Abstract
Annona squamosa has extensively been used in the traditional and folkloric medicine and found to possess many biological activities. Different solvents, petroleum ether, chloroform, ethyl acetate and methanol extracts of Annona squamosa seeds (ASPE, ASCH, ASEA, ASME) have been used to prepare plant extracts. The present investigations dealt with the free radical scavenging activity of four extracts using various techniques such as total reducing power estimation, total phenolic count, 1,1-diphenyl-2-picryl hydrazyl (DPPH) radical scavenging effect, evaluation of ABTS cation decolorisation capacity, FRAP assay, hdroxyl radical scavenging assay, super oxide assay and Nitric oxide radical scavenging assay of the extracts. The results showed that the four extracts of Annona squamosa showed significant reducing power in four extracts. The total phenolic contents in petroleum ether, chloroform, ethyl acetate, methanol extracts and positive control were 0.64±0.17, 0.54±0.27, 0.49±0.24, 0.57±0.22 and 0.66±0.33. The antioxidant capacity by ABTS assay of ASPE, ASCH, ASEA, ASME and positive control, trolox showed 77.75±0.5,73.25±1.7,78.5± 1.2, 80 ± 0.8 μg/ml and 94.2 ± 0.9 respectively. The (50 % scavenging activity) SA50 of ASPE and ASCH, ASEA and ASME was found to be 34.4 µg/ml, 43.8 µg/ml 34.7 µg/m and 28.8 µg/ml respectively by DPPH assay. The percentage of hydroxyl radical scavenging increased with the increasing concentration of the extracts. ASPE, ASCH, ASEA and ASME showed superoxide radical scavenging activity, as indicated by their values 66 ± 0.5, 68 ± 1, 63 ± 1 and 70 ± 0.5 μg/ml respectively compared to gallic acid which was 97 ± 0.5 μg/ml. The values for scavenging of nitric oxide for ASPE, ASCH, ASEA and ASME were 91.0 ± 1.0, 66.75 ± 0.5, 71.75 ± 1.1 and 75.75 ± 1.15 μg/ml while value for standard ascorbic acid was 91.0 ± 1.0 μg/ml. The results revealed strong antioxidants in four extracts may lead to the development of potent antioxidant agents from Annona squamosa seeds.
Keywords: Annona squamosa-antioxidant activity, free radical scavenging activity
Introduction
Annona squamosa (Annonaceae) is a small well-branched tree or shrub that bears edible fruits called custard apple. Different parts of Annona squamosa were used in folkloric medicine for the treatment of various diseases. Antioxidants in food play an important role as a health protecting factor. Antioxidant agents are closely associated to the prevention of degenerative diseases, such as cardiovascular and neurological illnesses, oxidative stress malfunctions and cancer (Diplock et al., 1995). Antioxidants when present in low concentrations relative to the oxidizable substrate significantly delays or reduces oxidation of the substrate (Halliwell et al., 1995). Over the past several years, nutritional antioxidants have attracted considerable interest in the popular press as potential treatment for a wide variety of disease states, including cancer and other causes e.g. atherosclerosis, chronic inflammatory diseases and aging (Delany et al., 1993). Fruits and vegetables that have been identified as sources of powerful antioxidants help people to counter the risk of heart ailments and different types of cancers.
Cancer chemoprevention by using antioxidant approaches has been suggested to offer a good potential in providing important fundamental benefits to public health, and is now considered by many clinicians and researchers as a key strategy for inhibiting, delaying, or even reversal of the process of carcinogenesis (Shureiqi et al., 2000; Tsao et al., 2004). Free radicals are shown to be deleterious to the body causing cellular damage, aging and certain diseases like cancer, atherosclerosis, inflammatory diseases, liver disorders etc (Halliwell et al., 1992; Kamat et al., 2000).
Antioxidant compounds in food play an important role as a health protecting factor. Antioxidant compounds like phenolic acids, polyphenols and flavonoids scavenge free radicals such as peroxide, hydroperoxide or lipid peroxyl and thus inhibit the oxidative mechanisms that lead to degenerative diseases (Miller et. al., 2000). Antioxidants with free-radical scavenging activities could have great importance as prophylactic and therapeutic agents in diseases in which oxidants or free radicals are implicated (Marnett et. al., 2000).
