Abstract
Sunguru virus (SUNV), a novel virus belonging to the highly diverse Rhabdoviridae family, was isolated from a domestic chicken in the district of Arua, Uganda, in 2011. This is the first documented isolation of a rhabdovirus from a chicken. SUNV is related to, but distinct from, Boteke virus and other members of the unclassified Sandjimba group. The genome is 11 056 nt in length and contains the five core rhabdovirus genes plus an additional C gene (within the ORF of a phosphoprotein gene) and a small hydrophobic protein (between the matrix and glycoprotein genes). Inoculation of vertebrate cells with SUNV resulted in significant viral growth, with a peak titre of 7.8 log10 p.f.u. ml−1 observed in baby hamster kidney (BHK) cells. Little to no growth was observed in invertebrate cells and in live mosquitoes, with Anopheles gambiae mosquitoes having a 47.4 % infection rate in the body but no dissemination of the virus to the salivary glands; this suggests that this novel virus is not arthropod borne as some other members of the family Rhabdoviridae.
INTRODUCTION
Members of the family Rhabdoviridae (order Mononegavirales) have been isolated from a wide range of organisms including mammals, marsupials, birds, reptiles, fish, insects and plants (Kuzmin et al., 2009), making it one of the most diverse groups of known viruses. The family has eleven established genera, eight that infect vertebrates (Lyssavirus, Vesiculovirus, Ephemerovirus, Novirhabdovirus, Perhabdovirus, Tibrovirus, Sprivivirus and Tupavirus), two that infect plants (Cytorhabdovirus and Nucleorhabdovirus) and one found in invertebrates (Sigmavirus). Additionally, because of the lack of available sequences and the low number of serogroup representatives, many of the vertebrate-associated rhabdoviruses are currently unclassified to genus level (Tordo et al., 2005; International Committee on Taxonomy of Viruses, 2014) or remain unassigned to any existing serogroup. Rhabdoviruses of the vertebrate genera can be transmitted by direct contact, as the prototypic rabies virus, or by arthropod vectors, as vesicular stomatitis virus. In addition, basic biological characteristics, including virus–host associations, geographical ranges and known arthropod vectors, remain unknown for virtually all of these rhabdoviruses (Allison et al., 2011).
Rabies virus (RABV), genus Lyssavirus, has the typical rhabdovirus genome consisting of five genes that are transcribed from a single-stranded, negative-sense RNA molecule (Dale & Peters, 1981): the genes for the nucleoprotein (N), the phosphoprotein (P), the matrix protein (M), the glycoprotein (G) and the polymerase (L). Genomic diversity is illustrated in the multiple genes that exist in other rhabdovirus genera, including the C and P′ genes, which exist as an overlapping reading frame in P (Vesiculovirus, Ephemerovirus and unclassified/unassigned group), the small hydrophobic (SH) gene that is transcribed after M (unclassified/unassigned group) and multiple designated small genes after the P, M or G genes (Ephemerovirus, Cytorhabdovirus, Hart Park and Tibrovirus group). For a review of rhabdovirus accessory genes see Walker et al. (2011). Each of the five primary genes is flanked by sequence that is conserved among viruses within a particular genus (Fu, 2005) and has an intergenic region composed of either a single or double nucleotide. The 3′ and 5′ genome termini display a high degree of complementarity and contain an untranslated leader sequence in front of the N gene and a trailer sequence after the L gene (Wertz et al., 1994).
In 2011, a novel virus genetically related to the unclassified group of viruses within the Rhabdoviridae family was isolated from the blood of a chicken (Gallus gallus domesticus) from a market in the village of Sunguru, Arua District, Uganda. This virus, provisionally named Sunguru virus (SUNV), is the first documented chicken isolate and is most closely related to other bird- and dipteran-associated viruses belonging to the proposed Sandjimba group (Kuzmin et al., 2006, 2009). In addition, SUNV contains the additional C and SH genes, as was previously described for Tupaia (TUPV) and Durham (DURV) viruses (Allison et al., 2011; Springfeld et al., 2005). In this study, we present the complete nucleotide sequence, phylogenetic relationship based on genetic analysis, antigenic cross-reactivity patterns and replication capacity of SUNV in several arthropod cell lines, as well as in Anopheles and Culex mosquitoes to evaluate possible vector-transmission capacity of the virus.
RESULTS
Virus isolation and screening by reverse transcriptase PCR
Cytopathic effects (CPE) were observed by day 5 post-inoculation on Vero cells inoculated with the serum from a healthy, 4-month-old chicken. The supernatant from infected cultures was harvested on day 9 post-inoculation when the cells displayed 50 % CPE. Reverse transcriptase PCR performed using alphavirus (genus), flavivirus (genus) and bunyavirus (various genera) degenerative group primers yielded no observable amplicons for DNA sequencing.
