Summary
Neurotransmission is a tightly regulated Ca2+-dependent process. Upon Ca2+ influx, Synaptotagmin1 (Syt1) promotes fusion of synaptic vesicles (SVs) with the plasma membrane. This requires regulation at multiple levels but the role of metabolites in SV release is unclear. Here, we uncover a role for isocitrate dehydrogenase 3a (idh3a), a Krebs cycle enzyme, in neurotransmission. Loss of idh3a leads to a reduction of the metabolite, alpha-ketoglutarate (αKG), causing defects in synaptic transmission similar to the loss of syt1. Supplementing idh3a flies with αKG suppresses these defects through an ATP or neurotransmitter independent mechanism. Indeed, αKG, but not glutamate, enhances Syt1 dependent fusion in a reconstitution assay. αKG promotes interaction between the C2-domains of Syt1 and phospholipids. The data reveal conserved metabolic regulation of synaptic transmission via αKG. Our studies provide role for αKG, a metabolite that has been proposed as a treatment for ageing and neurodegenerative disorders.
Keywords: Tricarboxylic acid (TCA) cycle, alpha-ketoglutarate, Synaptotagmin, exocytosis and endocytosis
eTOC Blurb
Ugur et al. find that loss of the mitochondrial enzyme IDH3A leads to a reduction in alpha-ketoglutarate (αKG) and impairs synaptic transmission through an ATP independent pathway. idh3a mutants phenocopy loss of the Ca2+ sensor syt1 at neuromuscular junctions and αKG promotes membrane fusion by enhancing Syt1-lipid interaction in vitro.

Introduction
Mitochondria perform a variety of functions including ATP production via the electron transport chain (ETC), lipid synthesis, heme biosynthesis, reactive oxygen species (ROS) production and carbohydrate metabolism (Calvo and Mootha, 2010). One of the key metabolic pathways that integrates different mitochondrial functions is the Krebs or tricarboxylic acid (TCA) cycle (Krebs and Johnson, 1937). Although the TCA cycle is known to generate electron carriers for the ETC, mutations in different TCA cycle enzymes result in phenotypic heterogeneity (Rustin et al., 1997) indicating that each step affects specific functions that remain to be elucidated (Brière et al., 2006). Indeed, TCA metabolites have been implicated in numerous cellular pathways that control ageing, neurodegeneration and cancer (Brière et al., 2006; Mishur et al., 2016). One of the most prominent TCA metabolites, the product of the Isocitrate Dehydrogenase complex (IDH), alpha-ketoglutarate (αKG), affects stem cell proliferation (TeSlaa et al., 2016), ageing (Chin et al., 2014) and DNA methylation (Yang et al., 2016) emphasizing the pleiotropy governed by TCA metabolites. Due to αKG’s roles in different contexts, it is a therapeutic target for a variety of diseases (Wu et al., 2016; Zdzisińska et al., 2016).
Despite the phenotypic heterogeneity associated with mutations in mitochondrial genes, mitochondrial diseases commonly affect neuronal function and cause the demise of neurons. The majority of these neuronal phenotypes are attributed to defects in ATP production or Ca2+ homeostasis (Vos et al., 2010). Yet, dysfunction of other mitochondrial pathways such as steroid synthesis (Sandoval et al., 2014; Schumacher et al., 2012) or iron metabolism (Chen et al., 2016; Hadzhieva et al., 2014; Liddell, 2015) also affect neuronal function. Overall, our understanding of how mitochondria and metabolites regulate synaptic function remains elusive.
Synaptic transmission relies on exocytotic release of neurotransmitters (Südhof, 2013). Upon stimulation, Ca2+ enters the presynaptic terminal and interacts with Synaptotagmin1 (Syt1), a transmembrane protein with two Ca2+-binding C2-domains, to initiate synaptic vesicle (SV) fusion with the plasma membrane (Chapman, 2008; Rizo and Xu, 2015). Syt1 promotes SV fusion by binding to both acidic phospholipids on the plasma membrane and SNAREs (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) (Rizo and Rosenmund, 2008). Syt1-SNARE interaction enhances the zippering of vesicle-SNARE (Synaptobrevin) with target-SNAREs (SNAP25 and Syntaxin) that brings the two membranes in close proximity and thereby leads to the opening of a fusion pore to release neurotransmitters (Han et al., 2017; Jahn and Scheller, 2006). Although this evolutionarily conserved synaptic transmission machinery has been studied for decades, the mechanism by which neural activity is integrated with metabolism is not clear.
Here we show that the metabolite, αKG is able to affect the dynamics of the Ca2+ sensor, Syt1 (Littleton et al., 1994). We isolated ethyl methanesulfonate (EMS) induced mutations in a fly homolog of human Isocitrate Dehydrogenase 3A (IDH3A) and document that a loss of idh3a causes loss of synaptic transmission in photoreceptors and larval neuromuscular junctions (NMJs). We found that, both the morphological and electrophysiological properties observed in idh3a mutants are similar to partial loss of function of syt1 in fly NMJs. We show that loss of idh3a results in reduced levels of its product, αKG, and supplementing idh3a mutant flies with αKG suppresses the synaptic transmission defects. Furthermore, we show that αKG improves the Ca2+ sensitivity of mammalian Syt1 and promotes its cytoplasmic domain to interact with membranes to trigger membrane fusion and neurotransmitter release. To our knowledge no other TCA metabolite has been shown to regulate Syt1 function and synaptic transmission.
Results
Loss of fly isocitrate dehydrogenase 3a (idh3a) causes loss of synaptic transmission
To isolate novel mutations affecting neuronal function, we conducted an unbiased forward genetic screen of essential genes on the fly X-chromosome (Haelterman et al., 2014; Yamamoto et al., 2014). From this screen we isolated four different mutations in CG12233, a homolog of human mitochondrial IDH3A (from now on idh3a, Figure 1A, Figures S1A, S1B, Tables S1 and S2). All four alleles cause larval lethality at the L3 stage, approximately 10 days after egg laying (Figure 1B). Lethality was rescued by two independent 80 Kb P[acman] genomic rescue clones (Venken et al., 2010) (GR) (Figures 1A and 1B), a ubiquitously overexpressed fly idh3a cDNA, as well as human IDH3A cDNA, showing that the chromosomes do not carry other mutations that cause lethality.
Figure 1. Loss of fly isocitrate dehydrogenase 3a (idh3a) causes loss of on and off transients.
(A) Schematic representation of isolated mutations that affect CG12233 (idh3a). Sanger sequencing identified 2 different splice site mutations idh3a1 and idh3a2, a nonsense mutation (Y198X), idh3a3, and a missense mutation (E168V), idh3a4. The diagram below shows the duplications spanned by genomic rescue (GR) construct as well as the position of the GFP in the genomic region. (B) These alleles fail to complement each other and a P-element insertion into the gene (P{lacW}l(1)G0156G0156) (Peter et al., 2002). The four alleles cause larval lethality at the L3 stage, approximately 10 days after egg laying. Lethality is rescued by two independent 80 Kb P[acman] duplications (GR). (C) Representative ERG traces of control (y w FRT19A), idh3a1 and idh3a1;; 80Kb genomic rescue (GR), the red arrows show on and off transients that are indicative of synaptic transmission. (D) ERG amplitude is significantly reduced in one day old idh3a1 when compared to control (y w FRT19A), n = 5. (E) On and off transients are significantly reduced in one day old animals in idh3a1, n = 5. (F) A schematic representation of a lamina cartridge that is composed of six photoreceptor terminals (R) surrounded by epithelial glia (red line) and two postsynaptic monopolar cells (L) that are centrally located. Green circles indicate amacrine and T1 cells. The structures where glial cells invaginate into the photoreceptor terminals are called capitate projections (cp). Each cartridge typically contains ~15 -20 mitochondria (m). (G) Representative transmission electron microscopy (TEM) images of PR terminal of idh3a1;;GR and idh3a1 at day one. Scale bar = 1 μm. (H) Quantification of number of capitate projections per cartridge, n = 14. Error bars indicate SD and p values were calculated using Student’s t test, *p < 0.05, **p < 0.01. See also Figures S1-S4 and Tables S1 and S2
To determine the expression pattern of the protein encoded by idh3a, we generated transgenic flies carrying a genomic construct (idh3aGFP) with a C-terminal GFP tagged idh3a (Sarov et al., 2016). idh3aGFP flies express GFP ubiquitously in larvae (Figure S1C) and this construct rescues the lethality of idh3a1 mutant animals. To assess if the protein localizes to mitochondria, we stained idh3aGFP larvae with a mitochondrial marker, Complex V. The GFP signal colocalizes with complex V in mitochondria in all tissues (Figure S1B–G).
