Version Changes
Revised. Amendments from Version 1
The Introduction has been rewritten and a new Discussion section has been added. The expository introduction to the NB linear modeling approach has been expanded to include an example with replicates and to explain the coefficient estimates more explicitly. New functions modelMatrixMeth, readBismark2DGE and nearestTSS have been added to streamline the code. New plots have been added based on distance to the TSS (Figures 3 and 4). The CpG filtering has been relaxed slightly. The calculation of M-values for the MDS plot has been improved and the MDS plot using beta-values has been removed. Other minor corrections have been made to improve the exposition. All software packages have been updated to Bioconductor 3.7.
Abstract
Cytosine methylation is an important DNA epigenetic modification. In vertebrates, methylation occurs at CpG sites, which are dinucleotides where a cytosine is immediately followed by a guanine in the DNA sequence from 5' to 3'. When located in the promoter region of a gene, DNA methylation is often associated with transcriptional silencing of the gene. Aberrant DNA methylation is associated with the development of various diseases such as cancer. Bisulfite sequencing (BS-seq) is the current "gold-standard" technology for high-resolution profiling of DNA methylation. Reduced representation bisulfite sequencing (RRBS) is an efficient form of BS-seq that targets CpG-rich DNA regions in order to save sequencing costs. A typical bioinformatics aim is to identify CpGs that are differentially methylated (DM) between experimental conditions. This workflow demonstrates that differential methylation analysis of RRBS data can be conducted using software and methodology originally developed for RNA-seq data. The RNA-seq pipeline is adapted to methylation by adding extra columns to the design matrix to account for read coverage at each CpG, after which the RRBS and RNA-seq pipelines are almost identical. This approach is statistically natural and gives analysts access to a rich collection of analysis tools including generalized linear models, gene set testing and pathway analysis. The article presents a complete start to finish case study analysis of RRBS profiles of different cell populations from the mouse mammary gland using the Bioconductor package edgeR. We show that lineage-committed cells are typically hyper-methylated compared to progenitor cells and this is true on all the autosomes but not the sex chromosomes. We demonstrate a strong negative correlation between methylation of promoter regions and gene expression as measured by RNA-seq for the same cell types, showing that methylation is a regulatory mechanism involved in epithelial linear commitment.
Keywords: Methylation, BS-seq, differential methylation analysis, Bioconductor
Introduction
Cytosine methylation is an important epigenetic DNA modification that is generally associated with transcriptional silencing 1. In vertebrates, methylation occurs at CpG sites, which are dinucleotides where a cytosine (C) is immediately followed by a guanine (G) in the DNA sequence from 5’ to 3’. CpG dinucleotides are relatively uncommon on the human genome but occur more frequently in gene promoters and exons 2. About 72% of human gene promoters are enriched for CpGs 2. CpGs in gene promoters tend to cluster in CpG islands (CGIs), which are regions of a few hundred to a couple of thousand base pairs with very strong enrichment of CpGs 3.
The relationship of DNA methylation to transcription in vertebrates is complex 4. Methylation of CGIs causes robust transcriptional repression and is required for long-term mono-allelic silencing including X inactivation and genomic imprinting 1. Methylation of CpG-poor promoters is more weakly associated with gene expression, and the mechanisms by which this occurs are unclear 1. Methylation of gene bodies on the other hand can be positively associated with gene expression 4, 5.
DNA methylation is relatively stable in that most CGIs do not change methylation state during normal cell development. Nevertheless DNA methylation is understood to play a regulatory role in differentiation and commitment in adult cell lineages 6, 7. Aberrant methylation patterns are also associated with the development of diseases such as cancer 8, 9.
Bisulfite sequencing (BS-seq) is increasingly used to profile DNA methylation 10, 11. Unmethylated cytosines (C) are converted to Uracils (U) by sodium bisulfite and then deaminated to thymines (T) during PCR amplification. Methylated Cs, on the other hand, remain intact after bisulfite treatment. This strategy produces whole genome bisulfite sequencing (WGBS) when combined with sequencing of the entire genome. WGBS is sometimes considered the “gold standard” for methylation profiling because it provides single-nucleotide resolution and whole-genome coverage 11. However it requires large quantities of DNA and is expensive because of the amount of sequencing required.
The fact that CpG islands constitute only a small percentage of the genome makes the WGBS approach inefficient in terms of information content per sequenced read. To improve efficiency and reduce costs, enrichment strategies have been developed and combined with BS-seq to target a specific fraction of the genome. A common targeted approach is reduced representation bisulfite sequencing (RRBS) that targets CpG-rich regions 11, 12. Under the RRBS strategy, small fragments that compose only 1% of the genome are generated using MspI digestion, which means fewer reads need to be sequenced in total to provide reasonable coverage of the targeted regions. The RRBS approach can capture approximately 70% of gene promoters and 85% of CpG islands, while requiring only small quantities of input sample 13. RRBS has great advantages in cost and efficiency, especially when CGI or gene-orientated results are required. RRBS is also applicable for single cell studies 14.
The first step of analyzing BS-seq data is to align short sequence reads to a reference genome. The number of C-to-T conversions are then counted for all the mapped reads. A number of software tools have been developed to facilitate read mapping and methylation calling, including Bismark 15, MethylCoder 16, BRAT 17, BSSeeker 18, 19 and BSMAP 20. Most of these tools rely on existing short read aligners, such as Bowtie 21, 22.
Typical downstream DNA methylation studies often involve finding differentially methylated regions (DMRs) between different experimental conditions. A number of statistical methods and software packages have been developed for detecting DMRs using the BS-seq technology. methylkit 23 and RnBeads 24 implement Fisher’s Exact Test, which is a popular choice for two-group comparisons with no replicates. In the case of complex experimental designs, regression methods are widely used to model methylation levels or read counts. RnBeads offers a linear regression approach based on the moderated t-test and empirical Bayes method implemented in limma 25. BSmooth 26 is another analysis pipeline that uses linear regression together with a local likelihood smoother. methylkit also has an option to apply logistic regression with overdispersion correction 23. Some other methods have been developed based on beta-binomial distribution to achieve better variance modeling. For example, DSS fits a Bayesian hierarchical beta-binomial model to BS-seq data and uses Wald tests to detect DMRs 27. Other software using beta-binomial model include BiSeq 28, MOABS 29 and RADMeth 30.
In this workflow, we demonstrate an edgeR pipeline for differential methylation analysis. edgeR is one of the most popular Bioconductor packages for assessing differential expression in RNA-seq data 31, 32. It is based on the negative binomial (NB) distribution and it models the variation between biological replicates through the NB dispersion parameter. Unlike other approaches to methylation sequencing data, the analysis explained in this workflow keeps the counts for methylated and unmethylated reads as separate observations. edgeR linear models are used to fit the total read count (methylated plus unmethylated) at each genomic locus, in such a way that the proportion of methylated reads at each locus is modeled indirectly as an over-dispersed binomial-like distribution. This approach has a number of advantages. First, it allows the differential methylation analysis to be undertaken using existing edgeR pipelines developed originally for RNA-seq differential expression analyses. The edgeR generalized linear model (GLM) framework offers great flexibility for analysing complex experimental designs while still accounting for the biological variability 33. Second, keeping methylated and unmethylated read count as separate data observations allows the inherent variability of the data to be modeled more directly and perhaps more realistically. Differential methylation is assessed by likelihood ratio tests so we do not need to assume that the log-fold-changes or other coefficient estimators are normally distributed.
This article presents an analysis of an RRBS data set generated by the authors containing replicated RRBS profiles of basal and luminal cell populations from the mouse mammary epithelium. Our primary interest is in gene-orientated and pathway-orientated interpretations of the result. It is of particular importance to relate methylation changes to RNA-seq expression changes for the same genes. We show how to analyze differential methylation changes either for individual CpGs or for pre-specified genomic regions, such as chromosomes or genes, and we particularly focus on methylation changes by promoter regions.
As with other articles in the Bioconductor Gateway series, our aim is to provide an example analysis with complete start to finish code. As with other Bioconductor workflow articles, we illustrate one analysis strategy in detail rather than comparing different pipelines. The analysis approach illustrated in this article can in principle be applied to any BS-seq data but is especially appropriate for RRBS data. The approach is designed for experiments that included biological replication but can be used without replication if the NB dispersion is preset. The results shown in this article were generated using Bioconductor Release 3.7.
The next section gives an expository introduction to the edgeR approach to methylation data. The analysis of the mammary epithelial data starts afterwards.
Introducing the NB linear modeling approach to BS-seq data
A very small example
To introduce the edgeR linear modeling approach to BS-seq data, consider a genomic locus that has mA methylated and u A unmethylated reads in condition A and m B methylated and u B unmethylated reads in condition B. Our approach is to model all four counts as NB distributed with the same dispersion but different means. Suppose the data is as given in Table 1. The counts can be entered into a matrix in R:
> counts <- matrix(c(2,12,11,0),1,4) > dimnames(counts) <- list("Locus", c("A.Me","A.Un","B.Me","B.Un")) > counts A.Me A.Un B.Me B.Un Locus 2 12 11 0
Table 1. A very small example data set.
| Sample | Condition | Methylated Count | Unmethylated Count |
|---|---|---|---|
| 1 | A | 2 | 12 |
| 2 | B | 11 | 0 |
The experimental design of the data can be summarized by indicating which counts correspond to each sample and each condition:
> design <- cbind(Sample1 = c(1,1,0,0), + Sample2 = c(0,0,1,1), + A = c(1,0,0,0), + B = c(0,0,1,0))
In the design matrix, the first two columns are indicators for samples 1 and 2 while the third and fourth columns indicate the methylated count columns for conditions A and B.
If this were a complete data set, then it could be analyzed in edgeR as follows. First we fit a negative binomial generalized linear model to the counts:
> library(edgeR) > fit <- glmFit(counts, design, lib.size=c(100,100,100,100), dispersion=0.0247)
The first two coefficients in the linear model are used to model the total number of reads (methylated or unmethylated) for samples 1 and 2, respectively. Coefficient 3 estimates the log ratio of methylated to unmethylated reads for condition A, a quantity that can also be viewed as the logit proportion of methylated reads in condition A. In mathematical terms, we have . (Note that edgeR adds 0.125 to each count when reporting the coefficients to avoid taking logarithms of zero.) Coefficient 4 estimates the log ratio of methylated to unmethylated reads for condition B, in mathematical terms .
Then we conduct a likelihood ratio test to compare the methylation rate in condition B to that in condition A:
> lrt <- glmLRT(fit, contrast = c(0,0,-1,1)) > topTags(lrt) Coefficient: -1*A 1*B logFC logCPM LR PValue FDR Locus 8.99 16.3 20.7 5.27e-06 5.27e-06
The contrast vector indicates that we wish to compare the fourth to the third coefficient, ignoring the two sample coverage coefficients. The estimated value for the contrast is the difference in logit methylation rates, which is estimated as , a quantity that is shown as the log2-fold-change (logFC) column of the results table. The chisquare likelihood ratio statistic (LR) is 20.7 and the P-value for differential methylation in condition B vs condition A is P = 5.27 × 10 −6.
