Abstract
Increasing evidence suggests that the human hippocampus contributes to a range of different behaviors, including episodic memory, language, short-term memory, and navigation. A novel theoretical framework, the Precision and Binding Model, accounts for these phenomenon by describing a role for the hippocampus in high-resolution, complex binding. Other theories like Cognitive Map Theory, in contrast, predict a specific role for the hippocampus in allocentric navigation, while Declarative Memory Theory predicts a specific role in delay-dependent conscious memory. Navigation provides a unique venue for testing these predictions, with past results from research with humans providing inconsistent findings regarding the role of the human hippocampus in spatial navigation. Here, we tested five patients with lesions primarily restricted to the hippocampus and those extending out into the surrounding medial temporal lobe cortex on a virtual water maze task. Consistent with the Precision and Binding Model, we found partially intact allocentric memory in all patients, with impairments in the spatial precision of their searches for a hidden target. We found similar impairments at both immediate and delayed testing. Our findings are consistent with the Precision and Binding Model of hippocampal function, arguing for its role across domains in high-resolution, complex binding.
Introduction
Navigation, particularly the ability to locate goal locations in one’s environment, is a critical skill for survival in many species, including humans. O’Keefe and Nadel (1978) proposed Cognitive Map Theory which argues that the hippocampus is necessary for allocentric representations, that is, combining multiple distal cues to infer ones’ location in space. In contrast, navigating in reference to the current viewpoint (egocentric navigation), does not depend on the hippocampus. In support of the role of this structure in allocentric spatial navigation, lesioning the rodent hippocampus results in profound impairments in spatial memory, specifically, locating a target location relative to distal cues (Eichenbaum et al., 1999; Morris et al., 1982). Importantly, however, lesions to the rodent hippocampus do not impair performance if a cue is placed at the target location or if the animal can use an already learned trajectory (D’Hooge & De Deyn, 2001; Morris, 1984; Morris et al., 1982; Moser et al.,1995). Together, these findings suggest a primary role for the hippocampus in allocentric navigation.
Replicating such findings in humans, though, has proven challenging. While several studies have shown impairments on the virtual Morris Water Maze (vMWM) following partial MTL damage (Astur et al., 2002; Bartsch et al., 2010; Goodrich-Hunsaker et al., 2010) other studies have not found the same pattern as found in rats (Bohbot & Corkin, 2007; Bohbot et al., 1998; Kolarik et al., 2016). As a means of reconciling such contradictory findings and incorporating findings regarding the effects of MTL lesions on other forms of perceptual processing, Yonelinas (2013) proposed the Precision and Binding Model (PBM), which argues that the hippocampus is necessary for complex high-resolution binding. According to PBM, the hippocampus becomes critical when a task requires binding multiple elements rather than simple associations and that the task becomes more dependent on the hippocampus as the resolution of that information increases. Recent work from both perceptual and short-term memory experiments provides support for this model (Aly et al., 2013; Goodrich & Yonelinas, 2016; Lee et al., 2012; Warren et al., 2012). Specifically, these data argue that the hippocampus is necessary only when the information to be remembered is complex (i.e. multiple elements) and high-resolution (requiring specificity).
Recent work from our lab has provided evidence consistent with PBM in the context of navigation. A patient with bilateral hippocampal lesions performed well above chance on a virtual water maze, but her search trajectories lacked the spatial precision exhibited by control participants (Kolarik et al., 2016). Although consistent with PBM, one potential criticism of that study is that the start position on probe trials was the same as one used during training, thus the patient could have used an egocentric strategy. Additionally, we administered only one probe trial for each of the two target locations, and single trials may provide noisy estimates of a participant’s spatial knowledge. Finally, some studies suggest that the hippocampus only comes online when the capacity of working memory is exceeded (Jeneson et al., 2011), yet our experimental design did not require information to be maintained over time.
To address these criticisms, we tested patients with bilateral (N=2) and unilateral (N=3) MTL damage on a vMWM that used novel start locations on probe trials. If the MTL is essential for all forms of allocentric representations, we should see severe impairments on trials starting from a novel position. However, if the hippocampus instead plays a role in spatial precision, we would expect some coarse allocentric memory to be preserved following hippocampal damage while observing impairments in spatial precision. Additionally, by including multiple probe trials for each location, we were able to compare performance on immediate and delay probe trials. If the hippocampus is only necessary for tasks requiring long-term memory, we should only observe impairments on probe trials after the delay. In contrast, if MTL is important for spatial precision at any time scale, we would anticipate precision deficits at both immediate and delayed testing.
Methods
Participants
We tested five amnestic patients (3 male) with a mean age of 38.8 years and mean education level of 15.6 years. We compared them with 10 age- and education-matched controls (mean age 36.7 years (range 26–58), mean education 16.5 years) from the greater Sacramento area. All five patients and nine of the ten controls underwent a neuropsychological test battery consisting of the Shipley (Shipley, 1940), WMS-R (Wechsler, 1987), and Doors and People Test (Baddeley et al., 1994). We were unable to test one control subject because they were unable to return to the lab for an additional testing session. We estimated WAIS-R IQ (Zachary et al., 1985) with the Shipley for patients (mean=98.6) and controls (mean=110.8). Table 1 lists each patients’ age, education and origin of MTL damage along with scores on neuropsychological tests.
Table 1.