Nucleotide sequencing
Next generation sequencing (NGS) generated 315 000 sequences (average length, 150 nt), which assembled into three large contigs that yielded sequence identity with members of the family Rhadoviridae in a GenBank (BLASTX) search. The maximum identity was 41 % for 1500 aa within the L gene for both Oak Vale (OVRV) and Maraba viruses. Of the 11 056 nt in SUNV, NGS covered 98 %. RACE and spot sequencing completed the genome, which has the schematic representation of 3′-Leader-N-P/C-M-SH-G-L-Trailer-5′ (Fig. 1a). Seven ORFs were identified, encoding a total of 3706 aa. Notable genome features include an internal ORF in the P gene encoding the C protein and an additional ORF between M and G, encoding the SH protein. The previously designated C protein, originally found in a paramyxovirus (order Mononegavirales, family Paramyxoviridae) (Giorgi et al., 1983), is 131 aa in length and its gene lies 5 nt downstream of the P gene start codon. Similar single overlapping ORFs have been described in the genomes of vesicular stomatitis New Jersey virus (VSNJV), TUPV and DURV (Allison et al., 2011; Spiropoulou & Nichol, 1993; Springfeld et al., 2005). The protein of the SH gene, originally discovered in the TUPV genome (Springfeld et al., 2005) and only observed in other members of the ‘unassigned’ or ‘unclassified’ rhabdoviridae (Allison et al., 2011; Gubala et al., 2010; Quan et al., 2011), is 78 aa in length in SUNV, which is one amino acid longer than that of TUPV and DURV.
Fig. 1.
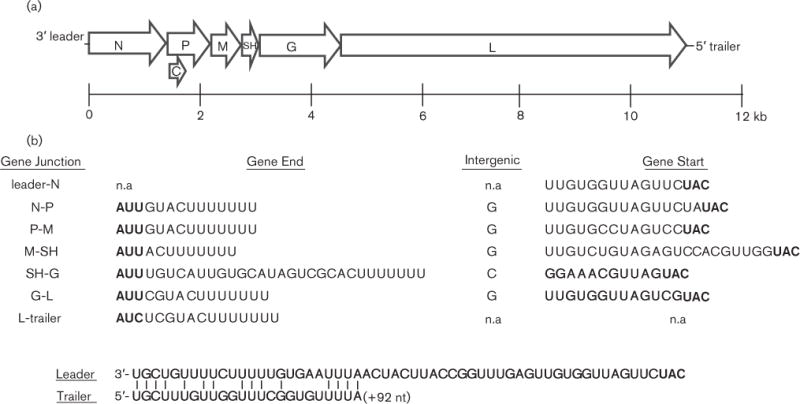
SUNV genome organization. (a) Schematic representation of the genome showing the seven open reading frames (ORFs) of the N, P, M, SH, G, L and overlapping C gene. (b) Transcription initiation, intergenic and transcription termination sequences. Start and stop codons are in bold type. Leader and trailer sequences are shown depicting end complementarity. n.a, Not applicable.
The transcription initiation and termination sequences of each ORF are believed to be 3′-UUGU, except at the SH-G junction (3′-GGA) and at the 3′ termini (3′-ACU7), respectively (Fig. 1b). The intergenic nucleotide between each ORF is a G, except at the SH-G junction where a C is observed. Rhabdovirus genomes are known to display partial complementarity between the 3′ and 5′ ends (Whelan et al., 2004), and the SUNV genome also showed this trait with the 3′ leader and 5′ trailer sequences having 15 of 23 terminal complementary nucleotides.
Genetic and serological relationships within Rhabdoviridae
Phylogenetic trees constructed from the available L gene aa sequence of other representative members of the Rhabdoviridae family suggested that SUNV (accession KF395226) is part of a divergent lineage that is related to members of the proposed Sandjimba group (Dacheux et al., 2010), and is most closely related to Boteke (BTKV) and OVRV viruses (Fig. 2). The phylogenetic analysis of the N gene indicated a grouping similar to that of the L gene, with SUNV forming a clade with Kolongo (KOLV), OVRV and Sandjimba (SJAV) viruses. However, because of limited availability of N gene sequence, viruses such as DURV and TUPV, which form a distinct branch for the L gene, group with SUNV. Interestingly, all known members of the Sandjimba group are bird or mosquito isolates from the Central African Republic (Dacheux et al., 2010) (Table 1).
Fig. 2.