Superoxide radical is known to be a very harmful species to cellular components as a precursor of more reactive species (Halliwell et al., 2007; Nanjo et al., 1996). The most important free radicals in the body are the radical derivatives of oxygen better known as reactive oxygen species (ROS) (Cheeseman et al., 1993). A nitrogen-centered radical also exists; for example the Phenyl diazine radical. It has been found that those reactive species also play a cardinal role in oxidative damage to cellular compartment which leads to cell injury and death. This has been associated with pathogenesis of various chronic diseases, e.g., carcinogenesis, coronary heart disease and many other health problems related to advancing age (Cadenas et al., 2000; Marnett et al., 2000, Stohs et al., 1995). Peroxisomal oxidation of fatty oxide is an important source of hydrogen peroxide production (Stohs et al., 1995). All the aerobic cells produce free radicals through the action of various membrane bound and soluble enzymes. The capacity of specific path ways to produce free radicals varies with the cell type (Kehrer et al., 1993). Annona squamosa Linn has been used as an antioxidant, antidiabetic, hepatoprotective, cytotoxic, genetoxic, antitumor and anti-lice agent. It contain alkaloids, carbohydrates, fixed oils, tannins and phenolic (Pandey et. al., 2011). The present study put forward the antioxidant activity of various organic extracts of Annona squamosa seeds.
Materials and Methods
Preparation of extracts
The seeds of Annonasquamosa were collected from Thiruvananthapuram district, Kerala state, authenticated by the taxonomist and a voucher specimen TBGT 57051 has been kept in the herbarium of Jawaharlal Nehru Tropical Botanical Garden and Research Institute, Palode, Thiruvananthapuram. The organic extract of the seed has been prepared as follows. For separation of secondary metabolites from the plant, the solvents were used. The shade dried and powdered seed materials (50g) were extracted at room temperature using a soxhlet apparatus with Petroleum ether (BP 60- 80°C), Chloroform (BP 61-62°C), Ethyl acetate (BP 77.1°C) and Methanol (BP 64.5-65.5°C). According to soxhlet procedure, the dried sample was ground into small particles and placed in a porous cellulose thimble. The thimble was placed in an extraction chamber, which was suspended above a flask containing the solvent and below a condenser. The flask was heated and the solvent evaporated and moved up in to the condenser, where it was converted into a liquid that trickles into the extraction chamber containing the sample. The extraction chamber was designed so that when the solvent surrounding the sample exceeds a certain level, it over flows and trickles back down into the boiling flask. At the end of the extraction process, which lasted a few hours, the flask containing the solvent and compound was removed. The petroleum ether (ASPE), chloroform (ASCH), Ethyl acetate (ASEA) and Methanol extracts (ASME) of Annona squamosa were employed for the study.
Total Reducing Power
The reducing power of the extract was determined according to the method of Naik et al., (2003). Different concentrations of the extracts in 1 ml of distilled water were mixed with phosphate buffer and potassium ferricyanide. The mixture was incubated at 50 °C for 20 min. A portion (2.5 ml) of trichloroacetic acid (10%) was added to the mixture, which was then centrifuged at 3,000 rpm for 10 min. The supernatant solution (2.5 ml) was mixed with distilled water (2.5 ml) and ferric chloride (0.5 ml of 0.1%) and the absorbance was measured at 700 nm. Increased absorbance of the reaction mixture indicated increased reducing power. Ascorbic acid was used as a reference standard; phosphate buffer (PH 6.6) was used as blank solution (Naik et. al., 2003). The absorbance of the final reaction mixture of two parallel experiments were taken was expressed as mean ± standard deviation.
Total Phenolic Content (TPC)
The Total Phenolic Content was determined using Folin- Ciocalteu method. Appropriately diluted extracts and standard gallic acid were made up to 3.5 ml using distilled water in a series of test tubes. Various solvent extracts and the standard were treated with 0.5 ml 2 Normal (N) Folin- Ciocalteu’s reagent and incubated for 3 minutes in room temperature. The reaction was then neutralized with the addition of 1ml of 20% Na2CO3. The reaction mixture was incubated at room temperature for 90 minutes and the absorbance of the blue colour developed was read at 760nm using a Spectrophotometer (Shimadzu UV Vis - Spectrophotometer, model 2450). The Total Phenolic Content was expressed as gallic acids equivalent in Microgram per ml of sample, using a standard curve generated with gallic acid.
Determination of antioxidant activity by DPPH method
The radical scavenging activities of the different extracts were determined by the method described by Kings and Berger (2001). To 1ml of 0.1mM solution of DPPH in ethanol, added 3 ml of extracts (25-800 µg/ml) in distilled water and kept for 30 minutes. The color change of the reaction mixture was then read at 517 nm. The percent DPPH decolourization of the sample was calculated as scavenged = (Absorbance of control-Absorbance of test)/absorbance of control x100.
ABTS Cation Decolorisation Capacity
The experiments were carried out using an improved ABTS decolorisation assayer et. al. 1999). The ABTS radical cation (ABTS+) was produced by reacting 7 mM stock solution ABTS with 2.45 mM potassium persulphate (final concentration) and allowed the mixture to stand in the dark for at least 6 hours at room temperature before use. The ABTS+ solution was diluted to an absorbance 0.7 ± 0.05 at 736nm. Absorbance was measured 7 minutes after the initial mixing of different concentrations of the extracts. The ABTS+ decolorisation capacity of the extracts were compared with the standard Trolox. A standard curve was prepared by measuring the reduction in the absorbance of ABTS+ solution at different concentration of Trolox over a period of 7 minutes.