Neuropsychological Test Scores
| Patient | Damage | Age | Education | WAIS-R | WMS-R (z-score) | D&P | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Est IQ | Verbal | Visual | Gen. | Attn. | Delay | Overall (%) | ||||
| 1001 | HPC | 57 | 16 | 110 | −0.87 | −1 | −1 | 1.3 | −0.47 | 25 |
| 1006 | HPC | 35 | 17 | 110 | −1.33 | 0.33 | −0.87 | 0.2 | −2.13 | 1 |
| 1009 | MTL | 41 | 17 | 97 | −1.6 | 0.4 | −1.13 | −0.67 | −0.6 | 50 |
| 1027 | MTL | 26 | 16 | 104 | −1.13 | 1.27 | −0.06 | 0.27 | −0.67 | 10 |
| 1028 | MTL | 35 | 12 | 72 | −1.53 | −1.46 | −1.67 | −1.87 | −2.27 | N/A |
|
| ||||||||||
| Amnesics (N=5) | 38.8 | 15.6 | 98.6 | −1.292 | −0.092 | −0.946 | −0.154 | −1.228 | 21.5 | |
| Controls (N=10) | 36.7 | 16.2 | 110.8 | −0.24 | 1.07 | 0.35 | 0.64 | 0.26 | 57.7 | |
WAIS-R (Wechsler Adult Intelligence Scale Revised) WMS-R (Wechsler Memory Scale Revised) D&P (Doors and People)
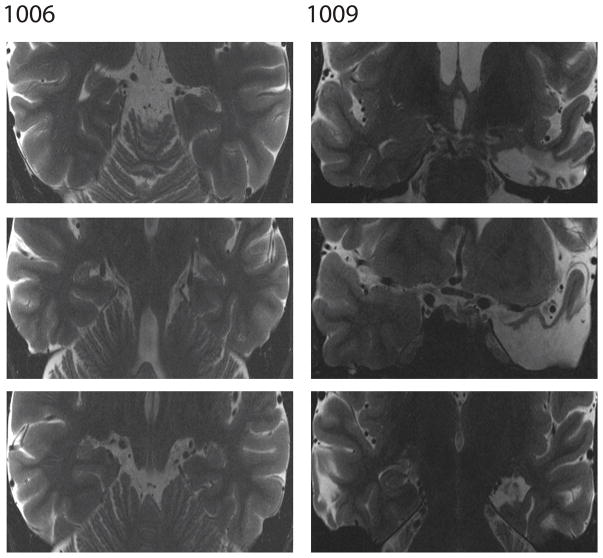
We briefly summarize patient neurological characteristics here and refer the reader to Figure 1 as well as additional papers in which more detail work-ups are available for patients. Patient 1001 displayed abnormal necrotic cavities as a result of Hashimoto’s encephalopathy. The cavities were visible in bilateral hippocampus though slightly less pronounced in the right hippocampus. The patient’s cavities were rounded and were consistent with cavities exhibited by individuals with hypoxia-related CA1 necrosis (Koen et al., 2016; Nakada et al., 2005). No damage was apparent in any other region. Patient 1006 suffered selective bilateral hippocampal damage following closed-head traumatic brain injury from a car accident. Both left and right hippocampus were significantly reduced in volume relative to control subjects while the rest of the brain appeared normal (see Addante et al., 2012; Aly et al., 2013 for a volumetric analysis). Patient 1009 underwent a left temporal lobectomy to treat epilepsy. Approximately 4 cm of the anterior temporal lobe including the anterior half of the hippocampus and anterior third of the parahippocampal cortex was removed. The rest of the brain appeared normal on a high-resolution structural MRI (Goodrich & Yonelinas, 2016). Patient 1027 had a right temporal lobectomy to alleviate epilepsy. The surgery was a standard right anterior temporal lobe resection with approximately 4cm of the anterior temporal lobe including the anterior half of the hippocampus, the amygdala and the anterior third of the parahippocampal gyrus were removed. The rest of the brain appeared normal on a clinical MRI scan (Goodrich & Yonelinas, 2016). Patient 1028 underwent a standard left temporal lobectomy to alleviate epilepsy. Approximately 4cm of the anterior temporal lobe neocortex and ~2cm of the anterior superior temporal gyrus were removed. The patient also had a complete resection of the left amygdala and 4.5cm of the left hippocampus including the entire hippocampal head and the anterior half of the body.
Figure 1.
Experimental Design
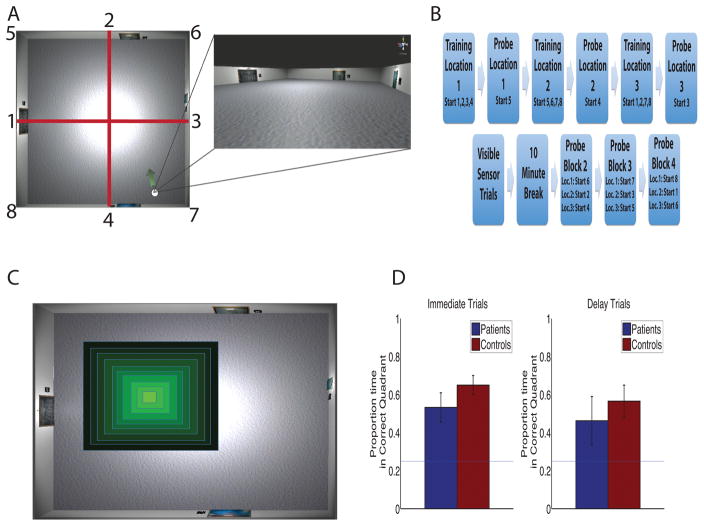
Participants performed a virtual reality analog of the Morris Water Maze created in Unity 3d (Unity Technologies, San Francisco) similar to the task used in Kolarik et al. (2016). The task was modeled on (Astur et al., 2002) which previously demonstrated chance levels of performance in MTL patients on the virtual Morris Water Maze with some important modifications that we detail below. The task required the participants to explore a virtual reality room presented on a computer screen using keyboard arrow keys to navigate the room in a first-person perspective (Fig. 2a). The room was 8 x 8 virtual meters, with 4 unique paintings, unevenly spaced, one on each wall. Participants were instructed to find a hidden invisible target located on the floor of the room. They were instructed to get back to the location of each target, one at a time, as quickly as possible. The hidden target was a .4 x.4 virtual meter square, occupying 0.25% of the total room area. When the participant virtually walked over the target, an onscreen prompt displayed ‘You found the hidden target’ and a 10-s countdown timer started in the corner of the screen during which time they were able to freely navigate. After the 10 s of free navigation, an inter-trial screen was presented and participants clicked on a button to begin the next trial. Participants completed 20 training trials in 5 blocks of 4 trials each for each target location. The starting position for the training trials was chosen from 8 positions around the perimeter of the room (see Figure 2a–b for task schematic).
