Abstract
p16INK4a is a tumor-suppressor protein and cyclin-dependent kinase (cdk) inhibitor that blocks cdk4- and cdk6-mediated pRb phosphorylation to inhibit E2F-dependent transcription and cell-cycle progression. Because the E7 protein of high-risk HPVs inactivates pRB, the resulting overexpression of p16INK4a may be a good marker for infection with high risk HPV types. Immunostaining of p16INK4a allows precise identification of even small CIN or cervical cancer lesions in biopsy sections and can help reduce inter-observer variation in the histopathological interpretation of cervical biopsy specimens. The aims of the present study were to evaluate the expression of p16 INK4a in cervical biopsies and to compare the grade of cervical neoplasia with intensity of staining. The study covered 110 cervical biopsy tissue blocks over a period of 2 years, (85 cases of CIN of varying grade and invasive cervical cancers and 25 of non-neoplastic lesions). Immunostaining with p16INK4a antibodies followed standard operating procedures. The results showed an increasing trend for p16INK4a immunoreactivity from benign to higher grade lesions. Out of 25 cases of non dysplasia (15 cervicitis &10 immature squamous metaplasia), 8%(2/25) showed P16INK4a expression (grade 1). Among low grade lesions like CIN1, 32% (8/25) cases demonstrated P16INK4a expression (grade 1). Some 52.3% (11/21) of CIN2 cases proved positive. The intensity of p16INK4a expression in CIN 2 was grade 1 in 33%, grade 2 in 14% and grade 3 in 4.7% of cases. All the CIN3 lesions and cervical squamous cell carcinomas exhibited grade 3 anti p16INK4a antibody staining. The association of p16INK4a expression with histologic grade of cervical pathology was highly significant (χ2-value:51.81, p<0.0001). The staining intensity increase with higher grade disease was also statistically significant (χ2-value :133.95, p<0.0001).
Keywords: p16INK4a, CIN, immature squamous metaplasia-invasive cervical carcinoma
Introduction
Cervical cancer is the fourth most common cancer in women, and the seventh overall. With an estimated 528,000 new cases and 266,000 death in 2012 (Faerly et al., 2015), it is the most common cause of death in developining countries. (Denny, 2012). Infection with human Papilloma Virus (especially by high risk HPV 16 and HPV18) has been established as etiological factor for development of cervical cancer (Walboomers et al,1999; Ferlay et al., 2010). Cervical cancer in India ranks as the 2nd most frequent cancer among women and the 2nd most frequent cancer among women between 15 and 44 years of age as stated by Summary report of ICO Information Centre on HPV and cancer in 2014. According to this report, in India, every year 122844 women are diagnosed with cervical cancer and 67477 die from the disease (Summary report of ICO Information Centre on HPV and cancer in 2014). Several Indian studies performing HPV detection tests in cervical samples showed that about 5.0% of women in the general population are estimated to harbour cervical HPV-16/18 infection at a given time, and 82.7% of invasive cervical cancers are attributed to HPVs 16 or 18 (Bruni et al., 2015). Since HPV infections supersede cell cycle controls, the immune detection of cell proteins that are differentially expressed in infected cells is currently being considered for use as tumour and prognostic marker, as well as for application in different modalities of cervical cancer screening (IARC,2007). p16INK4a is a tumor-suppressor protein and cyclin-dependent kinase (cdk) inhibitor that blocks cdk4- and cdk6-mediated pRb phosphorylation to inhibit E2F-dependent transcription and cell-cycle progression ((Ferlay et al., 2012). Hence the level of expression of the cyclin-dependent kinase inhibits or p16INK4a in the cervical biopsy has been recently evaluated by many (Kanthiyan et al., 2016; Sharon et al., 2016). An inverse relationship was found between the expression of p16INK4a and the presence of the normal retinoblastoma protein (pRB) in cancer cell lines in which the p16INK4a protein is detectable when pRB is mutated, deleted or inactivated, and is markedly reduced or absent in cell lines that contain normal pRB (IARC2007). pRB was shown to act as a negative regulator of p16INK4a gene transcription via repression of E2F activity (Li et al., 1994; Khleif et al., 1996). Because the E7 protein of high-risk HPVs inactivates pRB, the resulting over expression of p16INK4a may be a good marker for infection by these HPV types. Immunostaining of p16INK4a allows precise identification of even small CIN or cervical cancer lesions in biopsy sections and helped reduce inter-observer variation in the histopathological interpretation of cervical biopsy specimens (Doeberitz et al., 2001). So overexpression of P16 INK4a have been noted in CIN as well as in invasive cervical cancer and the intensity of P16 INK4 a expression gradually become higher in high grade lesion. AIM of the study was to -1)evaluate the expression of P16 INK4a in cervical biopsy, and 2) to compare the relationship between the grade of the cervical neoplasia and intensity of P16 INK4a.