FRAP (Ferric reducing/antioxidant power) assay
This procedure was done according to Benzie et al., (1996) with slight modification. The working FRAP reagent was produced by mixing 300mM acetate buffer (pH 3.6), 10 mM 2, 4, 6-tripyridyl-striazine (TPTZ) solution and 20 mM FeCl3.6H2O in a 10:1:1 ratio prior to use and heated to 37°C in water bath. A total of 3.0 ml FRAP reagent was added to a cuvette and blank reading was then taken at 593 nm using spectrophotometer. A total of 100 μl selected solvent extracts and 300 μl distilled water was added to the cuvette. After addition of the sample to the FRAP reagent, a second reading at 593 nm was performed after 4 min. The change in absorbance after 4 min from initial blank reading was compared with standard curve. The FRAP values for the samples were taken determined using this standard curve. The final result was expressed as the concentration of antioxidant having a ferric reducing ability.
Hydroxyl radical scavenging-
This was assayed as described by Elizabeth and Rao (1990) with a slight modification. The assay is based on quantification of the degradation product of 2-deoxyribose by condensation with TBA. Hydroxyl radical was generated by the Fe3+-ascorbate-EDTA-H2O2 system (the Fenton reaction). The reaction mixture contained, in a final volume of 1 ml, 2-deoxy-2-ribose (2.8 mM); KH2PO4-KOH buffer (20 mM, pH 7); FeCl3 (100 μM); EDTA (100 μM); H2O2 (1.0 mM); ascorbic acid (100 μM) and various concentrations (25–800 μg/ml) of the test sample or reference compound was added. After incubation for 1 h at 37°C, 0.5 ml of the reaction mixture was added to 1 ml 2.8% TCA, then 1 ml 1% aqueous TBA was added and the mixture was incubated at 90°C for 15 min to develop the color. After cooling, the absorbance was measured at 532 nm against an appropriate blank solution. All tests were performed for four times.
Superoxide radical scavenging activity
Superoxide anion scavenging activity was measured according to the modified method of Robak and GryglewskiPeter et. al., 2004). All the reagents were prepared in 100 mM phosphate buffer (pH 7.4). 1 ml of NBT (nitro bue tetrazolium) (156 μM), 1 ml of NADH (468μM) and different concentrations of extracts were mixed. The reaction was started by adding 100 μl of phenazine methosulphate (60 μM) and the mixture was then incubated at 25ºC for 5 min followed by measurement of absorbance at 560 nm. Gallic acid was used as a reference standard. The percentage inhibition was calculated using the following formula.
% Inhibition = [(A control - A test) / A control] X 100.
Nitric oxide radical scavenging activity
Sodium nitroprusside (5 mM) in phosphate– buffered saline (PBS) was mixed with 3.0 ml of different concentrations (25-800 μg /ml) of the extracts dissolved in the suitable solvent systems and incubated at 25°C for 150 min. The samples from the above were reacted with Griess reagent (1% sulphanilamide, 2% H3PO4 and 0.1% napthylethylenediamine dihydrochloride). The absorbance of the chromophore formed during the diazotization of nitrite with sulphanilamide and subsequent coupling with napthylethylenediamine was read at 546 nm and referred to the absorbance at standard solutions of potassium nitrite, treated in the same way with Greiss reagent.
Statistical analysis
The averages of the results were calculated along with standard deviation of four experiments by using Excel and Graph pad.
Results
The Annona squamosa seed solvent extracts were evaluated for their antioxidant activities.
Total Reducing Power
The Petroleum ether extract (ASPE) of Annona squamosa showed a higher reducing power than other extracts. Chloroform, ethyl acetate and methanol also showed significant reducing power compared to the standard gallic acid (Figure 1).
Figure 1.
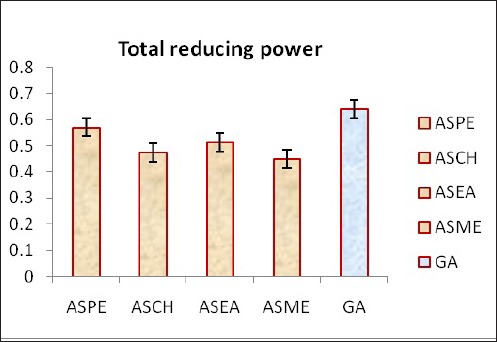
Total Reducing Power Estimation of Extracts of Annona squamosa Seeds
Total Phenolic Content
The total phenolic content was 0.64 ± 0.4 for the petroleum ether extract of Annona squamosa and for chloroform, it was 0.54 ± 0.27 g/g, ethyl acetate 0.49 ± 0.24, methanol 0.57 ± 0.22 gallic acid 0.66 ± 0.33. The total phenolic content of all other extracts were less and thus the activity was also less in comparison with the standard, gallic acid (Table 1).