Note that the specific parametrization used to account for the sample effects is not important. The test for differential methylation would be unchanged if we represented the two sample effects using an intercept model. Indeed, the first two columns of the design matrix could be any two columns spanning the sample effects:
> design <- cbind(Intercept = c(1,1,1,1), + Sample2 = c(0,0,1,1), + A = c(1,0,0,0), + B = c(0,0,1,0)) > fit <- glmFit(counts, design, lib.size=c(100,100,100,100), dispersion=0.0247) > lrt <- glmLRT(fit, contrast = c(0,0,-1,1)) > topTags(lrt) Coefficient: -1*A 1*B logFC logCPM LR PValue FDR Locus 8.99 16.3 20.7 5.27e-06 5.27e-06
The dispersion parameter controls the degree of biological variability 33. If we had set dispersion=0 in the above code, then the above analysis would be exactly equivalent to a logistic binomial regression, with the methylated counts as responses and the total counts as sizes, and with a likelihood ratio test for a difference in proportions between conditions A and B. Positive values for the dispersion produce over-dispersion relative to the binomial distribution. We have set the dispersion here equal to the value that is estimated below for the mammary epithelial data.
In the above code, the two library sizes for each sample should be equal. Otherwise, the library size values are arbitrary and any settings would lead to the same P-value.
Relationship to beta-binomial modeling
It is interesting to compare this approach with beta-binomial modeling. It is well known that if m and u are independent Poisson random variables with means µ m and µ u, then the conditional distribution of m given m + u is binomial with success probability p = µ m/( µ m + µ u). If the Poisson means µ m and µ u themselves follow gamma distributions, then the marginal distributions of m and u are NB instead of Poisson. If the two NB distributions have different dispersions, and have expected values in inverse proportion to the dispersions, then the conditional distribution of m given m + u follows a beta-binomial distribution. The approach taken in this article is closely related to the beta-binomial approach but makes different and seemingly more natural assumptions about the NB distributions. We instead assume the two NB distributions to have the same dispersion but different means. The NB linear modeling approach allows the means and dispersions of the two NB distributions to be estimated separately, in concordance with the data instead of being artificially linked.
A small example with replicates
edgeR linear modeling takes advantage of replicate libraries for each condition. Now we augment the small example above to include two replicates for each experimental condition. There are now four samples and eight counts ( Table 2). The eight counts can be represented in R as follows:
> counts <- matrix(c(2,12,4,20,11,0,15,3),1,8) > row.names(counts) <- "Locus" > colnames(counts) <- c("A1.Me","A1.Un","A2.Me","A2.Un", + "B1.Me","B1.Un","B2.Me","B2.Un") > counts A1.Me A1.Un A2.Me A2.Un B1.Me B1.Un B2.Me B2.Un Locus 2 12 4 20 11 0 15 3
Table 2. A slightly less small example data set with n = 2 replicates for each condition.
| Sample | Condition | Methylated Count | Unmethylated Count |
|---|---|---|---|
| 1 | A | 2 | 12 |
| 2 | A | 4 | 20 |
| 3 | B | 11 | 0 |
| 4 | B | 15 | 3 |
We can represent the sample information associated with the counts as follows:
> Sample <- gl(4,2,8) > Methylation <- gl(2,1,8, labels=c("Me","Un")) > Condition <- gl(2,4,8, labels=c("A","B")) > SampleInfo <- data.frame(Sample,Methylation,Condition) > row.names(SampleInfo) <- colnames(counts) > SampleInfo Sample Methylation Condition A1.Me 1 Me A A1.Un 1 Un A A2.Me 2 Me A A2.Un 2 Un A B1.Me 3 Me B B1.Un 3 Un B B2.Me 4 Me B B2.Un 4 Un B
The design matrix is a simple combination of two parts. First there is a matrix to model the sample coverages:
> design.samples <- model.matrix(~0+Sample)
Then there is a matrix to model the two treatment conditions.
> design.conditions <- model.matrix(~0+Condition)
The full matrix is constructed from the two parts, with the condition design matrix mediating the methylation effects:
> design <- cbind(design.samples, (Methylation=="Me") * design.conditions) > design Sample1 Sample2 Sample3 Sample4 ConditionA ConditionB 1 1 0 0 0 1 0 2 1 0 0 0 0 0 3 0 1 0 0 1 0 4 0 1 0 0 0 0 5 0 0 1 0 0 1 6 0 0 1 0 0 0 7 0 0 0 1 0 1 8 0 0 0 1 0 0
Now we can fit the GLM and conduct the statistical test for differential methylation:
> fit <- glmFit(counts, design, lib.size=100, dispersion=0.0247) > contBvsA <- makeContrasts(BvsA = ConditionB - ConditionA, levels=design) > lrt <- glmLRT(fit, contrast=contBvsA) > topTags(lrt) Coefficient: -1*ConditionA 1*ConditionB logFC logCPM LR PValue FDR Locus 5.4 16.6 34.3 4.84e-09 4.84e-09
The results for each condition have been averaged over the replicates. The change in methylation is now of smaller magnitude than for Table 1, as shown by the smaller logFC value, but the P-value is more significant because of the greater confidence that comes from the extra replicates.
Automatic construction of the design matrix
In this expository introduction we have demonstrated the construction of the design matrix from basic principles. In practice, edgeR provides a function modelMatrixMeth to automate the construction of the design matrix. The user can simply specify the treatment conditions associated with each DNA sample (as in the second column of Table 2):
> Condition <- factor(c("A","A","B","B"))
Then the design matrix is constructed by:
> design <- modelMatrixMeth(~0+Condition) > design Sample1 Sample2 Sample3 Sample4 ConditionA ConditionB 1 1 0 0 0 1 0 2 1 0 0 0 0 0 3 0 1 0 0 1 0 4 0 1 0 0 0 0 5 0 0 1 0 0 1 6 0 0 1 0 0 0 7 0 0 0 1 0 1 8 0 0 0 1 0 0
Alternatively, a user can construct any appropriate design matrix at the sample level (with 4 rows), exactly as one would do for a RNA-seq differential expression analysis, then the function will expand the sample-level design matrix to the appropriate observation-level matrix with 8 rows:
> designSL <- model.matrix(~0+Condition) > design <- modelMatrixMeth(designSL)
This gives exactly the same result as above.
Another way to make the design matrix
When there are just two treatment conditions to be compared, it can be slightly simpler to compute the log2-fold-change directly as a coefficient of the linear model, rather than forming it as a contrast. This can be done by including an intercept in the design matrix as follows:
> design <- modelMatrixMeth(~Condition) > design Sample1 Sample2 Sample3 Sample4 (Intercept) ConditionB 1 1 0 0 0 1 0 2 1 0 0 0 0 0 3 0 1 0 0 1 0 4 0 1 0 0 0 0 5 0 0 1 0 1 1 6 0 0 1 0 0 0 7 0 0 0 1 1 1 8 0 0 0 1 0 0
In this formulation, the second last coefficient still estimates the logit methylation level in condition A, but the last coefficient now estimates the log2-fold-change directly:
> fit <- glmFit(counts, design, lib.size=100, dispersion=0.0247) > lrt <- glmLRT(fit, coef="ConditionB") > topTags(lrt) Coefficient: ConditionB logFC logCPM LR PValue FDR Locus 5.4 16.6 34.3 4.84e-09 4.84e-09
Again, this gives the same results as the previous analysis.
Analogy with paired-samples expression analyses
The above design matrix might seem as if it is very special to methylation data, but in fact the same sort of design matrix would be used for any gene expression analysis when there are paired-samples and we wish to compare treatment effects between groups. To see this, consider the small RNA-seq experiment shown in Table 3. This experiment has four subjects, each of whom contributes both treated and untreated RNA samples. The subjects belong to two groups (A and B) and we wish to test whether the treatment effect differs between the groups.
Table 3. A hypothetical paired-samples RNA-seq experiment.
| Subject | Group | Treated | Untreated |
|---|---|---|---|
| 1 | A | 2 | 12 |
| 2 | A | 4 | 20 |
| 3 | B | 11 | 0 |
| 4 | B | 15 | 3 |
Readers will note that Table 3 is the same as Table 2 except that the columns have been relabeled. Here, the four subjects are analogous to the four DNA samples in Table 2, the groups are analogous to conditions, and treatment is analogous to methylation. Exactly the same design matrix, as used above for the methylation experiment, would be appropriate here for the expression experiment. In edgeR, the only difference between the methylation and expression analyses would be in the normalization steps. The linear modeling and testing steps would be identical.
This shows that BS-seq data has a structure that is already familiar from RNA-seq experiments with paired-samples. The theme of this article is that statistical analysis methods developed for RNA-seq can be beneficially applied to BS-seq data.
NB GLMs
In practice, the data will consist of many thousands of genomic loci and the counts matrix will have a row for each locus. The function glmFit fits a NB GLM to each row of read counts. If y gi is the ith read count for genomic locus g, then y gi is assumed to be NB distributed with expected value µ gi and dispersion φ g. The expected values are modeled by the design matrix,
where the x ij are the ith row of the design matrix, β gj are the regression coefficients and N i are the library sizes. In the small example above with four samples, the design matrix had 6 columns, so p = 6. In general, if we have an experiment comparing k treatment conditions using n samples, then the count matrix will have 2 n columns and the design matrix will have p = n + k columns. The first n coefficients capture sample effects in read abundance, while the remaining k coefficients compare the methylation proportions between the conditions.
Dispersion estimation
In the small example above we did not estimate the dispersion but simply used a preset value. In practice, there will not only be replicates for each genomic locus but also many thousands of loci. The edgeR package estimates a dispersion for each locus from the replicate variability, using an empirical Bayes procedure to pool information across all the loci while estimating locus-specific dispersion estimates 33– 35. The full data example on mouse mammary epithelial cells will illustrate this.
The variance of the read counts is assumed to follow a quadratic mean-variance relationship
where ϕ g is the dispersion for locus g. The first term follows from sequencing variability and the second from biological variability 33. The NB variance function captures the fact that larger counts have larger variances. The quadratic relationship captures the technical factors that affect variability, so that ϕ g itself reflects only the biological characteristics of each locus.
RRBS profiling of mammary epithelial cells
Aim of the study
We now describe the main data set to be analyzed in this article. The epithelium of the mammary gland exists in a highly dynamic state, undergoing dramatic morphogenetic changes during puberty, pregnancy, lactation and regression 36. Characterization of the lineage hierarchy of cells in the mammary epithelium is an important step toward understanding which cells are predisposed to oncogenesis. In this study, we profiled the methylation status of the two major functionally distinct epithelial compartments: basal and luminal cells. The basal cells were further divided into those showing high or low expression of the surface marker Itga5 as part of our investigation of heterogeneity within the basal compartment. We carried out global RRBS DNA methylation assays on two biological replicates of each of the three cell populations to determine whether the epigenetic machinery played a potential role in (i) differentiation of luminal cells from basal and (ii) any compartmentalization of the basal cells associated with Itga5. Of particular interest is to relate transcriptional changes to methylation changes for the same genes.
Sample preparation
Inguinal mammary glands (minus lymph node) were harvested from FVB/N mice. All animal experiments were conducted using mice bred at and maintained in our animal facility according to the Walter and Eliza Hall Institute of Medical Research Animal Ethics Committee guidelines. Epithelial cells were suspended and fluorescence-activated cell sorting (FACS) was used to isolate basal and luminal cell populations 37. Genomic DNA (gDNA) was extracted from freshly sorted cells using the Qiagen DNeasy kit. Around 25ng gDNA input was subjected to DNA methylation analysis by BS-seq using the Ovation RRBS Methyl-seq kit from NuGEN. The process includes MspI digestion of gDNA, sequencing adapter ligation, end repair, bisulfite conversion, and PCR amplification to produce the final sequencing library. The Qiagen EpiTect Bisulfite kit was used for bisulfite-mediated conversion of unmethylated cytosines.