Figure 2.
On trial 21 (the probe trial), unbeknownst to the participant, traversing the hidden location no longer resulted in an automatic end to the trial. The probe trial functioned just as the training trials with the exception that feedback was not given regarding whether they were in the correct position, allowing a more detailed analysis of spatial memory (Astur et al., 2002; Morris et al., 1982). On this trial, the starting position was fixed for every participant and different than those used for the training trials. Probe trials terminated after 30 s and the entire procedure was repeated for two more target locations.
Following the third probe trial, there were eight trials in which the hidden goal was visible and the participants simply had to navigate to it. This condition served to control for motivational or motoric deficits in performing the task. Following the visible target trials, participants took a 10 minute break to minimize fatigue. After the break participants performed an additional 9 probe trials, with 3 trials for each target location. Importantly, the starting positions for each of these was different than those used for the training trials and immediate probe trials. This ensured that they were not using a simple view matching strategy from encoding to remember the locations or remembering what they had learned during the visible target trials. These ‘delay trials,’ which were administered after the break, thus allow a direct comparison with ‘immediate trials,’ those that were administered immediately after the training trials. This allowed us to extend our previous experimental design by requiring the participants remember multiple hidden goal locations from novel start points both at immediate test and a delay. Throughout the entire session, the patient’s location within the environment was recorded to a text file at a rate of 20 samples per second.
Data analysis
Data were analyzed using a combination of custom written Matlab code and SPSS (Version 20, IBM Corp. Armonk, NY). Following previous methods of analyzing tasks of this nature (i.e., Astur et al., 2002; Morris et al., 1982), the room was divided into quadrants using the North–South and East–West axes (red lines Figure 2a). If participants had correctly encoded the location of the target, then they should spend significantly more time on the probe trials in the quadrant where the target had previously been located. The quadrant analysis, however, gives little information about the precision of spatial memory. Nonetheless, we provide this analysis approach to be consistent with past such studies using the real and virtual Morris Water Maze.
To better assay spatial precision, we calculated the amount of time spent within a sliding window centered on the location of the hidden target. For each probe trial, 10 individual precision windows were calculated, each of which was a square ranging from .4 to 4 virtual meters in size (Fig. 2c). Critically, each analysis square was centered on the hidden target’s location and ranged from the size of the target to the size of a quadrant. Thus, the “highest” precision square contained only the target (.4 x .4 m, the lightest green square in Figure 1C) while the largest square was 4 x 4 m (darkest outer square). Since the precision windows increase in size and are all centered on the target they are nested and larger areas contain the area of smaller windows. We therefore, calculated the proportion of time spent in the unique area encompassed by each of these sliding windows (different colored areas figure 1C) to give us proportion of time spent in each of these areas which increase in distance from the target location. This allowed us to determine the precision of the spatial memory using non-arbitrary metrics. See Kolarik et al. (2016) for detailed description of precision analysis.
We estimated chance performance for the precision analysis using a bootstrapping procedure in which we resampled every control participants’ trajectory throughout the room over the entire session (80 trials), resulting in a series of random trajectories through the environment (see Kolarik et al., 2016). This approach calculates every participant’s trajectory over all trials, giving a sample of all possible trajectories through the environment regardless of the goal. The 10 precision windows were then imposed on these trajectories, proportion of time in each of those windows was calculated, and then the windows shifted by 50 units in either the x or y dimension until the entire area of the room was covered. This resulted in 1600 separate calculations for each precision window for each participant that were then averaged to give an estimate of chance performance. This number represents the likelihood that a participant would spend a given amount of time in a precision window simply by chance, and thus provides a baseline estimate for a random search with no knowledge of the target. For more detail see Kolarik et al. (2016).
Results
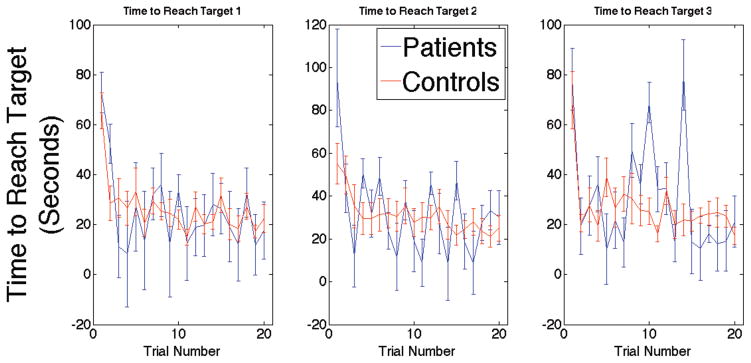
First, we assessed learning of the three different locations over the training trials to ensure that both patients and controls learned the task. We did this by analyzing acquisition trials, as is conventional in the Morris Water Maze (Astur et al., 2002; Morris et al., 1982). We compared the slope of the learning curve (time taken to find the target over all 20 training trials) for each of the three target locations for both patients and controls (Figure 3). We conducted a Group (Patient vs. Control) x Target (1-2-3) mixed ANOVA on the slopes, i.e., the rate of learning over the training trial as measured by time to reach the hidden target. This analysis revealed no statistically significant effects for either Group (F(1,13)=.427, p=.525) or Target (F(2,26)=2.03, p=.150). These findings suggested that both patients and controls learned the locations of each of the three targets at a similar rate over the training trials
Figure 3.