To assess phototransduction and synaptic transmission in idh3a mutant animals, we induced idh3a mutant eye clones (Newsome et al., 2000) and recorded electroretinograms (ERG) (Hardie and Raghu, 2001; Wu and Wong, 1977). Loss of idh3a causes an approximate 40% loss of amplitude, suggesting a defect in phototransduction in young animals (Figures 1C and 1D). In addition, we observe a reduction of ‘on’ and ‘off’ transients (Figures 1C, 1E and S2A), arguing that there is also a reduction in synaptic transmission in one day old photoreceptors (PRs). Defects in on and off transients often correlates with loss of capitate projections (cp) in the synaptic terminals of the lamina cartridges. These cps correspond to glial invaginations in PR terminals (R in Figure 1F) where neurotransmitter precursors secreted by glial cells are taken up (Fabian-Fine et al., 2003). We assessed the idh3a mutant PR terminal morphology via transmission electron microscopy (TEM) and observed severely reduced numbers of cps in the cartridges (Figure 1H and S2B). These data show that loss of idh3a affects synaptic transmission in fly PRs.
Loss of idh3a does not alter ATP levels
In addition to a reduction in cps, TEM also revealed bloated mitochondria with few cristae in the lamina of the fly eye (Figure 1G, S2B and S2C). To assess if the mitochondria are affected in third instar larvae, we stained muscles with Complex V. As shown in Figure S3A, muscles display highly aberrant mitochondria, suggesting that their function may be altered in idh3a1 animals. TEM of larval idh3a1 hemizygous muscles revealed mitochondria with highly aberrant cristae (Figure S4A and S4B). Interestingly, ATP levels in idh3a mutant larvae are similar to positive control animals whereas the ATP levels in MarfE (Mitochondrial assembly regulatory factor), a previously characterized mitochondrial mutant, had significantly reduced ATP levels (Figure S3B)(Sandoval et al., 2014). To assess the membrane potential in muscles we stained them with TMRE (tetramethylrhodamine, ethyl ester) (Burman et al., 2012), that stains polarized mitochondria. As shown in Figure S3C and S3D, idh3a mutant muscles maintain mitochondrial membrane polarization. We also assessed levels of reactive oxygen species (ROS) as they are often increased when mitochondria are dysfunctional (Liu et al., 2015; Redmann et al., 2016). The levels of Dihydroethidium (DHE), a dye that fluoresces in the presence of ROS (Owusu-Ansah et al., 2008), were comparable to controls (Figure S3E and S3F). Similarly, the levels of aconitase activity, a mitochondrial enzyme that is extremely sensitive to ROS (Gardner, 2002), were unaltered (Figure S3G). Finally, MitoSOX levels, a mitochondrial ROS sensitive dye similar to DHE, were unaltered, again showing that ROS is not increased (S3H). Moreover, we measured ATPase activity of complex V and did not observe a difference between controls (idh3a1; GR) and idh3a1 (Figure S3I). If the mitochondria are defective we would expect an increase in lactate, yet lactate levels were decreased in idh3a1 (Figure S3J). In summary, despite severe mitochondrial morphological defects, mitochondrial membrane potential, ATP and ROS levels are not altered in idh3a mutants.
Loss of idh3a causes reduced alpha-ketoglutarate levels
Because Idh3a is a TCA enzyme that is responsible for the oxidative decarboxylation of isocitrate into alpha-ketoglutarate (αKG) (Figure 2A), we performed targeted Liquid Chromatography-Mass Spectrometry (LC-MS) in control, idh3a1;;GR and idh3a1 larvae to assess levels of various TCA metabolites. Metabolomics analyses revealed that hydroxyl glutarate (a derivative of αKG) levels decreased by about 60% in mutants when compared to controls. However, the levels of other TCA metabolites (such as isocitrate) were unaffected (Figure 2B). As the metabolomics data measured hydroxyl glutarate, we performed direct enzymatic measurements of the levels of αKG. As shown in Figure 2C, loss of idh3a causes a significant decrease in αKG levels.
Figure 2. Loss of fly idh3a causes reduced alpha-ketoglutarate (αKG) levels.
(A) A simplified representation of the tricarboxylic acid (TCA) cycle. (B) Liquid Chromatography-Mass spectrometry (LC-MS) results for TCA metabolites in idh3a1 and GR animals individually compared to controls (y w FRT19A) shows a significant reduction in hydroxyglutarate, which is a derivative of alpha-ketoglutarate (αKG). Other TCA metabolites are not significantly altered when compared to control. n = 3 assays (30 animals per genotype per assay) (C) αKG levels are decreased in idh3a alleles; n = 3 assays (10 animals per genotype per assay). Error bars indicate SD and p values were calculated using Student’s t test by individually comparing metabolites to the control, *p < 0.05, **p < 0.01.
Feeding idh3a1 animals with αKG rescues on and off transients
Since αKG is the only TCA metabolite that was obviously affected, we hypothesized that a decrease in αKG levels may underlie the on and off transient phenotype observed in the ERGs. We therefore induced idh3a1 mutant clones in the eye and fed freshly emerged adult females with 0.1 mM αKG and recorded ERGs in one week aged animals. Mutants whose food was supplemented with αKG exhibited a remarkable improvement of on and off transients, suggesting that synaptic transmission was restored (Figures 3A and 3B). To assess whether αKG supplementation could also rescue the cp phenotype, we performed TEM in mutant PRs. As shown in Figures 3C–F, αKG supplementation rescued the number of cps. To determine if this suppression was specific to αKG, we supplemented the food with glutamate, a precursor for αKG. Feeding mutants with glutamate did not result in suppression of the on or off transient defects (Figures S5A and S5B) indicating that the synaptic transmission defects are specific to the reduction in αKG. Since glutamate can be converted to αKG via glutamate dehydrogenase, feeding animals with glutamate may restore αKG independent of Idh3a. However, we measured total glutamate levels in animals fed with normal food and to our surprise we observed an overall increase in glutamate levels in idh3a mutants when compared to control animals (Figure S5C). Hence, even though glutamate levels are high in the mutants, the αKG pool remains low. Together these data argue that αKG levels play a role in proper synaptic transmission.
Figure 3. Feeding idh3a animals with αKG rescues on and off transients.
(A) Representative ERG traces of one week old animals; red arrows indicate on and off transients. (B) Quantification of on and off transients in one week old flies, n = 5. Representative TEM lamina images of one week old flies, (C) idh3a1;;GR fed with 0.1mM αKG, (D) idh3a1 fed with normal food and (E) idh3a1 fed with 0.1mM αKG; scale bar = 1 μm. Note that αKG supplementation does not rescue mitochondrial (m) morphology. * indicates dying terminals. (F) Quantification of cp, n = 14. Error bars indicate SD and p values were calculated using Student’s t test, *p < 0.05, **p < 0.01. See also Figure S5.
Loss of idh3a impairs spontaneous and evoked vesicle release at larval NMJ
A loss of synaptic transmission in larvae may affect locomotion. Indeed, idh3a1 mutant larvae exhibit defects in peristalsis and locomotion that are alleviated upon αKG feeding (Figures S5D and S5E). To test if αKG feeding rescues αKG levels in idh3a mutant larvae, we measured αKG levels in idh3a mutants fed with normal or αKG food. αKG supplementation resulted in a significant increase in αKG levels, however it did not restore αKG to control levels (Figure S5F). Moreover, αKG feeding, did not delay the lethal phase, nor did it affect the developmental delay or alleviate mitochondrial morphology (Figure S4C and S4C’) in idh3a1, suggesting that the suppression of the synaptic transmission defect is selective.
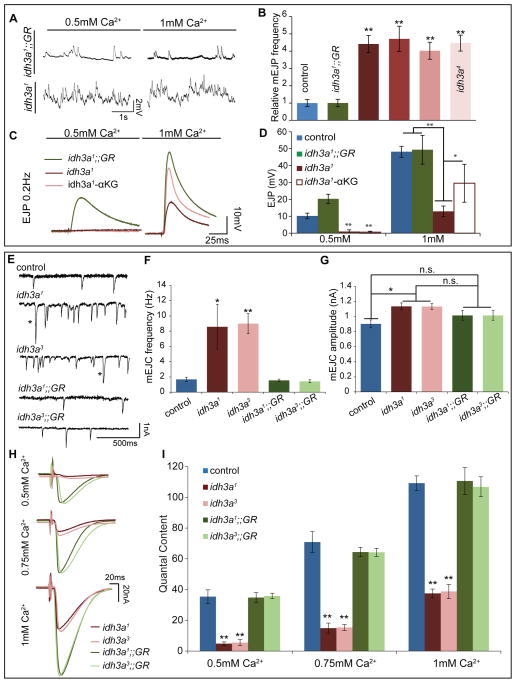
To determine the electrophysiological properties at the neuromuscular junction (NMJ) of third instar larvae, we measured spontaneous and evoked SV release. Surprisingly, the frequency of spontaneous release of SVs as indicated by miniature Excitatory Junction Potential (mEJP) was elevated more than five-fold in idh3a mutants (Figure 4A and 4B). This may be an underestimate as the mEJPs are superimposed and hence difficult to quantify precisely. In contrast, the amplitude of the evoked SV release (EJP) at 0.5mM extracellular Ca2+ was dramatically reduced or absent in mutants when compared to controls and GR animals (Figures 4C and 4D). These results indicated that although SVs are present in idh3a mutants, SV release upon stimulation is impaired.
