Abstract
Background:
Tumor associated neutrophils (TAN) are related to aggressiveness and a poor prognosis with human cancers. However, the relevance of TAN in breast cancer has not been previously investigated and here we sought to determine their presence among different subtypes.
Methods:
We analyzed patients with stage I-III breast cancers between 2006 and 2012. Tumors were divided into three subtypes: hormone-receptor [HR]-positive, HER2-negative (HR+, HER2-ve); HER2-positive and triple negative (TN). Hematoxylin and eosin stained sections were examined and the number of TAN per 10 high power fields (HPF, 40x) was recorded. Tumors with >1 TAN per 10 HPF were considered TAN-positive. Fisher’s exact test was used to test for independence between qualitative variables, and logistic regression models were applied for multivariate analysis.
Results:
A total of 133 patients were assessed for inclusion and 105 were analyzed (28 excluded on various criteria). Some 72 tumors (69%) were classified as HR+, HER2-ve, 15 (14%) as HER2+ and 18 (17%) as TN. Totals of 16 TN (88%), 8 HER2+ (53%) and 4 HR+, HER2-ve tumors (5%) were TAN+ (p<0.001), including 79% of HR-ve tumors (19 of 24), in contrast to 11% of their HR+ve counterparts (9 of 81) (p<0.001). HER2 expression (p=0.023) and tumor grade (p<0.001) were also associated with TAN positivity. On multivariate analysis, only HR negativity (OR 16.85; 95% CI 4.4-64.6, p=<0.0001) was associated with a higher likelihood of TAN positivity.
Conclusions:
TAN are present in most TN tumors. We found an absence of HR expression to be the only predictor of TAN positivity. These results raise the question as to whether TAN, as part of the tumor microenvironment, have a role in the aggressiveness and progression of TN tumors and thus warrant further investigation in this breast cancer subtype, particularly in relation to response to treatment and prognosis.
Keywords: Neutrophils, neutrophils, breast neoplasms, triple negative breast neoplasms, tumor microenvironment
Introduction
There is increasing evidence pointing towards the fundamental role of the immune system and its components in the development and progression of malignant tumors. Every human cancer induces an immune response in its microenvironment, which is generally ineffective in destroying malignant cells (de la Cruz-Merino et al., 2013). However, recent data show that the interaction between tumor cells and leukocytes is partly responsible for the response to chemotherapy (Denkert, 2013). The presence of tumor-infiltrating lymphocytes, for example, has been recognized as an independent predictor of response to treatment and improved prognosis in patients with breast cancer (Denkert et al., 2010), particularly in triple negative (TN) and HER2-overexpressing tumors (Loi et al., 2014; Loi et al., 2013) However, research regarding the role of other major cells of the immune system in the tumor microenvironment is lacking (Fridlender and Albelda, 2012).
Although neutrophils are commonly encountered within the tumor microenvironment, little is known about their role in the pathogenesis of cancer, and they have been traditionally considered bystanders (Gregory and Houghton, 2011). Neutrophils represent the most common circulating leukocyte population, and when they traffic into tumors, they are referred to as tumor associated neutrophils, or TAN (Fridlender and Albelda, 2012). Under the influence of cytokines within the tumor microenvironment, these TAN undergo polarization, becoming either pro-tumorigenic (N2 phenotype) or pro-inflammatory and antitumorigenic (N1 phenotype) (Fridlender et al., 2009). The exact mechanisms which generate this polarization, as well as the particular characteristics of each TAN phenotype, have not been fully elucidated. The presence of TAN has been shown to be an unfavorable prognostic factor in localized gastric (Zhao et al., 2012), colorectal (Rao et al., 2012), hepatocellular (Li et al., 2011), cervical (Carus et al., 2013) and renal cell carcinoma (Jensen et al., 2009). TAN have also been found to correlate with tumor grade in gliomas (Fossati et al., 1999) and with more aggressive pancreatic tumors (Reid et al., 2011). A recent metaanalysis of 20 studies looking at the prognostic value of TAN across different cancers found that their presence was associated with worse recurrence-free and overall survival (Shen et al., 2014). It is important to keep in mind the polarized phenotypes of TAN when interpreting the existing literature, and to consider which of the two possible phenotypes predominates in each specific tumor (Fridlender and Albelda, 2012).
