Version Changes
Revised. Amendments from Version 1
In this new version of the manuscript we have included the corrections of Dr. Rimstad and Dr. Bols along the manuscript, in order to improve manuscript understanding. The line of the manuscript has not changed, but the manuscript comprehension has been improved. We have corrected several mistyping and linguistic errors, as well as clarifying some points in the manuscript and eliminating some confusing citations. Therefore, some references have been eliminated and one reference previously unpublished is now updated. Besides, as Dr. Bols suggested, in Figure 3A, FFU/ml has been removed from the y axis and only mentioned in the legend of the figure.
Abstract
Background: Some fish viruses, such as piscine orthoreovirus and infectious salmon anemia virus, target red blood cells (RBCs), replicate inside them and induce an immune response. However, the roles of RBCs in the context of infectious pancreatic necrosis virus (IPNV) infection have not been studied yet.
Methods: Ex vivo rainbow trout RBCs were obtained from peripheral blood, Ficoll purified and exposed to IPNV in order to analyze infectivity and immune response using RT-qPCR, immune fluorescence imaging, flow cytometry and western-blotting techniques.
Results: IPNV could not infect RBCs; however, IPNV increased the expression of the INF1-related genes ifn-1, pkr and mx genes. Moreover, conditioned media from IPNV-exposed RBCs conferred protection against IPNV infection in CHSE-214 fish cell line.
Conclusions: Despite not being infected, rainbow trout RBCs could respond to IPNV with increased expression of antiviral genes. Fish RBCs could be considered as mediators of the antiviral response and therefore targets of new strategies against fish viral infections. Further research is ongoing to completely understand the molecular mechanism that triggers this antiviral response in rainbow trout RBCs.
Keywords: erythrocytes, IPNV, birnavirus, immune response, antiviral, trout, interferon
Introduction
Fish viral infections cause significant losses in aquaculture. Infectious pancreatic necrosis (IPN) is a highly contagious viral disease with a high impact on salmonid aquaculture industry. Infectious pancreatic necrosis virus (IPNV) is the causative agent of IPN and was the first fish virus isolated in cell culture 1. IPNV outbreaks are usually related to high mortality rates in salmonid aquaculture, especially in young individuals 2, 3, highlighting the urgent necessity for the development of efficient strategies in vaccination. IPNV belongs to the Aquabirnavirus genus within the Birnaviridae family. Viruses of this family are non-enveloped particles with a double stranded RNA genome. This genome consists of two segments: the A segment contains the information to encode a protein that is post-translationally cleaved into VP2, VP3 and VP4 viral proteins; the B segment encodes the viral RNA polymerase VP1 4. VP2 and VP3 are the major structural and immunogenic proteins, as they represent 64% of the total proteins of the virion 5.
In contrast to mammals, fish, reptiles and avian red blood cells (RBCs) are nucleated. Typically, the role associated with RBCs has been the transport of O 2 to different tissues and gas exchange. However, a whole set of biological processes related to the immune response has been recently reported for nucleated RBCs from different species: recognition of pathogen associated molecular patterns 6, 7 through expression of pattern recognition receptors, such as toll-like receptors (TLRs) 8; production of cytokine-like factors 7, 9– 11; phagocytosis 12; and formation of complement immune complexes 13. Fish RBCs are known to be the target of some viruses, such as infectious salmon anemia virus (ISAV) 11 and piscine orthoreovirus (PRV) 14, 15. Furthermore, both viruses can induce immune responses in infected RBCs, characterized by the expression of genes related to IFN-1 (type I interferon) pathway. Besides, recently it has been shown that viral hemorrhagic septicemia virus (VHSV) halted replication in rainbow trout RBCs could induce cytokine production 16.
In view of the aforementioned evidence, this study was aimed to evaluate the immune response of rainbow trout RBCs against IPNV, one of the most ubiquitous viral fish pathogens. To achieve this objective, we first analyzed the infectivity of IPNV in rainbow trout RBCs. Then, RBCs immune response was evaluated after ex vivo exposure to IPNV, by means of antiviral gene and protein expression analysis. Finally, we evaluated the ability of RBCs to confer protection against IPNV in CHSE-214 cells, which are susceptible to IPNV infection. To summarize, here we report the regulation of the immune response of rainbow trout RBCs by IPNV, a non-infective virus in this cell type. This immune response was characterized by the expression of genes related to the IFN-1 pathway, Mx production and induction of an antiviral state to IPNV in CHSE-214 cells.
Methods
Animals
Rainbow trout ( Oncorhynchus mykiss) individuals of approximately 10 g were obtained from a commercial fish farm (PISZOLLA S.L., CIMBALLA FISH FARM, Zaragoza, Spain). Fish were maintained at the University Miguel Hernandez (UMH) facilities with a re-circulating dechlorinated-water system, at a stocking density of 1 fish/3L, at 14°C, and fed daily with a commercial diet (SKRETTING, Burgos, Spain). Fish were acclimatized to laboratory conditions over 2 weeks before experimentation. The number of fish used is indicated for each experiment/figure.
RBCs purification
Rainbow trout were sacrificed by overexposure to tricaine methanesulfonate (Sigma-Aldrich, Madrid, Spain) at 0.2 g/L. Peripheral blood was sampled from the caudal vein using insulin syringes (NIPRO Bridgewater, NJ). Approximately 100 µL of blood was diluted in RPMI-1640 medium (Dutch modification) (Gibco, Thermo Fischer Scientific Inc., Carlsbad, CA) supplemented with 10% FBS (Cultek, Madrid, Spain), 1 mM pyruvate (Gibco), 2 mM L-glutamine (Gibco), 50 µg/mL gentamicin (Gibco), 2 µg/mL fungizone (Gibco) and 100 U/mL penicillin/streptomycin (Sigma-Aldrich). Then, RBCs were purified by two consecutive density gradient centrifugations with Histopaque 1077 (7206g, Ficoll 1.007; Sigma-Aldrich). Finally, RBCs were washed twice with RPMI 2% FBS. Purity of RBCs of 99.9% was estimated by optical microscopy evaluation. Then, purified RBCs were cultured in the above indicated medium at a density of 10 7 cells/mL, in cell culture flasks, at 14°C, overnight.
Viral infection assays
Ex vivo rainbow trout RBCs along with CHSE-214 cell line (Chinook Salmon Embryo, ATCC CRL-1681) were infected using IPNV Sp strain 17. IPNV was grown as previously described 18. Ex vivo RBCs exposure to IPNV was performed by incubating RBCs with diluted IPNV at the indicated MOI (multiplicity of infection) in RPMI 2% FBS. After three hours of incubation at 14°C, RBCs were centrifuged at 1600 rpm for 5 minutes and then washed with medium to completely eliminate the non-adsorbed excess of virus. Finally, RBCs were placed in 24 well plates (Corning Costar, Sigma-Aldrich, Madrid, Spain) with 500 µl of RPMI 2% FBS. The whole process was done at 14°C. Infection of the CHSE-214 cell line was done by incubating IPNV diluted in RPMI 2% FBS at the desired MOI for 1 hour at 14°C. After that, medium was removed and RPMI 2% FBS was added to each well. Infected CHSE-214 cells were maintained at 14°C 18.