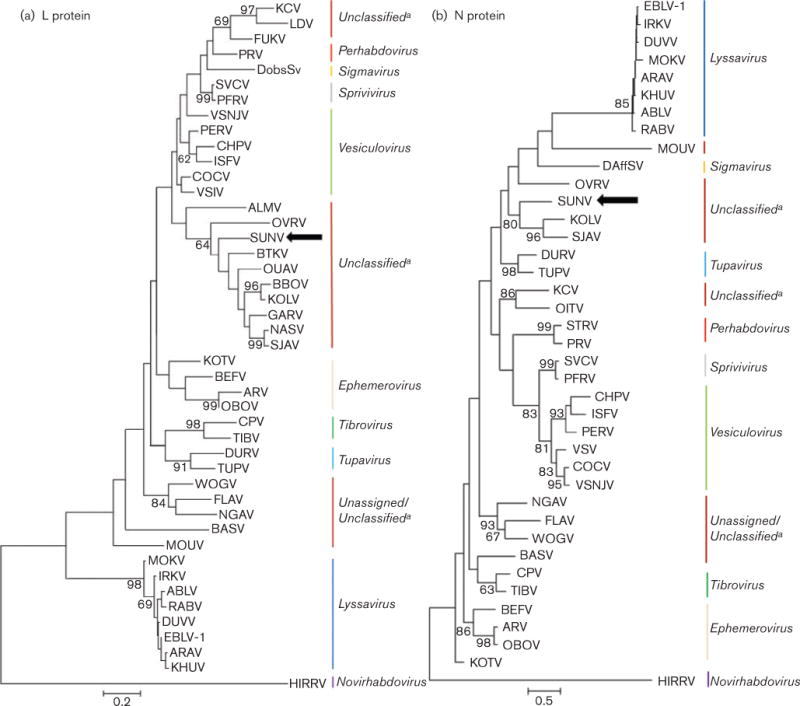
Phylogenetic relationship of SUNV (accession KF395226) with other representatives of the family Rhabdoviridae. The trees were generated by aligning the available amino acid sequences for the L (a) and N (b) proteins (see Fig. 2 Supplement available in the online Supplementary Material) using the maximum-likelihood method and Jones–Taylor–Thorton substitution model. Bootstrap values were determined using 1000 replicates and are listed at each node. Gaps in the alignment were analysed by partial deletion, which resulted in 136 and 298 positions for the L and N protein trees, respectively, in the final dataset. See Fig. 2 Supplement for accession numbers and abbreviations. The scale bars represent the number of amino acid substitutions per horizontal distance. aNot found in the ICTV database.
Table 1.
Isolation information for viruses of the Sandjimba group
| Virus | Abbr. | Principal host species/vector | Country | Year |
|---|---|---|---|---|
| Bimbo | BBOV | Bird, Euplectes afer (yellow-crowned bishop) | Central African Republic | 1970 |
| Boteke | BTKV | Dipteran, Coquillettidia maculipennis | Central African Republic | 1968 |
| Garba | GARV | Birds, Corythornis cristata (kingfisher), Nectarina pulchella (beautiful sunbird) | Central African Republic | 1970 |
| Kolongo | KOLV | Birds, Euplectes afer (yellow-crowned bishop), Polceus cucullatus (village weaver) | Central African Republic | 1970 |
| Nasoule | NASV | Bird, Andropadus virens (little greenbul) | Central African Republic | 1973 |
| Ouango | OUAV | Bird, Ploceus melanocephalus (black-headed weaver) | Central African Republic | 1970 |
| Sandjimba | SJAV | Bird, Acrocephalus schoenobaenus (sedge warbler) | Central African Republic | 1970 |
Cross-neutralization assays with mouse-generated antibodies from BTKV, OVRV, KOLV, TUPV, Bimbo virus (BBOV), Ouango virus (OUAV) and Nasoule virus (NASV) further suggest that SUNV exists distinctly from other rhabdoviruses as no cross-neutralization of SUNV with antibodies from the other viruses was observed (Table 2).
Table 2.
Cross-reactivity of SUNV to mouse-produced hyper-immune ascitic antibody fluid (MHIAF) of other members of the unassigned group of Rhabdoviridae. The values listed represent the antibody titers corresponding to 80% neutralization in duplicate tests
| Virus | Antibody (MHIAF)
|
||||||
|---|---|---|---|---|---|---|---|
| BTKV | OVRV | KOLV | TUPV | BBOV | OUAV | NASV | |
| SUNV | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 |
| BTKV | 360 | ND | ND | ND | ND | ND | ND |
| OVRV | ND | 80 | ND | ND | ND | ND | ND |
| KOLV | ND | ND | 1280 | ND | ND | ND | ND |
ND, not done.
Growth kinetics in vitro and in vivo
SUNV was inoculated into both vertebrate and invertebrate cell lines to analyse its replication patterns and evaluate SUNV’s potential as a vector-borne virus. In vertebrate cells, all three cell lines (Vero, DF-1 and BHK) supported replication of SUNV. Maximum titres were achieved at 48 h in the Vero cell line (6.6 log10 p.f.u. ml−1) and at 72 h in the BHK and DF-1 cell lines (7.8 log10 and 6.5 log10 p.f.u. ml−1, respectively; Fig. 3a). Compared with the Vero and DF-1 cell lines, replication was significantly higher (between 1 and 1.5 log10 p.f.u. ml−1) for the BHK cell line at 48, 72, 96 and 120 h. Among the invertebrate cell lines (Sua4, C6/36, HAE/CTVM9, Culex quinquefasciatus and AP-61), only the Sua4 Anopheles gambiae cells supported SUNV replication (Fig. 3b). An increase in titre was observed only for the Sua4 cells, with a maximum titre achieved at 7 days (5.85 log10 p.f.u. ml−1). The remaining cell lines decreased (by between 0.8 and 3.45 log10 p.f.u. ml−1) in titre through the course of the experiment; no significant increase in titre was detected with these four cell lines. On the basis of these results, Anopheles gambiae mosquitoes were exposed to SUNV to confirm the possibility that SUNV could replicate in vivo in Anopheles gambiae. Additionally, because other viruses in the Sandjimba group are known to be associated with birds and/or mosquitoes, Culex quinquefaciatus mosquitoes, which are known avian host seekers (Tempelis, 1975), were also tested.