Next, we assayed performance on the probe trials, which provided a measure of knowledge of the position of the hidden location (Morris et al, 1982). We thus compared the amount of time spent in the quadrant where the targets had previously been located on both immediate and delay trials (Figure 2d). Immediate trials are those that occurred right after the last training trial while delay trials are those that occurred after the 10 minute break. Note that each entry into the ANOVA involved the average of three different probe trials for immediate trials and nine probe trials for delay. No significant difference were observed for the amount of time spent in the correct quadrant for immediate trials (t(13)=1.57, p=.138) or for delay trials (t(13)=.391, p=.701). These results indicate that the MTL patients were not impaired in their spatial search relative to controls when using the coarse quadrant metric. We also compared the patient’s average distance from the correct target location on probe trials relative to the average distance to the other two target locations. On average, patients were closer to the correct target location than to the non-target locations (2.9 vs 3.9 virtual meters). A two-sample t-test showed that patients were on average significantly closer to the correct target location than to the other locations across probe trials t(10)=3.028, p=.0127. This distance analysis thus provides a statistically positive finding (rather than a null finding for the quadrant analysis) that patients indeed had a partially intact memory for the hidden target location. Together, these results indicate that patients were not simply confusing the multiple locations and were indeed searching the correct area of the target.
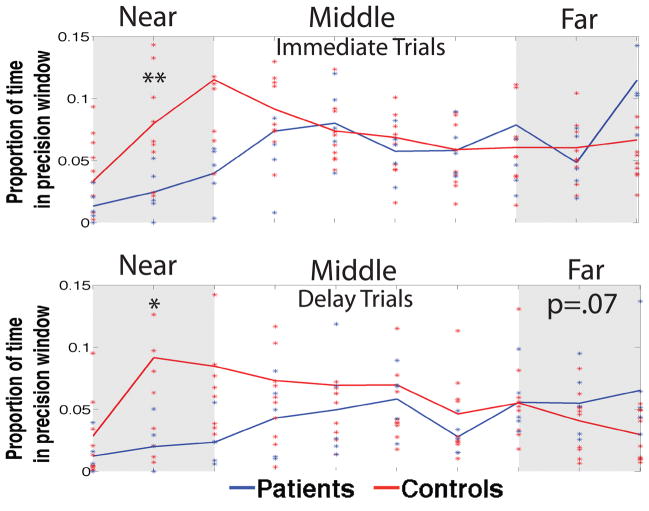
As we have previously noted, however, the quadrant analysis potentially misses information in probe trial search trajectories regarding the precision of their spatial search (Kolarik et al., 2016). We therefore conducted a 2-D sliding window analysis, with each window centered on the target, to better assay deficits in search patterns (see Methods and Kolarik et al., 2016 for more detail). To assess spatial precision, we first fit each participants data across all precision windows and calculated the area under the curve (AUC) separately for the closest 3 (near) the middle 4 (middle) and furthest 3 (far) precision windows separately for immediate and delay trials (Figure 4). We then conducted a Group (Patient vs. Control) x Window (Near-Mid-Far) x Time (Immediate vs. Delay) mixed effects ANOVA on the AUC data.
Figure 4.
We found a significant main effect of Window (F(2,26)=6.83, p=.004), a main effect of Time (F(1,13)=6.59, p=.023), and a Window x Group interaction (F(2,26)=5.64, p=.009) but no Group x Time interaction. Because of our a priori hypotheses about patient performance based on our previous findings (Kolarik et al. 2016), we conduced one-tailed post-hoc independent sample t-tests. Patients spent significantly less time in the smallest precision windows for both immediate (t(13)=−2.736, p=.008) and delay trials (t(13)=−2.170, p=.024). There was no significant group difference in the amount of time spent in the intermediate-distance windows and a trending effect for the patients to spend more time in the furthest windows on delay trials (t(13)=1.50, p=.078). These results highlight the specific impairment demonstrated by patients in which they spend less time in the windows near the target and more (though not quite statistically significant) time in the intermediate and further distanced areas around the hidden target.
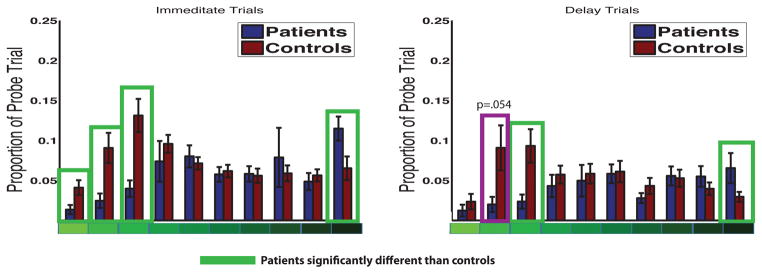
To more precisely assess the precision data, we performed a 2 x 2 x 10 (Group x Time (immediate vs delay) x Precision Window) mixed effects ANOVA using individual precision window proportions with Greenhouse-Geisser correction for a violation of sphericity (χ2(44)= 96.46, p=.00001 (Figure 5). We found a main effect of group (F(1,13)=4.98, P=.04), a main effect of precision window (F(2.9,38.7)=3.016, p=.042), main effect of time (F(1,13)=11.2, p=.005), and a precision window by group interaction (F(2.9,38.7) =4.72, p=.007). Post-hoc t-tests revealed significant group differences on immediate trials in window 1 (t(13)=1.96, p=.035), window 2 (t(13)=2.36, p=.017), window 3 (t(13)=2.97, p=.005) and window 10 (t(13)=−2.102, p=.025). On delay trials, there were significant group effects at window 3 (t(13) =2.26, p=.020) and window 10 (t(13)=−2.307, p=.019) and a trend level effect at window 2 (t(13)=1.72, p=.054). There was one high performing control participant at delay window 2 (z=2.3) which when removed from the analysis resulted in a significant difference between patients and controls at delay window 2 (t(13)=1.81, p=.047). These findings show that patients exhibited significant impairments in the smallest precision windows compared to controls, an effect present at both immediate and delay testing.
Figure 5.