Despite all the evidence presented above, the relevance of TAN in breast cancer has not been systematically investigated. In this study, our primary objective was to determine whether TAN were present in the breast tumor microenvironment, and to compare their presence among different breast cancer molecular subtypes. We hypothesized that the presence of TAN would correlate with more aggressive histologic subtypes, particularly TN tumors, where a high circulating neutrophil count has been associated with poor prognosis (Pistelli et al., 2014).
Materials and Methods
We conducted an observational retrospective cohort medical record review study. Between January 2006 and December 2013, consecutive cases of treatment-naive patients with the diagnosis of localized breast cancer at the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ) were retrieved from our archives. Ethical approval for all observational research was obtained from INCMNSZ Ethics Committee. As the study had no direct patient involvement and minimum risk, patient consent was not required. Patient characteristics including age, menopausal status, stage at diagnosis, histologic subtype, histologic grade, hormone receptor (HR) status and HER2 status were recorded. The number of cases treated at INCMNSZ during the study period determined the sample size.
All pathology assessments were performed at the Pathology Department of INCMNSZ by dedicated breast pathologists. Each sample was analyzed using the same procedures and standardized assays. Estrogen receptor (ER) and progesterone receptor (PR) were determined by immunohistochemistry (IHC). Cases were classified as HR positive in case of ER and/or PR positivity and HR negative in case of both ER and PR negativity according to the Allred score. HER2 was determined initially by IHC and considered negative in cases of 0 (no membrane staining) or 1+ (weak and incomplete membrane staining) scoring. Tumors were considered HER2+ in cases of 3+ IHC staining or amplified FISH (ratio of HER2 to CEP17 of >2.2 or average HER2 gene copy number >six signals/nucleus) and HER2- in cases with 0, 1+ and 2+ IHC plus negative FISH amplification. Tumors were divided into three subtypes according to receptor status: those expressing positive HR but negative HER2 were considered HR+, HER2-ve; those expressing HER2, regardless of HR status, were considered HER2+ and those not expressing any receptors were considered TN.
Clinical and radiographic staging procedures were used for all patients. T stage was defined using ultrasound measurements. Clinical N stage was defined by either the presence of palpable axillary lymph nodes or of abnormal lymph nodes upon ultrasound examination. Metastatic disease was excluded using tomographic assessment of the thorax and abdomen when indicated. Patients with an incorrect pathological diagnosis, found to have metastatic disease upon review of the records and/or lacking material for pathologic review were excluded from analysis.
The primary outcome was the absolute amount of TAN, defined as neutrophils with direct cell-cell contact with carcinoma cells, within each tumor (Figure 1). Hematoxylin & eosin stained sections were examined by a dedicated breast pathologist blinded to tumor subtype, and the absolute number of TAN in 10 consecutive high power fields (HPF, 40x) was recorded in a quantitative manner. Neutrophils not in direct contact with tumor cells and those within blood vessels or in the stroma were not counted as TAN. Granulocytes in necrotic zones or admixed with tumoral necrosis were excluded. To evaluate whether TAN measurements could predict HER2+ or TN tumors and to identify an optimal cut-off value for TAN positivity, ROC curve analysis was performed. Fisher’s exact test was used to test for independence between qualitative variables, and logistic regression models were used for quantitative variables and multivariate analysis. All data are presented as medians, means or proportions. Statistical analysis was carried out using XLSTAT (Addinsoft, Inc, v9x). All P values presented are 2 sided, and P values <0.05 were considered statistically significant.
Figure 1.
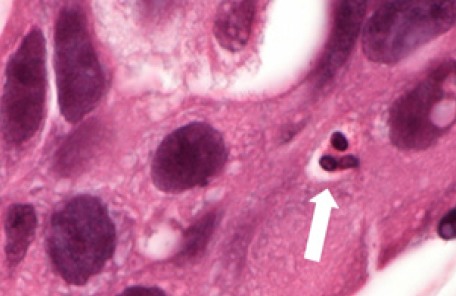
Triple Negative Infiltrating Ductal Carcinoma. Note the Presence of a Tumor Associated Neutrophil Adjacent to a Breast Cancer Cell (Hematoxylin and Eosin Stain, Magnification x 400) (arrow).
Results
A total of 133 patients with treatment-naïve localized breast cancer were identified. 28 were excluded because of missing material, incorrect staging or incorrect pathological diagnosis, and 105 were included for analysis. Median age was 54 years (range, 30 to 88 years) and 37% of patients were premenopausal at the time of diagnosis. 79% of patients had stage T1 and T2 tumors and 52% were node negative. 89% of the tumors were invasive ductal carcinoma, and 24% were considered to be grade 3. 77% of tumors were HR positive. According to subtype, 72 tumors (69%) were classified as HR+, HER2-ve, 15 (14%) as HER2+ and 18 (17%) as TN. The characteristics of the patients are summarized in Table 1.