Table 1.
Total Phenolic Content Estimation of Extracts of Annona squamosa
| Name of Standard/Extract | 25µg/ml |
|---|---|
| Gallic acid | 0.66±0.33 |
| ASPE | 0.64±0.17 |
| ASCH | 0.54±0.27 |
| ASEA | 0.49±0.24 |
| ASME | 0.57±0.22 |
Determination of antioxidant activity of the extracts
1,1-dipehenyl-2-picryl hydrazyl (DPPH) radical scavenging effect of the extracts was determined spectrophotometrically at 517nm. The in vitro antioxidant activities of ASPE, ASCH, ASEA and ASME at 800µg/ml were found to be 67.5±0.95, 70.5±1.73, 70.5±1.291, 80.5±0.5 respectively at concentration of 800µg/ml with reference to standard (Gallic acid) 97.5±0.5. (Figure 2). The (50 % scavenging activity) SA50 of ASPE and ASCH, ASEA and ASME was found to be 34.4 µg/ml (Figure 3), 43.8 µg/ml (Figure 4), 34.7 µg/m (Figure 5) and 28.8 µg/ml (Figure 6) respectively.
Figure 2.
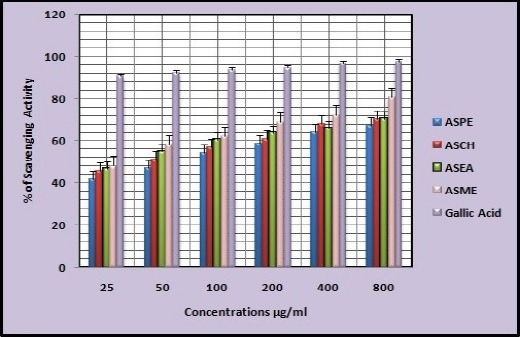
Free Radical Scavenging Activity of Annona Squamosa Seed Extracts by DPPH Assay.
Figure 3.
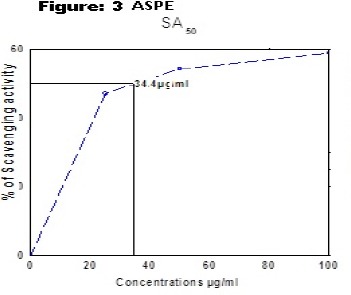
DPPH Free Radical Scavenging Activity (SA50) of ASPE
Figure 4.
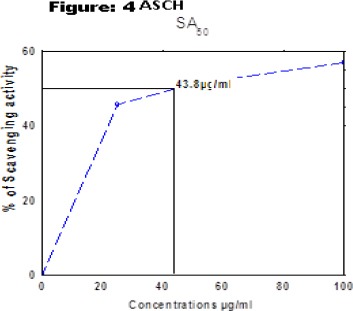
DPPH Free Radical Scavenging Activity (SA50) of ASCH
Figure 5.
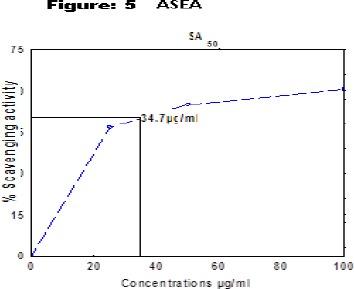
DPPH Free Radical Scavenging Activity (SA50) of ASEA
Figure 6.
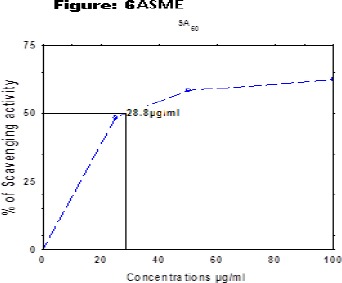
DPPH Free Radical Scavenging Activity (SA50) of ASME
ABTS cation decolorisation capacity
The concentrations for 25 to 800 μg/ml decolourisation were observed. Annona squamosa seed extracts ASPE, ASCH, ASEA, ASME and positive control, trolox showed 77.75 ± 0.5,73.25 ± 1.7,78.5 ± 1.2, 80 ± 0.8 μg/ml and 94.2 ± 0.9 (Figure 7 and 8). All values are mean+ standard deviation, n=4.
Figure 7.
