LVEF was 64% (range, 50% to 72%) at baseline, 64% (range, 50% to 73%) at 3 months, 63% (range, 49% to 69%) at 6 months, 63% (range, 47% to 73%) at 9 months, 64% (range, 44% to 70%) at 12 months, 62% (range, 55% to 69%) at 15 months, 61% (range, 55% to 69%) at 18 months, 64% (range, 54% to 74%) at 21 months, and 64% (range, 59% to 67%) at 24 months. One patient had an asymptomatic LVEF decline, a 61-year-old woman with a history of cardiomyopathy of unknown etiology in 2003 with LVEF of 20% at the time. Her prior heart failure resolved in 2005 with medical treatment that included carvedilol, furosemide, and lisinopril. She met the criteria for study enrollment with an LVEF of 57% at baseline. It remained 59% at 3 months and increased to 64% at 6 months but then fell to 47% at 9 months at which point dual antibody therapy was held. A repeat echocardiogram was performed 2 weeks later that showed LVEF of 37%, and she was withdrawn from the study. She was seen by a cardiologist but no adjustments in her medications were made because she was asymptomatic. Subsequent LVEF values were 44% at 12 months, 54% at 18 months, and 45% at 24 months. She remained asymptomatic throughout. She remains stable on standard palliative chemotherapy without any HER2-targeted agents.
DISCUSSION
In this study of paclitaxel given once per week and dual anti-HER2 antibody therapy, the Kaplan-Meier 6-month PFS was 86% (95% CI, 75% to 92%). This study met its a priori definition of success: a promising 6-month PFS rate of at least 65%. In this study, 74% of patients received treatment in the first-line setting and 26% received treatment in the second-line setting. Unlike the CLEOPATRA study in which only 10% of patients had received trastuzumab for the treatment of early-stage breast cancer, 32% of our patients received trastuzumab as part of adjuvant or neoadjuvant therapy, 20% received trastuzumab as part of one line of palliative therapy, and 9% received trastuzumab in both settings before enrolling onto our study. The fact that about a quarter of our patients were treated in the second-line setting and that a much higher percentage of patients had prior trastuzumab exposure could have accounted for the seemingly lower clinical benefit rate seen in our study of 84% (11% CR, 48% PR, and 25% SD) as opposed to 94.7% in CLEOPATRA (5.5% CR, 74.6% PR, and 14.6% SD). Although we cannot compare our data directly with the data from CLEOPATRA, despite this more extensive prior trastuzumab therapy, the results are promising. As would be expected, the median PFS was lower for those who were treated in the second-line setting. The median PFS was 19.5 months (95% CI, 14 to 26 months); it was 24.2 months (95% CI, 14 months to NR) for those without prior treatment and 16.4 months (95% CI, 8.5 months to NR) for those with prior treatment. This appeared similar to the CLEOPATRA data showing a median PFS of 18.5 months for those treated in the first-line setting.16,17 At 1 year, 70% (95% CI, 56% to 79%) of patients were alive and progression free. Of note, at 1 year, 71% (95% CI, 55% to 82%) of the first-line patients were alive and progression free, again promising when compared with the CLEOPATRA results. We will continue to observe our patients to obtain a more refined estimate of the median PFS and OS.
Paclitaxel given once per week with trastuzumab and pertuzumab was well tolerated. Perhaps most notably, the incidence of febrile neutropenia was 0% as opposed to 13.8% with docetaxel and dual anti-HER2 antibody therapy in the CLEOPATRA study.16 Grade 3 to 4 diarrhea incidence was low at 3%. Despite a minimum of 6 months of exposure to paclitaxel given once per week, the incidence of grade 3 to 4 peripheral neuropathy was only 3%. The results of our trial are consistent with previous data showing that paclitaxel given once per week is well tolerated and offers a favorable toxicity profile when compared with docetaxel.22 No patients experienced symptomatic LVSD (heart failure). Only one patient (with a prior history of cardiomyopathy) had a persistent but asymptomatic LVEF decline that led to study withdrawal. Overall, the median LVEF was well preserved throughout treatment. As in the CLEOPATRA study, the addition of pertuzumab was not associated with an increased signal of cardiac toxicity. In clinical practice, it is quite reasonable that infrequent LVEF monitoring in the absence of clinical symptoms should be considered. The substudy of cardiac biomarkers and echocardiogram strain imaging assessments will be reported separately. Our data on the efficacy of paclitaxel once per week with trastuzumab and pertuzumab in the second-line setting are informative for patients who were not treated with dual antibody therapy initially. In addition, Perez et al23 reported the safety results of vinorelbine with trastuzumab and pertuzumab, and we await the efficacy data. We also look forward to seeing the efficacy and safety data of other chemotherapies combined with these dual antibodies in ongoing trials.