Table 1.
TAN Positivity According to Baseline Patient Characteristics
| Variable | n | TAN - (n=77), n(%) | TAN + (n=28), n(%) | p |
|---|---|---|---|---|
| Age (median) | - | 56 (30-88) | 51 (32-80) | 0.12 |
| Menopausal status | ||||
| Premenopausal | 39 | 28 (36%) | 11 (39%) | |
| Posmenopausal | 66 | 49 (64%) | 17 (61%) | 0.822 |
| Clinical T stage | ||||
| 1 | 32 | 26 (34%) | 6 (21%) | |
| 2 | 51 | 37 (48%) | 14 (50%) | |
| 3 | 15 | 10 (13%) | 5 (18%) | |
| 4 | 7 | 4 (5%) | 3 (11%) | 0.478 |
| Clinical N stage | ||||
| 0 | 55 | 42 (54%) | 13 (46%) | |
| 1 | 42 | 32 (42%) | 10 (36%) | |
| 2 | 8 | 3 (4%) | 5 (18%) | 0.077 |
| Histology | ||||
| Lobular | 12 | 12 (15%) | 0 (0%) | |
| Grade 1 Ductal | 19 | 19 (25%) | 0 (0%) | |
| Grade 2 Ductal | 49 | 37 (48%) | 12 (43%) | |
| Grade 3 Ductal | 25 | 9 (12%) | 16 (57%) | <0.001 |
| Hormone Receptor status (HR) | ||||
| Negative | 24 | 5 (6%) | 19 (68%) | |
| Positive | 81 | 72 (94%) | 9 (32%) | <0.001 |
| HER2 status | ||||
| Negative | 90 | 70 (91%) | 20 (71%) | |
| Positive | 15 | 7 (9%) | 8 (29%) | 0.023 |
| Tumor Subtype | ||||
| HR+, HER2-ve | 72 | 68 (88%) | 4 (14%) | |
| HER2+ | 15 | 7 (9%) | 8 (29%) | |
| Triple negative | 18 | 2 (3%) | 16 (57%) | <0.001 |
The mean TAN count for the entire sample was 1.82 x 10 HPF (0-28). ROC analysis determined a cut-off value for positivity of >1 TAN x 10 HPF (AUC 0.85; 95% CI 0.76-0.93, p<0,001) for the detection of TN and HER2+ tumors, with a sensitivity of 73% (IC 95% 55-85) and a specificity of 94% (IC 95% 86-98). Tumors with >1 TAN x 10 HPF were considered TAN-positive, and those with ≤1 TAN x 10 HPF were considered TAN-negative. 27% of all tumors were TAN-positive (n=28).
Of the 18 tumors classified as TN, 16 (88%) were TAN-positive, with an average TAN count of 7 x 10 HPF (0-17). Of the 15 tumors classified as HER2+, 8 (53%) were TAN-positive, with an average TAN count of 4 x 10 HPF (0-28). Only 4 (5%) of the 72 tumors classified as HR+, HER2-ve were TAN-positive (p=<0.001). The difference between subtypes was statistically significant with a p value of <0.001 (Figure 2).
Figure 2.
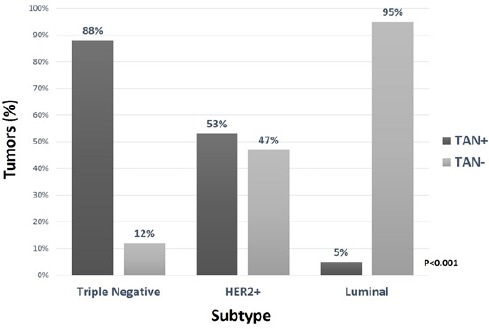
TAN Positivity According to Breast Cancer Subtype (HR+, HER2-ve subtype: HR+, HER2-ve; HER2+ subtype: HR+/-, HER2+; triple negative [TN]: HR-ve, HER2-ve).
79% of HR-ve tumors (19 of 24) were TAN-positive, in contrast with 11% of HR+ tumors (9 of 81) (p=<0.001). (Figure 3). On univariate analysis, other factors associated with TAN positivity were HER2 expression (p=0.023) and tumor grade (p=<0.001). Age, menopausal status and T and N stage were not significant for the presence of TAN (Table 1). On multivariate analysis, only HR negativity (OR 16.85; 95% CI 4.4-64.6, p<0.0001) was associated with a higher likelihood of TAN positivity (Table 2).