Materials and Methods
Study Design
It is a retrospective study of p16 INK4a protein expressions on cervical tissue, diagnosed as CIN of varying grade and cervical cancer using immunohistochemical method. The study was approved by Institutional Ethical committee vide No.233/2013/252. The study included 110 numbers of paraffin embedded cervical biopsy tissue blocks (either wedge/punch cervical biopsy/ hysterectomy) collected from the archive of Pathology Department, Gauhati Medical College for a period of almost 2 years, from June’ 2014 to May’ 2016 Out of 110 cases, 68 cases had initial diagnosis of CIN of varying grade and 27 had cervical cancer (SCC-26 and adenoca-1); 10 cases had chronic cervicitis and 5 had metaplastic change. Endocervical polyps were excluded. All the paraffin embedded tissue blocks were cut at 4 μM thickness and two sections were made ready from each block. One tissue section was placed on albuminized slides for routine hematoxylin and eosin stain and other set of tissue sections was mounted on poly L –lysine coated slides for immunostain. The poly L –lysine coated slides were placed in the oven for 10 minutes. Sections were then deparaffinized by passage through xylene and subsequently rehydrated in graded alcohol of decreasing concentration i.e. 100 %, 70% and 50% at five minutes interval per change. They were then rinsed in running water for five minutes. Antigen retrieval, in which the sections were placed in the target antigen retrieval buffer solution was performed using EZ-Retriever system v.2 (Biogenex) at temperature 60 °C for five minutes one cycle followed by setting the temperature at 90 °C for five minutes two cycle. Then the sections were allowed to cool at room temperature for 20 minutes. They were washed with running water and then rinsed with tris-buffer saline (TBS). Sections were incubated for 30 minutes at room temperature with mouse monoclonal anti- p16 INK4a antibody (Ready to use, from BioGenex, Fremont, CA). After washing thoroughly with TBS at pH 7.4, high definition amplifier was added on the slides and incubated for 30 minutes at room temperature in humidity chamber, followed by rinsing with TBS. High definition polymer HRP Label (secondary antibody) is added in the slides and incubated for 30 minutes at room temperature. Again the slides were washed with TBS at PH 7.4. A drop of diamino benzidene (DAB) was then spread over the sections for seven minutes and then it was rinsed in water. The sections were counter-stained with haematoxylin for 30-45 seconds before rinsing with running water for three minutes and dehydrated in increasing alcohol concentration and mounted.
Interpretation of Cervical biopsy
The sections were examined with the help of olympus microscope under 10X and 40 X power eye piece. The H and E stained slides were analysed by 2nd pathologist (U.S.) for the following features-
1) The presence of neoplasia and its grade
2) Degree of inflammation
3) The presence of immature metaplasia.
The IHC stained (anti p16INK4a antibody) sections were interpreted in semi quantitative manner (Negative or 1+ to 3 +) based on none, 5-25%, 25-75% and >75% of the cells immunostained in a lesion. The scoring of p16 generally includes both nuclear and cytoplasmic staining, graded as 0 (no staining), 1 (rare singly dispersed cells staining), 2 (patchy but strong staining, often not continuous from basement membrane), and 3 (strong and diffuse staining, usually continuous staining from basement membrane and extending upward in proportion to lesion grade).