Experimental design
There are three groups of samples: luminal population, Itga5- basal population and Itga5+ basal population. Two biological replicates were collected for each group. This experimental design is summarized in the table below.
> targets <- read.delim("targets.txt", row.names="Sample", stringsAsFactors=FALSE) > targets Population Description P6_1 P6 Luminal P6_4 P6 Luminal P7_2 P7 Basal_Itga5_neg P7_5 P7 Basal_Itga5_neg P8_3 P8 Basal_Itga5_pos P8_6 P8 Basal_Itga5_pos
The experiment has a simple one-way layout with three groups.
The sequencing was carried out on the Illumina NextSeq 500 platform. About 30 million 75bp paired-end reads were generated for each sample.
Reading and annotating the methylation counts
Processing the BS-seq FASTQ files with Bismark
The first step of the analysis is to map the sequence reads from the FASTQ files to the mouse genome and to perform methylation calls. This is the only step of the analysis that cannot currently be done in R.
Though many options are available, we used Bismark software ( https://www.bioinformatics.babraham.ac.uk/projects/bismark) to count the methylated and unmethylated reads at each genomic locus. Bismark was run using recommended default settings. We first ran trim_galore ( https://www.bioinformatics.babraham.ac.uk/projects/trim_galore/) to remove adapters and to trim poor quality reads. Then Bismark version v0.13.0 was used to align the reads to the mouse mm10 genome using Bowtie2 22. Finally, methylation calls were made using bismark_methylation_extractor.
The Bismark output consists of one coverage file for each sample. Readers wishing to reproduce the analysis presented in this article can download the coverage files produced by Bismark from http://bioinf.wehi.edu.au/edgeR/F1000Research2017.
Reading in the data
The Bismark coverage files are just tab-delimited text files and so can be read into a dataframe in R using read.delim. Each of the files has the following format:
> P6_1 <- read.delim("P6_1.bismark.cov.gz", header=FALSE, nrows=6) > P6_1 V1 V2 V3 V4 V5 V6 1 chr15 3051454 3051454 87.5 14 2 2 chr15 3051455 3051455 100.0 1 0 3 chr15 3051486 3051486 100.0 16 0 4 chr15 3051555 3051555 81.2 13 3 5 chr15 3051556 3051556 100.0 1 0 6 chr15 3051559 3051559 87.5 14 2
The columns in the coverage file represent: V1: chromosome number; V2: start position of the CpG site; V3: end position of the CpG site; V4: methylation proportion; V5: number of methylated Cs; V6: number of unmethylated Cs.
In practice, we only need to read in columns V1, V2, V5 and V6. Column V3 is the same as V2, and the methylation proportion V4 is just a function of V5 and V6.
We now read in the Bismark coverage files for all the samples. The edgeR function readBismark2DGE reads all the files and collates the counts for all the sample into one data object:
> Sample <- row.names(targets) > files <- paste0(Sample,".bismark.cov.gz") > yall <- readBismark2DGE(files, sample.names=Sample) Reading P6_1.bismark.cov.gz Reading P6_4.bismark.cov.gz Reading P7_2.bismark.cov.gz Reading P7_5.bismark.cov.gz Reading P8_3.bismark.cov.gz Reading P8_6.bismark.cov.gz Hashing ... Collating counts ... > dim(yall) [1] 3538117 12
The edgeR package stores the counts and associated annotation in a simple list-based data object called a “DGEList”. The count matrix is
> head(yall$counts) P6_1-Me P6_1-Un P6_4-Me P6_4-Un P7_2-Me P7_2-Un P7_5-Me P7_5-Un chr15-3051454 14 2 18 0 6 0 15 1 chr15-3051455 1 0 1 0 0 0 0 0 chr15-3051486 16 0 17 1 6 0 16 0 chr15-3051555 13 3 18 0 5 1 16 0 chr15-3051556 1 0 1 0 0 0 0 0 chr15-3051559 14 2 15 3 5 1 15 1 P8_3-Me P8_3-Un P8_6-Me P8_6-Un chr15-3051454 8 0 3 0 chr15-3051455 0 0 1 0 chr15-3051486 8 0 3 0 chr15-3051555 8 0 3 0 chr15-3051556 0 0 1 0 chr15-3051559 8 0 3 0
There is a row for each CpG locus found in any of the files. There columns of methylated and unmethylated counts for each sample. The chromosomes and genomic loci are stored in the annotation data.frame:
> head(yall$genes) Chr Locus chr15-3051454 chr15 3051454 chr15-3051455 chr15 3051455 chr15-3051486 chr15 3051486 chr15-3051555 chr15 3051555 chr15-3051556 chr15 3051556 chr15-3051559 chr15 3051559
Summary information on each sample is stored in yall$samples:
> yall$samples$group <- factor(targets$Population) > head(yall$samples) group lib.size norm.factors P6_1-Me P6 27803470 1 P6_1-Un P6 33444764 1 P6_4-Me P7 37579181 1 P6_4-Un P7 35595908 1 P7_2-Me P8 17902366 1 P7_2-Un P8 15609315 1
Filtering unassembled chromosomes
It is convenient to remove genomic segments that have not been assembled into any of the recognized chromosomes:
> keep <- rep(TRUE, nrow(yall)) > Chr <- as.character(yall$genes$Chr) > keep[ grep("random",Chr) ] <- FALSE > keep[ grep("chrUn",Chr) ] <- FALSE
For this analysis, we also remove the Y chromosome and mitochondrial DNA:
> keep[Chr=="chrY"] <- FALSE > keep[Chr=="chrM"] <- FALSE > table(keep) keep FALSE TRUE 6285 3531832
Then we can subset the DGEList data object to remove the rows corresponding to the unwanted chromosomes:
> yall <- yall[keep,, keep.lib.sizes=FALSE]
The option keep.lib.sizes=FALSE causes the library sizes to be recomputed.
Sort into genomic order
Just for convenience, we sort the DGEList so that all loci are in genomic order, from chromosome 1 to chromosome X:
> ChrNames <- paste0("chr",c(1:19,"X")) > ChrNames [1] "chr1" "chr2" "chr3" "chr4" "chr5" "chr6" "chr7" "chr8" "chr9" [10] "chr10" "chr11" "chr12" "chr13" "chr14" "chr15" "chr16" "chr17" "chr18" [19] "chr19" "chrX" > yall$genes$Chr <- factor(yall$genes$Chr, levels=ChrNames) > o <- order(yall$genes$Chr, yall$genes$Locus) > yall <- yall[o,]
Gene annotation
We now annotate the CpG loci with the identity of the nearest gene. We search for the gene transcriptional start site (TSS) closest to each our CpGs:
> TSS <- nearestTSS(yall$genes$Chr, yall$genes$Locus, species="Mm") > yall$genes$EntrezID <- TSS$gene_id > yall$genes$Symbol <- TSS$symbol > yall$genes$Strand <- TSS$strand > yall$genes$Distance <- TSS$distance > yall$genes$Width <- TSS$width > head(yall$genes) Chr Locus EntrezID Symbol Strand Distance Width chr1-3020689 chr1 3020689 497097 Xkr4 - -650809 457017 chr1-3020690 chr1 3020690 497097 Xkr4 - -650808 457017 chr1-3020708 chr1 3020708 497097 Xkr4 - -650790 457017 chr1-3020724 chr1 3020724 497097 Xkr4 - -650774 457017 chr1-3020725 chr1 3020725 497097 Xkr4 - -650773 457017 chr1-3020814 chr1 3020814 497097 Xkr4 - -650684 457017
Here EntrezID, Symbol, Strand and Width are the Entrez Gene ID, symbol, strand and width of the nearest gene. Distance is the genomic distance from the CpG to the TSS. Positive values means the TSS is downstream of the CpG and negative values means the TSS is upstream.
Differential methylation analysis at CpG loci
Filtering to remove low counts
We now turn to statistical analysis of differential methylation. Our first analysis will be for individual CpG loci.
CpG loci that have low coverage are removed prior to downstream analysis as they provide little information for assessing methylation levels. Filtering low-coverage CpGs also simplifies the subsequent analysis because of the reduction in data rows.
We sum up the counts of methylated and unmethylated reads to get the total read coverage at each CpG site for each sample:
> Coverage <- yall$counts[, Methylation=="Me"] + yall$counts[, Methylation=="Un"] > head(Coverage) P6_1-Me P6_4-Me P7_2-Me P7_5-Me P8_3-Me P8_6-Me chr1-3020689 16 21 4 13 6 2 chr1-3020690 37 47 18 46 15 22 chr1-3020708 0 2 0 1 0 0 chr1-3020724 16 21 4 14 6 2 chr1-3020725 37 47 18 46 15 22 chr1-3020814 0 0 0 1 0 0
The analysis needs to be restricted to CpG sites that have enough coverage for the methylation level to be measurable in a meaningful way at that site. As a conservative rule of thumb, we require a CpG site to have a total count (both methylated and unmethylated) of at least 8 in every sample before it is considered in the study.
> keep <- rowSums(Coverage >= 8) == 6 > table(keep) keep FALSE TRUE 3019830 512002
This filtering criterion could be relaxed somewhat in principle but the number of CpGs kept in the analysis is large enough for our purposes.
The DGEList object is subsetted to retain only the non-filtered loci:
> y <- yall[keep,, keep.lib.sizes=FALSE]
Again, the option keep.lib.sizes=FALSE causes the library sizes to be recomputed after the filtering. We generally recommend this, although the effect on the downstream analysis is usually small.
Normalization
A key difference between BS-seq and other sequencing data is that the pair of libraries holding the methylated and unmethylated reads for a particular sample are treated as a unit. To ensure that the methylated and unmethylated reads for the same sample are treated on the same scale, we need to set the library sizes to be equal for each pair of libraries. We set the library sizes for each sample to be the average of the total read counts for the methylated and unmethylated libraries:
> TotalLibSize <- y$samples$lib.size[Methylation=="Me"] + + y$samples$lib.size[Methylation=="Un"] > y$samples$lib.size <- rep(TotalLibSize, each=2) > y$samples group lib.size norm.factors P6_1-Me P6 27754786 1 P6_1-Un P6 27754786 1 P6_4-Me P7 41007143 1 P6_4-Un P7 41007143 1 P7_2-Me P8 21424225 1 P7_2-Un P8 21424225 1 P7_5-Me P6 26197143 1 P7_5-Un P6 26197143 1 P8_3-Me P7 18782105 1 P8_3-Un P7 18782105 1 P8_6-Me P8 15539660 1 P8_6-Un P8 15539660 1
Other normalization methods developed for RNA-seq data, such as TMM 38, are not required for BS-seq data.
Exploring differences between samples
In microarray methylation studies, a common measure of methylation level is the M-value, which is defined as M = log 2 {(Me + α) /(Un + α)} where Me and Un are the methylated and unmethylated intensities and α is some suitable offset to avoid taking logarithms of zero 39. The M-value can be interpreted as the base2 logit transformation of the proportion of methylated signal at each locus.
We compute the corresponding methylation summary from the methylated and unmethylated counts.