In time course experiments, the initial supernatant with IPNV was not removed. When each of the time points was reached, RBCs were washed with cell culture medium and CHSE-214 cells with PBS supplemented with calcium.
Viral titration assay
The virus titer in IPNV-exposed RBCs supernatants was quantified by TCID 50 and by RT-qPCR. Briefly, different dilutions of the supernatants (from 10 -1 to 10 -4) were added to CHSE-214 cell monolayers, and incubated at 14°C for 90 minutes. Then, the virus was removed and infected CHSE-214 cell monolayers covered with a solution of RPMI 2% FBS. Cell plates were incubated at 14°C for 7 days. For RT-qPCR titration, 30 µL of IPNV with known titer (10 9 TCID 50/mL) and 30 µL of IPNV-exposed RBCs supernatants were used to extract RNA and synthetize cDNA, as explained hereafter. Ten-fold serial dilutions from 10 8 to 10 2 TCID 50/mL were done to obtain IPNV cDNA and create a standard line.
RNA isolation and DNAse treatment
The E.Z.N.A.® Total RNA Kit (Omega Bio-Tek Inc., Norcross, GA) was used for total RNA extraction, in accordance with manufacturer’s instructions. DNAse treatment was done in order to eliminate residual genomic DNA using TURBO™ DNase (Ambion, Thermo Fischer Scientific Inc.), following the manufacturer’s instructions. RNA was quantified with a NanoDrop® 377 Spectrophotometer (Nanodrop Technologies, Wilmington, DE).
Gene expression by RT-qPCR
cDNA was synthesized from RNA using M-MLV reverse transcriptase (Invitrogen, Thermo Fischer Scientific Inc.), as previously described 19. Final concentration of cDNA was 6 ng/µL. RT-qPCR reactions were performed in a total volume of 20 μl using 12 ng of cDNA, 10 μl of TaqMan universal PCR master mix (Thermo Fischer Scientific), 900 nM final concentration of each primer (300 nM for IPNV segment A) and 300 nM of probe (150 nM for IPNV segment A). RT-qPCR was performed using the ABI PRISM 7300 System (Thermo Fischer Scientific). Cycling conditions were 50°C for 2 min and 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min.
Gene expression was analyzed by the 2 -ΔΔCt method 20. The eukaryotic 18S rRNA gene (Cat#4310893E, Thermo Fischer Scientific) was used as endogenous control. Primers and probes are listed in Table 1.
Table 1. List of primers and probes.
| Gene | Forward primer
(5’ – 3‘) |
Reverse primer
(5’ – 3‘) |
Probe
(5’ – 3‘) |
Reference or
accession number |
|---|---|---|---|---|
| IPNV
SA |
TCTCCCGGGCAGTTCAAGT | CGGTTTCACGATGGGTTGTT | CCAGAACCAGGTGACGAGTATGAGGACTACAT | 18 |
| tlr3 | ACTCGGTGGTGCTGGTCTTC | GAGGAGGCAATTTGGACGAA | CAAGTTGTCCCGCTGTCTGCTCCTG | NM_001124578.1 |
| irf7 | CCCAGGGTTCAGCTCCACTA | GGTCTGGCAACCCGTCAGT | TCGAGCCAAACACCAGCCCCT | AJ829673 |
| ifn1 | ACCAGATGGGAGGAGATATCACA | GTCCTCAAACTCAGCATCATCTATGT | AATGCCCCAGTCCTTTTCCCAAATC | AM489418.1 |
| mx1–3 | TGAAGCCCAGGATGAAATGG | TGGCAGGTCGATGAGTGTGA | ACCTCATCAGCCTAGAGATTGGCTCCCC | 28 |
| pkr | GACACCGCGTACCGATGTG | GGACGAACTGCTGCCTGAAT | CACCACCTCTGAGAGCGACACCACTTC | NM_001145891.1 |
| il8 | AGAGACACTGAGATCATTGCCAC | CCCTCTTCATTTGTTGTTGGC | 29 | |
| ifnγ | CAAACTGAAAGTCCACTATAAGATCTCCA | TCCTGAATTTTCCCCTTGACATATTT | 30 | |
| tnfα | AGCATGGAAGACCGTCAACGAT | ACCCTCTAAATGGATGGCTGCTT | 31 |
Antibodies
Several antibodies were used to stain cells for cytokines and to measure polypeptides in RBCs extracts by western blotting. They are briefly described below and their Research Resource Identifiers (RRIDs) given. For intracellular staining, mouse polyclonal antibodies against rainbow trout IL1β (RRID: AB_2716269) 21, 22, IL8 (RRID: AB_2716272) 23 and TNF-α (RRID: AB_2716270) 24 were produced at the laboratory of Dr. Luis Mercado. Rabbit polyclonal antibody against rainbow trout Mx3 (RRID: ABA_2716267) 25, 26 was produced at the laboratory of Dr. Amparo Estepa. Anti-IPNV-VP3 monoclonal antibody 2F12 (RRID: AB_2716296) was used for IPNV labelling 27. Anti-rabbit IgG (H+L) CF™ 488 antibody produced in goat and anti-mouse IgG (H+L) CF™ 488 antibody produced in goat were used as secondary antibodies for proteins and anti-mouse IgG (H+L) CF™ 647 produced in goat to detect 2F12 antibody.
For western blotting, rabbit polyclonal antibody against human eIF2α-P (Cat# E2152, RRID:AB_259283) and rabbit polyclonal antibody against human α-Actin (Cat#2066, RRID:AB_476693) were purchased from Sigma-Aldrich.
Western blot
Control and IPNV-exposed RBCs pellets (≈10 7 cells) were used for protein extraction. Cell pellets were washed 3 times with PBS and then resuspended in 30 µl of PBS with a cocktail of protease inhibitors (Sigma-Aldrich). Then, cells were frozen/thawed 3 times and lysed using an eppendorf micropistile (Eppendorf, Hamburg, Germany). Samples were loaded in Tris–Glycine sodium dodecyl sulfate 12% polyacrylamide gels under reducing conditions. Electrophoresis was performed at 200 V for 60 min. For blotting, the proteins in the gel were transferred for 80 min at 100 V in transfer buffer (2.5 mM Tris, 9 mM glycine, 20% methanol) to nitrocellulose membranes (BioRad, Madrid, Spain). Then, membranes were blocked with 8% dry milk and 1% Tween-20 in PBS and incubated with eIF2α-P or α-Actin antibodies, at the recommended dilutions in PBS containing 0.5% dry milk and 0.5% Tween-20 at 4°C and overnight. Incubation with secondary antibody GAR-Po (Sigma-Aldrich) was done in 0.5% milk 0.5% Tween-20 in PBS for 45 min. Membranes were washed 3 times with PBS containing 1% dry milk 0.5% Tween-20 for 15 min after every antibody incubation. Finally, the membrane was washed 3 times with PBS before analysis of the peroxidase activity. Peroxidase activity was detected using ECL chemiluminescence reagents (Amersham Biosciences, Buckinghamshire, UK) and revealed by exposure to X-ray. Protein bands from western blotting were analysed by densitometry using the Scion Image 4.0.2 Software (RRID: SCR_008673) ( www.scionorg.com).