Satistical analysis
The patients’ data was collected from medical records department and data entry was carried out by using MICROSOFT OFFICE EXCEL 2007. The associations between variables (The expression of P16INK4a and histological diagnosis of cervical tissue) was calculated by using Chi-Square (X2) test of significance; P values less than 0.5 was considered statistically significant. The Cohen’s Kappa (k) value was calculated to measure the interobserver reliability of initial and final diagnosis. We interpreted the kappa statistic to represent the following levels of agreement: < 0.0 = poor, 0.0 – 0.2 =slight, 0.2– 0.4 = fair, 0.4 – 0.6 = moderate, 0.6 – 0.8 = substantial, and 0.8 –1.0 = almost perfect (Fleiss JL ., 1981). The standard methods of statistical analysis was performed by adopting the statistical software Graph Pad InStat.
Results
The result of the re examined 110 cervical biopsy showed 25 cases to be non dysplasia (Chronic Cervicitis = 15, Squamous Metaplasia = 10), 58 cases had CIN of varying grade, 26 cases had invasive squamous cell carcinoma and 1 was adenocarcinoma. Exact agreement of both diagnosis was seen in higher grade lesion (CIN3, SCC and Adenoca). Only 10 (9.1%) cases had discrepancy in both diagnosis. Hence 88.50% of the total cases showed concordant diagnosis (k=0.8850). Five cases of cervicitis were initially over diagnosed as CIN1 and 5 cases of squamous metaplasia were initially put in CIN2 groups (Table 1).
Table 1.
Table Showing Comparison between Initial Diagnosis and Final Diagnosis of Cervical Biopsy (n=110)
| Final Diagnosis | ||||||||
|---|---|---|---|---|---|---|---|---|
| Non Dysplasia | CIN1 | CIN2 | CIN3 | SCC | Adenoca | Total n=110 | ||
| Initial Diagnosis | Non dysplasia | 15 | 0 | 0 | 0 | 0 | 0 | 15 |
| CIN1 | 5 | 25 | 0 | 0 | 0 | 0 | 30 | |
| CIN2 | 5 | 0 | 21 | 0 | 0 | 0 | 26 | |
| CIN3 | 0 | 0 | 0 | 12 | 0 | 0 | 12 | |
| SCC | 0 | 0 | 0 | 0 | 26 | 0 | 26 | |
| Adeno Ca | 0 | 0 | 0 | 0 | 0 | 1 | 1 | |
| Total | 25 | 25 | 21 | 12 | 26 | 1 | 110 | |
The bold cells highlight exact agreement between the two diagnoses. CIN, cervical intraepithelial neoplasia; SCC, squamous cell carcinoma; Adenoca, adenocarcinoma.(Kappa value =0.8850)
The expression of p16INK4a in cervical biopsy was correlated with the H and E stained routine histopathological diagnosis as stated in Table 2. Among the non dysplasia category, 8% (2/25) cases showed p16INK4a expression (grade1). On H and E stained preparation, these 8% cases of non dysplasia cases were diagnosed as immature squamous metaplasia. Among the Low grade lesion like CIN1, 32% (8/25) cases was showing P16INK4a expression (grade 1). The expression of P16 INK4a noted in 52.3% (11/21) cases of CIN2. The intensity of p16INK4a expression in CIN 2 is grade 1 in 33%, grade2 in 14% cases and grade 3 in 4.7% cases. All the CIN3 and cervical squamous cell carcinoma cases were stained positively by anti p16INK4a antibody and their staining intensity has been seen to be increased with the higher grade of the disease which is highly significant (p<0.0001) (Table 3 and Figure 1).
Table 3.