With regard to adjuvant use, the median dose intensity of paclitaxel given once per week was 80 mg/m2 during months 1 to 3 and 4 to 6 in our study. This suggests that full-dose uninterrupted paclitaxel given once per week should be deliverable with trastuzumab and pertuzumab in both the adjuvant and neoadjuvant settings. Our data support the use of paclitaxel given once per week (in addition to docetaxel) with trastuzumab and pertuzumab after an anthracycline-based treatment, such as the regimen described in the (now enrolled) large randomized adjuvant APHINITY (Adjuvant Pertuzumab and Herceptin in Initial Therapy) study as well as the recently opened KAITLIN (A Randomized, Multicenter, Open-Label, Phase III Trial Comparing Trastuzumab Plus Pertuzumab Plus a Taxane Following Anthracyclines Versus Trastuzumab Emtansine Plus Pertuzumab Following Anthracyclines As Adjuvant Therapy in Patients With Operable HER2-Positive Primary Breast Cancer) trial.24,25 We note that pertuzumab recently received an accelerated approval from the US Food and Drug Administration when used in combination with docetaxel and trastuzumab for three to six cycles in the neoadjuvant setting based on the results of the NeoSphere (Neoadjuvant Study of Pertuzumab and Herceptin in an Early Regimen Evaluation) and TRYPHAENA (Tolerability of Pertuzumab, Herceptin and Anthracyclines in Neo-Adjuvant Breast Cancer) studies.26–28
Overall, because of its efficacy and safety, this treatment has already changed practice in the metastatic setting, in which it has been considered a standard option in the first-line setting.29 It has also changed guidelines in the adjuvant and neoadjuvant settings.29 Finally, it has also influenced the design of other clinical trials, including the postmarketing neoadjuvant study (BERENICE [A Multicenter, Multinational, Phase II Study to Evaluate Pertuzumab in Combination With Trastuzumab and Standard Neoadjuvant Anthracycline-Based Chemotherapy in Patients With HER2-Positive, Locally Advanced, Inflammatory, or Early-Stage Breast Cancer]) to assess cardiac safety for the US Food and Drug Administration. The outcomes of patients with metastatic and early-stage HER2-positive breast cancer have been significantly improved because of effective HER2-directed agents, such as trastuzumab, lapatinib, pertuzumab, and, recently, trastuzumab emtansine. Our study provides additional evidence for a well-tolerated, taxane-based approach using paclitaxel given once per week with dual antibody therapy.
Footnotes
Supported by Roche/Genentech.
Presented at the 2012 Breast Cancer Symposium, San Francisco, CA, September 13-15, 2012; the 49th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 31-June 4, 2013; and the 36th Annual San Antonio Breast Cancer Symposium, San Antonio, TX, December 10-14, 2013.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Chau Dang, Sujata Patil, Clifford Hudis
Provision of study materials or patients: Chau Dang, Gabriella D'Andrea, Maria Theodoulou, Maura Dickler, Shari Goldfarb, Diana Lake, Julie Fasano, Monica Fornier, Theresa Gilewski, Shanu Modi, Devika Gajria, Mary Ellen Moynahan, Clifford Hudis
Collection and assembly of data: Chau Dang, Neil Iyengar, Farrah Datko, Gabriella D'Andrea, Maria Theodoulou, Maura Dickler, Shari Goldfarb, Diana Lake, Julie Fasano, Monica Fornier, Theresa Gilewski, Shanu Modi, Devika Gajria, Mary Ellen Moynahan, Nicola Hamilton, Sujata Patil, Maxine Jochelson, Larry Norton, Clifford Hudis
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase II Study of Paclitaxel Given Once per Week Along With Trastuzumab and Pertuzumab in Patients With Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Chau Dang
Consulting or Advisory Role: Pfizer
Research Funding: Roche/Genentch, GlaxoSmithKline
Neil Iyengar
No relationship to disclose
Farrah Datko