Figure 3.
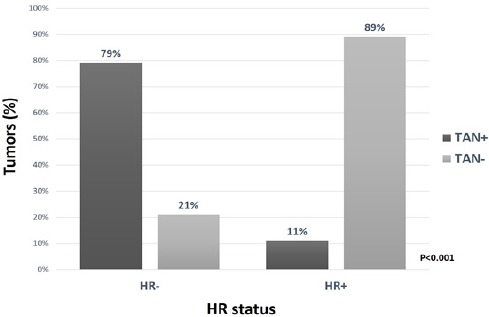
TAN Positivity in Relation with HR Status
Table 2.
Multivariate Analysis for TAN Positivity
| Variable | OR | 95% CI | p |
|---|---|---|---|
| Histology (Grade 3 ductal) | 2.72 | 0.74-9.9 | 0.13 |
| HR negative | 16.85 | 4.4-64.6 | <0.0001 |
| HER2 positive | 2.15 | 0.48-9.52 | 0.31 |
Discussion
The data presented in this paper represent, as far as we know, the first description of the presence of TAN in breast cancer. Our study suggests that there is a preferential chemotaxis of neutrophils into certain subtypes of breast tumors, and that TAN occur almost exclusively in HR-ve ductal adenocarcinoma. TAN were found more commonly in TN tumors, with 88% of them being TAN-positive. It is important to mention, however, that the two TN tumors which were found to be TAN-negative in this study were of non-ductal histology (one infiltrating lobular carcinoma and one medullary carcinoma). If we were to analyze exclusively those TN tumors with infiltrating ductal histology then TAN-positivity would reach 100%. On the other hand, TAN were seldom seen in HR+ tumors, and the largest absolute amount of TAN found this subtype was 2.
Regarding the group of HER2+ tumors included in our study, it is important to say that 9 out of 15 tumors (60%) were also HR+. Although there was no difference in the number of TAN-positive tumors in HER2+, HR+ cases compared to HER2+, HR-ve cases (55 vs 50%, p=1.0), this analysis is limited by the small sample size. However, it is important to stress that although HER2 positivity was associated with the presence of TAN on univariate analysis, this association was not relevant upon multivariate analysis, and only the expression of HR was found to correlate with TAN positivity.
The main question set forth by our results is why TAN show a predilection for a particular breast cancer subtype, in this case TN tumors. Previous research has shown that TN tumors are associated with a higher number of circulating neutrophils. Additionally, genomic analysis has shown that some TN breast cancer subtypes (mesenchymal and basal-like immune activated) show enrichment of pathways associated with leukocyte extravasation, adhesion and diapedesis (Burstein et al., 2014). This, alongside with our findings of a higher presence of TAN in this tumor subtype, suggests that tumor associated factors in TN breast cancer (including cytokines and colony-stimulating factors) may have direct or indirect effects on the production of neutrophils in the bone marrow and on their chemotaxis into tumors. One such cytokine may be transforming growth factor beta (TGF-β), which is highly expressed in TN breast cancer (Jovanović et al., 2014) and has been shown to promote the inflammatory process and to be a potent neutrophil chemotactic factor (Lagraoui and Gagnon, 1997). Moreover, there is evidence pointing towards the fact that TGF-β within the tumor microenvironment may induce a population of TAN with a protumor phenotype (the so called N2 TAN) (Fridlender et al., 2009). The presence of TAN within the TN breast cancer microenvironment could represent a source of proangiogenic factors, particularly certain proteases such as matrix metalloproteinases (MMP). One of those proteases is gelatinase B/MMP-9, which serves as a potent proangiogenic factor and a major matrix-degrading protease (Piccard et al., 2012). MMP-9 has been shown to have a role in switching on angiogenesis by counteracting antiangiogenic molecules and by promoting the release of vascular endothelial growth factor (VEGF) (Fridlender and Albelda, 2012).
Our study has some limitations. We have not been able to characterize the specific TAN polarization phenotype associated with these tumors. As such, it is still unclear whether the presence of TAN in TN breast cancer has a detrimental effect on tumor behavior or, on the contrary, represents an attempt to control the disease by anti-tumorigenic neutrophils, even if the known proinflammatory milieu of TN breast cancer and the aforementioned genomic landscape points toward an N2 phenotype. Additionally, the small sample size and short follow up make it impossible to draw conclusions regarding the influence of TAN on outcomes.