Association of Cervical Pathology and Intensity of p16INK4a
| HP Dg | Intensity of p16INK4A Expression | Total N=110 | d.f | χ2-value | p-value | |||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |||||
| Cervicitis | 15 | 0 | 0 | 0 | 15 | |||
| Metaplasia | 8 | 2 | 0 | 0 | 10 | |||
| CIN1 | 17 | 8 | 0 | 0 | 25 | |||
| CIN2 | 10 | 7 | 3 | 1 | 21 | 18 | 133.95** | P<0.0001 |
| CIN3 | 0 | 2 | 4 | 6 | 12 | |||
| SCC | 0 | 0 | 0 | 26 | 26 | |||
| Adenoca | 0 | 1 | 0 | 0 | 1 | |||
| Toal | 50 (45.45%) | 20 (18.18%) | 7 (6.36%) | 33 (30.00%) | 110 | |||
0, (no staining); 1, (rare singly dispersed cells staining); 2, (patchy but strong staining, often not continuous from basement membrane); 3, (strong and diffuse staining; usually continuous staining from basement membrane and extending upward in proportion to lesion grade)
Figure 1.
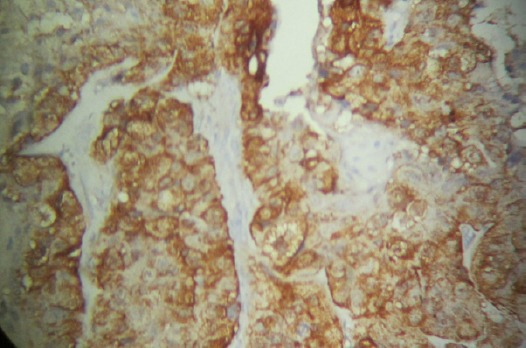
Diffuse Staining Pattern (Grade3) of P16INK4a in Cervical Cancer
Table 2.
Association of Cervical Pathology on Routine Histology and Expression of p16INK4a
| HP Dg | p16 +ve | P16- ve | Total N=110 | d.f | χ2-value | p-value |
|---|---|---|---|---|---|---|
| Cervicitis | 0 | 15 | 15 | |||
| Metaplasia | 2 | 8 | 10 | |||
| CIN1 | 8 | 17 | 25 | |||
| CIN2 | 11 | 10 | 21 | 6 | 60.47** | P<0.0001 |
| CIN3 | 12 | 0 | 12 | |||
| SCC | 26 | 0 | 26 | |||
| Adenoca | 1 | 0 | 1 | |||
| Toal | 57 (51.35) | 53 (48.65) | 110 |
Discussion
Routine histology is the gold standard for patient management. However, it needs interobserver reliability to judge qualitative test. In this study we have found that 88.50% of the total cases showed concordant diagnosis (K=0.8850). There was a better agreement in interpretation of higher grade lesion. But, in 10 non dysplasia cases, there was discrepancy in differentiating them from CIN (CIN1=5/110) and CIN2 =5/110) which might be due to either 1) presence of mild nuclear atypia associated with underlying infammation 2) reparative process is going on that may show nuclear hyperchromasia as well as cytoplasmic halos, 3) presence of binuclation or 4) post menopausal squamous atypia.