We compute the corresponding methylation summary from the methylated and unmethylated counts.
> Me <- y$counts[, Methylation=="Me"] > Un <- y$counts[, Methylation=="Un"] > M <- log2(Me + 2) - log2(Un + 2) > colnames(M) <- Sample
Here M contains the empirical logit methylation level for each CpG site in each sample. We have used a prior count of 2 to avoid logarithms of zero. The exact value of the prior count is unimportant, but a value of 2 is common in other contexts such as RNA-seq.
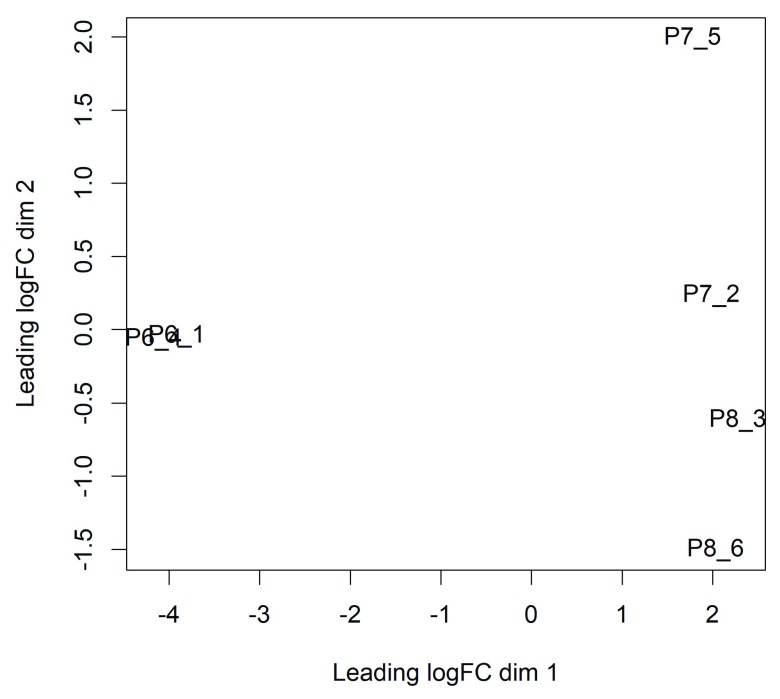
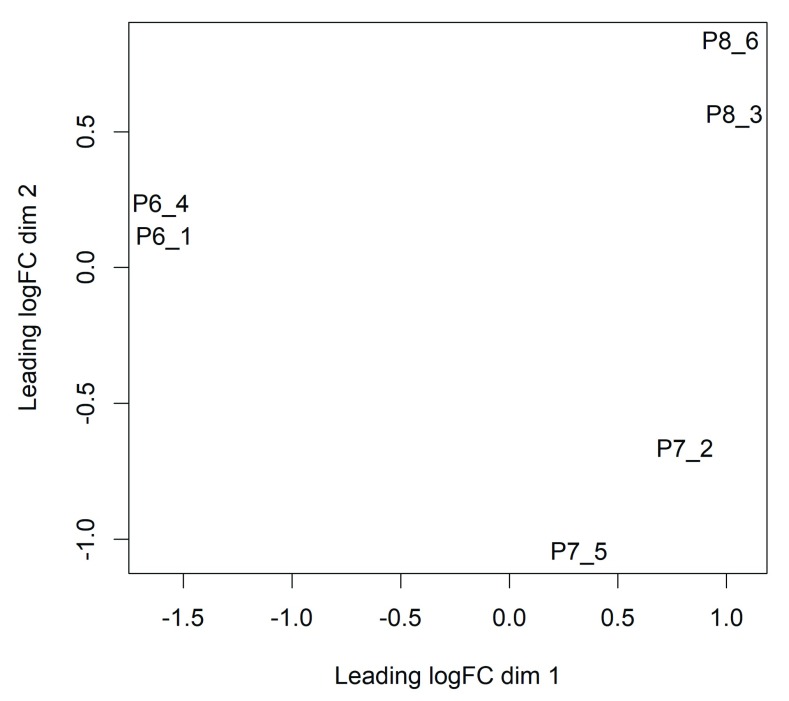
Now we can generate a multi-dimensional scaling (MDS) plot to explore the overall differences between the methylation levels of the different samples ( Figure 1):
Figure 1. MDS plots showing overall differences in methylation levels between the samples.
Replicate samples from the same population cluster together. The first dimension separates the luminal cells from the basal cells. The second dimension separates the Itga+ basal cells at the bottom from the Itga- basal cells at the top.
> plotMDS(M)
In this plot the distance between each pair of samples represents the average logit change between the samples for the top most differentially methylated CpG loci between that pair of samples. (We call this average the leading log-fold-change.) The two replicate samples from the luminal population (P6) are seen to be well separated from the four basal samples (populations P7 and P8), with a leading change of about 6 logit units.
Design matrix
One aim of this study is to identify differentially methylated (DM) loci between the different cell populations. In edgeR, this can be done by fitting linear models under a specified design matrix and testing for corresponding coefficients or contrasts. A basic sample-level design matrix can be made as follows:
> designSL <- model.matrix(~0+Population, data=targets) > colnames(designSL) <- c("P6","P7","P8") > designSL P6 P7 P8 P6_1 1 0 0 P6_4 1 0 0 P7_2 0 1 0 P7_5 0 1 0 P8_3 0 0 1 P8_6 0 0 1 attr(,"assign") [1] 1 1 1 attr(,"contrasts") attr(,"contrasts")$Population [1] "contr.treatment"
The we expand this to the full design matrix modeling the sample and methylation effects:
> design <- modelMatrixMeth(designSL) > design Sample1 Sample2 Sample3 Sample4 Sample5 Sample6 P6 P7 P8 1 1 0 0 0 0 0 1 0 0 2 1 0 0 0 0 0 0 0 0 3 0 1 0 0 0 0 1 0 0 4 0 1 0 0 0 0 0 0 0 5 0 0 1 0 0 0 0 1 0 6 0 0 1 0 0 0 0 0 0 7 0 0 0 1 0 0 0 1 0 8 0 0 0 1 0 0 0 0 0 9 0 0 0 0 1 0 0 0 1 10 0 0 0 0 1 0 0 0 0 11 0 0 0 0 0 1 0 0 1 12 0 0 0 0 0 1 0 0 0
The first six columns represent the sample coverage effects. The last three columns represent the methylation levels (in logit units) in the three cell populations.
Dispersion estimation
With the design matrix specified, we can now proceed to the standard edgeR pipeline and analyze the data in the same way as for RNA-seq data. Similar to the RNA-seq data, the variability between biological replicates has also been observed in bisulfite sequencing data. This variability can be captured by the NB dispersion parameter under the generalized linear model (GLM) framework in edgeR.
The mean-dispersion relationship of BS-seq data has been studied in the past and no apparent mean-dispersion trend was observed 27. This is also verified through our own practice. Therefore, we would not consider a mean-dependent dispersion trend as we normally would for RNA-seq data. A common dispersion estimate for all the loci, as well as an empirical Bayes moderated dispersion for each individual locus, can be obtained from the estimateDisp function in edgeR:
> y <- estimateDisp(y, design, trend="none") > y$common.dispersion [1] 0.0275 > y$prior.df [1] Inf
This returns a DGEList object with additional components ( common.dispersion and tagwise.dispersion) added to hold the estimated dispersions. Here the estimation of trended dispersion has been turned off by setting trend="none". For this data, the estimated prior degrees of freedom (df) are infinite for all the loci, which implies all the CpG-wise dispersions are equal to the common dispersion. A BCV plot is often useful to visualize the dispersion estimates, but is not informative in this case.
Testing for differentially methylated CpG loci
We first fit NB GLMs for all the CpG loci using the glmFit function in edgeR.
> fit <- glmFit(y, design)
Then we can proceed to testing for differentially methylated CpG sites between different populations. One of the most interesting comparisons is between the basal (P7 and P8) and luminal (P6) populations. The contrast corresponding to any specified comparison can be constructed conveniently using the makeContrasts function:
> contr <- makeContrasts(LvsB=P6-0.5*(P7+P8), levels=design)
The actual testing is performed using likelihood ratio tests (LRT) in edgeR:
> lrt <- glmLRT(fit, contrast=contr)
The top set of most differentially methylated (DM) CpG sites can be viewed with topTags:
> topTags(lrt) Coefficient: 1*P6 -0.5*P7 -0.5*P8 Chr Locus EntrezID Symbol Strand Distance Width logFC chr16-76326604 chr16 76326604 268903 Nrip1 - -46445 85647 -8.87 chr13-45709467 chr13 45709467 66355 Gmpr + -202023 38943 7.60 chr10-40387375 chr10 40387375 78334 Cdk19 + -38067 134511 -8.13 chr11-100144651 chr11 100144651 16669 Krt19 - 952 2889 -8.19 chr17-46572098 chr17 46572098 20807 Srf - 15936 9324 9.10 chr13-45709489 chr13 45709489 66355 Gmpr + -202045 38943 7.47 chr3-54724012 chr3 54724012 69639 Exosc8 - -11352 6686 -8.40 chr13-45709480 chr13 45709480 66355 Gmpr + -202036 38943 7.70 chr8-120068504 chr8 120068504 102193 Zdhhc7 - -32968 20378 7.42 chr2-69631013 chr2 69631013 72569 Bbs5 + 16242 20315 -7.30 logCPM LR PValue FDR chr16-76326604 1.52 321 8.35e-72 4.27e-66 chr13-45709467 1.98 319 2.62e-71 6.70e-66 chr10-40387375 1.77 312 6.46e-70 1.10e-64 chr11-100144651 1.58 306 1.40e-68 1.79e-63 chr17-46572098 1.61 302 1.44e-67 1.48e-62 chr13-45709489 1.97 294 5.46e-66 4.66e-61 chr3-54724012 1.31 292 1.56e-65 1.14e-60 chr13-45709480 1.97 292 2.01e-65 1.28e-60 chr8-120068504 1.73 285 7.12e-64 4.05e-59 chr2-69631013 2.03 277 3.66e-62 1.88e-57
Here positive log-fold-changes represent CpG sites that have higher methylation level in the luminal population compared to the two basal populations. The Benjamini-Hochberg multiple testing correction is applied to control the false discovery rate (FDR).