Figure 4. Loss of idh3a causes increase in spontaneous vesicle release frequency and decrease in evoked release in larval neuromuscular junction (NMJ).
(A) Representative mini Excitatory Junction Potential (mEJP) traces for idh3a1;;GR and idh3a1 in 0.5mM and 1mM extracellular Ca2+. (B) Quantification of the mEJP frequency, n= 5 animals, 0.5mM Ca2+. (C) Representative EJP traces for idh3a1;;GR and idh3a1 in 0.5mM and 1mM extracellular Ca2+. (D) Quantification of EJP amplitudes at indicated [Ca2+] (E) Representative mEJC traces at indicated at 0.5mM Ca2+, * indicates rare mEJCs with high amplitude (>1.5 nA). (F) Quantification of mEJC frequency at 0.5mM Ca2 (G) Quantification of mEJC amplitude at 0.5mM Ca2+. (H) Representative EJC traces at indicated at indicated [Ca2+]. (I) Quantification of quantal content at 0.5mM, 0.75mM and 1mM Ca2+. Error bars indicate SD for B and D; SEM for F, G and I; p values were calculated using Student’s t test, *p < 0.05, **p < 0.001, n= 5 animals or more per assay. See also Figures S5 and S6.
To test if the decrease in evoked response is due to a cell autonomous role of idh3a, we performed tissue specific rescue experiments with idh3a cDNA. Ubiquitous and neuronal overexpression rescued all the observed electrophysiological defects (Figure S6A and S6B) yet only ubiquitous overexpression rescued lethality. In contrast, although muscle specific overexpression rescued mitochondrial morphology defects in the muscle (data not shown) it failed to rescue electrophysiological defects (Figure S6A and S6B) indicating that idh3a is required cell autonomously in neurons for synaptic transmission.
To test how the EJP amplitudes respond to various extracellular [Ca2+], we varied the extracellular [Ca2+]. We observed a significant increase in EJP response when we record in 1mM extracellular Ca2+ in the same animals that showed no EJP at 0.5mM Ca2+ (Figure 4D). However, the responses at 1mM Ca2+ were still significantly lower than control animals. Similar to PRs, αKG supplementation alleviated the EJP phenotype at 1mM Ca2+ (Figure 4D). However, at low [Ca2+] (0.5mM), αKG did not alleviate the EJP loss. Moreover, at 10mM Ca2+, a concentration that is well above physiological levels, idh3a and control animals had indistinguishable EJPs (Figure S6C). Hence, raising the [Ca2+] alone can suppress the loss of synaptic transmission in idh3a mutants.
Although the intracellular recordings were very informative, due to the increase in mEJP frequency and problems with non-linear summation at high [Ca2+], we could not accurately determine the quantal content in idh3a mutants. To circumvent these issues, we recorded two-electrode voltage clamp (TEVC) in idh3a mutants and controls. As shown in Figure 4E and 4F, mEJC (miniature Excitatory Junction Current) traces also conferred a five fold increase in spontaneous SV release in idh3a mutants. Moreover, idh3a mutants displayed a subtle increase in mEJC amplitude (Figure 4G). Similar to intracellular recordings, EJCs were significantly reduced in idh3a mutants, and increasing the extracellular Ca2+ levels alleviated the reduction in EJCs (Figure 4H and S6D). Although increasing the extracellular Ca2+ (0.75 and 1mM) partially suppressed the phenotype, the quantal content in idh3a mutant animals was highly reduced at 0.5, 0.75 and 1mM Ca2+ when compared to controls.
Loss of idh3a causes enhanced facilitation upon repetitive stimulation and increases presynaptic Ca2+ levels upon stimulation
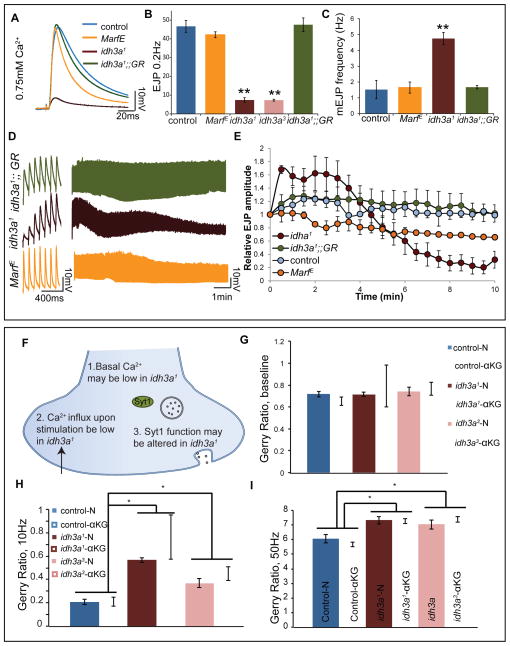
The aforementioned observations regarding spontaneous and evoked SV release are in contrast with previous observations made in other mitochondrial mutants for which NMJ recordings have been reported in Drosophila including Marf (Sandoval et al., 2014), drp1 (Verstreken et al., 2005), dmiro (Guo et al., 2005), shawn (SLC25A39) (Slabbaert et al., 2016) and porin (Graham et al., 2010). None of these mitochondrial mutants are associated with a dramatic decrease in evoked SV release upon low frequency stimulation. Mutations in Marf, lead to a decrease in ATP production (Figure S3B) and yet do not show a decrease in evoked release (Figure 5A and 5B) (Sandoval et al., 2014) and exhibit normal mini frequency (Figure 5C), suggesting that low frequency stimulation does not rely on ATP levels. A defect in synaptic ATP production is, however, associated with a decrease in response to high frequency stimulation in Marf, dmiro, and drp1 mutants (Guo et al., 2005; Sandoval et al., 2014; Verstreken et al., 2005). To test the response of idh3a mutants to high frequency stimulation, we performed 10Hz stimulation for 10 minutes. In idh3a1 mutants, we observed a dramatic facilitation followed by a striking rundown (Figure 5D). To better document the facilitation in idh3a mutants we performed paired pulse stimulation (Figure S6E) and observed a significant increase in the paired pulse ratio in idh3a mutants when compared to controls (Figure S6F). Overall, these observations once again indicate that uptake of Ca2+ alleviates the evoked release in idh3a1.
Figure 5. Loss of idh3a causes enhanced facilitation upon repetitive stimulation and increase presynaptic Ca2+ uptake upon stimulation.
(A) Representative EJP traces for indicated genotypes, (B) quantification of EJP amplitude and (C) quantification of mini EJP frequency, n= 5. (D) Representative high frequency stimulation traces, first seven traces are shown on the left, combined traces for 10minutes stimulation are shown on the right. (E) EJP amplitudes normalized to the average of first five traces of each genotype show a depletion of the amplitude in idh3a. Error bars indicate SD and p values were calculated using Student’s t test, *p < 0.05, **p < 0.01. (F) Possible ways in which loss of idh3a impairs evoked release. (G-I) Real time Ca2+ imaging using GCaMP6m-mCherry fusion protein, Gerry. (G) The ratios show that cytoplasmic Ca2+ levels are not low in idh3a mutant terminal boutons, n=6. The changes in Gerry ratio upon nerve stimulation in 0.5mM Ca2+ at (H) 10 Hz and (I) 50 Hz, show that idh3a mutant animals have elevated Ca2+ levels upon stimulation, n=6, Error bars indicate SEM, two-way ANOVA *p < 0.05. See also Figures S7-S9.