The present study showed that the expression of P16INK4a in cervical biopsy is directly correlated with higher grade lesion, the result of which is comparable with data generated by Kanthiya et al., 2016. Kanthyia et al., (2016) reported expression of P16INK4a in 9.4 % cases of non dysplasia. Queroz et al., (2013) also reported p16INK4a immunoreactivity in 9.1% cases of non dysplasia. The current study revealed p16INK4a expression in 8% of non dysplasia (Immature squamous metaplasia). Keating et al., (2001) found that p16INK4a positivity in lesions with squamous metaplastic atypia correlated significantly with the presence of high-risk HPV types. In a study by Nicholas et al., (2003), of the atypical lesions (n = 39), 6 (15%) of the original diagnosis showed 1+ p16INK4a expression compared with 4 (31%) of the recut diagnosis. Two of these were originally coded as atypical squamous metaplasia (ASM), one atypical favor low-grade dysplasia, one atypical not otherwise specified, and two atypical favor reactive Nicholas et al ., (2003). Of the atypical lesions with p16INK4a expression, most (4 of 6) had either a history of significant dysplasia, concurrent dysplasia, were high-risk HPV positive, and/or had dysplasia on follow-up (Nicholas et al., 2003). One case of atypical squamous metaplasia that was p16INK4apositive had a previous biopsy and Pap that were diagnosed as high-grade dysplasia but only showed atypical squamous metaplasia on the LEEP specimen. The other case of p16INK4a positive atypical squamous metaplasia lacked high-risk HPV, and the concurrent Pap was negative; unfortunately no follow-up was available for this case (Nicholas et al., 2003). In our study also, 2.5% (2/8) cases of immature squamous metaplasia cases showed 1+ P16ink4a expression. Though the HPV status of these cases is not known, even than it may indicate for persistent infection with high risk HPV and these cases should undergo regular follow-up with repeat Pap smear and colposcopic evaluation. The study by Alshenawy et al., (2015) revealed no expression of P16 INK4a in normal tissue (no expression) while 100% cases of invasive cervical cancer showed P16 INK4a expression. Our study also revealed the ascending pattern of p16 INK4a expression as the grade of CIN increases. Studies have shown that P16INK4a positive low grade lesion (CIN1) have a higher risk to progress to full blown cancer than negative lesions (Negri et al., 2004). According to Klaes et al., (2001) immunohistological expression of P16INK4a has been associated with dysplastic or neoplastic cells, but not seen in normal cervical epithelium and it is related to degree of histologic dysplasia. A study done by Tsoumpou et al., (2009) reported that over expression of p16INK4a increased with the degree of cytological or histological abnormality, and showed immunoreactivity in 38% of CIN1, 68% of CIN2 and 82% of CIN3cases, where as the present study reported p16INK4a immunoreactivity in 32 % (8/25) of CIN1, 52.3% (11/21) of CIN2 and 100% positive stain in CIN3 & invasive cervical cancer. Similarly Kory et al., (2016) studied 40 cases of cervical biopsy and found that p16 expression is directly related to higher grade lesion. The normal or non neoplastic cervical tissue were not expressed p16 immunostain but as the degree of lesion becomes higher, the intensity of p16 immunostain is also becoming stronger (Kory et al., 2016).
In conclusion, the result of the present study shows strong association of cervical neoplasia of different grades with staining pattern of p16INK4a. The staining intensity also increases with the increasing grade of the lesion which is strongly significant. The P16INK4a positive low grade lesion may suggest persistent infection by high grade human papilloma virus.
The limitation of the study is that only one biomarker is used. Combination of another marker like Ki67 would give a better understanding of proliferating potential of these lesions. The cases could not be followed up due to retrospective nature of the study. As 100% expression of p16INK4a in cervical squamous cell carcinomas and CIN3, studying the expression of status of p16INK4a in biopsy would support the histopathological features of CIN and also predict the clinical behavior of the lesion and their possibility of progression to higher grade lesion.
Conflict of Interest
Nil.