Intracellular immunofluorescence stain and flow cytometry
RBCs were fixed with 4% paraformaldehyde (PFA; Sigma-Aldrich) and 0.08% glutaraldehyde (Sigma-Aldrich) diluted in RPMI medium for 20 minutes. Then, RBCs were incubated with permeabilization buffer containing 0.05% saponin (Sigma-Aldrich) in RPMI, for 15 minutes. Primary antibodies were used at 1/50 dilution for IL-1β, IL-8 and TNF-α, 1/300 for Mx and 1/500 for 2F12 in permeabilization buffer and incubated for 60 minutes at room temperature. Secondary antibodies were incubated for 30 minutes at 1/200 dilution. RBCs were washed with permeabilization buffer after antibody incubations. Finally, RBCs were kept in PFA 1% in PBS. For nuclear staining, RBCs were stained with 1 μg/mL of 4′-6-408 Diamidino-2-phenylindole (DAPI; Sigma-Aldrich) for 5 minutes. Flow cytometry (FC) analysis was done in a BD FACSCanto™ II (BD Biosciences) flow cytometer. Immunofluorescence (IF) images were performed in the INCell Analyzer 6000 Cell imaging system (GE Healthcare, Little Chalfont, UK).
Antiviral activity of conditioned medium
Conditioned medium (CM) was obtained from control and IPNV-exposed RBCs at MOI 0.5, during 3 days. The CMs were clarified at 1600 rpm for 5 min. IPNV titer in the supernatants of IPNV-exposed RBCs resulted in 10 TCID 50/mL or less, therefore viral presence in the supernatants was obviated. To test the antiviral activity of the CM, confluent CHSE-214 cells (7.8×10 4 cells/well), seeded in 96 well plates, were pre-treated with 100 µL of each supernatant at the indicated dilutions for 24 hours. After that, CHSE-214 cells were infected, as described previously, with IPNV at MOI 0.05, for 24 hours. Finally, intracellular staining of IPNV foci was carried out.
Intracellular staining of IPNV foci
CHSE-214 cells were fixed with PFA diluted at 4% in PBS followed by a second fixation with cold methanol. Each fixation step lasted 15 minutes. Cells were washed with PBS after each fixation step. Blocking buffer containing 5% goat serum (Sigma-Aldrich) and 0.3% Triton X-100 (Sigma-Aldrich) was added to each well with the cells for 1 hour. Then, anti-VP3 2F12 antibody was diluted 1/500 in antibody dilution buffer (1% BSA (Sigma-Aldrich), 0.3% Triton X-100) and was incubated for 1 hour. FITC-labelled goat anti-rabbit was used as secondary antibody at 1/300 dilution. Cells were washed three times after each antibody incubation with PBS. IF images were taken INCell Analyzer 6000 imaging system. IN Cell Developer Toolbox 1.9.2 (RRID: SCR_015790; GE Healthcare, Little Chalfont, UK) was used to count number of IPNV foci (positive areas after image segmentation were selected when >21000 fluorescence units and >2500 µm 2 criteria was reached).
MTT assays
Cell viability was tested using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) colorimetric assay 32. Briefly, 25 µl of MTT at a final concentration of 1.9 mg/mL were added to previously treated CHSE-214 cells monolayers, seeded in 96 well plates. Cells were incubated for 3 hours at 21°C with the reagent. Then, the medium was removed from the wells. Formazan crystals were dissolved in 100 µl of 100% DMSO, incubated for 30 minutes. Absorbance was read at 570 nm in the EON™ microplate spectrophotometer (Biotek, Winooski, VT). Percentage of viable cells was calculated as follows: absorbance treated cells/absorbance non-treated cells) x100.
Software and statistics
All the figures and graphics show the mean and standard deviation of the data. P-values associated with each graphic are represented by the legends: *, p-value < 0.05; **, p-value < 0.01; ***, p-value < 0.001, ****, p-value < 0.0001. Graphpad Prism 6 (RRID: SCR_002798, www.graphpad.com) (Graphpad Software Inc., San Diego, A) was used for preparing graphs and preforming statistical calculations. FC data were analyzed using Flowing Software 2.5.1 (RRID: SCR_015781)( www.flowingsoftware.com) to obtain Mean Fluorescence Intensity (MFI) and Mean Relative Fluorescence Intensity (MRFI) (relative to control cells) values.
Ethics approval
Methodology was carried out in accordance with the Spanish Royal Decree RD 53/2013 and EU Directive 2010/63/EU for animals used in research experimentation. All experimental protocols involving animal handling were also reviewed and approved by the Animal Welfare Body and the Research Ethics Committee at the Miguel Hernandez University (approval number 2014.205.E.OEP; 2016.221.E.OEP) and performed by qualified research personnel.
Results
IPNV did not infect rainbow trout RBCs
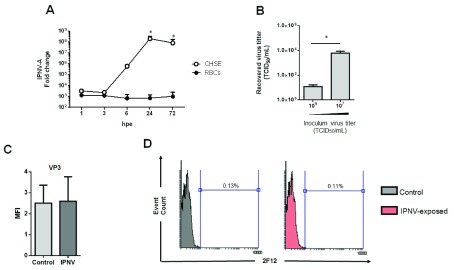
To evaluate the infectivity of IPNV in rainbow trout RBCs, RBCs were exposed to IPNV at MOI 0.5 and the viral RNA was evaluated by RT-qPCR in the cell pellet at different times post-exposure. IPNV infectivity was also evaluated in parallel in the CHSE-214 cell line, used as a positive control of infection. IPNV segment A (IPNV-A) RNA levels inside RBCs and CHSE-214 cell line were similar at 1 and 3 hours post-exposure (hpe) ( Figure 1A). After 6 hpe, IPNV-A RNA level was 3 logarithms lower in RBCs in comparison with CHSE-214 cells. On the other hand, the titer of IPNV in the supernatants from IPNV-exposed RBCs at a MOI of 0.5 and 5, was evaluated by TCID 50, at 3 days post-exposure (dpe), and showed a recovered titer of 5 and 4 logarithms lower, respectively ( Figure 1B). Furthermore, the supernatants titrated by RT-qPCR, were below the lowest limit of detection 10 2 TCID 50 ( Table 2). Moreover, FC analysis of control and IPNV-exposed RBCs for IPNV VP3 protein did not show significant differences ( Figure 1C and D). Therefore, IPNV did not infect rainbow trout RBCs.