Based on these findings we propose three alternatives to explain how idh3a loss is causing a defect in evoked SV release (Figure 5F). First, the cytoplasmic resting [Ca2+] in terminal boutons may be low, possibly due to increased Ca2+ uptake by mitochondria or ER. Second, the Ca2+ entry via Ca2+-channels in the bouton may be reduced. Third, there may be a defect in Ca2+ sensing by the Ca2+ sensor, Synaptotagmin1 (Syt1) (Südhof, 2013). To compare cytoplasmic Ca2+ levels under resting and stimulated conditions we used a genetically encoded Ca2+ indicator (Gerry). Gerry consists of GCaMP6m (Chen et al., 2013) fused with mCherry to permit pseudo-ratiometric measurements when expressed under the control of neuronal-Synaptobrevin-Gal4. To obtain [Ca2+] at rest, we measured the Gerry fluorescence ratio over a five second period. To induce robust Ca2+ entry, we electrically stimulated the nerve at 10 Hz for two seconds and again at 50Hz after a ten second rest. As shown in Figure 5G, at 0.5mM extracellular Ca2+, [Ca2+] at rest in idh3a mutant boutons is similar to control animals. When we stimulated idh3a mutant boutons, we observed a significant increase in [Ca2+] compared to control animals (Figure 5H and 5I). Supplementing idh3a animals with αKG, however, did not restore the increase in [Ca2+] upon stimulation (Figure 5H and 5I). These data indicate that the first two hypotheses are highly unlikely. These results are further supported by our measurements of mitochondrial function within these boutons, using a matrix-targeted ratiometric fluorescent indicator of [Ca2+] (mito-TN-XXL) (Ivannikov and Macleod, 2013). Mitochondria in both idh3a1 and control animals had similar baseline Ca2+ levels (Figure S7B) and took up Ca2+ in response to 50 Hz nerve stimulation (Figure S7A). However, idh3a1 mitochondria did not show a greater rise in [Ca2+]. In sum, the idh3a1 mitochondrial responses observed here are indistinguishable from those expected in control mitochondria under similar conditions.
Syt1 is a low affinity Ca2+ sensor anchored to SVs and loss of syt1 severely impairs synaptic transmission upon Ca2+ influx (Chapman, 2008). Mutations in syt1 also display increase in spontaneous mini frequency as well as a decrease in evoked release (Littleton et al., 1994). Our recordings in idh3a mutants showed that elevated levels of extracellular Ca2+ are required to elicit proper SV release (Figures 4D, 4H and S6D), indicating that the Ca2+ sensitivity of neurotransmitter release is severely impaired in idh3a mutants, similar to what has been observed in syt1.
To test if idh3a genetically interacts with syt1, we recorded evoked release and spontaneous release frequency in animals that have loss of one copy of idh3a with loss of one copy of syt1 and did not observe any phenotypes (Figures S7C and S7D). Next we assessed if overexpressing syt1 suppresses the phenotypes associated with loss of idh3a. Syt1 overexpression does not cause a phenotype when overexpressed in wt background (Lee et al., 2013). Similarly, we did not observe an alleviation of the idh3a phenotypes when we overexpressed syt1 in idh3a background (Figure S7C and S7D) indicating that Syt1 levels are not the limiting factor in idh3a mutants.
In addition to Syt1, another SNARE-binding molecule, Complexin (Cpx), also activates evoked release and clamps spontaneous release (Trimbuch and Rosenmund, 2016). Similar to syt1 and idh3a, mutations in cpx also display an increase in spontaneous mini frequency as well as decrease in evoked release (Huntwork and Littleton, 2007; Xue et al., 2009). To determine if the idh3a phenotypes may be caused by a decrease in Cpx, we tested the Cpx levels in idh3a mutants. As shown in Figure S8A and S8B, we observed a three-fold increase of Cpx in the idh3a mutants when compared to controls. Moreover, Cpx intensity at NMJ boutons was significantly increased but the Cpx localization was not altered (Figure S8C and S8D). These data suggest that the electrophysiological phenotypes in idh3a are not likely to be associated with a decrease in Cpx levels or an altered subcellular localization of Cpx.
Loss of idh3a causes reduced number of synaptic vesicles with heterogeneous size at NMJ
Since we observed similar electrophysiological features in idh3a, as those described for syt1 and cpx mutant animals, we assessed the morphological features of the idh3a mutant NMJs. The gross NMJ morphology is normal in syt1 null animals, however the number of SVs per bouton is reduced by more than 50% when compared to controls (Lee et al., 2013). Moreover, numerous SVs are enlarged in syt1 mutants (Lee et al., 2013). The decrease in number and the increase in SV size is a robust indicator of a defect in endo-exocytosis (Stevens et al., 2012; Zhang et al., 1998) and loss of syt1 has been implicated in both endocytosis and exocytosis (Koh and Bellen, 2003; Poskanzer et al., 2003; Yao et al., 2011). In contrast, cpx mutant NMJs are vastly expanded when compared to syt1 (Huntwork and Littleton, 2007). In addition, TEM revealed no obvious difference in SV size and number in cpx mutants (Jorquera et al., 2012). To test if loss of idh3a affects the overall morphology of the NMJs, we performed immunohistochemistry and did not observe obvious changes in bouton morphology based on staining with the neuronal marker HRP, presynaptic marker EPS15, or the postsynaptic markers, Discs large (Parnas et al., 2001) and the glutamate receptor GluRIIA (Kurusu et al., 2012) (Figures S9A–C), similar to what has been described in syt1 mutants (Lee et al., 2013). TEM analysis revealed that loss of idh3a causes a loss of SVs of approximately 50% (Figures 6A–6D). Similar to what has been described for numerous endocytic mutants (Bellen et al., 2010), loss of idh3a also causes an increase in SV size (Figures 6F and 6G). Moreover, αKG supplementation of idh3a1 partially suppresses some of the ultrastructural phenotypes, including the number of docked SVs at the T-bar and the size of SVs (Figures 6E–6G). In summary, idh3a and syt1 mutants share striking electrophysiological and ultrastructural similarities that are not observed in other mutations that affect synaptic transmission.
Figure 6. Loss of idh3a causes reduced number of synaptic vesicles with heterogeneous size at NMJ.
Representative TEM images of 3rd instar muscle 6/7 terminal boutons of (A) control (y w FRT19A), (B) idh3a1;;GR and (C) idh3a1 raised in normal and 0.1mM αKG supplemented food (‘). (D) Quantification of SV density shows that idh3a1 boutons have fewer SVs per bouton area when compared to control. (E) The number of SVs at active zones is reduced in idh3a1, and αKG supplementation rescues the number of SVs at the T-bar. idh3a1 animals have fewer SVs with (F) small size (≤ 44nm) and (G), more large size SVs (≤ 45nm) in boutons. N = 10 boutons for D, F, G and n = 10 active zones for E. Error bars indicate SD and p values were calculated using Student’s t test, *p < 0.05, **p < 0.01. See also Figures S9 and S10.
Together, the decrease in evoked response that can be rescued by increasing the extracellular Ca2+, the approximate five-fold increase in mEJP frequency, and the compelling similar phenotypes at the ultrastructural level argue that the loss of idh3a or the decrease in αKG may affect the proper function of syt1. To exclude a defect in transcription of syt1, we performed RT-qPCR in 3rd instar larvae of idh3a mutants and show that the Syt1 mRNA levels are unchanged in idh3a mutants when compared to controls (Figure S10A). To assess Syt1 protein levels we performed Western blots and did not observe a reduction in Syt1 levels in idh3a mutants (Figure S10B). Collectively, these data argue that loss of idh3a does not cause a reduction in Syt1 mRNA or protein levels.
To determine if the protein localization of Syt1 is altered, we performed immunohistochemistry of 3rd instar larval NMJs using the DSYT2 antibody (Littleton et al., 1993a). Given that Syt1 is associated with SVs, most of the protein localizes within boutons. However, during SV fusion, the protein redistributes to the presynaptic membrane, prior to its retrieval by endocytosis. As shown in Figure S10C, Syt1 localizes to the cytoplasm and the bouton membranes, where it colocalizes with another presynaptic marker CSP (Figure S10D). In idh3a mutant NMJs, although the Syt1 colocalization with CSP is unaltered, we observe a relative decrease in Syt1 at the membrane and an increase in the cytoplasm. To test if Syt motility in idh3a mutants is affected, we overexpressed Syt1-eGFP in neurons and photobleached a small region in boutons. We then recorded fluorescence recovery after photobleach (FRAP) to determine the rate of SV recycling (Figure S10E) (Seabrooke et al., 2010). We observed a significant decrease in fluorescence recovery in idh3a mutants (Figure S10E) and αKG supplementation of idh3a1 mutants significantly restored fluorescence recovery (Figure S10E). This observation indicates that αKG may enhance Syt1 and/or SV motility.