The total number of DM CpG sites identified at an FDR of 5% can be shown with decideTests. There are in fact more than 50,000 differentially methylated CpGs in this comparison:
> summary(decideTests(lrt)) 1*P6 -0.5*P7 -0.5*P8 Down 20302 NotSig 451589 Up 40111
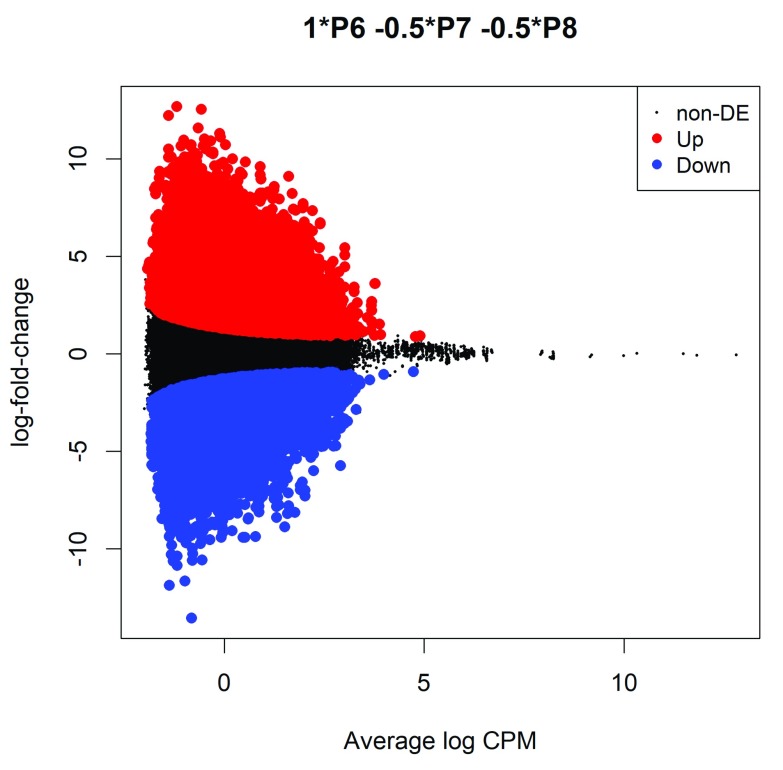
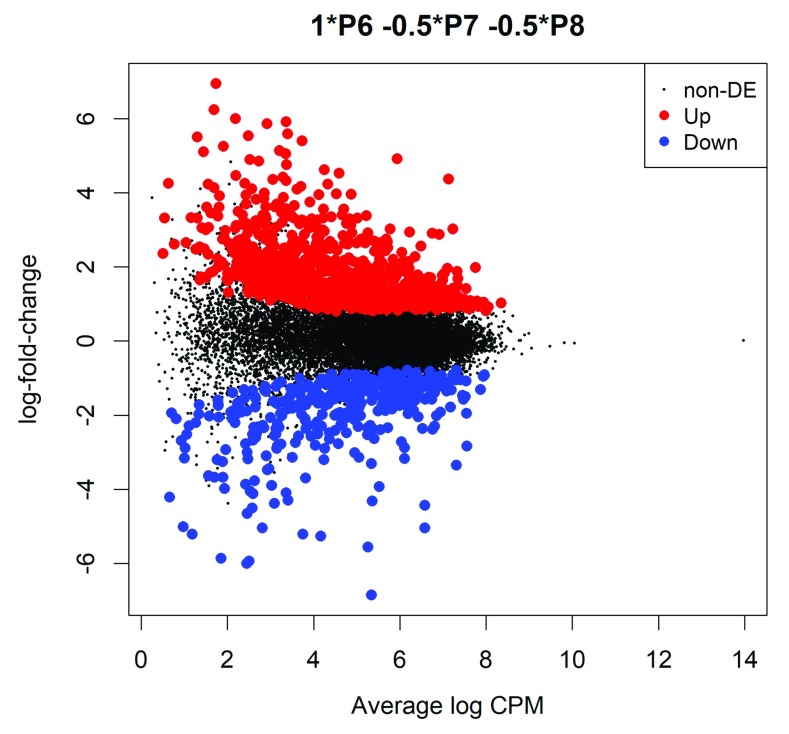
The differential methylation results can be visualized with an MD plot (see Figure 2):
Figure 2. MD plot showing the log-fold-change of the methylation level and average abundance of each CpG site.
Significantly hyper- and hypomethylated CpGs are highlighted in red and blue, respectively.
> plotMD(lrt)
The logFC of the methylation level for each CpG site is plotted against the average abundance in log2-CPM. Significantly differentially methylated CpGs are highlighted.
Differential methylation by chromosome
We now explore overall methylation patterns by chromosome. To do this we make a list of index vectors, with each vector identifying the loci that belong to that chromosome:
> ChrIndices <- list() > for (a in ChrNames) ChrIndices[[a]] <- which(y$genes$Chr==a)
Then we can conduct “fry” gene set tests to evaluate overall changes in methylation in each chromosome:
> fry(y, index=ChrIndices, design=design, contrast=contr) NGenes Direction PValue FDR PValue.Mixed FDR.Mixed chr18 16963 Up 0.00110 0.00543 1.02e-04 1.15e-04 chr15 22338 Up 0.00165 0.00543 8.53e-05 1.15e-04 chr10 27969 Up 0.00264 0.00543 1.03e-04 1.15e-04 chr7 31266 Up 0.00276 0.00543 1.08e-04 1.15e-04 chr2 36289 Up 0.00284 0.00543 7.08e-05 1.15e-04 chr3 24889 Up 0.00339 0.00543 1.22e-04 1.22e-04 chr9 26363 Up 0.00365 0.00543 6.43e-05 1.15e-04 chr8 29208 Up 0.00369 0.00543 5.90e-05 1.15e-04 chr11 35826 Up 0.00371 0.00543 4.70e-05 1.15e-04 chr13 22202 Up 0.00372 0.00543 8.61e-05 1.15e-04 chr14 18362 Up 0.00377 0.00543 6.85e-05 1.15e-04 chr12 20927 Up 0.00390 0.00543 8.22e-05 1.15e-04 chr1 30567 Up 0.00400 0.00543 7.50e-05 1.15e-04 chr6 24880 Up 0.00412 0.00543 1.09e-04 1.15e-04 chr5 37115 Up 0.00436 0.00543 8.93e-05 1.15e-04 chr19 15758 Up 0.00460 0.00543 8.50e-05 1.15e-04 chr16 16025 Up 0.00462 0.00543 6.52e-05 1.15e-04 chr4 34681 Up 0.00532 0.00591 8.54e-05 1.15e-04 chr17 23812 Up 0.01027 0.01082 7.03e-05 1.15e-04 chrX 16562 Up 0.93464 0.93464 4.27e-06 8.55e-05
This shows that methylation increases overall in committed luminal cells for every chromosome except for Chromosome X. This is consistent with the expectation that methylation is associated with silencing of genes not needed in a committed lineage. The column heading NGenes gives the number of CpG loci in each chromosome.
The results for Chromosome X are interesting. The mixed P-value shows that the X chromosome is actually enriched for DM CpGs, but the direction of change is not consistent with hyper and hypomethylated CpGs balancing each other.
Global methylation patterns around TSS
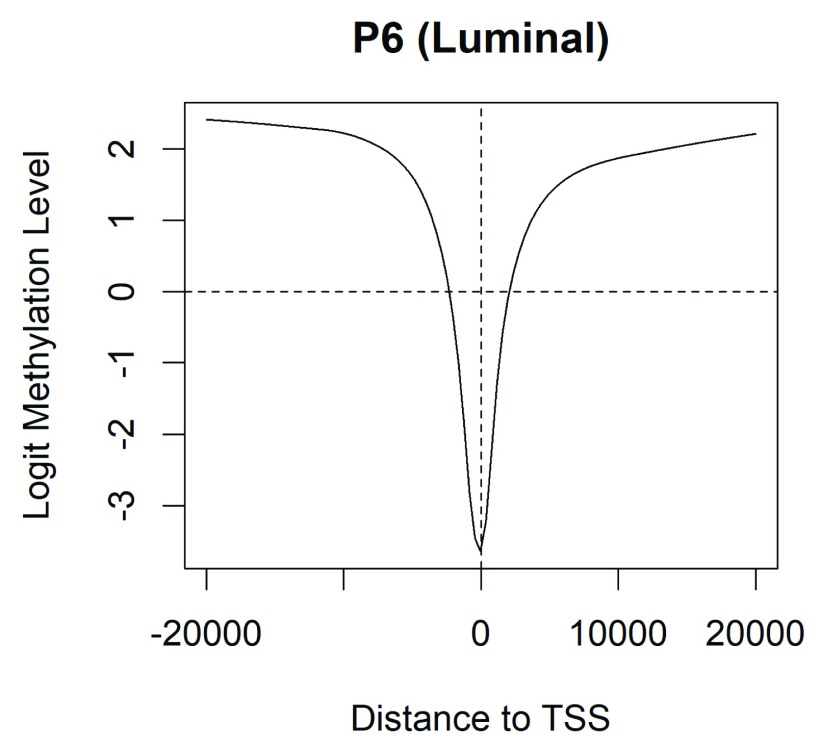
Now we explore methylation patterns to relative to gene TSSs. The coefficients of the fitted model fit record methylation levels at each CpG in each cell population. We now relate methylation in the P6 (luminal) population versus distance to the nearest TSS:
> i <- abs(fit$genes$Distance) < 20000 > lo <- lowess(fit$genes$Distance[i], fit$coefficient[i,"P6"], f=0.3) > plot(lo, type="l", xlab="Distance to TSS", ylab="Logit Methylation Level", + main="P6 (Luminal)") > abline(h=0, lty=2) > abline(v=0, lty=2)
Figure 3 shows that CpGs near a TSS tend to be unmethylated but CpGs elsewhere are predominately methylated. Cell populations P7 and P8 show the same patterns.
Figure 3. Methylation level by distance to the nearest gene TSS.
Results are averaged over all genes. Negative distances are downstream of TSS.
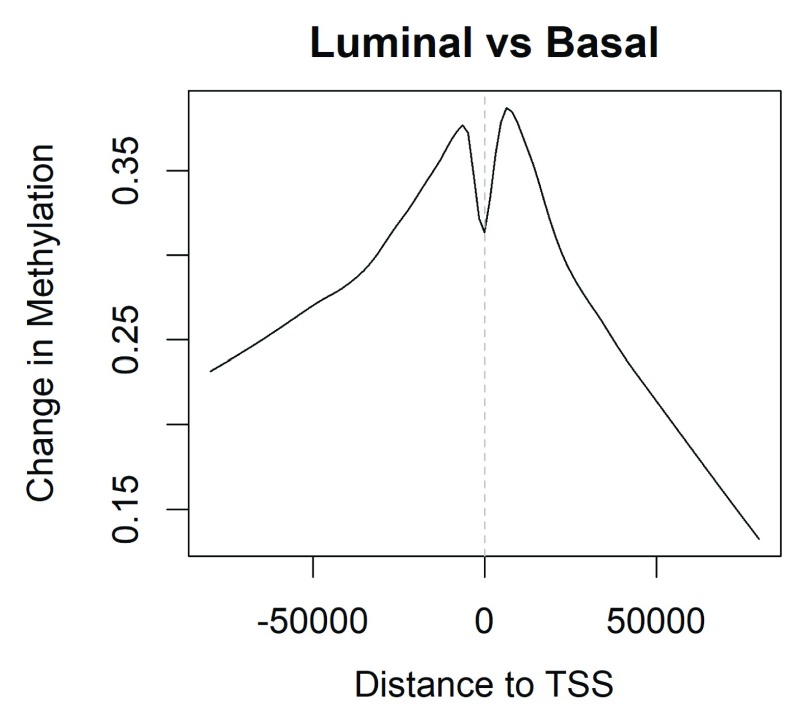
Now we examine the methylation changes stored in lrt and relate these to TSS position.
> i <- abs(lrt$genes$Distance) < 80000 > lo <- lowess(lrt$genes$Distance[i], lrt$table$logFC[i], f=0.3) > plot(lo, type="l", xlab="Distance to TSS", ylab="Change in Methylation", + main="Luminal vs Basal") > abline(v=0, lty=2, col="grey")
Figure 4 shows that methylation tends to be added around the TSS in luminal vs basal cells. CpGs further from TSS tend to be unchanged.