Figure 1. Infectivity of IPNV in RBCs.
( A) Time-course experiment of the expression of IPNV segment A (IPNV-A) in RBCs (●) (n = 6) and CHSE-214 cells (○) (n = 2) at MOI 0.5. Data is represented as mean±SD. Kruskal-Wallis Test with Dunn´s Multiple Comparison post-hoc test was performed among all time-points post-exposure in comparison with control time point (0 hpi) (*, p-value < 0.05). ( B) Recovered virus titer in supernatants from IPNV-exposed RBCs with an inoculum titer of 10 6 (MOI 0.5) and 10 7 (MOI 5) TCID 50/mL obtained after 72 hpe (n = 5). Data is represented as mean±SD. Mann-Whitney test was performed among both conditions (*, p-value < 0.05). ( C) MFI (mean fluorescence intensity) of viral protein VP3 in control and IPNV-exposed RBCs at MOI 0.5 and 3 dpe (n = 6) Mann-Whitney test was performed among both conditions. ( D) Representative flow cytometry histograms of IPNV VP3 protein detection in control and IPNV-exposed RBCs at MOI 0.5 and 3 dpi.
Table 2. Rt-qPCR virus titration.
Ct value ± standard deviation from standard line points (10 8 to 10 2 dilutions) and supernatants from IPNV-exposed RBCs at MOI 0.5, at 3 and 6 dpe. (n=7 individuals).
| Sample | Ct value ± SD |
|---|---|
| 10 8 TCID 50 | 25,885 ± 0,052 |
| 10 7 TCID 50 | 29,856 ± 0,117 |
| 10 6 TCID 50 | 33,165 ± 0,168 |
| 10 5 TCID 50 | 36,057 ± 0,11 |
| 10 4 TCID 50 | 39,126 ± 0.873 |
| 10 3 TCID 50 | Undetected |
| 10 2 TCID 50 | Undetected |
| RBCs #1 3 dpe | Undetected |
| RBCs #1 6 dpe | Undetected |
| RBCs #2 3 dpe | Undetected |
| RBCs #2 6 dpe | Undetected |
| RBCs #3 3 dpe | Undetected |
| RBCs #3 6 dpe | Undetected |
| RBCs #4 3 dpe | Undetected |
| RBCs #4 6 dpe | Undetected |
| RBCs #5 3 dpe | Undetected |
| RBCs #5 6 dpe | Undetected |
| RBCs #6 3 dpe | Undetected |
| RBCs #6 6 dpe | Undetected |
| RBCs #7 3 dpe | Undetected |
| RBCs #7 6 dpe | Undetected |
| NTC | Undetected |
IPNV exposure increased the expression of interferon-related antiviral genes and proteins in rainbow trout RBCs
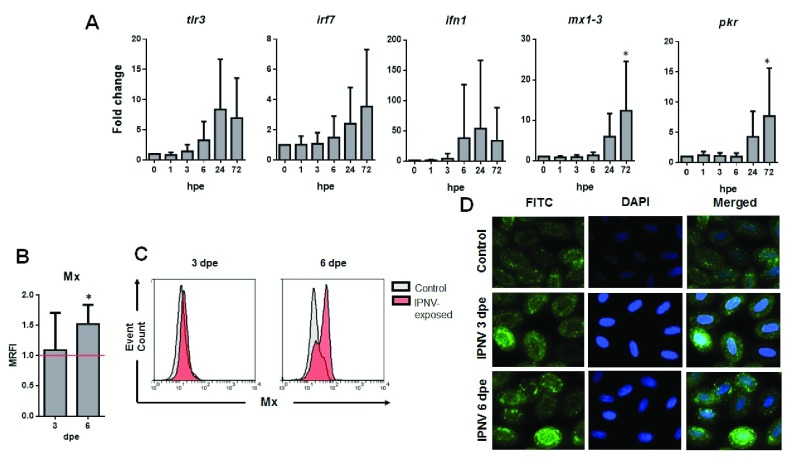
To determine if IPNV would induce an antiviral response in RBCs, RT-qPCR analysis of IFN-related antiviral genes was performed for IPNV-exposed RBCs. The results showed that mx1–3 and pkr genes were significantly expressed at 72 hpe. On the other hand, ifn1 gene presented a tendency to increase its expression after 6 hpe, having a peak at 24 hpe. Also, tlr3 gene expression tended to be upregulated at 24 hpe, whereas irf7 expression was upregulated at 72 hpe ( Figure 2A). Three and six dpe with IPNV, RBCs were stained intracellularly with an anti-Mx antibody and analyzed by FC and immunofluorescence imaging (IF). The results showed a significant increment in the expression of Mx protein at 6 dpe by both FC an IF ( Figure 2B and D). FC histograms showed, at 6 dpe, that RBCs depicted distinct peaks of Mx expression, showing that the expression of Mx in RBCs was heterogeneous in the total RBCs population ( Figure 2C).
Figure 2. RBCs IFN-related antiviral response against IPNV.
( A) Gene expression of tlr3, irf7, inf1, mx1–3 and pkr in IPNV-exposed RBCs at the indicated times post-infection and MOI 0.5, measured by RT-qPCR. Data represent mean±SD (n = 6). Kruskal-Wallis Test with Dunn´s Multiple Comparison post-hoc test was performed among all time-points post-exposure in comparison with control time point (0 hpi) (*, p-value < 0.05). ( B) Mx protein MRFI (mean relative fluorescent intensity, relative to control cells) in IPNV-exposed RBCs at MOI 0.5 (n = 5). ( C) Flow cytometry histograms of Mx protein expression from control (grey) and IPNV-exposed (red) RBCs at MOI 0.5 and the indicated days post-exposure (dpe). ( D) Representative immunofluorescence images of Mx protein expression in control and IPNV-exposed RBCs at MOI 0.5 (FITC – Mx protein expression, DAPI - Nuclei) (IF representative of 40 images).