Our data shows that a relevant presence of TAN in breast cancer is limited almost exclusively to TN breast cancer. As was the case with the demonstration of tumor infiltrating lymphocytes, our results should represent a stepping stone for further investigation into the functional and molecular characterization of TAN associated with TN breast cancer, as well as their relationship with treatment response and survival.
Declaration of conflict of interest None.
References
- Burstein MD, Tsimelzon A, Poage GM, et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res. 2015;21:1688–98. doi: 10.1158/1078-0432.CCR-14-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carus A, Ladekarl M, Hager H, Nedergaard BS, Donskov F. Tumour-associated CD66b+neutrophil count is an independent prognostic factor for recurrence in localised cervical cancer. Br J Cancer. 2013;108:2116–22. doi: 10.1038/bjc.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cruz-Merino L, Barco-Sánchez A, Henao Carrasco F, et al. New insights into the role of the immune microenvironment in breast carcinoma. Clin Dev Immunol. 2013;2013:785317. doi: 10.1155/2013/785317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denkert C, Loibl S, Noske, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–13. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- Denkert C. Diagnostic and therapeutic implications of tumor-infiltrating lymphocytes in breast cancer. J Clin Oncol. 2013;31:836–7. doi: 10.1200/JCO.2012.47.1698. [DOI] [PubMed] [Google Scholar]
- Fossati G, Ricevuti G, Edwards SW, et al. Neutrophil infiltration into human gliomas. Acta Neuropathol. 1999;98:349–54. doi: 10.1007/s004010051093. [DOI] [PubMed] [Google Scholar]
- Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–94. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis. 2012;33:949–55. doi: 10.1093/carcin/bgs123. [DOI] [PubMed] [Google Scholar]
- Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res. 2011;71:2411–16. doi: 10.1158/0008-5472.CAN-10-2583. [DOI] [PubMed] [Google Scholar]
- Jensen HK, Donskov F, Marcussen N, et al. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J Clin Oncol. 2009;27:4709–17. doi: 10.1200/JCO.2008.18.9498. [DOI] [PubMed] [Google Scholar]
- Jovanović B, Beeler JS, Pickup MW. Transforming growth factor beta receptor type III is a tumor promoter in mesenchymal-stem like triple negative breast cancer. Breast Cancer Res. 2014;16:R69. doi: 10.1186/bcr3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagraoui M, Gagnon L. Enhancement of human neutrophil survival and activation by TGF-beta 1. Cell Mol Biol (Noisy-le-grand) 1997;43:313–8. [PubMed] [Google Scholar]
- Li YW, Qiu SJ, Fan J, et al. Intratumoral neutrophils: a poor prognostic factor for hepatocellular carcinoma following resection. J Hepatol. 2011;54:497–505. doi: 10.1016/j.jhep.2010.07.044. [DOI] [PubMed] [Google Scholar]
- Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31:860–7. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- Loi S, Michiels S, Salgado R, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25:1544–50. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- Piccard H, Muschel RJ, Opdenakker G. On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Crit Rev Oncol Hematol. 2012;82:296–309. doi: 10.1016/j.critrevonc.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Pistelli M, De Lisa M, Ballatore Z, et al. Pretreatment neutrophil to lymphocyte ratio may be an useful tool in predicting survival in early triple-negative breast cancer patients. J Clin Oncol. 2014;32:5s. doi: 10.1186/s12885-015-1204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao HL, Chen JW, Li M, et al. Increased intratumoral neutrophil in colorectal carcinomas correlates closely with malignant phenotype and predicts patients'adverse prognosis. PLoS One. 2012;7:e30806. doi: 10.1371/journal.pone.0030806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MD, Basturk O, Thirabanjasak D, et al. Tumor-infiltrating neutrophils in pancreatic neoplasia. Mod Pathol. 2011;24:1612–9. doi: 10.1038/modpathol.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, Hu P, Donskov F, et al. Tumor-associated neutrophils as a new prognostic factor in cancer: a systematic review and meta-analysis. PLoS One. 2014;9:e98259. doi: 10.1371/journal.pone.0098259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Pan K, Wang W, et al. The prognostic value of tumor-infiltrating neutrophils in gastric adenocarcinoma after resection. PLoS One. 2012;7:e33655. doi: 10.1371/journal.pone.0033655. [DOI] [PMC free article] [PubMed] [Google Scholar]