Figure 4. Average methylation change by genomic position relative to TSS.
Changes are averaged over all genes. Negative distances are downstream of TSS. Vertical axis shows base 2 logit differences.
Differential methylation in gene promoters
Pre-defined gene promoters
The majority of CpGs are methylated in mammals. On the other hand, unmethylated CpGs tend to group into clusters of CpG islands, which are often enriched in gene promoters. CpG methylation in promoter regions is often associated with silencing of transcription and gene expression 3. Therefore it is of great biological interest to examine the methylation level within the gene promoter regions.
For simplicity, we define the promoter of a gene as the region from 2kb upstream to 1kb downstream of the transcription start site of that gene.
> InPromoter <- yall$genes$Distance >= -1000 & yall$genes$Distance <= 2000
We subset the CpGs to those contained in a promoter region:
> yIP <- yall[InPromoter,,keep.lib.sizes=FALSE]
Summarizing counts in promoter regions
One simple and effective way to conduct a gene-orientated analysis of the methylation changes is to collapse all the CpGs in each promotor into one locus, i.e., to compute the total number of methylated and unmethylated reads within each promoter. This strategy focuses on the aggregate methylation level within each promoter and allows us to test for an overall increase or decrease in methylation for each gene.
First we compute the total counts for each gene promoter:
> ypr <- rowsum(yIP, yIP$genes$EntrezID, reorder=FALSE) > ypr$genes$EntrezID <- NULL
The rowsum function here operated on the DGEList object yIP and produces a new DGEList object ypr with a row for each EntrezID. The integer matrix ypr$counts contains the total numbers of methylated and unmethylated CpGs observed within the promoter of each gene. Same as before, ypr$counts has 12 columns, two for each sample. The odd-numbered columns contain the numbers of methylated Cs, whereas the even-numbered columns contain the numbers of unmethylated Cs. The only difference is that each row of ypr$counts now represents a gene promoter instead of an individual CpG site.
Filtering to remove low counts
Filtering is performed in the same way as before. We sum up the read counts of both methylated and unmethylated Cs at each gene promoter within each sample.
> Coveragepr <- ypr$counts[,Methylation=="Me"] + + ypr$counts[,Methylation=="Un"]
Since each row represents a 3,000-bps-wide promoter region that contains multiple CpG sites, we would expect less filtering than before.
> keeppr <- rowSums(Coverageprm >= 10) == 6 > table(keeppr) keeppr FALSE TRUE 1949 16837 > ypr <- ypr[keeppr,,keep.lib.sizes=FALSE]
Same as before, we do not perform normalization but set the library sizes for each sample to be the average of the total read counts for the methylated and unmethylated libraries.
> TotalLibSizepr <- 0.5*ypr$samples$lib.size[Methylation=="Me"] + + 0.5*ypr$samples$lib.size[Methylation=="Un"] > ypr$samples$lib.size <- rep(TotalLibSizepr, each=2) > ypr$samples group lib.size norm.factors P6_1-Me P6 11620642 1 P6_1-Un P6 11620642 1 P6_4-Me P7 11740775 1 P6_4-Un P7 11740775 1 P7_2-Me P8 4781385 1 P7_2-Un P8 4781385 1 P7_5-Me P6 10426283 1 P7_5-Un P6 10426283 1 P8_3-Me P7 3854829 1 P8_3-Un P7 3854829 1 P8_6-Me P8 3329192 1 P8_6-Un P8 3329192 1
Exploring differences between samples
Same as before, we measure the methylation levels of gene promoter regions using M-values. A prior count of 2 is added to the calculation to avoid undefined values and to reduce the variability of M-values for gene promoters with low counts. Then a MDS plot is produced to examine the overall differences between the methylation levels of the different samples.
> Me <- ypr$counts[, Methylation=="Me"] > Un <- ypr$counts[, Methylation=="Un"] > M2 <- log2(Me + 2) - log2(Un + 2) > colnames(M2) <- Sample > plotMDS(M2)
The resulting Figure 5 shows that the two replicate samples from the luminal population (P6) are well separated from the four replicate samples from the basal population (P7 and P8).
Figure 5. MDS plot showing differences in methylation profiles at gene promoters.
Basal and luminal cell populations are well separated by the first dimension.
Dispersion estimation
We estimate the NB dispersions using the estimateDisp function in edgeR. For the same reason, we do not consider a mean-dependent dispersion trend as we normally would for RNA-seq data.
> ypr <- estimateDisp(ypr, design, trend="none") > ypr$common.dispersion [1] 0.0304 > ypr$prior.df [1] 10.7
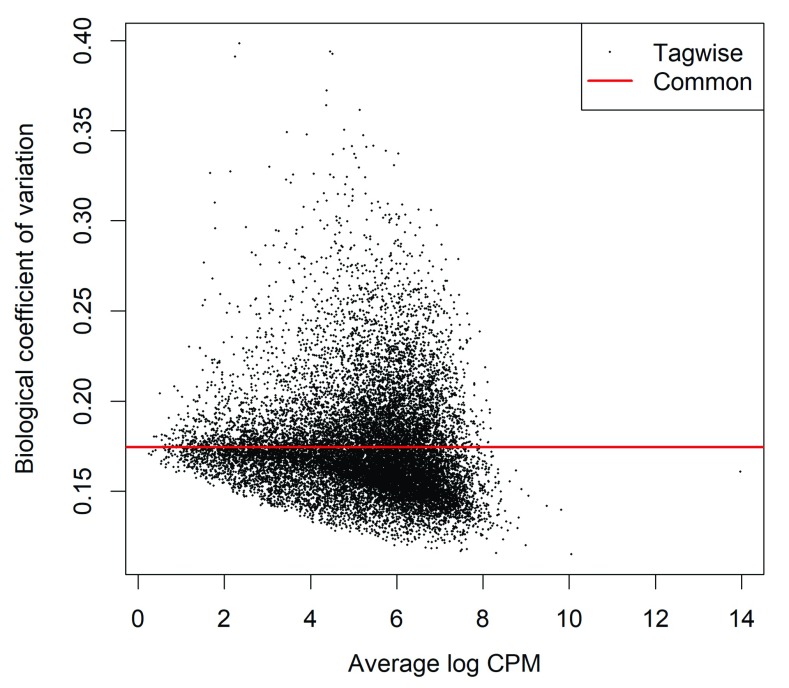
The dispersion estimates ( ϕ g ) can be visualized with a BCV plot (see Figure 6):
Figure 6. Scatterplot of the biological coefficient of variation (BCV) against the average abundance of each gene.
The plot shows the square-root estimates of the common and tagwise NB dispersions.
> plotBCV(ypr)
Testing for differential methylation in gene promoters
We first fit NB GLMs for all the gene promoters using glmFit.
> fitpr <- glmFit(ypr, design)
Then we can proceed to testing for differential methylation in gene promoter regions between different populations. Suppose the comparison of interest is the same as before. The same contrast can be used for the testing.
> lrtpr <- glmLRT(fitpr, contrast=contr)
The top set of most differentially methylated gene promoters can be viewed with topTags:
> topTags(lrtpr, n=20) Coefficient: 1*P6 -0.5*P7 -0.5*P8 Chr Symbol Strand logFC logCPM LR PValue FDR 16924 chr5 Lnx1 - -6.85 5.34 313 4.92e-70 8.29e-66 238161 chr12 Akap6 + -5.26 4.16 248 6.55e-56 5.51e-52 64082 chr16 Popdc2 + 4.91 5.94 225 7.96e-51 4.47e-47 11601 chr8 Angpt2 - 5.58 3.40 208 3.45e-47 1.45e-43 108168987 chr4 Gm13205 - 4.37 7.13 196 1.57e-44 5.29e-41 12740 chr5 Cldn4 - -5.56 5.26 189 6.49e-43 1.82e-39 16669 chr11 Krt19 - -5.05 6.58 187 1.65e-42 3.96e-39 73644 chr12 2210039B01Rik + -4.31 5.36 180 6.00e-41 1.26e-37 387514 chr6 Tas2r143 + 4.52 4.59 177 2.10e-40 3.94e-37 321019 chr14 Gpr183 - 5.86 2.92 152 5.36e-35 9.02e-32 76509 chr9 Plet1 + -6.00 2.45 146 1.43e-33 2.18e-30 100043766 chr2 Gm14057 - 5.40 3.74 145 2.04e-33 2.87e-30 68386 chr2 0610039K10Rik + -4.43 6.58 143 4.70e-33 6.09e-30 110308 chr15 Krt5 - 4.09 3.62 140 2.60e-32 3.13e-29 59091 chr2 Jph2 - 3.56 4.70 139 3.46e-32 3.89e-29 244198 chr7 Olfml1 + 5.91 3.36 133 1.04e-30 1.10e-27 18291 chr6 Nobox - 4.75 3.37 130 5.22e-30 5.17e-27 21345 chr9 Tagln - 4.62 4.25 127 1.54e-29 1.44e-26 653016 chr17 Mymx - 2.75 5.69 127 1.71e-29 1.52e-26 229277 chr3 Stoml3 + 4.85 2.73 126 3.09e-29 2.60e-26
Here positive log-fold-changes represent gene promoters that have higher methylation level in the luminal population compared to the basal population. The Benjamini-Hochberg multiple testing correction is applied to control the false discovery rate (FDR).
The total number of DM gene promoters identified at an FDR of 5% can be shown with decideTests. There are in fact about 1,200 differentially methylated gene promoters in this comparison:
> summary(decideTests(lrtpr)) 1*P6 -0.5*P7 -0.5*P8 Down 368 NotSig 15627 Up 842
For future reference we make a dataframe of all the DM genes:
> topME <- topTags(lrtpr, n=Inf, p=0.05)$table > dim(topME) [1] 1210 8
The differential methylation results can be visualized with an MD plot (see Figure 7):
Figure 7. MD plot showing the log-fold-change of the methylation level and average abundance of CpG sites in each gene promoter.
Significantly hyper and hypomethylated gene promoters are highlighted in red and blue, respectively.
> plotMD(lrtpr)
Correlate with RNA-seq profiles
RNA-seq profiles of mouse epithelium luminal and basal cells
To explore whether hypermethylation of promoter regions is associated with repressed gene expression, we relate the differential methylation results to differential expression results from RNA-seq for similar cell populations. The RNA-seq data used here is from a study of the epithelial cell lineage in the mouse mammary gland, in which the expression profiles were generated from basal stem-cell enriched cells and committed luminal cells in the mammary glands of virgin, pregnant and lactating mice 40. The complete differential expression analysis of the data is described in Chen et al. 32.
The RNA-seq data is stored as a DGEList object y_rna and saved in a RData file rna.RData. The object y_rna contains the count matrix, sample information, gene annotation, design matrix and dispersion estimates of the RNA-seq data. The gene filtering, normalization and dispersion estimation were performed in the same way as described in Chen et al. 32. The rna.RData file is available for download at http://bioinf.wehi.edu.au/edgeR/F1000Research2017.