Conditioned medium from IPNV-exposed RBCs protected CHSE-214 cells against IPNV infection
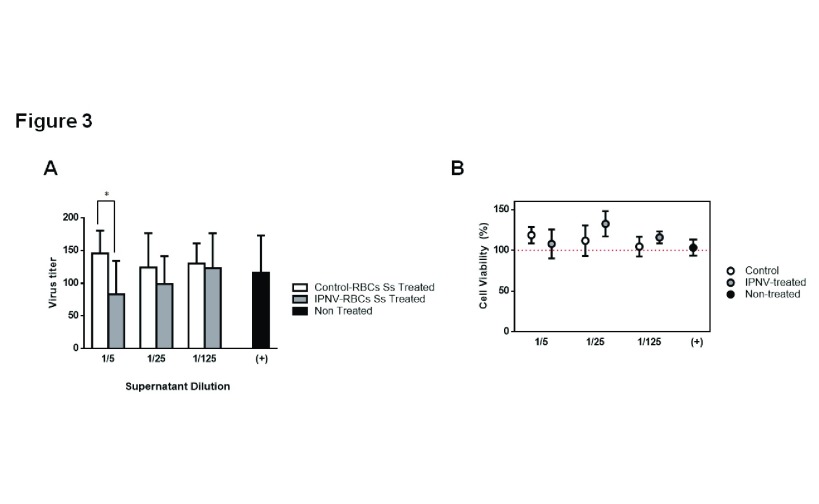
To analyze if IPNV-exposed RBCs could secrete factors that were capable to protect other fish cells against IPNV infection, conditioned medium (CM) from control and IPNV-exposed RBCs (with IPNV titer <10 TCID 50/mL) were added to CHSE-214 cells prior to infection. Figure 3A shows a significant decrease in the number of IPNV infective focus forming units (FFU/mL) when pre-treating with 1/5 diluted CM from IPNV-exposed RBCs. CHSE-214 cells viability, by means of an MTT colorimetric assay, was not affected by the exposure to CM ( Figure 3B).
Figure 3. Antiviral activity of the conditioned media from IPNV-exposed RBCs.
( A) Viral titers (FFU/mL) in CHSE-214 cells infected with IPNV at MOI 0.05 previously non-treated (black) or treated with either supernatants from control RBCs (white) or IPNV-exposed RBCs (grey), during 24 hours, at the indicated dilutions (n = 4, performing triplicates from each individual). Two-way ANOVA, with Sidak´s multiple comparison test, was performed among the different dilutions and conditions to test statistical differences. ( B) Percentage of viable CHSE-214 cells pre-treated with conditioned medium from control and IPNV-exposed RBCs, during 24 hours, and relative to non-treated CHSE-214 cells. Percentage of viable cells was calculated as follows: absorbance treated cells/absorbance non-treated cells) x100.
IPNV exposure decreased the expression of cytokines in rainbow trout RBCs
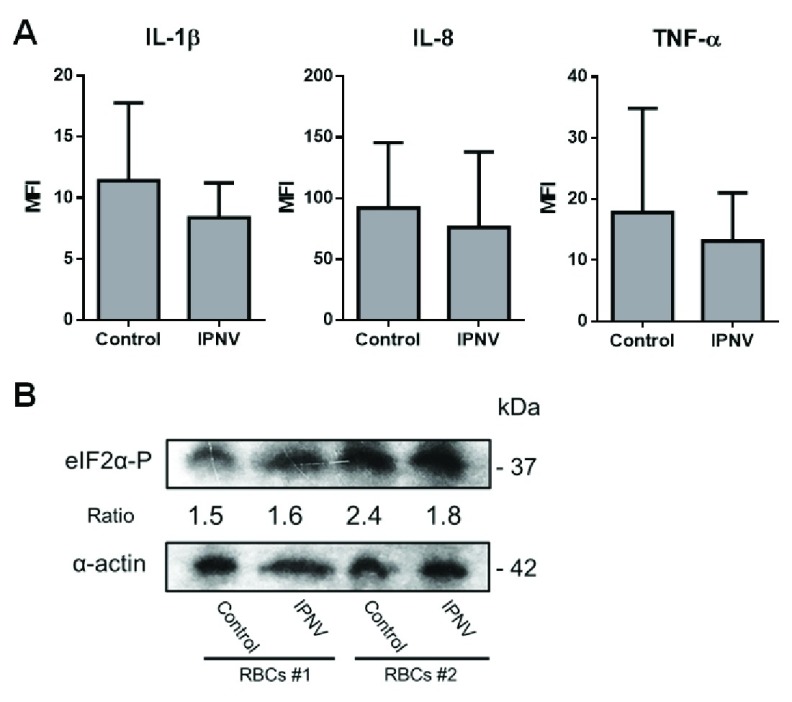
To evaluate whether ex vivo rainbow trout RBCs could produce cytokines in response to IPNV exposure, RBCs were exposed to IPNV and IL-1 β, IL-8 and TNF-α protein levels were evaluated by means of FC and IF in control and IPNV-exposed cell cultures. The results showed a decrease in the protein expression of IL-1β, IL-8 and TNFα in IPNV-exposed RBCs ( Figure 4A).
Figure 4. IPNV-exposure decreased cytokine levels in rainbow trout RBCs.
( A) Intracellular MFI (mean fluorescent intensity) values of IL-1β, IL-8 and TNFα from control and IPNV-exposed RBCs at MOI 0.5 and 3 dpe measured by FC (flow cytometry)(n = 6). Mann-Whitney test was performed among both conditions. ( B) Phosphorylation of translation initiation factor eIF2α in IPNV-exposed RBCs. Representative western blot of eIF2α-P in control and IPNV-exposed RBCs from two individuals at MOI 0.5, 3 dpe. Densitometry ratios were done relativizing to α-actin. Mann-Whitney test was performed among both conditions.
IPNV exposure did not induce phosphorylation of the α-subunit of the eukaryotic translational initiation factor 2 (eIF2α) in rainbow trout RBCs
The phosphorylation of the translation initiation factor eIF2α is a key mechanism of global inhibition of translational initiation 33 and it has been described to happen after IPNV infection in the permissive cell line CHSE-214 cells 34. In this sense, since IPNV-exposed RBCs depicted a small downregulation of the evaluated cytokines protein levels, we further investigated whether IPNV exposure could reduce protein translation in RBCs by triggering the phosphorylation of eIF2α. However, the results revealed no changes in the phosphorylation of eIF2α ( Figure 4B).
Each sheet contains the raw Ct values for the indicated figure numbers, organized by samples (rows) and genes (columns).
Copyright: © 2017 Nombela I et al.
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Contains the virus titer (TCID 50/mL) results of the indicated figure number.
Copyright: © 2017 Nombela I et al.
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Each folder contains the Flow Cytometry Standard (.fcs) format files. Source data files are organized by figure number, and then by sample number, condition and antibody.
Copyright: © 2017 Nombela I et al.
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Copyright: © 2017 Nombela I et al.
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Copyright: © 2017 Nombela I et al.
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Related uncropped blots are included.
Copyright: © 2017 Nombela I et al.
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Discussion
Previously, we have demonstrated that rainbow trout RBCs can respond to VHSV, a ssRNA virus not targeting RBCs, halting its replication, downregulating type I interferon-related genes, showing global protein downregulation in the cell and phosphorylation of the translation initiation factor eIF2α 16.