αKG regulates Ca2+ sensitivity of Synaptotagmin and the interaction of its tandem C2-domains with acidic phospholipids
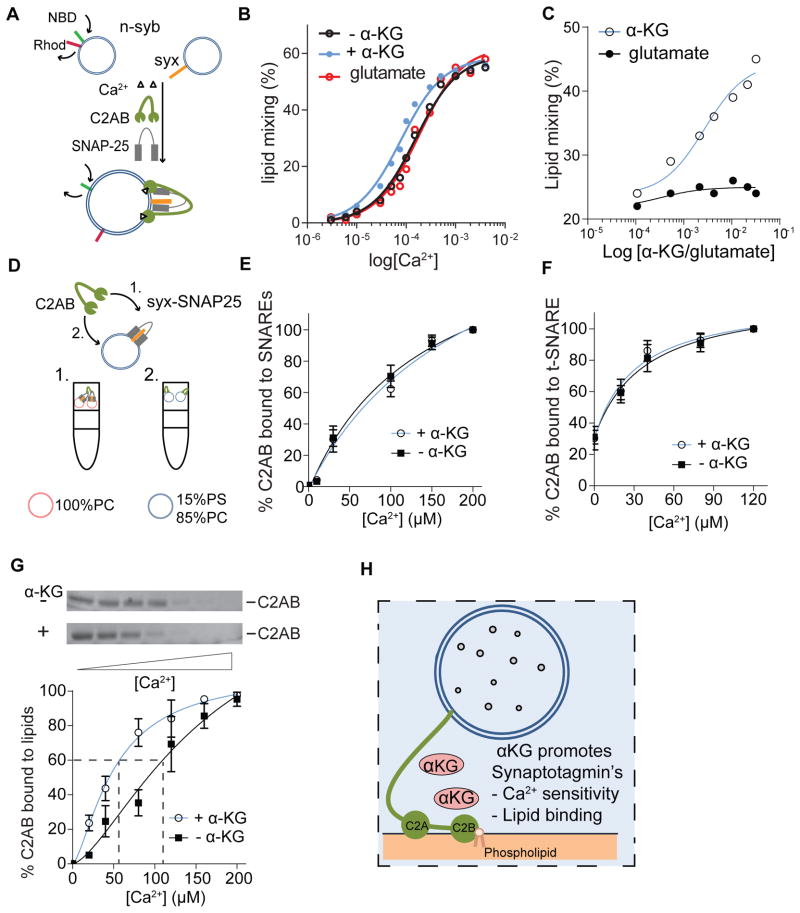
To understand if αKG regulates Syt1 function, we measured whether αKG directly affects Ca2+-Syt1-stimulated membrane fusion using a reconstituted membrane fusion assay (Bhalla et al., 2006; Tucker et al., 2004). This assay relies on proteoliposomes bearing mouse v-SNARE, t-SNAREs and the cytoplasmic domain (C2AB) of mouse Syt1 (Figure 7A) and measures lipid mixing as a readout for SV fusion. In the presence of αKG, we observed a two-fold increase in the Ca2+ sensitivity of Syt1-stimulated fusion (Figure 7B). Although the affinity of αKG for Syt1 is low based on isothermal titration calorimetry (data not shown), the stimulatory effect is specific to αKG because addition of glutamate had little to no effect (Figure 7B). In the presence of a low [Ca2+] that barely promotes lipid mixing (~25%), increasing concentrations of αKG caused a robust increase in fusion between v-SNARE and t-SNARE vesicles (~45%) (Figure 7C). Hence, αKG promotes Syt1-stimulated fusion at low [Ca2+].
Figure 7. αKG regulates Ca2+ sensitivity of Synaptotagmin1 and the interaction of its tandem C2-domains with acidic phospholipids.
(A) Schematic representation of reconstituted fusion assay based on the FRET between NBD and rhod (see below). Lipid mixing is measured between labeled liposomes that have Syb and unlabeled liposomes that have Syx in the presence of Ca2+, cytoplasmic Ca2+ sensing domain of Syt1 (C2AB) and cytoplasmic t-SNARE SNAP-25. (B) Adding αKG in a reconstituted fusion assay enhances lipid mixing at low Ca2+. (C) At 200 μM Ca2+, increasing the concentrations of αKG promotes lipid mixing whereas glutamate does not. (D) Syt1 can promote membrane fusion either by binding to (1) t-SNAREs or (2) lipids, pink circle indicate liposome composed of 100% PC. Blue circle indicate liposome composed of 15% PS and 85% PC. αKG does not alter the Ca2+ dependence of Syt1 binding to (E), ternary SNARE complex or (F) t-SNAREs. (G) Coflotation assay at 0, 10, 30, 100, 150, 200 μM Ca2+ show that αKG promotes Syt1-lipid interaction at low Ca2+. (H) αKG promotes membrane fusion by enhancing lipid binding of Syt1 . NBD: N-7-nitro-2,1,3-benzoxadiazol-4-yl, PC: phosphatidylcholine, PS: phosphatidylserine, rhod: rhodamine, syb: synaptobrevin, syx: syntaxin, t-SNARE: target-N-ethylmaleimide-sensitive factor (NSF) attachment protein receptors. Error bars indicate SD and p values were calculated using Student’s t test, *p < 0.05, **p < 0.01.
Ca2+-Syt1 stimulates membrane fusion by binding to lipids and t-SNAREs (Figure 7D) (Hui et al., 2011). To determine whether αKG enhances t-SNARE interaction with Syt1, we performed co-flotation with membrane embedded SNAREs and the Syt C2AB domain in the absence and presence of Ca2+. In this assay, the liposomes are constituted of 100% phosphatidylcholine (PC), which does not permit the binding of Syt1 to the membrane but allows binding to t-SNAREs (Tucker et al., 2004). These co-flotation assays and GST pull down experiments revealed that αKG did not affect the interaction between C2AB and SNAREs (Figures 7E and 7F). To assess whether αKG enhances lipid interaction, we performed co-flotation assays with C2AB and protein-free liposomes that harbored 15% phosphatdylserine (PS) that permits Syt1 binding (Tucker et al., 2004). In this assay, αKG enhanced C2AB-PS interaction at 25–100 μM [Ca2+] (Figure 7G). Hence, the effect of αKG is specific for the Syt1-lipid interactions, which have been shown to be essential for exocytosis in vivo (Bai et al., 2016; Liu et al., 2014).
Discussion
We show that the loss of idh3a causes a severe increase in mEJP/mEJC frequency and a severe reduction of the EJP/EJC amplitudes when extracellular [Ca2+] are below 2mM (Figures 4A–H). A comparison of the electrophysiological properties at the NMJ of the idh3a mutants with other mitochondrial mutants for which NMJ recordings have been reported in Drosophila reveal that loss of Marf (Sandoval et al., 2014), drp1 (Verstreken et al., 2005), dmiro (Guo et al., 2005), shawn (SLC25A39) (Slabbaert et al., 2016) and porin (Graham et al., 2010) cause phenotypes that are very different from what we observe for idh3a. In addition, the electrophysiological properties of idh3a are also very different from mutations that affect exocytosis or endocytosis at NMJs (Bellen et al., 2010) with two exceptions: synaptotagmin1 (Littleton et al., 1994) and complexin (cpx) (Xue et al., 2009). Partial loss of function mutations in syt1 exhibit severely reduced EJP amplitudes at low extracellular [Ca2+] and increased spontaneous SV release frequencies (Littleton et al., 1994; 1993b) very similar to what is observed in idh3a mutant animals (Figures 4A–H). Moreover, syt1 partial loss of function mutants exhibit reduced SV density along with enlarged SV size in boutons at the NMJs based on TEM (Lee et al., 2013), akin to idh3a mutants (Figures 6D and 6G). In contrast, although cpx mutants display comparable properties at the electrophysiological level, they show striking differences at the morphological level. Their NMJs are vastly expanded when compared to syt1 and idh3a mutants (Huntwork and Littleton, 2007). In addition, TEM revealed no obvious difference in SV size and number (Jorquera et al., 2012), unlike what we observe in syt1 and idh3a. In addition, the levels of Cpx are elevated three-fold in different idh3a mutant animals, arguing that loss of Cpx is not playing a role in the idh3a phenotype, and the electrophysiological and morphological defects associated with idh3a are very similar to those observed in partial loss-of-function mutations of syt1. Finally, food supplementation of αKG is able to suppress some of the synaptic transmission defects associated with loss of idh3a both in photoreceptor terminals and at NMJs (Figures 3A–3F, 4C and 4D). Overall, these results indicate that αKG may regulate Syt1-mediated SV fusion.
To assess how αKG may regulate Syt1, we modulated the αKG levels in a defined reconstituted membrane fusion assay. Syt1 interaction with lipids and tSNAREs is essential for proper membrane fusion to promote neurotransmitter release (Chapman, 2008). The Ca2+ dependent Syt1-lipid interaction is well documented but links to metabolic regulation are ill-defined. We show that αKG promotes the fusion of vesicles in the presence of mouse SNAREs and mouse Syt1 at low [Ca2+] in vitro (Figure 7B and 7C). However, glutamate, a precursor of αKG that has a very similar structure, is unable to induce this effect, emphasizing the specificity of αKG (Figure 7B and 7C). Moreover, by using a coflotation assay that consists of mouse C2AB (cytoplasmic Syt1) and SNARE embedded vesicles with a lipid composition that does not interact with Syt1, we show that the enhanced vesicle fusion in the presence of αKG is not due to Syt1-tSNARE interaction (Figures 7E and 7F). In contrast, performing coflotation assays with C2AB and vesicles with a lipid composition that enables Syt1 interaction in the absence of SNAREs, indicates that αKG enhances vesicle fusion by allowing Syt1 to bind to lipids at low [Ca2+] (Figure 7G). We therefore explored whether αKG is able to enhance this interaction by directly binding to Syt1. However we failed to detect binding of KG to Syt1 using ICT (data not shown), but cannot exclude that αKG may transiently bind to Syt1. Alternatively, αKG may alter the presynaptic membrane properties to enhance Syt1 lipid interaction. Probing precisely how αKG regulates Syt1-lipid interaction will require more research.