No relationship to disclose
Gabriella D'Andrea
No relationship to disclose
Maria Theodoulou
Consulting or Advisory Role: Genentech
Speakers' Bureau: Genentech
Maura Dickler
Consulting or Advisory Role: Bristol-Myers Squibb, AstraZeneca, Novartis, Merrimack Pharmaceuticals, Eisai, Syndax Pharmaceuticals, Genentech/Roche
Research Funding: Novartis (Inst), Eli Lilly (Inst), Genentech/Roche (Inst), AVEO Pharmaceuticals (Inst)
Shari Goldfarb
No relationship to disclose
Diana Lake
Honoraria: Genentech
Consulting or Advisory Role: Genentech
Julie Fasano
Consulting or Advisory Role: Magellan Rx Management
Monica Fornier
No relationship to disclose
Theresa Gilewski
No relationship to disclose
Shanu Modi
Research Funding: Roche/Genetech
Devika Gajria
No relationship to disclose
Mary Ellen Moynahan
Consulting or Advisory Role: Bristol-Myers Squibb
Patents, Royalties, Other Intellectual Property: Licensing patent pending for Biomarkers in Response to PI3K Inhibitors
Nicola Hamilton
No relationship to disclose
Sujata Patil
No relationship to disclose
Maxine Jochelson
No relationship to disclose
Larry Norton
No relationship to disclose
José Baselga
Leadership: Infinity Pharmaceuticals, Aura Biosciences
Stock or Other Ownership: Infinity Pharmaceuticals, Juno Therapeutics, Apogen, Seragon, Aragon
Honoraria: PMV Pharma, Juno Therapeutics, Infinity Pharmaceuticals, GRAIL, Seragon
Consulting or Advisory Role: Novartis, Eli Lilly, AstraZeneca, Verastem, Chugai, Bayer and Sanofi
Research Funding: Roche/Genentech
Travel, Accommodations, Expenses: Roche/Genentech
Clifford Hudis
Other Relationship: Breast Cancer Research Foundation
REFERENCES
- 1.Hudis CA: Trastuzumab: Mechanism of action and use in clinical practice N Engl J Med 357:39–51,2007 [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ Clark GM Wong SG, etal: Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene Science 235:177–182,1987 [DOI] [PubMed] [Google Scholar]
- 3.Agus DB Akita RW Fox WD, etal: Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth Cancer Cell 2:127–137,2002 [DOI] [PubMed] [Google Scholar]
- 4.Slamon DJ Leyland-Jones B Shak S, etal: Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2 N Engl J Med 344:783–792,2001 [DOI] [PubMed] [Google Scholar]
- 5.Marty M Cognetti F Maraninchi D, etal: Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: The M77001 study group J Clin Oncol 23:4265–4274,2005 [DOI] [PubMed] [Google Scholar]
- 6.Jelovac D, Emens LA: HER2-directed therapy for metastatic breast cancer Oncology 27:166–175,2013 [PubMed] [Google Scholar]
- 7.Romond E Suman VJ Jeong J-H, etal: Trastuzumab plus adjuvant chemotherapy for HER2-positive breast cancer: Final planned joint analysis of overall survival (OS) from NSABP B-31 and NCCTG N9831 Presented at the 35th Annual CTRC-AACR San Antonio Breast Cancer Symposium December 4-8, 2012 San Antonio, TX (abstr S5-5) [Google Scholar]
- 8.Goldhirsch A Piccart-Gebhart MJ Procter M, etal: HERA Trial: 2 years versus 1 year of trastuzumab after adjuvant chemotherapy in women with HER2-positive early breast cancer at 8 years of median follow up Presented at the 35th Annual CTRC-AACR San Antonio Breast Cancer Symposium December 4-8, 2012 San Antonio, TX (abstr S5-2) [Google Scholar]
- 9.Slamon DJ Eiermann W Robert N, etal: Adjuvant trastuzumab in HER2-positive breast cancer N Engl J Med 365:1273–1283,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joensuu H Kellokumpu-Lehtinen PL Bono P, etal: Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer N Engl J Med 354:809–820,2006 [DOI] [PubMed] [Google Scholar]
- 11.Spielmann M Roche H Humblet Y, etal: 3 year follow-up of trastuzumab following adjuvant chemotherapy in node positive HER2-positive breast cancer patients: Results of the PACS-04 trial Breast Cancer Res Treat 106:S72,2007 [Google Scholar]