It is known that IPNV primarily targets pancreatic and liver cells 35. It has been also reported that IPNV was detectable in kidney hematopoietic tissue, corpuscles of Stannius, in Islets of Langherhans, in the lamina propria of the pyloric caeca, the gill arch connective tissue, the ventricle of the heart and dermis of the skin 35. Our results showed that IPNV did not replicate in RBCs, although small amounts of IPNV were persistently found inside RBCs after 3 dpe (≈ 10 3 TCID 50/mL). Similarly, IPNV has been shown to enter mammalian cells, without significant levels of replication, being this entry suggested to be receptor mediated 36. From our results, the persistence of IPNV in RBCs after 72 hpe could point out the entry of the virus inside RBCs. However, we could not further verify the presence of the IPNV inside RBCs ( Figure 1).
Nevertheless, despite the lack of replication of IPNV in RBCs, IPNV could induce an antiviral gene expression mediated by the IFN pathway, as it has been observed in RBCs productive infections with ISAV 11 and PRV 14. As shown by our results, ifn1 and IFN-1 related genes ( irf7, pkr and mx) expression levels were increased time-dependently in response to IPNV-exposure. High inter-individual variability was observed, similarly to that found in the RBCs from salmons challenged with PRV 37. In addition, although we could not verify the entry and uncoating of IPNV inside RBCs, we could observe an increment in the expression of the tlr3 gene in parallel to the expression of the other IFN-related genes in IPNV-exposed RBCs. This could indicate the activation of the TLR3/IFN pathway by the presence of intracellular viral dsRNA.
IFN-1 leads to the expression of interferon stimulated genes (ISGs) 38. Among ISGs, the antiviral protein Mx has a well characterized antiviral role. Confirming those expectations, our results showed the significant upregulation of the Mx protein 6 dpe, after having a peak of its gene expression at 3 dpe. Previously, a positive correlation between the expression of Mx protein and the inhibition of IPNV in CHSE-214 cells has been established 39. Therefore, Mx protein production in IPNV-exposed RBCs could be involved in the low IPNV titers observed. The high basal levels of Mx protein detected inside RBCs ( Figure 2D), much elevated than those for CHSE-214 cells ( Figure S1), could be implicated in the early disappearance of IPNV inside RBCs. A similar hypothesis has been made in the abortive infection of VHSV in the RTS-11 cell line 40 and in rainbow trout RBCs 16, where upregulation or high constitutive expression of mx genes was speculated to be related to the inhibition of the virus.
Moreover, our results showed that CM from RBCs exposed to IPNV could partially protect CHSE-214 cells from IPNV infection. Similar to other cell types, this antiviral activity has been also observed in CM of RTS11 and RTG-2 cells exposed to Poly (I:C) (polyinosinic:polycytidylic acid) and/or infected with chum salmon reovirus 41. The fact that RBCs can secrete factors that confer protection against IPNV infection in other cell lines could indicate that RBCs, despite not being permissive to IPNV infection, may exhibit an antiviral response. Besides, we evaluated the production of cytokines in IPNV-exposed RBCs. Previously, the expression of IL-1β in salmon gill and head kidney tissues 42, IL-8 in rainbow trout head kidney tissue 43 and TNFα in zebrafish embryonic cells 44 have been implicated in the immune response against IPNV; therefore, we chose these cytokines to evaluate the immune response of rainbow trout RBCs to IPNV exposure. However, our results showed a reduction trend of these proteins in IPNV-exposed RBCs.
A shutdown in protein synthesis by phosphorylation of eIF2α has been reported in CHSE-214 cells infected with IPNV 34. So far, in rainbow trout RBCs exposed to IPNV, although a trend to cytokine protein reduction was observed, no phosphorylation of eIF2α was detected and Mx protein expression was increased. IFN-1 has been reported to inhibit the production of IL-1β 45, therefore, the cytokine reduction trend observed could have been a result of the related IFN-1 pathway upregulation. In contrast, in rainbow trout RBCs, VHSV rhabdovirus induced phosphorylation of eIF2α and a cell shut-off characterized by the downregulation of the proteome 16.
Further studies are needed to completely understand the molecular mechanism through which IPNV triggers this immune response in rainbow trout RBCs. However, the lack of commercial antibodies against fish proteins involved in cell signaling networks limits the study of this area. The implication of RBCs during in vivo IPNV infection and the response against different strains of IPNV remains to be evaluated.
Finally, one of the potential applications of these results is that fish RBCs could be considered mediators of the antiviral response and therefore targets of novel DNA vaccines and of new strategies against fish viral infections.
Data availability
The data referenced by this article are under copyright with the following copyright statement: Copyright: © 2017 Nombela I et al.
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication). http://creativecommons.org/publicdomain/zero/1.0/
Dataset 1. Excel file containing qPCR data. Each sheet contains the raw Ct values for the indicated figure numbers, organized by samples (rows) and genes (columns). doi, 10.5256/f1000research.12994.d182842 46
Dataset 2. Excel file containing the virus titration data. Contains the virus titer (TCID 50/mL) results of the indicated figure number. doi, 10.5256/f1000research.12994.d182843 47
Dataset 3. Flow cytometry data. Each folder contains the Flow Cytometry Standard (.fcs) format files. Source data files are organized by figure number, and then by sample number, condition and antibody. doi, 10.5256/f1000research.12994.d182844 48
Dataset 4. Excel file containing the Focus Forming Units (FFU) counting for Figure 3A. doi, 10.5256/f1000research.12994.d182845 49
Dataset 5. Excel file containing MTT absorbance raw data. doi, 10.5256/f1000research.12994.d182846 50
Dataset 6. Excel file containing the densitometry raw data of eIF2α-P and α-Actin western blots. Related uncropped blots are included. doi, 10.5256/f1000research.12994.d182847 51
Acknowledgments
Special thanks to Remedios Torres and Efren Lucas for their technical assistance.
Funding Statement
This work was supported by the European Research Council (ERC starting grant 2014 GA639249).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; referees: 2 approved]
Supplementary material
Figure S1. qPCR Amplification plot of mx1-3 gene expression in RBCs and CHSE-214 cells..