Fig. 3.
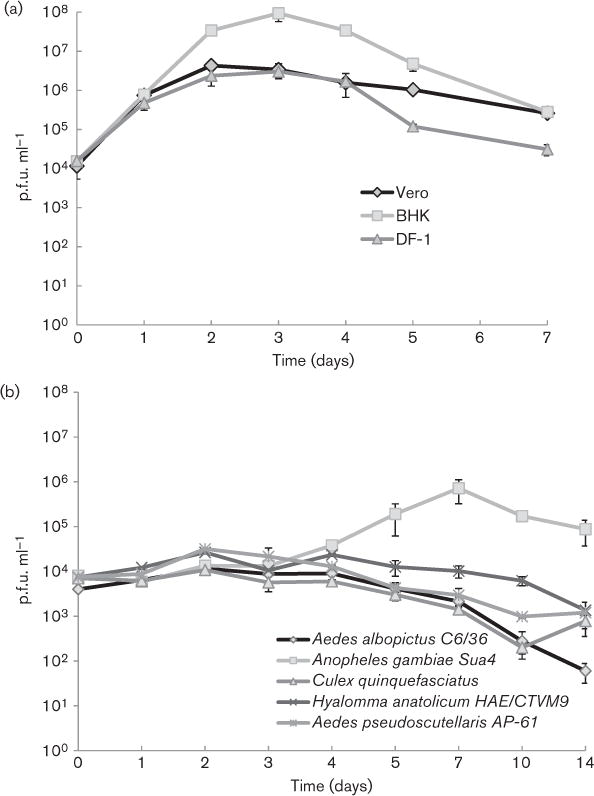
Growth of SUNV in (a) vertebrate and (b) invertebrate cell lines. Each point on the graph represents the mean of two replicates and the error bars signify one standard deviation.
SUNV growth observed in vivo was similar to the growth patterns observed in the cell culture studies. After feeding on a blood meal that contained SUNV, none of the Culex quinquefasciatus mosquitos had midgut infections, while 47.4 % of the Anopheles gambiae became infected. However, neither species showed any indication of disseminated infections (Table 3). Analysis by real-time RT-PCR of those Anopheles gambiae with infected bodies showed extremely low levels of virus-specific nucleic acid (range of 1–37 p.f.u. ml−1), indicating poor replication of SUNV in this mosquito species (Table 3).
Table 3. Mosquito infectivity, dissemination and virus titres (p.f.u. ml−1) after exposure to SUNV by artificial blood meal.
Mosquitoes were collected on day 8 (An. gambiae) or day 12 (Cx. quinquefasciatus). Titres were determined by TaqMan real-time RT-PCR. NA, not applicable.
| Mosquito (Strain) | Rep. | Titre | % infection (no.)
|
Avg. titre (range)
|
||
|---|---|---|---|---|---|---|
| Body | Head | Body | Head | |||
| Cx.quinquefasciatus (Sebring) | 1 | 5.9 | 0 (29) | 0 | NA | NA |
| 2 | 6.4 | 0 (26) | 0 | NA | NA | |
| An. gambiae (G3) | 1 | 5.5 | 47.4 (38) | 0 | 8 (1–37) | NA |
DISCUSSION
Rhabdoviruses are an exceptionally diverse group of viruses that include pathogens affecting humans, livestock, birds, fish and even plants. Transmission patterns are equally varied as the viruses can be transmitted via arthropod bite, mammalian bite, direct contact (plant viruses) or water (fish viruses). Curiously, despite such biological diversity, the members all appear to have emerged from a monophyletic origin (International Committee on Taxonomy of Viruses, 2014) and have been shown to exhibit clear antigenic relationships (Calisher et al., 1989).
Upon isolation of SUNV, initial genetic characterization efforts utilizing primers specific to the alphavirus, flavivirus and bunyavirus groups were unsuccessful. Thus, we used deep sequencing methodologies because the wide availability of this approach allowed a more thorough examination of the genetic nature of the isolate. Our sequencing analysis clearly revealed an organization of the SUNV genome similar to that of other members of the Rhabdoviridae and most similar to that of OVRV and BTKV viruses. Additionally, the genetic analyses suggested a relationship to the members of the dimarhabdovirus (dipteran–mammal-associated rhabdovirus) supergroup. However, bootstrap values for separate clades representing distinct genera (e.g. vesiculoviruses, ephemeroviruses, etc.) were extremely poor, making more distant evolutionary relationships in the family difficult to discern, as has been previously recognized (Palacios et al., 2013). This difficulty could be due to lack of sampling of the intermediate genotypes or caused by extinction events.