We load the RData file:
> load("rna.RData") > dim(y_rna) [1] 15641 12 > y_rna$samples group lib.size norm.factors MCL1.DG B.virgin 23137472 1.235 MCL1.DH B.virgin 21687755 1.213 MCL1.DI B.pregnant 23974787 1.125 MCL1.DJ B.pregnant 22545375 1.069 MCL1.DK B.lactating 21420532 1.036 MCL1.DL B.lactating 19916685 1.087 MCL1.LA L.virgin 20273585 1.370 MCL1.LB L.virgin 21568458 1.368 MCL1.LC L.pregnant 22117517 1.006 MCL1.LD L.pregnant 21877287 0.924 MCL1.LE L.lactating 24657903 0.529 MCL1.LF L.lactating 24600304 0.535
We keep only the genes that are also included in our methylation analysis:
> haveME <- row.names(y_rna) %in% row.names(ypr) > y_rna <- y_rna[haveME,] > dim(y_rna) [1] 13210 12
We assess differential expression between the luminal and basal virgin samples:
> fitrna <- glmFit(y_rna) > Contrastrna <- makeContrasts(L.virgin-B.virgin, levels=y_rna$design) > lrtrna <- glmLRT(fitrna, contrast=Contrastrna)
Correlate methylation and expression
We can add the expression log-fold-changes to our methylation results:
> topME$logFC.RNA <- lrtrna$table[row.names(topME),"logFC"] > topME[1:30,c("Symbol","logFC","logFC.RNA")] Symbol logFC logFC.RNA 16924 Lnx1 -6.85 2.2748 238161 Akap6 -5.26 -3.2258 64082 Popdc2 4.91 -7.6743 11601 Angpt2 5.58 -2.1021 108168987 Gm13205 4.37 NA 12740 Cldn4 -5.56 5.0985 16669 Krt19 -5.05 4.5266 73644 2210039B01Rik -4.31 NA 387514 Tas2r143 4.52 -3.1034 321019 Gpr183 5.86 -1.9598 76509 Plet1 -6.00 4.8335 100043766 Gm14057 5.40 -0.8902 68386 0610039K10Rik -4.43 2.1650 110308 Krt5 4.09 -8.9403 59091 Jph2 3.56 -7.2598 244198 Olfml1 5.91 NA 18291 Nobox 4.75 NA 21345 Tagln 4.62 -6.4003 653016 Mymx 2.75 NA 229277 Stoml3 4.85 NA 16846 Lep 3.96 -6.1556 231253 9130230L23Rik -5.21 3.9109 16012 Igfbp6 3.02 -2.4216 675921 Tnk2os -3.69 2.0039 11529 Adh7 5.53 -0.0508 100526468 Mir3063 3.56 NA 78896 1500015O10Rik 6.94 -6.6807 217143 Gpr179 5.05 -2.9424 17242 Mdk 2.93 -4.5784 100526559 Mir3103 6.24 NA
The negative correlation between methylation and expression is immediately apparent. Of the top 30 DM genes, eight have NA expression fold-changes because the genes were not expressed at a high enough level to be included in the analysis. Of the rest, all but Akap6 have methylation and expression logFCs of opposite signs.
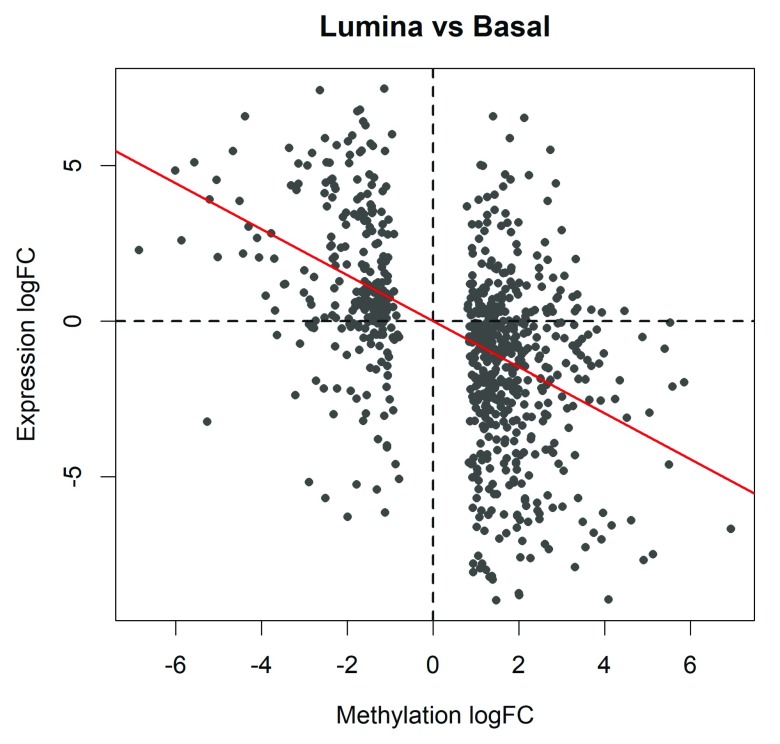
We can explore this correlation further by plotting the expression logFC vs the methylation logFC for all DM genes (see Figure 8):
Figure 8. Scatter plot of the log-fold-changes of methylation levels in gene promoters (x-axis) vs the log fold-changes of gene expression (y-axis).
The plot shows results for the genes of which the promoters are significantly differentially methylated between basal and luminal. The red line shows the least squares line with zero intercept. A strong negative correlation is observed.
> plot(topME$logFC, topME$logFC.RNA, main="Lumina vs Basal", + xlab="Methylation logFC", ylab="Expression logFC", + pch=16, cex=0.8, col="gray30") > abline(h=0, v=0, col="gray10", lty=2, lwd=2)
The horizontal axis of the scatterplot shows the log-fold-change in methylation level for each gene promoter while the vertical axis shows the log-fold-change in gene expression. To assess the correlation, we fit a least squares regression line through the origin and compute the P-value:
> RNAvsME <- lm(topME$logFC.RNA ~ 0 + topME$logFC) > coef(summary(RNAvsME)) Estimate Std. Error t value Pr(>|t|) topME$logFC -0.738 0.0472 -15.7 6.17e-48 > abline(RNAvsME, col="red", lwd=2)
The negative association is highly significant ( P = 6 × 10 −48). The last line of code adds the regression line to the plot ( Figure 8).
Gene set testing
The correlation in Figure 8 is convincing, but the above P-value computation assumes that genes are statistically independent of one another. We can perform a gene set test to get around this assumption.
First we make a dataframe of the logFCs of the DM genes, keeping only the genes for which we have RNA-seq results:
> ME <- data.frame(ID=row.names(topME), logFC=topME$logFC, stringsAsFactors=FALSE) > inRNA <- ME$ID %in% row.names(y_rna) > ME <- ME[inRNA,]
Then we test whether the DM genes are up or down-regulated using a “fry” gene set test. This analysis weights the DM genes by their methylation logFCs so that the test evaluates whether the expression changes are positively or negatively associated with the methylation changes:
> fry(y_rna, index=ME, contrast=Contrastrna) NGenes Direction PValue PValue.Mixed set1 740 Down 1.28e-09 5e-11
The result Down in the Direction column indicates negative correlation between the methylation and expression changes. The small PValue confirms a highly significant result.
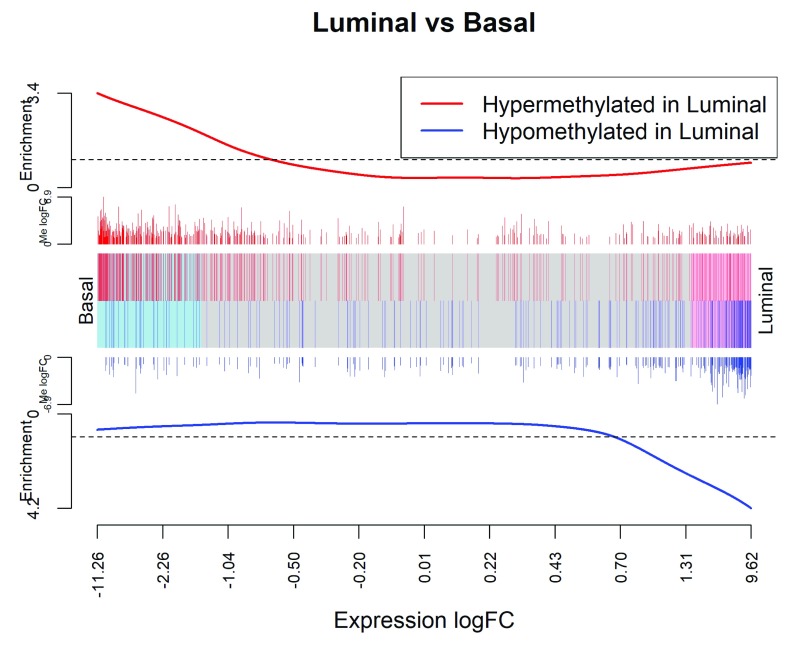
We can visualize the gene set test result with a barcode plot (see Figure 9):
Figure 9. Barcode plot showing strong negative correlation between expression changes and methylation changes when luminal cells are compared to basal.
The plot is analogous to Figure 8 with the DM genes now displayed as gene sets and the methylation logFCs now displayed as gene weights.
> logFC.ME <- rep_len(0,nrow(y_rna)) > names(logFC.ME) <- row.names(y_rna) > logFC.ME[ME$ID] <- ME$logFC > barcodeplot(lrtrna$table$logFC, gene.weights=logFC.ME, + labels=c("Basal","Luminal"), main="Luminal vs Basal", + xlab="Expression logFC", weights.label="Me logFC") > legend("topright", col=c("red","blue"), lty=1, lwd=2, + legend=c("Hypermethylated in Luminal", "Hypomethylated in Luminal"))
In the barcode plot, genes are sorted left to right from most down-regulated to most up-regulated in luminal vs basal. The x-axis shows the expression log2-fold-change. The vertical red bars indicate genes hypermethylated in luminal and vertical blue bars indicate genes hypomethylated in luminal. The variable-height vertical bars show the methylation log-fold-changes. The red and blue worms measure relative enrichment, showing that hypermethylation is associated with decreased regulation and hypomethylation is associated with up-regulation. In other words, there is a strong negative association between methylation of promoter regions and expression of the corresponding genes.
Discussion
This article has presented a complete start to finish analysis of an RRBS dataset from our own practice. The analysis demonstrates how BS-seq data can be analyzed using software designed for RNA-seq, thus benefiting from a large reservoir of highly-developed RNA-seq methodology. At first sight, BS-seq data is fundamentally different to RNA-seq because of the need to focus on the proportion of methylated reads at each locus rather than on the absolute number of reads at each locus. The link is achieved by conducting the statistical inference conditional on the read coverage at each locus, and this in turn is achieved by including sample-specific locus effects in the linear model.
We have concentrated on analysing methylation changes for pre-defined genomic regions. At the highest resolution, conducted differential methylation tests for each distinct CpG site. Our main analysis was gene-level, whereby we aggregated counts over a putative promoter region around the TSS for each gene. This gives a “big picture” analysis and facilities a correlation of DM results with differential expression results from RNA-seq for the same cell populations. We also illustrated the use of gene set tests to examine whether there were overall methylation changes at the whole chromosome level.
The analysis presented here was designed for reduced representation BS-seq and was tuned to our own research interests. While the same analysis approach could be fruitfully applied to WGBS data, researchers with WGBS data may also want to discover DMRs de novo without the use of gene annotation or pre-specified genomic regions, something we haven’t explored in this article. The edgeR approach presented here could in principle be extended to discover DMRs in a de novo fashion using similar methods to those developed for ChIP-seq data 41– 43.