References
- 1. Wolf K, Snieszko SF, Dunbar CE, et al. : Virus nature of infectious pancreatic necrosis in trout. Proc Soc Exp Biol Med. 1960;104:105–8. 10.3181/00379727-104-25743 [DOI] [PubMed] [Google Scholar]
- 2. Zhu L, Wang X, Wang K, et al. : Outbreak of infectious pancreatic necrosis virus (IPNV) in farmed rainbow trout in China. Acta Trop. 2017;170:63–9. 10.1016/j.actatropica.2017.02.025 [DOI] [PubMed] [Google Scholar]
- 3. Snieszko SF: Your fishes’ health: Diseases of fishes. Trop Fish Hobbyist. 1981;30(3). Reference Source [Google Scholar]
- 4. Dobos P, Hill BJ, Hallett R, et al. : Biophysical and biochemical characterization of five animal viruses with bisegmented double-stranded RNA genomes. J Virol. 1979;32(2):593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dobos P: The molecular biology of infectious pancreatic necrosis virus (IPNV). Ann Rev Fish Dis. 1995;5:25–54. 10.1016/0959-8030(95)00003-8 [DOI] [Google Scholar]
- 6. Baum J, Ward RH, Conway DJ: Natural selection on the erythrocyte surface. Mol Biol Evol. 2002;19(3):223–9. 10.1093/oxfordjournals.molbev.a004075 [DOI] [PubMed] [Google Scholar]
- 7. Morera D, Roher N, Ribas L, et al. : RNA-Seq reveals an integrated immune response in nucleated erythrocytes. PLoS One. 2011;6(10):e26998. 10.1371/journal.pone.0026998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rodriguez MF, Wiens GD, Purcell MK, et al. : Characterization of Toll-like receptor 3 gene in rainbow trout ( Oncorhynchus mykiss). Immunogenetics. 2005;57(7):510–9. 10.1007/s00251-005-0013-1 [DOI] [PubMed] [Google Scholar]
- 9. Passantino L, Massaro MA, Jirillo F, et al. : Antigenically activated avian erythrocytes release cytokine-like factors: a conserved phylogenetic function discovered in fish. Immunopharmacol Immunotoxicol. 2007;29(1):141–52. 10.1080/08923970701284664 [DOI] [PubMed] [Google Scholar]
- 10. Passantino L, Altamura M, Cianciotta A, et al. : Maturation of fish erythrocytes coincides with changes in their morphology, enhanced ability to interact with Candida albicans and release of cytokine-like factors active upon autologous macrophages. Immunopharmacol Immunotoxicol. 2004;26(4):573–85. 10.1081/IPH-200042323 [DOI] [PubMed] [Google Scholar]
- 11. Workenhe ST, Kibenge MJ, Wright GM, et al. : Infectious salmon anaemia virus replication and induction of alpha interferon in Atlantic salmon erythrocytes. Virol J. 2008;5:36. 10.1186/1743-422X-5-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Passantino L, Altamura M, Cianciotta A, et al. : Fish immunology. I. Binding and engulfment of Candida albicans by erythrocytes of rainbow trout ( Salmo gairdneri Richardson). Immunopharmacol Immunotoxicol. 2002;24(4):665–78. 10.1081/IPH-120016050 [DOI] [PubMed] [Google Scholar]
- 13. Schraml B, Baker MA, Reilly BD: A complement receptor for opsonized immune complexes on erythrocytes from Oncorhynchus mykiss but not Ictalarus punctatus. Mol Immunol. 2006;43(10):1595–603. 10.1016/j.molimm.2005.09.014 [DOI] [PubMed] [Google Scholar]
- 14. Finstad OW, Dahle MK, Lindholm TH, et al. : Piscine orthoreovirus (PRV) infects Atlantic salmon erythrocytes. Vet Res. 2014;45:35. 10.1186/1297-9716-45-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wessel Ø, Olsen CM, Rimstad E, et al. : Piscine orthoreovirus (PRV) replicates in Atlantic salmon ( Salmo salar L.) erythrocytes ex vivo. Vet Res. 2015;46:26. 10.1186/s13567-015-0154-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nombela I, Puente-Marin S, Chico V, et al. : Identification of diverse defense mechanisms in trout red blood cells in response to VHSV halted viral replication [version 1; referees: 3 approved with reservations]. F1000Res. 2017;6:1958 10.12688/f1000research.12985.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hill BJ, Way K: Serological classification of infectious pancreatic necrosis (IPN) virus and other aquatic birnaviruses. Annu Rev Fish Dis. 1995;5:55–77. 10.1016/0959-8030(95)00011-9 [DOI] [Google Scholar]
- 18. Marroquí L, Estepa A, Perez L: Inhibitory effect of mycophenolic acid on the replication of infectious pancreatic necrosis virus and viral hemorrhagic septicemia virus. Antiviral Res. 2008;80(3):332–8. 10.1016/j.antiviral.2008.07.007 [DOI] [PubMed] [Google Scholar]
- 19. Chico V, Gomez N, Estepa A, et al. : Rapid detection and quantitation of viral hemorrhagic septicemia virus in experimentally challenged rainbow trout by real-time RT-PCR. J Virol Methods. 2006;132(1–2):154–9. 10.1016/j.jviromet.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 20. Livak KJ, Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 21. Rojas V, Camus-Guerra H, Guzmán F, et al. : Pro-inflammatory caspase-1 activation during the immune response in cells from rainbow trout Oncorhynchus mykiss (Walbaum 1792) challenged with pathogen-associated molecular patterns. J Fish Dis. 2015;38(11):993–1003. 10.1111/jfd.12315 [DOI] [PubMed] [Google Scholar]
- 22. Schmitt P, Wacyk J, Morales-Lange B, et al. : Immunomodulatory effect of cathelicidins in response to a β-glucan in intestinal epithelial cells from rainbow trout. Dev Comp Immunol. 2015;51(1):160–9. 10.1016/j.dci.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 23. Santana P, Palacios C, Narváez E, et al. : Anti-peptide antibodies: A tool for detecting IL-8 in salmonids. Electron J Biotechnol. 2012;15(5). 10.2225/vol15-issue5-fulltext-15 [DOI] [Google Scholar]
- 24. Rojas V, Morales-Lange B, Guzmán F, et al. : Immunological strategy for detecting the pro-inflammatory cytokine TNF-alpha in salmonids. Electron J Biotechnol. 2012;15(5). 10.2225/vol15-issue5-fulltext-19 [DOI] [Google Scholar]
- 25. Chico V, Martinez-Lopez A, Ortega-Villaizan M, et al. : Pepscan mapping of viral hemorrhagic septicemia virus glycoprotein G major lineal determinants implicated in triggering host cell antiviral responses mediated by type I interferon. J Virol. 2010;84(14):7140–50. 10.1128/JVI.00023-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martinez-Lopez A, Garcia-Valtanen P, Ortega-Villaizan M, et al. : VHSV G glycoprotein major determinants implicated in triggering the host type I IFN antiviral response as DNA vaccine molecular adjuvants. Vaccine. 2014;32(45):6012–9. 10.1016/j.vaccine.2014.07.111 [DOI] [PubMed] [Google Scholar]