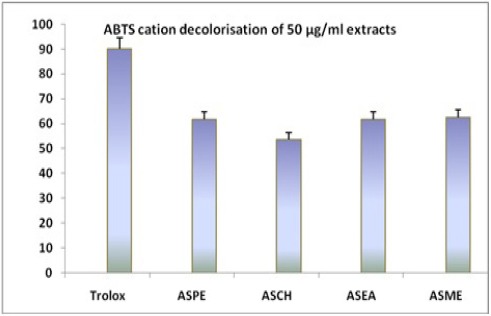
ABTS Decolorisation Capacity of Annona squamosa Seed Extracts at 50µg/ml
Figure 8.
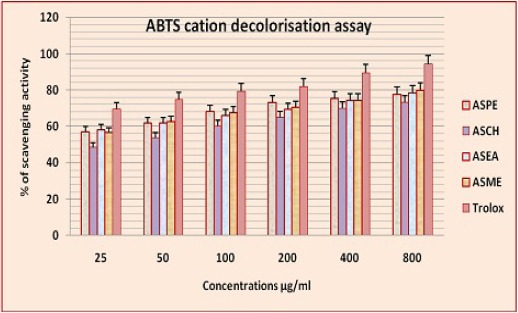
ABTS Decolorisation Capacity of Annona Squamosa Seed Extracts at Different Concentrations
FRAP Assay
The results of Annona squamosa seed extracts ASPE, ASCH, ASEA and ASME and positive control trolox showed 75. ± 0.6 µg/ml, 74 ± 1.3µg/ml,70.5 ± 1.2µg/ml, 67 ± 0.9 μg/ml), 63 ± 0.9µg/ml respectively. All values are mean+ standard deviation, n=4 (Figure 9).
Figure 9.
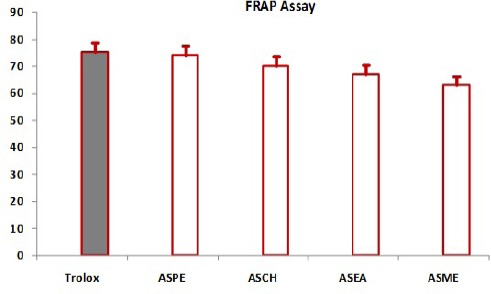
FRAP Assay of Annona squamosa Seed Extracts at 50µg/ml
Hydroxyl radical scavenging
The percentage of hydroxyl radical scavenging increased with the increasing concentration of the extracts. The percentage of H2O2 scavenging activity of 800 µg/ml ASPE, ASCH ASEA and ASME were found to be 73.25 ± 3.4, 62.5 ± 0.5, 65.5 ± 1.15 and 71 ± 0.5 respectively and ascorbic acid 91 ± 1 (Figure 10).
Figure 10.
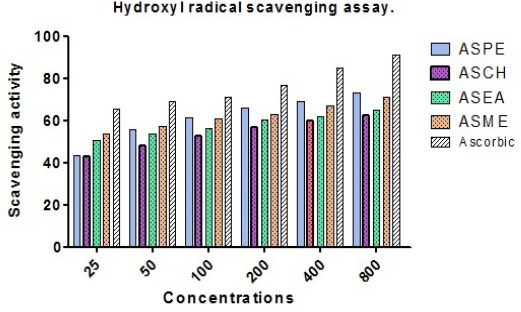
Hydroxyl Radical Scavenging Assay of Annonasquamosa Seed Extracts ASPE, ASCH, ASEA and ASME at Different Concentrations.
Superoxide radical scavenging activity
The superoxide radical scavenging activity of Annona squamosa seeds extracts were studied and compared with gallic acid. Petroleum ether, Chloroform, Ethyl acetate and Methanol, extracts showed superoxide radical scavenging activity, as indicated by their values 66 ± 0.5, 68 ± 1, 63 ± 1 and 70 ± 0.5 μg/ml respectively compared to gallic acid which showed 97+0.5 μg/ml (Figure 11).
Figure 11.
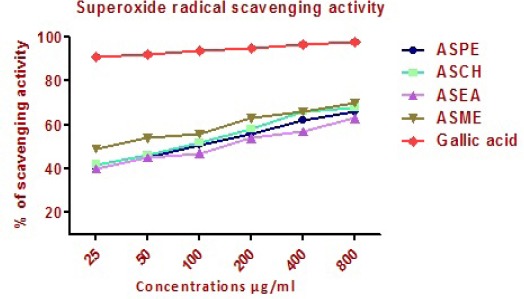
Super Oxide Radical Scavenging Activity of Annonasquamosa Seed Extracts ASPE, ASCH, ASEA and ASME at Different Concentrations
Nitric oxide scavenging activity
ASPE, ASCH, ASEA and ASME showed moderately good nitric oxide scavenging activity between 25 and 800μg/ml (Figure 12). The values for scavenging of nitric oxide for ASPE, ASCH, ASEA and ASME were 91.0 ± 1.0, 66.75 ± 0.5, 71.75 ± 1.1 and 75.75 ± 1.15 μg/ml while value for standard ascorbic acid was 91.0 ± 1.0 μg/ml.