These observations may provide a link between mitochondrial dysfunction and some neuronal disorders. IDH3 is a heterotetrameric enzyme complex consisting of 2α, 1β and 1γ subunits (Kim et al., 1995). Very recently, a homozygous mutation in IDH3A has been associated with a severe encephalopathy and intractable epilepsy of infantile onset (Fattal-Valevski et al., 2017). Although the authors claim that the disease is clearly defined as a mitochondrial encephalopathy and the symptoms are confined to the nervous system, the patient differs from canonical mitochondrial cases in key features. Of note, epileptic encephalopathy syndromes have also been associated with mutations in different genes required for synaptic transmission, including SNAP25 (Rohena et al., 2013; Shen et al., 2014), Syntaxin1B (syx1B) (Schubert et al., 2014), Syntaxin1 binding proteins Munc18/STX1BP (Saitsu et al., 2008; Stamberger et al., 2016) and Munc13 (Engel et al., 2016). The loss of the respective fly homologues of these genes including syntaxin1A (Schulze et al., 1995), Rop (STX1BP) (Schulze et al., 1994; Wu et al., 1998), and dunc13 (Aravamudan et al., 1999) all cause a severe decrease in evoked SV release at NMJs. We therefore speculate that the epileptic encephalopathy in IDH3A patient may also be associated with a decrease in synaptic transmission.
In summary we demonstrate that loss of a TCA cycle enzyme, idh3a leads to defects in synaptic transmission due to a reduction in αKG levels. Collectively, our data show that (1) αKG plays a regulatory role in synaptic transmission in vitro and in vivo (2) αKG directly enhances Syt1-lipid interaction and (3) αKG-Syt1-lipid regulation is an evolutionarily conserved mechanism (Figure 7H). Overall, our results reveal that there is a unique, conserved mitochondrial regulation of synaptic transmission at the metabolite level.
Experimental Procedures
Intracellular Recording from Larval Neuromuscular Junction
L3 NMJ recordings were performed as described previously (Verstreken et al., 2002, 2003). Briefly, EJPs were evoked by directly stimulating the segmental nerve innervating a hemisegment A3 through a glass capillary electrode at 0.2 Hz. Stimulus pulses (0.3 ms duration). We recorded mEJPs for 2 minutes and collected 20 EJPs from each larvae and recorded from the single muscle per animal. Paired-pulse stimuli were performed 50 ms apart and 8 responses were collected from each larvae. Data were processed with Mini Analysis Program by Synaptosoft, Clampfit and Excel.
Presynaptic Cytosolic Ca2+ Imaging
Changes in cytosolic Ca2+ levels were measured through relative changes in the fluorescence of the two fluorophores that comprise the fusion protein Gerry. Gerry was created as a UAS construct with mCherry linked to the carboxy terminus of GCaMP6m (Chen et al., 2013) using the 17 amino acid linker from Clophensor (Arosio et al., 2010). Cytosolic Ca2+ levels in the 0.1–1μM range are proportional to the ratio of the fluorescence emissions of GCaMP6m (470/24 nm, ex.; 525/50 nm em.) and mCherry (550/15 nm ex.; 617/73 nm em.). Resting Ca2+ levels, and changes in Ca2+ levels, can be compared between terminals and preparations due to the pseudo-ratiometric advantages of Gerry. Imaging was performed on a Nikon Eclipse FN1 wide-field microscope using a water-dipping 100X 1.10NA objective. The microscope was fitted with a TuCam beam-splitter and two Andor Technology EMCCD cameras (iXonX3 DU897). Excitation was provided by a Lumencor LED light source and separate cameras were dedicated to capturing emission from separate flurophores. Images were collected at 10Hz for each flurophore, yielding ratio measurements at 10Hz.
Reconstituted Membrane Fusion and Coflotation
Reconstituted membrane fusion and coflotation assays were performed as described previously in (Bai et al., 2016).
Statistical Analysis
Two-tailed Student's t tests were used to analyze the data. All data are presented as ± SD unless otherwise mentioned.
Supplementary Material
Highlights.
The TCA cycle enzyme IDH3A is required for synaptic transmission
Loss of idh3a leads to reduced αKG and phenocopies loss of synaptotagmin1 (syt1)
The product of IDH3A, αKG, enhances Syt1-phospholipid interaction in vitro
Acknowledgments
We thank Vivekananda Shetty and the Metabolomics Core Facility in Alkek Center for Molecular Discovery at Baylor College of Medicine for performing the LC/MS. We thank Yuchun He for the injections. We thank Karen Schulze, Manish Jaiswal, Shinya Yamamoto, Nele Haelterman, Kai Li Tan, Megan Campbell, Hsiao-Tuan Chao, Mingshan Xue and Hector Sandoval for their comments on the manuscript. We thank Frieda Li and Brett Graham for the help with aconitase activity assay. We thank Penelope Bonnen for the human IDH3A cDNA. We thank Troy Littleton for fly reagents. Microscopy at Baylor College of Medicine is supported by IDDRC grant number U54 HD083092 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study. H.B. was supported by a postdoctoral fellowship from the Human Frontier Science Program. GTM and MS were supported by NIH NINDS award NS061914. ERC was supported by NIMH RO1 MH061876. HJB was funded by the Robert and Renee Belfer Family Foundation, the Huffington Foundation, and the National Institutes of Health (1RC4GM096355-01). HJB and ERC are Investigators of the Howard Hughes Medical Institute.
Footnotes
Author Contributions: B.U, H.B, G. T. M, E.R.C. and H. J. B. designed the experiments. B. U. H.B. M.S., and Y.Q.L. performed the experiments. L. D and Z.Z. performed TEM imaging. B.U. and H.J.B. wrote the manuscript.