We next compared patient precision to estimated chance precision at each of the 10 precision windows. Levene’s test indicated unequal variances in our two samples (patients and estimated chance) at several precision windows. Therefore, for comparisons at those windows we used Welch’s test (Ruxton, 2006; Welch, 1938), a t-test for unequal variances which uses pooled degrees of freedom (in our case df=4) calculated with the Welch-Satterthwaite equation (Satterthwaite, 1946). In the case of equal variance assumptions being met, independent 2-sample t-tests were conducted. On immediate trials, patient precision was not different than that of chance in window 1 (t(4) =1.91, p=.128), window 2 (t(4)=1.92, p=.126), window 3 (t(4)=2.73, p=.052), window 4 (t(4)=2.30, p=.083) and window 8 (t(4)=1.36, p=.245). However, patients were significantly above chance in window 5 (t(4)=4.45, p=.011) window 6 (t(4)=3.86, p=.018), window 7 (t(4)=3.40, p=.027), window 9 t(6403)=2.153, p=.031 and window 10 (t(4)=5.40, p=.006). On delay trials, patient performance was no different than chance in window 1 (t(4)=1.33, p=.252), window 2 (t(4)=1.35, p=.248), window 3 (t(4)=1.36, p=.243), window 4 (t(4)=1.99, p=.117), window 5 (t(4)=1.56, p=.193), window 7 (t(4)=.385, p=.720) and window 10 (t(4)=1.63, p=.166). However, patients were significantly above chance on delay window 6 (t(4)=3.080, p=.037) window 8 (t(6403)=3.64, p<.00001) and window 9 (t(6403)=2.94, p=.003). Together, these results again highlight the precision impairment resulting from medial temporal lobe damage by demonstrating chance performance in the precision windows immediately surrounding target. Importantly, since patients were different from chance performance in the larger precision windows, these findings converge with our earlier quadrant and distance analyses to suggest that patient trajectories were not random but rather lacking the precision seen in controls.
While we found deficits in the precision of spatial searches by patients compared to controls, importantly, there was no significant Group x Time interaction. We also found impairments in precision of comparable extents at both immediate and delayed test for patients, both compared to controls and chance. These findings indicated that patients and controls did not differ on their spatial searches as a function of delay. While there was a main effect of time, as noted above, this indicated that overall performance for both groups dropped after the 10-minute break, consistent with the idea that memory typically worsens over a delay. These results highlight the impairment in precision demonstrated by patients during their spatial search where they spend less time relative to controls in the precision windows closest to the target on both immediate and delay probe trials, while spending more time in the furthest windows from the target. These results replicate our previous findings in a single MTL patient (Kolarik et al., 2016) by demonstrating similar spatial precision deficits in a larger (non-overlapping) cohort of medial temporal lobe lesion patients. At the same time, they extend our previous findings by showing that the results generalize to novel start locations during probe trials and do not differ between groups as a function of delay.
Discussion
Our results show that five amnestic patients were able to learn target locations in a virtual Morris Water Maze (vMWM) as well as a group of matched controls, specifically when using a coarse metric to assess spatial memory. Overall, these findings are consistent with previous reports of at least some intact spatial memory following MTL damage (Bohbot & Corkin, 2007; Bohbot et al., 1998; Goodrich-Hunsaker et al., 2010; Kolarik et al., 2016). As we have previously reported (Kolarik et al., 2016), however, the quadrant analysis imposes arbitrary boundaries on the environment and therefore can miss vital information present in the search trajectory. We addressed this issue by assessing the precision of trajectories using sliding windows at parametrically greater distances from the target that were nonetheless all centered on the target location. This in turn provided a precise 2-D estimate of the approximate distance that the patient searched from the target, with the smallest precision windows indicating the most precise search and the furthest ones indicating searches most distant from the target.
Using this analysis in the vMWM, novel start locations, and both immediate and delay testing, we showed that patients exhibited impaired precision on search trajectories both immediately after training and after a 10 minute delay. Compared to controls, patients spent less time searching the area closest to the target location on probe trials. Importantly, though, patients did not search randomly through the environment, as demonstrated in both the distance and 2-D window analyses, again suggesting some intact allocentric memory. While previous studies have analyzed data in a similar way by using center-middle-periphery zones around the target (Moser et al., 1993), our analysis method coupled with the high sampling rate of position allowed us to look at the continuous trajectory at a much finer scale to detect potentially overlooked behavioral impairments. Additionally, while it has been reported that proximity to the target is the most sensitive measure of detecting impairments in the water maze (Maei et al., 2009; Tomas Pereira & Burwell, 2015), we believe that a proximity metric can miss information in the data by averaging the position over the entire probe trial. Together these results suggest that MTL damage does not completely abolish ones’ ability to locate a target using distal cues, as has been argued (Astur et al., 2002; Morris et al., 1982; O’Keefe and Nadel, 1978). Instead, our findings suggest allocentric spatial memory is partially preserved in patients with MTL damage although not as precise as neurologically intact controls.
In addition, by testing at both immediate and delay intervals, we addressed the issue of whether temporal delay resulted in additional impairments in spatial memory. Declarative Memory Theory (Squire et al., 2004) would predict impairments at delay but not immediate testing, particularly when information exceeds the capacity of working memory over the delay (Jeneson et al., 2011). Our patients, however, demonstrated precision impairments on the immediate trials, even when only one target location was to be remembered, and no Group x Time interaction was found indicating that overall performance dropped after the delay. In addition, patient performance was not completely abolished after a delay even when our task required that patients distinguish multiple target locations. Instead, we found that patients’ coarse spatial memory was comparable to controls, while still exhibiting the precision deficit we observed on immediate trials. Our findings thus also contrast with previous results indicating that hippocampal lesions impair performance as memory load increases (Shrager et al., 2007). Together, these results indicate that the role of the hippocampus cannot simply be characterized by a working- versus long-term memory distinction but that the hippocampus is necessary when the information to be retained is sufficiently complex and high-resolution (Yonelinas, 2013).
Another issue we tested here was the starting positions on probe trials. Past studies have argued that MTL patients might use an egocentric view-matching strategy to overcome any deficits in allocentric memory (Banta-Lavenex et al., 2014). In our paradigm, starting positions on probe trials were different than those used during training, making it difficult to use a view-matching strategy to find the target. Nonetheless, patients did exhibit some intact coarse spatial memory. Indeed, studies of human episodic memory suggest that the hippocampus is crucial for representing distinct yet highly similar events in memory (Chadwick et al., 2011). Because our design required that the target location be referenced relative to the cues (paintings) around the room rather than following a previously learned route from start to target, our findings suggests that even following hippocampal damage, some allocentric memory persists. This contrasts with the idea that the hippocampus is critical for representing an environment as a ‘cognitive map’ as well as the results of numerous human and rodent lesion studies (Astur et al., 2002; Bartsch et al., 2010; Morris et al., 1982; O’Keefe, 1991; O’Keefe & Dostrovsky, 1971).