Figure 12.
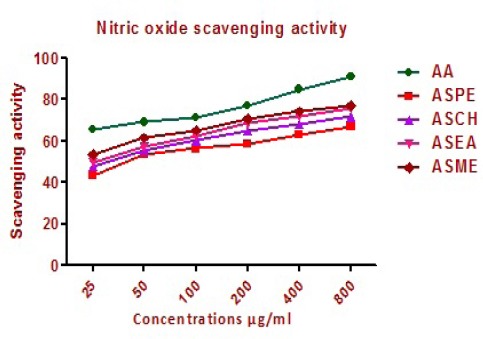
Nitric Oxide Scavenging Activity of Annonasquamosa Seed Extracts ASPE, ASCH, ASEA and ASME at Different Concentrations
Discussion
Antioxidant activity of Annona squamosa seed extracts have been evaluated taking total reducing power, total phenolic content, DPPH, ABTS scavenging activity, FRAP assay, hydroxyl radical scavenging and superoxide radical scavenging activity, nitric oxide scavenging activity as parameters. Results showed that all the extracts have significant antioxidant activity.
Our earlier observation on the preliminary phytochemical studies indicated the presence of flavanoids, coumarines, alkaloids, and terpenoids in Annona squamosa seeds (Biba et al., 2013). Many such compounds are known to possess potent antitumor properties (Kintzios et al., 2006). Terpenoids, falvanoids, alkaloids and tannins are considered to possess high antioxidant activities, which prevent or can be used in the treatment of many diseases, including cancer (Madhuri et al., 2009). Therefore the presence of appreciable to moderate amounts of these phytochemicals can be correlated with the possible significant medicinal potential of the plant. Several plant species rich in flavanoids are reported to reduce disease risk and have therapeutic properties. This observation is of particular importance since flavanoids are ingredients of many vegetables and fruits. Their consumption can reduce the cancer risk (Ferguson et al., 2000).
Reducing power assay shows the reductive capabilities of the solvent extracts compared to gallic acid. The reducing power of ASPE, ASCH, ASEA and ASME was very potent and the power of the extract was increased with quality of sample. The plant extract could reduce the most Fe3+ ions, which had a lesser reductive activity than the standard of gallic acid. Increased absorbance of the reaction indicated increased reducing power. Reducing power is to measure the reductive ability of antioxidant, and it is evaluated by the transformation of Fe (III) to Fe (II) in the presence of the sample extract (Gulcin et al., 2003). In this study the data showed that all the samples increased their reducing ability when the concentrations of extracts were increased. The ability of reducing power of extracts of A. squamosa showed significance compared with standard antioxidant trolox to reduce Fe (III) to Fe (II). The total phenolic content of all other extracts were determined and compared with standard gallic acid. We have determined the levels of total phenols in the crude ASPE, ASCH, ASEA, ASME and standard Gallic acid and have significant levels. The total polyphenol and radical scavenging activity have been determined in some fruits of the genus Annona, namely A. Muricata (Hassimotto et al., 2005) A. cherimolia (Vasco et al., 2008). The phenolic compounds influence the fruit quality contributing both to their sensory and health-promoting properties (Scalzo et al., 2005). Several authors demonstrated a strong positive correlation between phenolic content and the antioxidant capacity of fruits (Abidille et al., 2005).
The DPPH radical scavenging activity test showed remarkable radical scavenging activities of the extracts in a dose dependent manner with the percentage of inhibition compared to the positive control gallic acid. Many antioxidant based drug formulations are used for the prevention and treatment of complex diseases like atherosclerosis, stroke, diabetes, Alzheimer’s disease and cancer (Mosquera et al., 2007). Extracts of Annona squamosa had significant scavenging effect showed on ABTS assay free radical which increased with increasing concentration from 25-800 μg/ml. The experiments were carried out using an improved ABTS decolorisation assay (Re et al., 1999) and it involves the generation of ABTS+ chromophore by the oxidization of ABTS with potassium persulphate. The antioxidant capacity of Annona squamosa, determined by the FRAP assays was expressed as equivalents of the standard antioxidant trolox, which is a hydro soluble analog of vitamin E. Both the ferric-reducing power capacity was higher for the Annona squamosa extracts. Hydroxyl radicals are the major active oxygen species that cause lipid oxidation and enormous biological damage (Aurand et al., 1977). Extracts of Annona squamosa seeds are found to posses scavenging effect on hydroxyl radical in a concentration dependent manner. H2O2 itself is not very reactive, but it can sometimes be toxic to the cell because it may give rise to hydroxyl radical in the cells. Thus, removal of H2O2 is very important. The highly reactive hydroxyl radicals can cause oxidative damage to DNA, lipids and proteins (Spencer et al., 1984). In superoxide radical scavenging activity among the four extracts, methanol extract was found to be an efficient scavenger of superoxide radical generated in riboflavin-NBT-light system in vitro.