Phylogenetic analyses of both the N and L gene sequences indicated relationships between SUNV and unclassified viruses in the proposed Sandjimba group (Kuzmin et al., 2006, 2009). Because of the close genetic relationships noted among the Sandjimba group of viruses and the fact that, like SUNV, many of the viruses in this group were isolated from birds (Sandjimba, Garba, Nasoule, Kolongo and Bimbo) (Dacheux et al., 2010), we postulated SUNV might be antigenically related to these unclassified viruses. However, we found no evidence that related virus antisera neutralized SUNV, suggesting that even though a close genetic relationship exists, SUNV is distinct from other known rhabdoviruses, which supports the classification of SUNV as a new viral species.
As viruses in one of the most closely related genera (Vesiculovirus) and the ungrouped virus BTKV are known to be arthropod borne, we examined the possibility that SUNV might be an arbovirus. Generally, in vitro growth patterns with cultured mosquito or tick cell lines indicated limited or no growth of SUNV. This was in stark contrast with growth in vertebrate cell lines, including lines derived from both mammals and birds, where a rapid increase in virus titre was detected. Interestingly, since the Anopheles gambiae cell line (Sua4) supported a low level of virus replication from days 4 to 7 per os, in vivo infections of Anopheles gambiae were undertaken as anophelines have been shown to vector other arboviruses in the families Togaviridae (e.g. o’nyong nyong virus) and Bunyaviridae (e.g. Koongol virus). Likewise, a culex species mosquito (Culex quinquefasciatus) was also evaluated for infectivity with SUNV since other rhabdoviruses have been shown to infect culex species mosquitoes (e.g. Perinet virus infects Culex antennatus) (Dacheux et al., 2010). Our results indicated no infection capacity in Culex quinquefasciatus but an interestingly high infection rate (approx. 50 %) in Anopheles gambiae. However, since no evidence of dissemination in Anopheles gambiae was found, it would be highly unlikely that SUNV is vectored by this mosquito species. On the basis of all these findings, it appears unlikely at this time that SUNV is an arbovirus, and further studies are needed to determine its actual mode of transmission.
Finally, as for numerous other known rhabdoviruses, the capacity of SUNV to cause either human or animal illness has not been determined. There was no indication of illness in the chicken from which the virus was isolated, but a single sample cannot give a valid assessment of animal or human risk. To determine if this agent is capable of causing disease, extensive studies such as human serosurveys or acute febrile illness studies would be required. As more novel viruses are detected and characterized in the laboratory, these biological studies become increasingly important to perform. Extensive characterization of each new isolated virus is the critical first step in determining the scope of additional biological studies to be eventually undertaken.
METHODS
Specimen collection and virus isolation
Blood collections were performed on domestic animals from 14 January to 19 January 2011 in the Arua District, north-western Uganda. Samples were collected from goats, sheep, cattle and chickens from the Ejupala market in the village of Sunguru (02°80′40″ N 30°89′07″ E). Whole blood was collected by venipuncture in serum microtainer separator tubes (Becton Dickinson), kept on dry ice until freezing and stored at −80 °C before shipment and analysis.
Upon thawing, tubes were spun for 10 min at 1650 × g in a table top centrifuge. A 5 μl aliquot of serum was added to 245 μl of complete Dulbecco’s Minimum Essential Medium (DMEM; Gibco) in a 1.7 μl Costar tube (Corning). The full 250 μl of diluted serum was pipetted onto a 50 % confluent monolayer of Vero cells in one well of a cell culture-treated six-well plate (Corning). Four millilitres of complete DMEM were then added to the wells to ensure that the cells did not dry out. Inoculated cells were stored in an incubator at 37 °C and 5 % CO2 and were checked daily for the presence of CPE. Supernatant was removed from any positive wells, diluted 1 : 2 in heat-inactivated FBS (Atlas Biologicals) and stored in 2 μl cryovials (Sarstedt) at −70 °C until use.
Nucleic acid amplification
Viral RNA was extracted from both 140 μl of Vero cell culture supernatant and 100 μl of experimentally infected mosquito homogenates (see below) using the QIAamp viral RNA protocol (Qiagen). Total RNA was eluted from the columns using 60 μl elution buffer and the resulting RNA was stored at −70 °C until use. RT-PCR was used for both the detection of viral nucleic acid and to generate PCR amplicons for classical sequencing. A 5 μl aliquot of purified RNA was subjected to amplification using the OneStep RT-PCR protocol (Qiagen) and combined with the flavivirus (Kuno et al., 1998), bunyavirus (Lambert & Lanciotti, 2009) or alphavirus group mix primers, or those specifically designed for the SUNV genome (Table S1, available in the online Supplementary Material). The manufacturer’s protocol was followed with no modifications. The reactions were analysed by gel electrophoresis and correctly sized products were extracted and cleaned using the MinElute Gel Extraction kit (Qiagen) and sequenced.