Rather than a strict role for the hippocampus in allocentric spatial representations, we instead suggest a more specific role in representing complex high-resolution information across multiple cognitive domains. Compared to Cognitive Map Theory, we believe that the Precision and Binding Model can better account for our data in that it can explain why hippocampal lesions can leave some coarse spatial memory intact while impairing the precision of that memory. However, as is the nature of non-experimental lesions, all five of our patients have some intact hippocampal tissue. It is possible that the remaining tissue is what supports their coarse spatial memory, although previous studies have reported impairments on water maze tasks even with partial hippocampal lesions, particularly those extending into the posterior hippocampus (Moser et al., 1993; Moser et al., 1995). Indeed, some models suggest a gradient for representational precision along the long axis of the hippocampus with higher precision being represented more posteriorally and coarse representations anteriorally (Fanselow & Dong, 2010; Nadel et al., 2013). These models would therefore predict a loss of precision as a result of damage to the posterior hippocampus. In contrast, we observed precision impairments in a group of patients with primarily anterior hippocampal damage (resection patients, N=3) and both anterior and posterior damage (bilateral hippocampal patients, N=2). There are also models that would predict that right hippocampal damage would disproportionately impair performance compared to left hippocampal damage (Burgess et al., 2002). Our patients, however, had mixtures of damage to both right and left hippocampi. Overall, we observed similar patterns of results in unilateral left, unilateral right and bilateral hippocampal damage. Our findings are thus consistent with previous data showing that after right hippocampal lesions, some spatial memory can persist even after a delay (Bohbot et al., 1998). Thus, while partial preservation of the hippocampus could possibly account for our findings, our results are not consistent with theories suggesting differential impairments for anterior vs. posterior nor for right vs. left hippocampus.
The Precision and Binding model also differs from and makes slightly different predictions than Transformation Theory (Winocur, Moscovitch and Sekeres, 2007; Winocur, Moscovitch and Bontempi, 2010). Transformation Theory suggests that initially, all memories are dependent on the hippocampus, but that a memory is transformed during consolidation into a less detailed and more ‘gist-like’ representation. Once this transformation has occurred, the memory contains little details, but can be maintained independently of the hippocampus. Importantly, Transformation Theory suggests that a hippocampally-dependent richly detailed memory still remains after transformation. The Precision and Binding Model differs from this account by not assuming hippocampal dependence for all memories initially. PBM maintains that simple, less precise memories can be initially encoded independently of the hippocampus.
The Precision and Binding Model also makes predictions that differ slightly from Relational Memory Theory (RMT: Cohen & Eichenbaum, 1991; Eichenbaum & Cohen, 2014; Eichenbaum, Otto, & Cohen, 1992). Similarly to PBM, RMT assumes that the hippocampus is critical for relating or ‘binding’ features or objects present in one’s environment. PBM, however, posits that the hippocampus is particularly important for binding high-resolution information, and therefore will not be equally involved in all relational memory tasks. The current results show that relational information for the location of the target relative to the distal cues (paintings) is, in some capacity, intact in our patients. Importantly, the precise location of that target relative to the cues has been impaired while a more coarse relational representation persists. PBM therefore seems to better account for the current results rather than a general relational memory impairment.
The question remains as to what mechanism contributes to precise representations of spatial information and how MTL lesions disrupt that mechanism. A core feature of Cognitive Map Theory is that place cells form the neural basis of a “map” for an environment by firing at specific spatial locations, supported by the findings of place cells in humans in virtual reality (Ekstrom et al., 2003; Miller et al., 2013). One possibility, then, is that lesions to the MTL, particularly the hippocampus, reduce the number of place cells available for representing the environment in the vMWM. This would result in sparser coding of the environment as not every location would be coded by a place cell. Given this situation, the general area of a target could be represented coarsely but the exact location of the target would be missing, consistent with previous findings showing that water maze performance decreases nearly linearly with the amount of hippocampal tissue resected (Moser et al., 1993).
It is also possible that structures outside of the hippocampus compensated for the damage. The MTL is not a neurologically isolated structure, but rather is highly connected via inputs and outputs to other cortical areas (Libby et al., 2012). Damage to the MTL can therefore result in functional lesions whereby information cannot be sent to or received from extra-MTL structures. This could impact the information that is available for representing information during navigation, regardless of the location of the lesion. Thus, another possibility, rather than compensatory changes within the hippocampus, is that extra-hippocampal brain areas like retrosplenial cortex and prefrontal cortex compensated for lost function, allowing for some coarse allocentric memory. Indeed, some human studies argue for their importance in some forms of allocentric memory (Spiers & Maguire, 2007; Wolbers & Buchel, 2005; Zhang & Ekstrom, 2013), consistent with recent proposals of non-aggregate coding within networks of brain regions dedicated to cognition (Bassett & Gazzaniga, 2011) and allocentric spatial memory more specifically (Ekstrom et al., 2014).
Could the reduction in memory precision observed in the patients be due to swap errors or misbindings to distal landmarks? For example, if a patient were to misremember which target was in which location, this may reduce the apparent precision of their searches, even if their memory precision was quite good. The finding that the patients exhibited reduced precision even in the immediate test condition suggests the impairments were not due to swap errors, however, to further assess this possibility we examined performance for the very first location they learned in the first block. We found that they exhibited significant impairments relative to controls in 3 of the smallest precision windows on the very first probe trials when only one location had been learned [two-sample t-tests: precision window 2 t(13) = −2,45 p = .027, window 2 t(13) = −2.56, p = .0234, window 4 t(13) = −2.20, p = .046] ) and were no different from controls in the larger windows. This additional analysis rules against the likelihood of swap errors accounting for their impaired search precision.