In the present study, Chloroform, Ethyl acetate, Methanol has also showed significant Nitric oxide scavenging activity. Nitric oxide was generated from nitroprusside and measured by the Greiss reaction. Sodium nitroprusside in aqueous solution at physiological pH spontaneously generates nitric oxide (Green et al., 1982) which interacts with oxygen to produce nitric oxide which, interacts with oxygen to produce nitric ions that can be estimated by use of Griess reagent. Ethanolic extract of the bark of Annona squamosa showed significant antioxidant activity using in vitro antioxidant models like DPPH radical scavenging activity, hydroxyl radical scavenging activity and superoxide radical scavenging activity (Pandey et al 2011).
The free radical scavenging potential of the leaves of Annona squamosa L. was studied by using different antioxidant models of screening (Shirwaikar et al., 2004; Bhaskar 2007). The results obtained from the antioxidant activity of this plant seeds revealed that the extracts posses strong antioxidants and further investigations may lead to the development of potent antioxidant agents from Annona squamosa seeds.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
The authors gratefully acknowledge the Indian Council of Medical Research, New Delhi, India and Kerala State Council for Science, Technology& Environment, Govt. of Kerala, India for the financial support.
References
- Abidille MDH, et al. Antioxidant activity of the extracts from Dillenia indica fruits. Food Chem. 2005;90:891–6. [Google Scholar]
- Aurand LW, Boone NH. Giddings GG Superoxide and singlet oxygen in milk lipid peroxidation. J Dairy Sci. 1977;60:363–9. [Google Scholar]
- Baskar R, Rajeswari V, Kumar TS. In vitro antioxidant studies in leaves of Annona species. Indian J Exp Biol. 2007;45:480–5. [PubMed] [Google Scholar]
- Benzie IFF, Strain JJ. Ferric reducing ability of plasma (FRAP) aa measure of antioxidant power: The FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Biba VS, Lakshmi S, Dhanya GS, Remani P. Phytochemical analysis of Annona squamosa seed extracts. Int Res J Pharm App Sci. 2013;3:29–31. [Google Scholar]
- Cadenas E, Davies KJA. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29:222–30. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- Cheeseman CL, Mallinson PJ, Ryan J, Wilesmith JW. Recolonisation by badgers in Gloucestershire. In: Hayden T J F, editor. Royal Irish Academy. Dublin: The badger; 1993. pp. 787–93. [Google Scholar]
- Delany MF, Moore CT, Rogulske DRP. Survival and longevity of adult male Florida Grasshopper Sparrows. proceedings of the annual conference of the south eastern association of fish and wildlife agencies. 1993;47:366–9. [Google Scholar]
- Diplock A. Antioxidant nutrients- efficacy in disease prevention and safety. Biochemist. 1995;17:16–8. [Google Scholar]
- Elizabeth K, Rao MWA. Oxygen radical scavenging activity of Curcumin. Int J Pharmaceu. 1990;58:237–40. [Google Scholar]
- Ferguson LR, Pearson AE, Esteves Souza A, et al. Cytotoxic activities against ehrlich carcinoma and human K-562 leukaemia of alkaloids and flavanoids from two Solanum species. J Braz Chem Soc. 2000;13:838–42. [Google Scholar]
- Green LC, Wagner DA, Glogowski J, et al. Analysis of nitrate, nitrite and 15N in biological fluids. Anal Biochem. 1982;126:131–6. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Gulcin I, Buyukokuroglu ME, Oktay M, Kufrevioglu IO. Antioxidant and analgesic activities of turpentine of Pinus nigra Arn. Subsp. Pallsiana (Lamb.) Holmboe. J Ethnopharmacol. 2003;86:51–8. doi: 10.1016/s0378-8741(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Aeschblach R, Loliger J, Aruoma OI. The characterization of antioxidants. Food Chem Toxicol. 1995;33:610–7. doi: 10.1016/0278-6915(95)00024-v. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 4th ed. Oxford; 2007. pp. 23–4. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC, Cross CE. Free-radicals, antioxidants and human diseases: Where are we now? J Lab Clin Med. 1992;119:598–620. [PubMed] [Google Scholar]