Extracted RNA from the mosquito homogenates was tested for viral replication by the TaqMan QuantiTect Probe RT-PCR protocol (Qiagen). The SUNV oligonucleotide set was designed with the Primer Select software program (DNASTAR) (Table S1). A 50 μl total reaction volume consisted of kit components, 10 μl RNA, 400 nM primer and 150 nM probe. The reactions were subjected to 45 cycles of amplification in an iQ5 Real-Time PCR detection system (Bio-Rad) according to the recommended conditions. The limits of detection were found to be a Ct of 37.5, which is equivalent to 1.0 p.f.u. ml−1, using techniques previously described by Linnen et al. (2008). In addition, each run included a standard RNA curve. The standard curve was completed by serially diluting virus stock and extracting the RNA from each dilution according to the previously mentioned RNA extraction protocol while simultaneously titrating each dilution in a standard plaque assay (as p.f.u. ml−1). A curve correlation coefficient of ⩾0.950 and a 90–100 % PCR efficiency was used to validate each detection assay. The RNA amounts were correlated with p.f.u. ml−1.
Sequencing
NGS, using the Ion Torrent Personal Genome Machine system (Life Technologies) and associated protocols, was used to collect sequence information from viral RNA purified from the Vero cell supernatant. Briefly, a cDNA library was prepared using the Ovation RNA-Seq System V2 (NuGEN) and the IonExpress Plus gDNA and Amplicon Library Preparation kit (Life Technologies) according to the manufacturer’s recommendations. To assess the size profile and quantity of the cDNA, the sheared and purified cDNA library was analysed on a Bioanalyzer (Agilent Technologies). The cDNA template was then diluted to the appropriate molar concentration in distilled water. To prepare and enrich template-positive particles from the cDNA library, the Ion OneTouch System Template kit (Life Technologies) was used. The enriched ion spheres were then sequenced using the Ion Torrent Personal Genome Machine Sequencer and the Ion Sequencing kit, with a 314 Chip (Life Technologies). NGen 4 (DNASTAR) and CLC Genomics Workbench 5.1.1 (CLC Bio) software were used to assemble the NGS data into contigs using the de novo assembly parameters. To identify and establish genome orientation, the assembled contigs (>1000 nt) were entered into the BLASTX search function of the GenBank sequence database.
Gaps in the NGS-generated contigs were covered using classical Sanger sequencing. The 5′ and 3′ genome termini were obtained using the 5′/3′ RACE kit protocol following the manufacturer’s instructions (Roche). Sequencing reactions were performed on the PCR products using the Big Dye v3.1 kit on an ABI 3130 xl Genetic Analyzer (Applied Biosystems) and SUNV-specific primers (Table S1). Sequencing chromatograms were analysed for sequence quality and genome coverage using the Lasergene 9 Core suite software (DNASTAR).
Phylogenetic analysis
Trees were constructed based on 45 and 39 published amino acid sequences of the L and N genes of multiple rhabdoviruses, respectively. Sequences were aligned using the CLUSTALW software algorithm and the phylogenetic trees constructed under partial deletion of gaps. This resulted in 298 and 136 positions analysed in the final dataset for the N and L proteins, respectively. The evolutionary history was inferred using the maximum-likelihood method based on the Jones–Taylor–Thorton (JTT) matrix-based model with 1000 bootstrap iterations and nearest-neighbour-interchange (NNI) heuristic method (MEGA 5.05) (Tamura et al., 2011).
Antigenic relationship by cross-neutralization
The plaque reduction neutralization assay (PRNT) (Dulbecco et al., 1956; Lindsey et al., 1976) was used to determine the antigenic relationships of members of the unassigned Rhabdoviridae [BTKV, KOLV, OVRV, NASV, BBOV and TUPV] to SUNV. Briefly, 25 μl of mouse-generated hyper-immune ascitic fluid (MHIAF) was diluted twofold in DMEM, supplemented with 10 % FBS, 100 U penicillin ml−1, 100 mg streptomycin ml−1 and then combined with a suspension of 100 p.f.u. SUNV per 125 μl medium. The mixture was allowed to incubate for 1 h at 37 °C. An aliquot was then inoculated onto Vero cells, allowed to incubate at 37 °C for 1 h, covered with agarose media overlay and returned to the incubator. The plates were monitored for plaques and fixed with a fixative/staining solution (40 % methanol and 0.25 % crystal violet) at the appropriate time. PRNT80 titres were calculated for the homologous virus-antibody pairs and for SUNV crossed with the listed MHIAF.
In vitro growth kinetics
Vertebrate cells lines including chicken embryo fibroblasts (DF-1), African green monkey (Vero-E6) and BHK, as well as invertebrate cells lines Aedes albopictus (C6/36-mosquito) (Igarashi, 1978), Anopheles gambiae (Sua4-mosquito), Culex quinquefasciatus (mosquito), Hyalomma anatolicum (HAE/CTVM-tick) (Bell-Sakyi, 1991; Ruzek et al., 2008) and Aedes pseudoscutellaris (AP-61) (Varma et al., 1974) were inoculated with SUNV (Vero passage 2) at 80–90 % confluency and an m.o.i. of 0.1 for vertebrate cells or 0.05 for insect cells. Maintenance conditions and growth media varied for several of the cell types (Table S2). CPEs were monitored by microscopy and cell supernatants were collected every 24 h for 7 (vertebrate cells) or 14 (invertebrate cells) days. Supernatants were titrated in Vero cells by plaque assay with the results recorded as p.f.u. per millilitre.