- 12.Perez EA Suman VJ Davidson NE, etal: Sequential versus concurrent trastuzumab in adjuvant chemotherapy for breast cancer J Clin Oncol 29:4491–4497,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pivot X Romieu G Bonnefoi H, etal: PHARE trial results of subset analysis comparing 6 to 12 months of trastuzumab in adjuvant early breast cancer Presented at the 35th Annual CTRC-AACR San Antonio Breast Cancer Symposium December 4-8, 2012 San Antonio, TX (abstr S5-3) [Google Scholar]
- 14.Dang C Fornier M Sugarman S, etal: The safety of dose-dense doxorubicin and cyclophosphamide followed by paclitaxel with trastuzumab in HER-2/neu overexpressed/amplified breast cancer J Clin Oncol 26:1216–1222,2008 [DOI] [PubMed] [Google Scholar]
- 15.Franklin MC Carey KD Vajdos FF, etal: Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex Cancer Cell 5:317–328,2004 [DOI] [PubMed] [Google Scholar]
- 16.Baselga J Cortés J Kim SB, etal: Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer N Engl J Med 366:109–119,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swain SM Kim S-B Corés J, etal: Final overall survival (OS) analysis from the CLEOPATRA study of first-line (1L) pertuzumab (Ptz), trastuzumab (T), and docetaxel (D) in patients with HER2-positive metastatic breast cancer (MBC) Presented orally at the European Society of Medical Oncology September 26-30, 2014 Madrid, Spain [Google Scholar]
- 18.US Food and Drug Administration. Drug Approvals and Databases: Pertuzumab. www.fda.gov/drugs/informationondrugs/./ucm307592.htm.
- 19.Eniu A, Palmieri FM, Perez EA: Weekly administration of docetaxel and paclitaxel in metastatic or advanced breast cancer Oncologist 10:665–685,2005 [DOI] [PubMed] [Google Scholar]
- 20.De Laurentiis M Cancello G D'Agostino D, etal: Taxane-based combinations as adjuvant chemotherapy of early breast cancer: A meta-analysis of randomized trials J Clin Oncol 26:44–53,2008 [DOI] [PubMed] [Google Scholar]
- 21.Peto R Davies C, etal: Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials Lancet 379:432–444,2012Early Breast Cancer Trialists' Collaborative Group (EBCTCG) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sparano JA Wang M Martino S, etal: Weekly paclitaxel in the adjuvant treatment of breast cancer N Engl J Med 358:1663–1671,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez EA López-Vega JM Del Mastro L, etal: Safety of pertuzumab plus trastuzumab plus vinorelbine for first-line treatment of patients with HER2-positive locally advanced or metastatic breast cancer Presented at the 36th Annual CTRC-AACR San Antonio Breast Cancer Symposium December 10-14, 2013 San Antonio, TX (abstr P2-16-10) [Google Scholar]
- 24.ClinicalTrials.gov. A Study of Pertuzumab in Addition to Chemotherapy and Herceptin (Trastuzumab) as Adjuvant Therapy in Patients With HER2-Positive Primary Breast Cancer. http://clinicaltrials.gov/show/NCT01358877.
- 25.ClinicalTrials.gov. A Study of Kadcyla (Trastuzumab Emtansine) Plus Perjeta (Pertuzumab) Following Anthracyclines in Comparison With Herceptin (Trastuzumab) Plus Perjeta and a Taxane Following Anthracyclines as Adjuvant Therapy in Patients With Operable HER2-Positive Primary Breast Cancer. http://clinicaltrials.gov/ct2/show/NCT01966471.
- 26.National Cancer Institute. FDA Approval for Pertuzumab: Brand name—Perjeta. http://www.cancer.gov/cancertopics/druginfo/fda-pertuzumab.
- 27.Gianni L Pienkowski T Im YH, etal: Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial Lancet Oncol 13:25–32,2012 [DOI] [PubMed] [Google Scholar]
- 28.Schneeweiss A Chia S Hickish T, etal: Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA) Ann Oncol 24:2278–2284,2013 [DOI] [PubMed] [Google Scholar]
- 29.National Cancer Institute. FDA Approval for Ado-Trastuzumab Emtansine: Brand name(s)—Kadcyla. http://www.cancer.gov/cancertopics/druginfo/fda-ado-trastuzumab-emtansine.