- Hassimotto NMA, Genovese MI, Lajolo FM. Antioxidant activity of dietary fruits, vegetables, and commercial frozen fruit pulps. J Agric Food Chem. 2005;53:28–35. doi: 10.1021/jf047894h. [DOI] [PubMed] [Google Scholar]
- Kamat JP, Boloor KK, Devasagayam TP. Chlorophyllin as an effective antioxidant against membrane damage in vitro and ex vivo. Biochem Biophys Acta. 2000;1487:113–27. doi: 10.1016/s1388-1981(00)00088-3. [DOI] [PubMed] [Google Scholar]
- Kehrer JP. Free radicals as mediators of tissue injury and disease. Crit. Rev. Toxicol. 1993;23:21–48. doi: 10.3109/10408449309104073. [DOI] [PubMed] [Google Scholar]
- Kintzios E. Terrestrial plant-derived anticancer agents and plant species used in anticancer research. CRC Crit Rev Plant Sci. 2006;25:79–113. [Google Scholar]
- Krings U, Berger RG. Antioxidant activity of some roasted foods. Food Chem. 2001;72:223–9. [Google Scholar]
- Madhuri S, Pandey G. Some anticancer medicinal plants of foreign origin. Curr Sci. 2009;96:779–83. [Google Scholar]
- Marnett LJ. Oxiradicals and DNA damage. Carcinogenisis. 2000;21:361–70. doi: 10.1093/carcin/21.3.361. [DOI] [PubMed] [Google Scholar]
- Miller WJ, Nagel A, Bachmann J, Bachmann L. Evolutionary dynamics of the SGM transposon family in the Drosophila obscura species group. Mol Biol Evol. 2000;17:1597–609. doi: 10.1093/oxfordjournals.molbev.a026259. [DOI] [PubMed] [Google Scholar]
- Mosquera OM, Correa YM, Buitrago DC, Nio J. Antioxidant activity of twenty five plants from Colombian biodiversity. Mem Inst Oswaldo Cruz. 2007;102:631–4. doi: 10.1590/s0074-02762007005000066. [DOI] [PubMed] [Google Scholar]
- Naik GH, Priyadarshini KI, Satav JG, Banavalikar MM, Sohani DP. Comparative antioxidant activity of individual herbal components used in Ayurvedic medicine. Phyto Chem. 2003;63:104. doi: 10.1016/s0031-9422(02)00754-9. [DOI] [PubMed] [Google Scholar]
- Nanjo F, Goto K, Seto R, et al. Scavenging effects of tea catechins and their derivatives on 1,1-diphenyl-2-picrylhydrazyl radical. Free Radic Biol Med. 1996;21:895–902. doi: 10.1016/0891-5849(96)00237-7. [DOI] [PubMed] [Google Scholar]
- Neha Pandey, Mahadevan Dushyant, Barve Antioxidant activity of ethanolic extract of Annona squamosa Linn bark. Int J Pharm Biol Sci. 2011;2:1692–7. [Google Scholar]
- Peter CH, Hollman Absorption, bioavailability, and metabolism of flavonoids. Pharm Bio. 2004;42:74–83. [Google Scholar]
- Re R, Pellegrini N, Proteggente A, et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–7. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–7. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Scalzo J, Politi A, Pellegrini N, Mezzetti B, Battino M. Plant genotype affects total antioxidant capacity and phenolic contents in fruit. Nutrition. 2005;21:207–13. doi: 10.1016/j.nut.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Shirwaikar A, Rajendran K, Kumar CD, Bodla R. Antidiabetic activity of aqueous leaf extracts of Annona squamosa in streptozotocin–nicotinamide type 2 diabetic rats. J Ethnopharmacol. 2004;21:171–5. doi: 10.1016/j.jep.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Shureiqi I, Reddy P, Brenner DE. Chemoprevention: general perspective. Crit Rev Oncol Hematol. 2000;33:157–67. doi: 10.1016/s1040-8428(99)00072-4. [DOI] [PubMed] [Google Scholar]
- Spencer JP, Jenner A, Aruoma OI, et al. Intense oxidative DNA damage promoted by L-dopa and its metabolites: Implications for neurodegenerative disease. FEBS Lett. 1984;353:246–50. doi: 10.1016/0014-5793(94)01056-0. [DOI] [PubMed] [Google Scholar]
- Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Rad Biol Med. 1995;18:321–6. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- Tsao AS, Kim ES, Hong WK. Chemoprevention of Cancer. CA Cancer J Clin. 2004;54:150–80. doi: 10.3322/canjclin.54.3.150. [DOI] [PubMed] [Google Scholar]
- Uchida K. Role of reactive aldehyde in cardiovascular diseases. Free Radic Biol Med. 2000;28:1685–196. doi: 10.1016/s0891-5849(00)00226-4. [DOI] [PubMed] [Google Scholar]
- Vasco C, Ruales J, Kamal-Eldin A. Total phenolic compound sand antioxidant capacities of major fruits from Ecuador. Food Chem. 2008;111:816–23. [Google Scholar]