Mosquito experiments
Three- to four-day-old adult Culex quinquefasciatus (Sebring strain) and Anopheles gambiae (G3 strain) mosquitoes were fed on a blood meal containing SUNV. The blood meal contained equal parts of virus, FBS with 10 % sucrose and goose (Culex quinquefasciatus) or calf (Anopheles gambiae) red blood cells (Colorado Serum CO) washed with PBS and packed by centrifugation. A Haemotek feeding system (Discovery Workshops) was used to deliver the blood meal to the mosquitoes at 37 °C. The mosquitoes were allowed to feed for 1 h. The fully engorged females were separated and placed into a humidified environmental chamber (Thermo Scientific) and held at 28 °C for 8 (Anopheles gambiae) or 12 (Culex quinquefasciatus) days until processing. Additionally, a portion of the blood meal was collected and titrated by plaque assay to determine the infecting dose.
After the holding period, cold-anaesthetized mosquitoes were decapitated, placing the heads and bodies into separate, 1.7 ml tubes (Eppendorf). A 400 μl aliquot of DMEM supplemented with 10 % FBS, 100 U ml−1 penicillin and streptomycin, and 1 U ml−1 fungizone and gentamicin was placed into each tube and the sample was homogenized using a pestle (Kontes). The supernatant was clarified by filtration through a 0.2 μm syringe filter (Pall) into a clean tube and frozen at −70 °C until use. A 100 μl sample was then deposited into a single well of a 96-well plate (Corning) with 50 μl of a Vero cell suspension. The plate was placed into a 37 °C, 5 % CO2 incubator and each well monitored daily for CPE. The sample was considered virus positive if CPE was observed.
Supplementary Material
Acknowledgments
We thank Dr Lesley Bell-Sakyi and the Tick Cell Biobank, University of Edinburgh, for providing the HAE/CTVM9 tick cell line and editing this manuscript, Andrea Peterson (CDC) for supplying the mosquitoes and Jason Velez (CDC) for providing critical guidance and production of the cell lines. Additional thanks go out to the Vurra subcounty, Arua District, Uganda, for supporting and granting specimen collections. This work was funded by the USAID Emerging Pandemic Threats Program and the United States Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors only and do not necessarily reflect the views of their respective institutions.
Footnotes
The GenBank/EMBL/DDBJ accession number for the full-length genome sequence of Sunguru virus is KF395226.
Two supplementary tables and supplementary information for Fig. 2 are available with the online version of this paper.
References
- Allison AB, Palacios G, Travassos da Rosa A, Popov VL, Lu L, Xiao SY, DeToy K, Briese T, Lipkin WI, et al. Characterization of Durham virus, a novel rhabdovirus that encodes both a C and SH protein. Virus Res. 2011;155:112–122. doi: 10.1016/j.virusres.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell-Sakyi L. Continuous cell lines from the tick Hyalomma anatolicum anatolicum. J Parasitol. 1991;77:1006–1008. [PubMed] [Google Scholar]
- Calisher CH, Karabatsos N, Zeller H, Digoutte JP, Tesh RB, Shope RE, Travassos da Rosa AP, St George TD. Antigenic relationships among rhabdoviruses from vertebrates and hematophagous arthropods. Intervirology. 1989;30:241–257. doi: 10.1159/000150100. [DOI] [PubMed] [Google Scholar]
- Dacheux L, Berthet N, Dissard G, Holmes EC, Delmas O, Larrous F, Guigon G, Dickinson P, Faye O, et al. Application of broad-spectrum resequencing microarray for genotyping rhabdoviruses. J Virol. 2010;84:9557–9574. doi: 10.1128/JVI.00771-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale JL, Peters D. Protein composition of the virions of five plant rhabdoviruses. Intervirology. 1981;16:86–94. doi: 10.1159/000149252. [DOI] [PubMed] [Google Scholar]
- Dulbecco R, Vogt M, Strickland AG. A study of the basic aspects of neutralization of two animal viruses, western equine encephalitis virus and poliomyelitis virus. Virology. 1956;2:162–205. doi: 10.1016/0042-6822(56)90017-4. [DOI] [PubMed] [Google Scholar]
- Fu ZF. Genetic comparison of the rhabdoviruses from animals and plants. Curr Top Microbiol Immunol. 2005;292:1–24. doi: 10.1007/3-540-27485-5_1. [DOI] [PubMed] [Google Scholar]
- Giorgi C, Blumberg BM, Kolakofsky D. Sendai virus contains overlapping genes expressed from a single mRNA. Cell. 1983;35:829–836. doi: 10.1016/0092-8674(83)90115-0. [DOI] [PubMed] [Google Scholar]
- Gubala A, Davis S, Weir R, Melville L, Cowled C, Walker P, Boyle D. Ngaingan virus, a macropod-associated rhabdovirus, contains a second glycoprotein gene and seven novel open reading frames. Virology. 2010;399:98–108. doi: 10.1016/j.virol.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Igarashi A. Isolation of a Singh’s Aedes albopictus cell clone sensitive to Dengue and Chikungunya viruses. J Gen Virol. 1978;40:531–544. doi: 10.1099/0022-1317-40-3-531. [DOI] [PubMed] [Google Scholar]
- International Committee on Taxonomy of Viruses. 2014 www.ictvonline.org.