Acknowledgements
The authors have acknowledged the financial grant offered by Indian Council of Medical Research, New Delhi, India vide letter No. 5/7/1169/2014-RCH
References
- Agoff NP, Lin C, Janice Morihara, et al. p16INK4a expression correlates with degree of cervical neoplasia: A comparison with Ki-67 expression and detection of high-risk HPV types. Mod Pathol. 2003;16:665–73. doi: 10.1097/01.MP.0000077518.78046.0C. [DOI] [PubMed] [Google Scholar]
- Alshenawy HA. Evaluation of P16, human papillomavirus capsid protein L1 and Ki67 in cervical intraepithelial lesions: potential utility in diagnosis and prognosis. Cervical cancer: recent research and review studies |www.smgebooks. 2015:1–12. doi: 10.1016/j.prp.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Aslani FS, Safaei A, Pourjabali M, Momtahan M. Evaluation of Ki67, P16 and CK17 markers in differentiating cervical intraepithelial neoplasia and benign lesions. Iran J Med Sci. 2013;31:15–21. [PMC free article] [PubMed] [Google Scholar]
- Bruni L, Barrionuevo-Rosas L, Albero G, et al. Human papillomavirus and related diseases in India. Summary report. ICO information centre on HPV and cancer (HPV information Centre) 2016:3–20. [Google Scholar]
- Denny L. Cervical cancer: prevention and treatment. Discov Med. 2012;14:125–31. [PubMed] [Google Scholar]
- Doeberitz MVK. New molecular tools for efficient screening of cervical cancer. Dis Markers. 2001;17:123–8. doi: 10.1155/2001/249506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–7. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Ervik M, et al. Cancer incidence and mortality worldwide. Globocan. 2012;1:11. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in Globocan 2012. Int J Cancer. 2015;136:359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- Fleiss JL. Statistical methods for rates and proportions. 2nd ed. New York: John Wiley and Sons; 1981. [Google Scholar]
- International Agency for Research on Cancer (IARC) IARC Monograph on the evaluation of carcinogenics risks to human – Human Papillomavirus, World Health Organization. 2007 ISBN 978-92-832-1290-4. [Google Scholar]
- ICO. Information Centre on HPV and cancer (Summary Report 2014-08-22) Human Papillomavirus and Related Diseases in India. 2014;2014 [Google Scholar]
- Kanthiya K, Khunnarong J, Tangjitgamol S, Puripat N, Tanvanich S. Expression of p16 and Ki67 in cervical squamous intraepithelial lesions and cancer. Asian Pac J Cancer Prev. 2016;17:3201. [PubMed] [Google Scholar]
- Keating JT, Cviko A, Riethdorf S, et al. Ki-67, cyclin E, and p16INK4 are complimentary surrogate biomarkers for human papilloma virus-related cervical neoplasia. Am J Surg Pathol. 2001;25:884–91. doi: 10.1097/00000478-200107000-00006. [DOI] [PubMed] [Google Scholar]
- Khleif SN, DeGregori J, Yee CL, et al. Inhibition of cyclin D-CDK4/CDK6 activity is associated with an E2F-mediated induction of cyclin kinase inhibitor activity. Proc Natl Acad Sci. 1996;93:4350–4. doi: 10.1073/pnas.93.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaes R, Friedrich T, Spitkovsky D, et al. Overexpression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer. 2001;92:276–84. doi: 10.1002/ijc.1174. [DOI] [PubMed] [Google Scholar]
- Kory S, Shantala PR, Ramdas N, Chanabasappa Ch, Aijaz MN. Immunohistochemical study of p16 expression in cervical carcinoma and dysplasia in correlation with histopathology. Int J Recent Trends Sci Technol. 2016;18:493–7. [Google Scholar]
- Li Y, Nichols MA, Shay JW, Xiong Y. Transcriptional repression of the D-type cyclin-dependent kinase inhibitor p16 by the retinoblastoma susceptibility gene product pRb. Cancer Res. 1994;54:6078–82. [PubMed] [Google Scholar]
- Queriroz C, Silva TC, Venancio AF. P16 expression as a potential prognostic marker in cervical pre-neoplastic and neoplatic lesion. Pathol Res Pract. 2006;202:77–83. doi: 10.1016/j.prp.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Sharon L, Lee MJ, Cho I, Hong R, Lim SC. Efficacy of p16 and Ki-67 immunostaining in the detection of squamous intraepithelial lesions in a high –risk HPV group. Oncol Lett. 2016;11:1447–52. doi: 10.3892/ol.2015.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoumpou I, Arbyn M, Kyrgiou M, et al. p16(INK4a) immunostaining in cytological and histological specimens from the uterine cervix: a systematic review and meta-analysis. Cancer Treat Rev. 2009;35:210–20. doi: 10.1016/j.ctrv.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]