Another possibility is that the patients may misbind to distal cues. For example, patients may remember that the target location was in front of the blue painting, but may forget part of that binding (i.e. they remember it was in front of a painting but forget if it was the blue or red painting). This would lead them to appear to have less memory precision, when in fact their impairment was due to a reduction in the number of bindings. Overall, we believe that such misbindings would lead to chance performance for both the first trial and when averaged over probe trials, and the fact that patients searched with significantly greater likelihood near the correct target compared to other targets would argue that they were likely using at least some of the distal cues to remember the correct hidden location. Future experiments will be needed to address this possibility in more depth.
In conclusion, we have provided evidence that the MTL is crucial for representing and maintaining high-resolution information, here in the service of spatial navigation. Although partially consistent with CMT, we believe that these findings fit better with the specific predictions of the Precision and Binding Model of hippocampal function, thus providing new insight into the workings of the human MTL and the intersection between episodic memory and navigation. Future research should explore this model in order to determine the limits of what constitutes ‘high-resolution’ both within a spatial navigation framework as well as broader cognitive domains.
Significance Statement.
Remembering goal locations in one’s environment is a critical skill for survival. How this information is represented in the brain is still not fully understood, but is believed to rely in some capacity on structures in the medial temporal lobe. Contradictory findings from studies of both humans and animals have been difficult to reconcile with regard to the role of the MTL, specifically the hippocampus. By assessing impairments observed during navigation to a goal in patients with medial temporal lobe damage we can better understand the role these structures play in such behavior. Utilizing virtual reality and novel analysis techniques, we have more precisely assessed the impact that medial temporal lobe damage has on spatial memory and navigation.
Acknowledgments
Funding Sources: 1R03NS093052 (A.D.E)
Footnotes
The authors declare no competing financial interests.
References
- Addante RJ, Ranganath C, Olichney J, Yonelinas AP. Neurophysiological evidence for a recollection impairment in amnesia patients that leaves familiarity intact. Neuropsychologia. 2012;50(13):3004–3014. doi: 10.1016/j.neuropsychologia.2012.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly M, Ranganath C, Yonelinas AP. Detecting changes in scenes: the hippocampus is critical for strength-based perception. Neuron. 2013;78(6):1127–1137. doi: 10.1016/j.neuron.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astur RS, Taylor LB, Mamelak AN, Philpott L, Sutherland RJ. Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behavioural brain research. 2002;132(1):77–84. doi: 10.1016/s0166-4328(01)00399-0. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Emslie H, Nimmo-Smith I. Doors and people: A test of visual and verbal recall and recognition. Thames Valley Test Company; 1994. [Google Scholar]
- Banta-Lavenex P, Colombo F, Ribordy-Lambert F, Lavenex P. The human hippocampus beyond the cognitive map: Evidence from a densely amnesic patient. Frontiers in Human Neuroscience. 2014;8(711):1–18. doi: 10.3389/fnhum.2014.00711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch T, Schonfeld R, Muller FJ, Alfke K, Leplow B, Aldenhoff J, … Koch JM. Focal lesions of human hippocampal CA1 neurons in transient global amnesia impair place memory. Science. 2010;328(5984):1412–1415. doi: 10.1126/science.1188160. 328/5984/1412 [pii] [DOI] [PubMed] [Google Scholar]
- Bassett DS, Gazzaniga MS. Understanding complexity in the human brain. Trends Cogn Sci. 2011;15(5):200–209. doi: 10.1016/j.tics.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohbot VD, Corkin S. Posterior parahippocampal place learning in H.M. Hippocampus. 2007;17(9):863–872. doi: 10.1002/hipo.20313. [DOI] [PubMed] [Google Scholar]
- Bohbot VD, Kalina M, Stepankova K, Spackova N, Petrides M, Nadel L. Spatial memory deficits in patients with lesions to the right hippocampus and to the right parahippocampal cortex. Neuropsychologia. 1998;36(6) doi: 10.1016/s0028-3932(97)00161-9. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Chadwick MJ, Hassabis D, Maguire EA. Decoding overlapping memories in the medial temporal lobes using high-resolution fMRI. Learn Mem. 2011;18(12):742–746. doi: 10.1101/lm.023671.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. The theory that wouldn’t die: a critical look at the spatial mapping theory of hippocampal function. Hippocampus. 1991;1(3):265–268. doi: 10.1002/hipo.450010312. [DOI] [PubMed] [Google Scholar]
- D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. Can we reconcile the declarative memory and spatial navigation views on hippocampal function? Neuron. 2014;83(4):764–770. doi: 10.1016/j.neuron.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Dudchencko P, Wood E, Shapiro M, Tanila H. The hippocampus, memory, and place cells: Is it spatial memory or a memory space? Neuron. 1999;23(2):209–226. doi: 10.1016/s0896-6273(00)80773-4. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Otto T, Cohen NJ. The hippocampus – what does it do? Behavioral and neuroal biology. 1992;57(1):2–36. doi: 10.1016/0163-1047(92)90724-i. [DOI] [PubMed] [Google Scholar]
- Ekstrom AD, Arnold AE, Iaria G. A critical review of the allocentric spatial representation and its neural underpinnings: Toward a network-based perspective. Frontiers in Human Neuroscience. 2014 doi: 10.3389/fnhum.2014.00803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Kahana MJ, Caplan JB, Fields TA, Isham EA, Newman EL, Fried I. Cellular networks underlying human spatial navigation. Nature. 2003;425(6954):184–188. doi: 10.1038/nature01964. [DOI] [PubMed] [Google Scholar]
- Fanselow M, Dong M. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65(1):7–19. doi: 10.1016/j.neuron.2009.11.031. doi:0.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich RI, Yonelinas AP. The Medial Temporal Lobe Supports Sensing-Based Visual Working Memory. Neuropsychologia. 2016 doi: 10.1016/j.neuropsychologia.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Livingstone SA, Skelton RW, Hopkins RO. Spatial deficits in a virtual water maze in amnesic participants with hippocampal damage. Hippocampus. 2010;20(4):481–491. doi: 10.1002/hipo.20651. [DOI] [PubMed] [Google Scholar]