- Kuno G, Chang GJ, Tsuchiya KR, Karabatsos N, Cropp CB. Phylogeny of the genus Flavivirus. J Virol. 1998;72:73–83. doi: 10.1128/jvi.72.1.73-83.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin IV, Hughes GJ, Rupprecht CE. Phylogenetic relationships of seven previously unclassified viruses within the family Rhabdoviridae using partial nucleoprotein gene sequences. J Gen Virol. 2006;87:2323–2331. doi: 10.1099/vir.0.81879-0. [DOI] [PubMed] [Google Scholar]
- Kuzmin IV, Novella IS, Dietzgen RG, Padhi A, Rupprecht CE. The rhabdoviruses: biodiversity, phylogenetics, and evolution. Infect Genet Evol. 2009;9:541–553. doi: 10.1016/j.meegid.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Lambert AJ, Lanciotti RS. Consensus amplification and novel multiplex sequencing method for S segment species identification of 47 viruses of the Orthobunyavirus, Phlebovirus, and Nairovirus genera of the family Bunyaviridae. J Clin Microbiol. 2009;47:2398–2404. doi: 10.1128/JCM.00182-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey HS, Calisher CH, Mathews JH. Serum dilution neutralization test for California group virus identification and serology. J Clin Microbiol. 1976;4:503–510. doi: 10.1128/jcm.4.6.503-510.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnen JM, Vinelli E, Sabino EC, Tobler LH, Hyland C, Lee TH, Kolk DP, Broulik AS, Collins CS, et al. Dengue viremia in blood donors from Honduras, Brazil, and Australia. Transfusion. 2008;48:1355–1362. doi: 10.1111/j.1537-2995.2008.01772.x. [DOI] [PubMed] [Google Scholar]
- Palacios G, Forrester NL, Savji N, Travassos da Rosa AP, Guzman H, Detoy K, Popov VL, Walker PJ, Lipkin WI, et al. Characterization of Farmington virus, a novel virus from birds that is distantly related to members of the family Rhabdoviridae. Virol J. 2013;10:219. doi: 10.1186/1743-422X-10-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan PL, Williams DT, Johansen CA, Jain K, Petrosov A, Diviney SM, Tashmukhamedova A, Hutchison SK, Tesh RB, et al. Genetic characterization of K13965, a strain of Oak Vale virus from Western Australia. Virus Res. 2011;160:206–213. doi: 10.1016/j.virusres.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Růžek D, Bell-Sakyi L, Kopecký J, Grubhoffer L. Growth of tick-borne encephalitis virus (European subtype) in cell lines from vector and non-vector ticks. Virus Res. 2008;137:142–146. doi: 10.1016/j.virusres.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Spiropoulou CF, Nichol ST. A small highly basic protein is encoded in overlapping frame within the P gene of vesicular stomatitis virus. J Virol. 1993;67:3103–3110. doi: 10.1128/jvi.67.6.3103-3110.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springfeld C, Darai G, Cattaneo R. Characterization of the Tupaia rhabdovirus genome reveals a long open reading frame overlapping with P and a novel gene encoding a small hydrophobic protein. J Virol. 2005;79:6781–6790. doi: 10.1128/JVI.79.11.6781-6790.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempelis CH. Host-feeding patterns of mosquitoes, with a review of advances in analysis of blood meals by serology. J Med Entomol. 1975;11:635–653. doi: 10.1093/jmedent/11.6.635. [DOI] [PubMed] [Google Scholar]
- Tordo N, Benmansour A, Calisher C, Dietzgen RG, Fang RX, Jackson AO, Kurath G, Nadin-Davis S, Tesh RB, Walker PJ. Family Rhabdoviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus Taxonomy: Eighth report of the International Committee on Taxonomy of Viruses. San Diego, CA: Elsevier; 2005. pp. 623–644. [Google Scholar]
- Varma MG, Pudney M, Leake CJ. Cell lines from larvae of Aedes (Stegomyia) malayensis Colless and Aedes (S) pseudoscutellaris (Theobald) and their infection with some arboviruses. Trans R Soc Trop Med Hyg. 1974;68:374–382. doi: 10.1016/0035-9203(74)90152-7. [DOI] [PubMed] [Google Scholar]
- Walker PJ, Dietzgen RG, Joubert DA, Blasdell KR. Rhabdovirus accessory genes. Virus Res. 2011;162:110–125. doi: 10.1016/j.virusres.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz GW, Whelan S, LeGrone A, Ball LA. Extent of terminal complementarity modulates the balance between transcription and replication of vesicular stomatitis virus RNA. Proc Natl Acad Sci U S A. 1994;91:8587–8591. doi: 10.1073/pnas.91.18.8587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan SP, Barr JN, Wertz GW. Transcription and replication of nonsegmented negative-strand RNA viruses. Curr Top Microbiol Immunol. 2004;283:61–119. doi: 10.1007/978-3-662-06099-5_3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