- 27. Domínguez J, Hedrick RP, Sánchez-Vizcaino JM: Use of monoclonal-antibodies for detection of infectious pancreatic necrosis virus by the enzyme-linked-immunosorbent-assay (ELISA). Dis Aquat Organ. 1990;8:157–63. 10.3354/dao008157 [DOI] [Google Scholar]
- 28. Ortega-Villaizan M, Chico V, Martinez-Lopez A, et al. : In vitro analysis of the factors contributing to the antiviral state induced by a plasmid encoding the viral haemorrhagic septicaemia virus glycoprotein G in transfected trout cells. Vaccine. 2011;29(4):737–43. 10.1016/j.vaccine.2010.11.021 [DOI] [PubMed] [Google Scholar]
- 29. Wang T, Bird S, Koussounadis A, et al. : Identification of a novel IL-1 cytokine family member in teleost fish. J Immunol. 2009;183(2):962–74. 10.4049/jimmunol.0802953 [DOI] [PubMed] [Google Scholar]
- 30. Wang T, Diaz-Rosales P, Costa MM, et al. : Functional characterization of a nonmammalian IL-21: rainbow trout Oncorhynchus mykiss IL-21 upregulates the expression of the Th cell signature cytokines IFN-gamma, IL-10, and IL-22. J Immunol. 2011;186(2):708–21. 10.4049/jimmunol.1001203 [DOI] [PubMed] [Google Scholar]
- 31. Purcell MK, Nichols KM, Winton JR, et al. : Comprehensive gene expression profiling following DNA vaccination of rainbow trout against infectious hematopoietic necrosis virus. Mol Immunol. 2006;43(13):2089–106. 10.1016/j.molimm.2005.12.005 [DOI] [PubMed] [Google Scholar]
- 32. Roehm NW, Rodgers GH, Hatfield SM, et al. : An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J Immunol Methods. 1991;142(2):257–65. 10.1016/0022-1759(91)90114-U [DOI] [PubMed] [Google Scholar]
- 33. Levin D, London IM: Regulation of protein synthesis: activation by double-stranded RNA of a protein kinase that phosphorylates eukaryotic initiation factor 2. Proc Natl Acad Sci U S A. 1978;75(3):1121–5. 10.1073/pnas.75.3.1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gamil AA, Mutoloki S, Evensen Ø: A piscine birnavirus induces inhibition of protein synthesis in CHSE-214 cells primarily through the induction of eIF2α phosphorylation. Viruses. 2015;7(4):1987–2005. 10.3390/v7041987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ellis AE, Cavaco A, Petrie A, et al. : Histology, immunocytochemistry and qRT-PCR analysis of Atlantic salmon, Salmo salar L., post-smolts following infection with infectious pancreatic necrosis virus (IPNV). J Fish Dis. 2010;33(10):803–18. 10.1111/j.1365-2761.2010.01174.x [DOI] [PubMed] [Google Scholar]
- 36. Ørpetveit I, Küntziger T, Sindre H, et al. : Infectious pancreatic necrosis virus (IPNV) from salmonid fish enters, but does not replicate in, mammalian cells. Virol J. 2012;9:228. 10.1186/1743-422X-9-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dahle MK, Wessel Ø, Timmerhaus G, et al. : Transcriptome analyses of Atlantic salmon ( Salmo salar L.) erythrocytes infected with piscine orthoreovirus (PRV). Fish Shellfish Immunol. 2015;45(2):780–90. 10.1016/j.fsi.2015.05.049 [DOI] [PubMed] [Google Scholar]
- 38. Schoggins JW, Rice CM: Interferon-stimulated genes and their antiviral effector functions. Curr Opin Virol. 2011;1(6):519–25. 10.1016/j.coviro.2011.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Larsen R, Røkenes TP, Robertsen B: Inhibition of infectious pancreatic necrosis virus replication by atlantic salmon Mx1 protein. J Virol. 2004;78(15):7938–44. 10.1128/JVI.78.15.7938-7944.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pham PH, Lumsden JS, Tafalla C, et al. : Differential effects of viral hemorrhagic septicaemia virus (VHSV) genotypes IVa and IVb on gill epithelial and spleen macrophage cell lines from rainbow trout ( Oncorhynchus mykiss). Fish Shellfish Immunol. 2013;34(2):632–40. 10.1016/j.fsi.2012.12.004 [DOI] [PubMed] [Google Scholar]
- 41. DeWitte-Orr SJ, Leong JA, Bols NC: Induction of antiviral genes, Mx and vig-1, by dsRNA and Chum salmon reovirus in rainbow trout monocyte/macrophage and fibroblast cell lines. Fish Shellfish Immunol. 2007;23(3):670–82. 10.1016/j.fsi.2007.01.017 [DOI] [PubMed] [Google Scholar]
- 42. Ingerslev HC, Rønneseth A, Pettersen EF, et al. : Differential expression of immune genes in Atlantic salmon ( Salmo salar L.) challenged intraperitoneally or by cohabitation with IPNV. Scand J Immunol. 2009;69(2):90–8. 10.1111/j.1365-3083.2008.02201.x [DOI] [PubMed] [Google Scholar]
- 43. Reyes-Cerpa S, Reyes-López F, Toro-Ascuy D, et al. : Induction of anti-inflammatory cytokine expression by IPNV in persistent infection. Fish Shellfish Immunol. 2014;41(2):172–82. 10.1016/j.fsi.2014.08.029 [DOI] [PubMed] [Google Scholar]
- 44. Wang WL, Liu W, Gong HY, et al. : Activation of cytokine expression occurs through the TNFα/NF-κB-mediated pathway in birnavirus-infected cells. Fish Shellfish Immunol. 2011;31(1):10–21. 10.1016/j.fsi.2011.01.015 [DOI] [PubMed] [Google Scholar]
- 45. Guarda G, Braun M, Staehli F, et al. : Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34(2):213–23. 10.1016/j.immuni.2011.02.006 [DOI] [PubMed] [Google Scholar]
- 46. Nombela I, Carrion A, Puente-Marin S, et al. : Dataset 1 in: Piscine birnavirus triggers antiviral immune response in trout red blood cells, despite not being infective. F1000Research. 2017. Data Source [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nombela I, Carrion A, Puente-Marin S, et al. : Dataset 2 in: Piscine birnavirus triggers antiviral immune response in trout red blood cells, despite not being infective. F1000Research. 2017. Data Source [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nombela I, Carrion A, Puente-Marin S, et al. : Dataset 3 in: Piscine birnavirus triggers antiviral immune response in trout red blood cells, despite not being infective. F1000Research. 2017. Data Source [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nombela I, Carrion A, Puente-Marin S, et al. : Dataset 4 in: Piscine birnavirus triggers antiviral immune response in trout red blood cells, despite not being infective. F1000Research. 2017. Data Source [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nombela I, Carrion A, Puente-Marin S, et al. : Dataset 5 in: Piscine birnavirus triggers antiviral immune response in trout red blood cells, despite not being infective. F1000Research. 2017. Data Source [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nombela I, Carrion A, Puente-Marin S, et al. : Dataset 6 in: Piscine birnavirus triggers antiviral immune response in trout red blood cells, despite not being infective. F1000Research. 2017. Data Source [DOI] [PMC free article] [PubMed] [Google Scholar]