Packages used
This workflow depends on various packages from version 3.7 of the Bioconductor project, running on R version 3.5.0 or higher. For all the code to work as presented, edgeR 3.22.2 or later is required. A complete list of the packages used for this workflow is shown below:
> sessionInfo() R version 3.5.1 (2018-07-02) Platform: x86_64-w64-mingw32/x64 (64-bit) Running under: Windows 10 x64 (build 15063) Matrix products: default locale: [1] LC_COLLATE=English_Australia.1252 LC_CTYPE=English_Australia.1252 [3] LC_MONETARY=English_Australia.1252 LC_NUMERIC=C [5] LC_TIME=English_Australia.1252 attached base packages: [1] stats graphics grDevices utils datasets methods base other attached packages: [1] edgeR_3.22.5 limma_3.36.5 knitr_1.20 loaded via a namespace (and not attached): [1] Rcpp_0.12.18 AnnotationDbi_1.42.1 magrittr_1.5 [4] IRanges_2.14.11 BiocGenerics_0.26.0 hms_0.4.2 [7] bit_1.1-14 lattice_0.20-35 R6_2.2.2 [10] rlang_0.2.2 blob_1.1.1 stringr_1.3.1 [13] highr_0.7 tools_3.5.1 parallel_3.5.1 [16] grid_3.5.1 Biobase_2.40.0 DBI_1.0.0 [19] bit64_0.9-7 digest_0.6.17 tibble_1.4.2 [22] crayon_1.3.4 org.Mm.eg.db_3.6.0 readr_1.1.1 [25] S4Vectors_0.18.3 codetools_0.2-15 memoise_1.1.0 [28] evaluate_0.11 RSQLite_2.1.1 stringi_1.1.7 [31] compiler_3.5.1 pillar_1.3.0 stats4_3.5.1 [34] locfit_1.5-9.1 pkgconfig_2.0.2
Data and software availability
All software packages used in this workflows are publicly available as part of Bioconductor 3.7. The data and analysis code used in this workflow are available from http://bioinf.wehi.edu.au/edgeR/F1000Research2017. The data and analysis code as at time of publication of this article has been archived at http://doi.org/10.5281/zenodo.1052870 44.
Acknowledgments
The authors thank Andrew Keniry for help on Bismark.
Funding Statement
This work was supported by the National Health and Medical Research Council (Fellowship 1058892 and Program 1054618 to G.K.S, Independent Research Institutes Infrastructure Support to the Walter and Eliza Hall Institute) and by a Victorian State Government Operational Infrastructure Support Grant.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; referees: 2 approved
References
- 1. Schübeler D: Function and information content of DNA methylation. Nature. 2015;517(7534):321–326. 10.1038/nature14192 [DOI] [PubMed] [Google Scholar]
- 2. Saxonov S, Berg P, Brutlag DL: A genome-wide analysis of CpG dinucleotides in the human genome distinguishes two distinct classes of promoters. Proc Natl Acad Sci U S A. 2006;103(5):1412–1417. 10.1073/pnas.0510310103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bird AP: CpG-rich islands and the function of DNA methylation. Nature. 1986;321(6067):209–213. 10.1038/321209a0 [DOI] [PubMed] [Google Scholar]
- 4. Jones PA: Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13(7):484–492. 10.1038/nrg3230 [DOI] [PubMed] [Google Scholar]
- 5. Lister R, Pelizzola M, Dowen RH, et al. : Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462(7271):315–22. 10.1038/nature08514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ji H, Ehrlich LI, Seita J, et al. : Comprehensive methylome map of lineage commitment from haematopoietic progenitors. Nature. 2010;467(7313):338–342. 10.1038/nature09367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith ZD, Meissner A: DNA methylation: roles in mammalian development. Nat Rev Genet. 2013;14(3):204–220. 10.1038/nrg3354 [DOI] [PubMed] [Google Scholar]
- 8. Hao X, Luo H, Krawczyk M, et al. : DNA methylation markers for diagnosis and prognosis of common cancers. Proc Natl Acad Sci U S A. 2017;114(28):7414–7419. 10.1073/pnas.1703577114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stieglit E, Mazor T, Olshen AB, et al. : Genome-wide DNA methylation is predictive of outcome in juvenile myelomonocytic leukemia. Nat Commun. 2017;8(1): 2127. 10.1038/s41467-017-02178-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Frommer M, McDonald LE, Millar DS, et al. : A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc Natl Acad Sci U S A. 1992;89(5):1827–1831. 10.1073/pnas.89.5.1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stirzaker C, Taberlay PC, Statham AL, et al. : Mining cancer methylomes: prospects and challenges. Trends Genet. 2014;30(2):75–84. 10.1016/j.tig.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 12. Meissner A, Gnirke A, Bell GW, et al. : Reduced representation bisulfite sequencing for comparative high-resolution DNA methylation analysis. Nucleic Acids Res. 2005;33(18):5868–5877. 10.1093/nar/gki901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gu H, Smith ZD, Bock C, et al. : Preparation of reduced representation bisulfite sequencing libraries for genome-scale DNA methylation profiling. Nat Protoc. 2011;6(4):468–81. 10.1038/nprot.2010.190 [DOI] [PubMed] [Google Scholar]
- 14. Guo H, Zhu P, Guo F, et al. : Profiling DNA methylome landscapes of mammalian cells with single-cell reduced-representation bisulfite sequencing. Nat Protoc. 2015;10(5):645–659. 10.1038/nprot.2015.039 [DOI] [PubMed] [Google Scholar]
- 15. Krueger F, Andrews SR: Bismark: a flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics. 2011;27(11):1571–1572. 10.1093/bioinformatics/btr167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pedersen B, Hsieh TF, Ibarra C, et al. : MethylCoder: software pipeline for bisulfite-treated sequences. Bioinformatics. 2011;27(17):2435–2436. 10.1093/bioinformatics/btr394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harris EY, Ponts N, Levchuk A, et al. : BRAT: bisulfite-treated reads analysis tool. Bioinformatics. 2010;26(4):572–573. 10.1093/bioinformatics/btp706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen PY, Cokus SJ, Pellegrini M: BS Seeker: precise mapping for bisulfite sequencing. BMC Bioinformatics. 2010;11(1):203. 10.1186/1471-2105-11-203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang KYY, Huang YJ, Chen PY: BS-Seeker3: ultrafast pipeline for bisulfite sequencing. BMC Bioinformatics. 2018;19(1):111. 10.1186/s12859-018-2120-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xi Y, Li W: BSMAP: whole genome bisulfite sequence MAPping program. BMC Bioinformatics. 2009;10(1):232. 10.1186/1471-2105-10-232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Langmead B, Trapnell C, Pop M, et al. : Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10(3):R25. 10.1186/gb-2009-10-3-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Langmead B, Salzberg SL: Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357–359. 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Akalin A, Kormaksson M, Li S, et al. : methylKit: a comprehensive R package for the analysis of genome-wide DNA methylation profiles. Genome Biol. 2012;13(10):R87. 10.1186/gb-2012-13-10-r87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Assenov Y, Müller F, Lutsik P, et al. : Comprehensive analysis of DNA methylation data with RnBeads. Nat Methods. 2014;11(11):1138–1140. 10.1038/nmeth.3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ritchie ME, Phipson B, Wu D, et al. : limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. 10.1093/nar/gkv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hansen KD, Langmead B, Irizarry RA: BSmooth: from whole genome bisulfite sequencing reads to differentially methylated regions. Genome Biol. 2012;13(10):R83. 10.1186/gb-2012-13-10-r83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Feng H, Conneely KN, Wu H: A Bayesian hierarchical model to detect differentially methylated loci from single nucleotide resolution sequencing data. Nucleic Acids Res. 2014;42(8):e69. 10.1093/nar/gku154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hebestreit K, Dugas M, Klein HU: Detection of significantly differentially methylated regions in targeted bisulfite sequencing data. Bioinformatics. 2013;29(13):1647–1653. 10.1093/bioinformatics/btt263 [DOI] [PubMed] [Google Scholar]
- 29. Sun D, Xi Y, Rodriguez B, et al. : MOABS: model based analysis of bisulfite sequencing data. Genome Biol. 2014;15(2):R38. 10.1186/gb-2014-15-2-r38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dolzhenko E, Smith AD: Using beta-binomial regression for high-precision differential methylation analysis in multifactor whole-genome bisulfite sequencing experiments. BMC Bioinformatics. 2014;15(1):215. 10.1186/1471-2105-15-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Robinson MD, McCarthy DJ, Smyth GK: edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–140. 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen Y, Lun AT, Smyth GK: From reads to genes to pathways: differential expression analysis of RNA-Seq experiments using Rsubread and the edgeR quasi-likelihood pipeline [version 2; referees: 5 approved]. F1000Res. 2016;5:1438. 10.12688/f1000research.8987.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McCarthy DJ, Chen Y, Smyth GK: Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 2012;40(10):4288–4297. 10.1093/nar/gks042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Robinson MD, Smyth GK: Moderated statistical tests for assessing differences in tag abundance. Bioinformatics. 2007;23(21):2881–7. 10.1093/bioinformatics/btm453 [DOI] [PubMed] [Google Scholar]
- 35. Chen Y, Lun AT, Smyth GK: Differential expression analysis of complex RNA-seq experiments using edgeR. In Somnath Datta and Daniel S Nettleton, editors, Statistical Analysis of Next Generation Sequencing Data. Springer, New York,2014;51–74. 10.1007/978-3-319-07212-8_3 [DOI] [Google Scholar]
- 36. Visvader JE: Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes Dev. 2009;23(22):2563–2577. 10.1101/gad.1849509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shackleton M, Vaillant F, Simpson KJ, et al. : Generation of a functional mammary gland from a single stem cell. Nature. 2006;439(7072):84–8. 10.1038/nature04372 [DOI] [PubMed] [Google Scholar]
- 38. Robinson MD, Oshlack A: A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010;11(3):R25. 10.1186/gb-2010-11-3-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Du P, Zhang X, Huang CC, et al. : Comparison of Beta-value and M-value methods for quantifying methylation levels by microarray analysis. BMC Bioinformatics. 2010;11(1):587. 10.1186/1471-2105-11-587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fu NY, Rios AC, Pal B, et al. : EGF-mediated induction of Mcl-1 at the switch to lactation is essential for alveolar cell survival. Nat Cell Biol. 2015;17(4):365–75. 10.1038/ncb3117 [DOI] [PubMed] [Google Scholar]
- 41. Lun AT, Smyth GK: De novo detection of differentially bound regions for ChIP-seq data using peaks and windows: controlling error rates correctly. Nucleic Acids Res. 2014;42(11):e95. 10.1093/nar/gku351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lun AT, Smyth GK: From reads to regions: a Bioconductor workflow to detect differential binding in ChIP-seq data [version 2; referees: 2 approved, 1 approved with reservations]. F1000Res. 2015;4:1080. 10.12688/f1000research.7016.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lun AT, Smyth GK: csaw: a Bioconductor package for differential binding analysis of ChIP-seq data using sliding windows. Nucleic Acids Res. 2016;44(5):e45. 10.1093/nar/gkv1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen Y, Pal B, Visvader JE, et al. : Data and code for “differential methylation analysis of reduced representation bisulfite sequencing experiments using edgeR” [data set]. Zenodo. 2017. 10.5281/zenodo.1052871 [DOI] [PMC free article] [PubMed] [Google Scholar]