- Jeneson A, Mauldin KN, Hopkins RO, Squire LR. The role of the hippocampus in retaining relational information across short delays: the importance of memory load. Learn Mem. 2011;18(5):301–305. doi: 10.1101/lm.2010711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koen JD, Borders AA, Petzold MT, Yonelinas AP. Visual short-term memory for high resolution associations is impaired in patients with medial temporal lobe damage. Hippocampus. 2016 doi: 10.1002/hipo.22682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolarik BS, Shahlaie K, Hassan B, Borders AA, Kaufman K, Gurkoff G, … Ekstrom AD. Impairments in precision, rather than spatial strategy, characterize performance on the virtual Morris Water Maze: A case study. Neuropsychologia. 2016;80:90–101. doi: 10.1016/j.neuropsychologia.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AC, Yeung LK, Barense MD. The hippocampus and visual perception. Front Hum Neurosci. 2012;6:91. doi: 10.3389/fnhum.2012.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby LA, Ekstrom AD, Ragland JD, Ranganath C. Differential connectivity of perirhinal and parahippocampal cortices within human hippocampal subregions revealed by high-resolution functional imaging. Journal of Neuroscience. 2012;32:6550–6560. doi: 10.1523/JNEUROSCI.3711-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maei HR, Zaslavsky K, Teixeira CM, Frankland PW. What is the Most Sensitive Measure of Water Maze Probe Test Performance? Front Integr Neurosci. 2009;3:4. doi: 10.3389/neuro.07.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JF, Neufang M, Solway A, Brandt A, Trippel M, Mader I, … Schulze-Bonhage A. Neural activity in human hippocampal formation reveals the spatial context of retrieved memories. Science. 2013;342(6162):1111–1114. doi: 10.1126/science.1244056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure or studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Garrud P, Rawlins JNP, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Moser EI, Moser MB, Anderson P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci. 1993;13:3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Moser EI, Forrest E, Anderson P, Morris R. Spatial learning with a minislab in the dorsal hippocampus. Proc Natl Acad Sci U S A. 1995;92:9697–9701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel L, Hoscheidt S, Ryan L. Spatial cognition and the hippocampus:the anterior-posterior axis. J Cogn Neurosci. 2013;25(1):22–28. doi: 10.1162/jocn_a_00313. [DOI] [PubMed] [Google Scholar]
- Nakada T, Kwee I, Fujii Y, Knight RT. High-field T2 reversed MRI of the hippocampus in transient global amnesia. Neurology. 2005;64(April):1170–1174. doi: 10.1212/01.WNL.0000156158.48587.EA. [DOI] [PubMed] [Google Scholar]
- O’Keefe J. An allocentric spatial model for the hippocampal cognitive map. Hippocampus. 1991;1(3):230–235. doi: 10.1002/hipo.450010303. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34(1):171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford: Clarendon Press; 1978. [Google Scholar]
- Ruxton GD. The unequal variance t-test is an underused alternative to Student’s t-test and the Mann-Whitney U test. Behavioral Ecology. 2006;17(4):688–690. doi: 10.1093/beheco/ark016. [DOI] [Google Scholar]
- Satterthwaite F. An approximate distribution of estimates of variance components. Biometrics Bulletin. 1946;2:110–114. [PubMed] [Google Scholar]
- Shipley WC. A Self-Administering Scale for Measuring Intellectual Impairment and Deterioration. The Journal of Psychology. 1940;9(2):371–377. doi: 10.1080/00223980.1940.9917704. [DOI] [Google Scholar]
- Shrager Y, Bayley PJ, Bontempi B, Hopkins RO, Squire LR. Spatial memory and the human hippocampus. Proc Natl Acad Sci U S A. 2007;104(8):2961–2966. doi: 10.1073/pnas.0611233104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiers HJ, Maguire EA. A navigational guidance system in the human brain. Hippocampus. 2007;17(8):618–626. doi: 10.1002/hipo.20298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1038/nrn2154. [DOI] [PubMed] [Google Scholar]
- Tomas Pereira I, Burwell RD. Using the spatial learning index to evaluate performance on the water maze. Behav Neurosci. 2015;129(4):533–539. doi: 10.1037/bne0000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DE, Duff MC, Jensen U, Tranel D, Cohen NJ. Hiding in plain view: lesions of the medial temporal lobe impair online representation. Hippocampus. 2012;22(7):1577–1588. doi: 10.1002/hipo.21000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Revised manual. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- Welch B. The significance of the difference between two means when the population variances are unequal. Biometrika. 1938;29:350–362. [Google Scholar]
- Winocur G, Moscovitch M, Bontempi B. Memory formation and long-term retention in humans and animals: Convergence towards a transformation account of hippocampal-neocortical interactions. Neuropsychologia. 2010;48(8):2339–56. doi: 10.1016/j.neuropsychologia.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Winocur G, Moscovitch M, Sekeres M. Memory consolidation or transformation: Context manipulation and hippocampal representations of memory. Nature Neuroscience. 2007;10(5):555–557. doi: 10.1038/nn1880. [DOI] [PubMed] [Google Scholar]
- Wolbers T, Buchel C. Dissociable retrosplenial and hippocampal contributions to successful formation of survey representations. J Neurosci. 2005;25(13):3333–3340. doi: 10.1523/JNEUROSCI.4705-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. The hippocampus supports high-resolution binding in the service of perception, working memory and long-term memory. Behav Brain Res. 2013;254:34–44. doi: 10.1016/j.bbr.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary R, Crumpton E, Spiegel D. Estimating WAIS-R IQ from the Shipley Institue of Living Scale. J Clin Psychol. 1985;41(4):532–540. doi: 10.1002/1097-4679(198511)41:6<820::aid-jclp2270410616>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Zhang H, Ekstrom AD. Human neural systems underlying rigid and flexible forms of allocentric spatial representation. Human Brain Mapping. 2013;34(5):1070–1087. doi: 10.1002/hbm.21494. [DOI] [PMC free article] [PubMed] [Google Scholar]