Supplemental Information includes Supplemental Experimental Procedures, ten figures, RRID numbers of materials used in this study and can be found with this article online at doi:10.17632/4j7g9854ph.1
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aravamudan B, Fergestad T, Davis WS, Rodesch CK, Broadie K. Drosophila UNC-13 is essential for synaptic transmission. Nat Neurosci. 1999;2:965–971. doi: 10.1038/14764. [DOI] [PubMed] [Google Scholar]
- Bai H, Xue R, Bao H, Zhang L, Yethiraj A, Cui Q, Chapman ER. Different states of synaptotagmin regulate evoked versus spontaneous release. Nat Commun. 2016;7:10971. doi: 10.1038/ncomms10971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellen HJ, Tong C, Tsuda H. 100 years of Drosophila research and its impact on vertebrate neuroscience: a history lesson for the future. Nature Reviews Neuroscience. 2010;11:514–522. doi: 10.1038/nrn2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla A, Chicka MC, Tucker WC, Chapman ER. Ca(2+)-synaptotagmin directly regulates t-SNARE function during reconstituted membrane fusion. Nat Struct Mol Biol. 2006;13:323–330. doi: 10.1038/nsmb1076. [DOI] [PubMed] [Google Scholar]
- Brière JJ, Favier J, Gimenez-Roqueplo AP, Rustin P. Tricarboxylic acid cycle dysfunction as a cause of human diseases and tumor formation. Am J Physiol, Cell Physiol. 2006;291:C1114–C1120. doi: 10.1152/ajpcell.00216.2006. [DOI] [PubMed] [Google Scholar]
- Burman JL, Yu S, Poole AC, Decal RB, Pallanck L. Analysis of neural subtypes reveals selective mitochondrial dysfunction in dopaminergic neurons from parkin mutants. Proc Natl Acad Sci USa. 2012;109:10438–10443. doi: 10.1073/pnas.1120688109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo SE, Mootha VK. The Mitochondrial Proteome and Human Disease. Annual Review of Genomics and Human Genetics. 2010;11:25–44. doi: 10.1146/annurev-genom-082509-141720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman ER. How does synaptotagmin trigger neurotransmitter release? Annu Rev Biochem. 2008;77:615–641. doi: 10.1146/annurev.biochem.77.062005.101135. [DOI] [PubMed] [Google Scholar]
- Chen K, Lin G, Haelterman NA, Ho TSY, Li T, Li Z, Duraine L, Graham BH, Jaiswal M, Yamamoto S, et al. Loss of Frataxin induces iron toxicity, sphingolipid synthesis, and Pdk1/Mef2 activation, leading to neurodegeneration. Elife. 2016;5:e34863. doi: 10.7554/eLife.16043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin RM, Fu X, Pai MY, Vergnes L, Hwang H, Deng G, Diep S, Lomenick B, Meli VS, Monsalve GC, et al. The metabolite α-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature. 2014;510:397–401. doi: 10.1038/nature13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AG, Selcen D, Shen XM, Milone M, Harper CM. Loss of MUNC13-1 function causes microcephaly, cortical hyperexcitability, and fatal myasthenia. Neurol Genet. 2016;2:e105. doi: 10.1212/NXG.0000000000000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian-Fine R, Verstreken P, Hiesinger PR, Horne JA, Kostyleva R, Zhou Y, Bellen HJ, Meinertzhagen IA. Endophilin promotes a late step in endocytosis at glial invaginations in Drosophila photoreceptor terminals. J Neurosci. 2003;23:10732–10744. doi: 10.1523/JNEUROSCI.23-33-10732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattal-Valevski A, Eliyahu H, Fraenkel ND, Elmaliach G, Hausman-Kedem M, Shaag A, Mandel D, Pines O, Elpeleg O. Homozygous mutation, p. Pro304His, in IDH3A, encoding isocitrate dehydrogenase subunit is associated with severe encephalopathy in infancy. Neurogenetics. 2017;18:57–61. doi: 10.1007/s10048-016-0507-z. [DOI] [PubMed] [Google Scholar]
- Graham BH, Li Z, Alesii EP, Versteken P, Lee C, Wang J, Craigen WJ. Neurologic Dysfunction and Male Infertility in Drosophila porin Mutants: A NEW MODEL FOR MITOCHONDRIAL DYSFUNCTION AND DISEASE. Journal of Biological Chemistry. 2010;285:11143–11153. doi: 10.1074/jbc.M109.080317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Macleod GT, Wellington A, Hu F, Panchumarthi S, Schoenfield M, Marin L, Charlton MP, Atwood HL, Zinsmaier KE. The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron. 2005;47:379–393. doi: 10.1016/j.neuron.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Hadzhieva M, Kirches E, Mawrin C. Review: iron metabolism and the role of iron in neurodegenerative disorders. Neuropathol Appl Neurobiol. 2014;40:240–257. doi: 10.1111/nan.12096. [DOI] [PubMed] [Google Scholar]
- Haelterman NA, Jiang L, Li Y, Bayat V, Sandoval H, Ugur B, Tan KL, Zhang K, Bei D, Xiong B, et al. Large-scale identification of chemically induced mutations in Drosophila melanogaster. Genome Res. 2014;24:1707–1718. doi: 10.1101/gr.174615.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Pluhackova K, Böckmann RA. The Multifaceted Role of SNARE Proteins in Membrane Fusion. Front Physiol. 2017;8:5. doi: 10.3389/fphys.2017.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie RC, Raghu P. Visual transduction in Drosophila. Nature. 2001;413:186–193. doi: 10.1038/35093002. [DOI] [PubMed] [Google Scholar]
- Hui E, Gaffaney JD, Wang Z, Johnson CP, Evans CS, Chapman ER. Mechanism and function of synaptotagmin-mediated membrane apposition. Nat Struct Mol Biol. 2011;18:813–821. doi: 10.1038/nsmb.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntwork S, Littleton JT. A complexin fusion clamp regulates spontaneous neurotransmitter release and synaptic growth. Nat Neurosci. 2007;10:1235–1237. doi: 10.1038/nn1980. [DOI] [PubMed] [Google Scholar]
- Ivannikov MV, Macleod GT. Mitochondrial Free Ca2+ Levels and Their Effects on Energy Metabolism in Drosophila Motor Nerve Terminals. Biophysical Journal. 2013;104:2353–2361. doi: 10.1016/j.bpj.2013.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Scheller RH. SNAREs — engines for membrane fusion. Nature Reviews Molecular Cell Biology. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- Jorquera RA, Huntwork-Rodriguez S, Akbergenova Y, Cho RW, Littleton JT. Complexin controls spontaneous and evoked neurotransmitter release by regulating the timing and properties of synaptotagmin activity. J Neurosci. 2012;32:18234–18245. doi: 10.1523/JNEUROSCI.3212-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YO, Oh IU, Park HS, Jeng J, Song BJ, Huh TL. Characterization of a cDNA clone for human NAD(+)-specific isocitrate dehydrogenase alpha-subunit and structural comparison with its isoenzymes from different species. Biochem J. 1995;308(Pt 1):63–68. doi: 10.1042/bj3080063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh TW, Bellen HJ. Synaptotagmin I, a Ca2+ sensor for neurotransmitter release. Trends Neurosci. 2003;26:413–422. doi: 10.1016/S0166-2236(03)00195-4. [DOI] [PubMed] [Google Scholar]
- Krebs HA, Johnson WA. Metabolism of ketonic acids in animal tissues. Biochem J. 1937;31:645–660. doi: 10.1042/bj0310645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurusu M, Katsuki T, Zinn K, Suzuki E. Developmental changes in expression, subcellular distribution, and function of Drosophila N-cadherin, guided by a cell-intrinsic program during neuronal differentiation. Dev Biol. 2012;366:204–217. doi: 10.1016/j.ydbio.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Guan Z, Akbergenova Y, Littleton JT. Genetic analysis of synaptotagmin C2 domain specificity in regulating spontaneous and evoked neurotransmitter release. J Neurosci. 2013;33:187–200. doi: 10.1523/JNEUROSCI.3214-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddell JR. Targeting mitochondrial metal dyshomeostasis for the treatment of neurodegeneration. Neurodegener Dis Manag. 2015;5:345–364. doi: 10.2217/nmt.15.19. [DOI] [PubMed] [Google Scholar]
- Littleton JT, Bellen HJ, Perin MS. Expression of synaptotagmin in Drosophila reveals transport and localization of synaptic vesicles to the synapse. Development. 1993a;118:1077–1088. doi: 10.1242/dev.118.4.1077. [DOI] [PubMed] [Google Scholar]
- Littleton JT, Stern M, Perin M, Bellen HJ. Calcium dependence of neurotransmitter release and rate of spontaneous vesicle fusions are altered in Drosophila synaptotagmin mutants. Proc Natl Acad Sci USa. 1994;91:10888–10892. doi: 10.1073/pnas.91.23.10888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littleton JT, Stern M, Schulze K, Perin M, Bellen HJ. Mutational analysis of Drosophila synaptotagmin demonstrates its essential role in Ca(2+)-activated neurotransmitter release. Cell. 1993b;74:1125–1134. doi: 10.1016/0092-8674(93)90733-7. [DOI] [PubMed] [Google Scholar]
- Liu H, Bai H, Xue R, Takahashi H, Edwardson JM, Chapman ER. Linker mutations reveal the complexity of synaptotagmin 1 action during synaptic transmission. Nat Neurosci. 2014;17:670–677. doi: 10.1038/nn.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishur RJ, Khan M, Munkácsy E, Sharma L, Bokov A, Beam H, Radetskaya O, Borror M, Lane R, Bai Y, et al. Mitochondrial metabolites extend lifespan. Aging Cell. 2016;15:336–348. doi: 10.1111/acel.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome TP, Asling B, Dickson BJ. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development. 2000;127:851–860. doi: 10.1242/dev.127.4.851. [DOI] [PubMed] [Google Scholar]
- Parnas D, Haghighi AP, Fetter RD, Kim SW, Goodman CS. Regulation of postsynaptic structure and protein localization by the Rho-type guanine nucleotide exchange factor dPix. Neuron. 2001;32:415–424. doi: 10.1016/s0896-6273(01)00485-8. [DOI] [PubMed] [Google Scholar]
- Peter A, Schöttler P, Werner M, Beinert N, Dowe G, Burkert P, Mourkioti F, Dentzer L, He Y, Deak P, et al. Mapping and identification of essential gene functions on the X chromosome of Drosophila. EMBO Rep. 2002;3:34–38. doi: 10.1093/embo-reports/kvf012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poskanzer KE, Marek KW, Sweeney ST, Davis GW. Synaptotagmin I is necessary for compensatory synaptic vesicle endocytosis in vivo. Nature. 2003;426:559–563. doi: 10.1038/nature02184. [DOI] [PubMed] [Google Scholar]
- Rizo J, Rosenmund C. Synaptic vesicle fusion. Nat Struct Mol Biol. 2008;15:665–674. doi: 10.1038/nsmb.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizo J, Xu J. The Synaptic Vesicle Release Machinery. Annual Review of Biophysics. 2015;44:339–367. doi: 10.1146/annurev-biophys-060414-034057. [DOI] [PubMed] [Google Scholar]
- Rohena L, Neidich J, Truitt Cho M, Gonzalez KD, Tang S, Devinsky O, Chung WK. Mutation in SNAP25 as a novel genetic cause of epilepsy and intellectual disability. Rare Dis. 2013;1:e26314. doi: 10.4161/rdis.26314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustin P, Bourgeron T, Parfait B, Chretien D, Munnich A, Rötig A. Inborn errors of the Krebs cycle: a group of unusual mitochondrial diseases in human. Biochim Biophys Acta. 1997;1361:185–197. doi: 10.1016/s0925-4439(97)00035-5. [DOI] [PubMed] [Google Scholar]
- Saitsu H, Kato M, Mizuguchi T, Hamada K, Osaka H, Tohyama J, Uruno K, Kumada S, Nishiyama K, Nishimura A, et al. De novo mutations in the gene encoding STXBP1 (MUNC18-1) cause early infantile epileptic encephalopathy. Nat Genet. 2008;40:782–788. doi: 10.1038/ng.150. [DOI] [PubMed] [Google Scholar]
- Sandoval H, Yao CK, Chen K, Jaiswal M, Donti T, Lin YQ, Bayat V, Xiong B, Zhang K, David G, et al. Mitochondrial fusion but not fission regulates larval growth and synaptic development through steroid hormone production. Elife. 2014;3:211. doi: 10.7554/eLife.03558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarov M, Barz C, Jambor H, Hein MY, Schmied C, Suchold D, Stender B, Janosch S, KJVV, Krishnan RT, et al. A genome-wide resource for the analysis of protein localisation in Drosophila. Elife. 2016;5:e12068. doi: 10.7554/eLife.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert J, Siekierska A, Langlois M, May P, Huneau C, Becker F, Muhle H, Suls A, Lemke JR, de Kovel CGF, et al. Mutations in STX1B, encoding a presynaptic protein, cause fever-associated epilepsy syndromes. Nat Genet. 2014;46:1327–1332. doi: 10.1038/ng.3130. [DOI] [PubMed] [Google Scholar]
- Schulze KL, Broadie K, Perin MS, Bellen HJ. Genetic and electrophysiological studies of Drosophila syntaxin-1A demonstrate its role in nonneuronal secretion and neurotransmission. Cell. 1995;80:311–320. doi: 10.1016/0092-8674(95)90414-x. [DOI] [PubMed] [Google Scholar]
- Schulze KL, Littleton JT, Salzberg A, Halachmi N, Stern M, Lev Z, Bellen HJ. rop, a Drosophila homolog of yeast Sec1 and vertebrate n-Sec1/Munc-18 proteins, is a negative regulator of neurotransmitter release in vivo. Neuron. 1994;13:1099–1108. doi: 10.1016/0896-6273(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Hussain R, Gago N, Oudinet JP, Mattern C, Ghoumari AM. Progesterone synthesis in the nervous system: implications for myelination and myelin repair. Front Neurosci. 2012;6:10. doi: 10.3389/fnins.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrooke S, Qiu X, Stewart BA. Nonmuscle Myosin II helps regulate synaptic vesicle mobility at the Drosophila neuromuscular junction. BMC Neurosci. 2010;11:37. doi: 10.1186/1471-2202-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen XM, Selcen D, Brengman J, Engel AG. Mutant SNAP25B causes myasthenia, cortical hyperexcitability, ataxia, and intellectual disability. Neurology. 2014;83:2247–2255. doi: 10.1212/WNL.0000000000001079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabbaert JR, Kuenen S, Swerts J, Maes I, Uytterhoeven V, Kasprowicz J, Fernandes AC, Blust R, Verstreken P. Shawn, the Drosophila Homolog of SLC25A39/40, Is a Mitochondrial Carrier That Promotes Neuronal Survival. J Neurosci. 2016;36:1914–1929. doi: 10.1523/JNEUROSCI.3432-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamberger H, Nikanorova M, Willemsen MH, Accorsi P, Angriman M, Baier H, Benkel-Herrenbrueck I, Benoit V, Budetta M, Caliebe A, et al. STXBP1 encephalopathy: A neurodevelopmental disorder including epilepsy. Neurology. 2016;86:954–962. doi: 10.1212/WNL.0000000000002457. [DOI] [PubMed] [Google Scholar]
- Stevens RJ, Akbergenova Y, Jorquera RA, Littleton JT. Abnormal synaptic vesicle biogenesis in Drosophila synaptogyrin mutants. J Neurosci. 2012;32:18054-67–18067a. doi: 10.1523/JNEUROSCI.2668-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC. Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron. 2013;80:675–690. doi: 10.1016/j.neuron.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TeSlaa T, Chaikovsky AC, Lipchina I, Escobar SL, Hochedlinger K, Huang J, Graeber TG, Braas D, Teitell MA. α-Ketoglutarate Accelerates the Initial Differentiation of Primed Human Pluripotent Stem Cells. Cell Metab. 2016;24:485–493. doi: 10.1016/j.cmet.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimbuch T, Rosenmund C. Should I stop or should I go? The role of complexin in neurotransmitter release. Nature Reviews Neuroscience. 2016;17:118–125. doi: 10.1038/nrn.2015.16. [DOI] [PubMed] [Google Scholar]
- Tucker WC, Weber T, Chapman ER. Reconstitution of Ca2+-regulated membrane fusion by synaptotagmin and SNAREs. Science. 2004;304:435–438. doi: 10.1126/science.1097196. [DOI] [PubMed] [Google Scholar]
- Venken KJT, Popodi E, Holtzman SL, Schulze KL, Park S, Carlson JW, Hoskins RA, Bellen HJ, Kaufman TC. A molecularly defined duplication set for the X chromosome of Drosophila melanogaster. Genetics. 2010;186:1111–1125. doi: 10.1534/genetics.110.121285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreken P, Ly CV, Venken KJT, Koh TW, Zhou Y, Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Vos M, Lauwers E, Verstreken P. Synaptic mitochondria in synaptic transmission and organization of vesicle pools in health and disease. Front Synaptic Neurosci. 2010;2:139. doi: 10.3389/fnsyn.2010.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CF, Wong F. Frequency characteristics in the visual system of Drosophila: genetic dissection of electroretinogram components. J Gen Physiol. 1977;69:705–724. doi: 10.1085/jgp.69.6.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MN, Littleton JT, Bhat MA, Prokop A, Bellen HJ. ROP, the Drosophila Sec1 homolog, interacts with syntaxin and regulates neurotransmitter release in a dosage-dependent manner. Embo J. 1998;17:127–139. doi: 10.1093/emboj/17.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N, Yang M, Gaur U, Xu H, Yao Y, Li D. Alpha-Ketoglutarate: Physiological Functions and Applications. Biomol Ther (Seoul) 2016;24:1–8. doi: 10.4062/biomolther.2015.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Lin YQ, Pan H, Reim K, Deng H, Bellen HJ, Rosenmund C. Tilting the balance between facilitatory and inhibitory functions of mammalian and Drosophila Complexins orchestrates synaptic vesicle exocytosis. Neuron. 2009;64:367–380. doi: 10.1016/j.neuron.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Jaiswal M, Charng WL, Gambin T, Karaca E, Mirzaa G, Wiszniewski W, Sandoval H, Haelterman NA, Xiong B, et al. A drosophila genetic resource of mutants to study mechanisms underlying human genetic diseases. Cell. 2014;159:200–214. doi: 10.1016/j.cell.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Liang X, Sun X, Zhang L, Fu X, Rogers CJ, Berim A, Zhang S, Wang S, Wang B, et al. AMPK/α-Ketoglutarate Axis Dynamically Mediates DNA Demethylation in the Prdm16 Promoter and Brown Adipogenesis. Cell Metab. 2016 doi: 10.1016/j.cmet.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Kwon SE, Gaffaney JD, Dunning FM, Chapman ER. Uncoupling the roles of synaptotagmin I during endo- and exocytosis of synaptic vesicles. Nat Neurosci. 2011;15:243–249. doi: 10.1038/nn.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdzisińska B, Żurek A, Kandefer-Szerszeń M. Alpha-Ketoglutarate as a Molecule with Pleiotropic Activity: Well-Known and Novel Possibilities of Therapeutic Use. Archivum Immunologiae Et Therapiae Experimentalis. 2016:1–16. doi: 10.1007/s00005-016-0406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Koh YH, Beckstead RB, Budnik V, Ganetzky B, Bellen HJ. Synaptic vesicle size and number are regulated by a clathrin adaptor protein required for endocytosis. Neuron. 1998;21:1465–1475. doi: 10.1016/s0896-6273(00)80664-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.