Abstract
Background:
Limited data are currently available regarding the psychological well-being and quality of life of breast cancer patients after active treatment in Lebanon and the Arab region in general. The objective of this study was to determine the prevalence of anxiety and depression among Arab breast cancer patients and assess the quality of life with reference to socio-demographic and clinical characteristics.
Methods:
This cross-sectional study was conducted among female breast cancer patients diagnosed between January 2009 and March 2014, who were recruited from the outpatient clinics of Naef K. Basile Cancer Institute at the American University of Beirut Medical Center (AUBMC) from November 2015 till December 2016. An interview was conducted utilizing two validated questionnaires: the Hospital Anxiety and Depression Scale (HADS) and the Functional Assessment of Cancer Therapy-Breast (FACT-B). Socio-demographic and clinical characteristics that might predict patient quality of life were collected and summarized.
Results:
A total of 150 patients were interviewed (median age 53.5±10.4 years). Most were assessed 3 to 5 years (68.7%) after initial diagnosis and had undergone surgery, chemotherapy, radiation, or hormonal therapy (97.3%, 79.3%, 80.7% and 86.0%, respectively). The median total HADS score was 10.0 ± 8.0, with approximately 41.3% of study participants having abnormal scores on the anxiety subscale and 24.7% on the depression subscale. Significant predictors of total HADS score were nationality and level of education (p=0.001, p=0.001 respectively; R2=0.181). Participants who were Iraqi, had stage IV disease, had a household monthly income below 1000 USD, or had received chemotherapy exhibited significantly lower total FACT-B scores, these being highly negatively correlated with total HADS scores (rs= -0.73, p=0.001).
Conclusion:
There is a vital need for the development of individualized interventions and psychosocial support programs tailored to the physical and psychological well-being of breast cancer patients in the Levant region.
Keywords: Quality of life, breast cancer, anxiety, depression, Levant
Introduction
Breast cancer remains the most commonly diagnosed cancer among women worldwide (WHO, 2017). In the Arab world, breast cancer represents 14% to 42% of all female cancers (Rahou et al., 2016). In Lebanon, this disease accounts for 20% of all malignant tumors (Shamseddine and Musallam, 2010). Unlike Western countries, a significant number of women in Lebanon are diagnosed at a younger age with a median age at diagnosis of 50 years, as compared to 63 years in the West (El Saghir et al., 2002; El Saghir et al., 2007). Moreover, age-standardized incidence rates have been on the rise over the years, going from a reported 78.3 cases per 100,000 in 2003 to 95.7 cases per 100,000 in 2008 (Shamseddine and Musallam, 2010; Shamseddine et al., 2014).
Great progress has been made in the diagnosis and management of this disease, with cure rates reaching around 90% in early stage disease (American Cancer Society, 2015). However, advances in therapy often come with long-standing effects on the patient’s quality of life. Nowadays, an important goal in healthcare is to sustain the long-term physical health of patients, as well as their mental and psychological well- being (Rahou et al., 2016).
Breast cancer and its subsequent treatment are a great source of anxiety and depression in patients (Jones et al., 2015). One can expect a patient to experience a decline in his/her perceived quality of life during cancer therapy. Surgery, radiation, chemotherapy and other kinds of interventions carry an array of side effects. Some of these adverse events are well tolerated by patients but many can be debilitating. This makes the goal discussed earlier difficult to maintain during active treatment (Burgess et al., 2005). However, it remains unclear whether this decrease in mental and physical health is temporary or if long-term effects would appear once the patient is well out of these stressful times. Previous studies from Western countries have found the annual prevalence of depression and anxiety among women during their first year of diagnosis to be 48% compared to 15% later on in the disease course, specifically in the fifth year after diagnosis (Burgess et al., 2005; Jones et al., 2015; Stafford et al., 2015).
Many studies have highlighted the importance of providing early management for depression and anxiety in breast cancer patients. This can help improve survival rates and quality of life, as well as reduce healthcare costs (Kroenke et al., 2006; Frick et al., 2007; Matsuda et al., 2014). Thus, in order to decrease patient suffering and improve their quality of life, it is important to estimate the psychological burden caused by breast cancer and its treatment and try to find useful measures to reduce it.
Although information is available regarding the immediate effects of breast cancer treatment, limited data is found regarding the psychological well- being and quality of life of breast cancer patients post-active treatment in Lebanon and the Arab region. Moreover, results from studies conducted previously have been widely inconsistent (Rahou et al., 2016). The objective of this study is to determine the prevalence and severity of undiagnosed anxiety and depression among breast cancer patients in the Arab region and assess the quality of life based on socio-demographic and clinical characteristics.
Materials and Methods
Study Design
This is a cross-sectional study conducted among female breast cancer patients diagnosed between January 2009 and March 2014 at the American University of Beirut Medical Center (AUBMC), a tertiary care academic center in Lebanon. Interviews were conducted at the Naef K. Basile Cancer Institute of AUBMC during the period extending from November 2015 till December 2016. This cancer center was chosen due to its load of inpatient and outpatient visits as well as its reputation as one of the best cancer centers in the Middle Eastern region.
Eligibility Criteria
Patients were included in the study if they fulfilled the following inclusion criteria: female breast cancer patients diagnosed at least 6 months prior to the date of assessment, aged 18 and above, able to communicate in Arabic or English, signed an informed consent form, negative history of other malignancies and absence of any temporary acute illness affecting psychological well-being while filling the questionnaire. Patients were excluded if they were unable to attend or complete the interview due to time constraints, refused to participate in the study or chose later to withdraw from it, had a history of psychiatric disorder prior to breast cancer diagnosis, or had a history of metastatic brain disease.
Study Instruments
Participants were interviewed once the aim of the study was explained and written informed consent was obtained. Two questionnaires were employed in our study: the Hospital Anxiety and Depression Scale (HADS) for assessment of anxiety and depression, and the Functional Assessment of Cancer Therapy- Breast (FACT-B Version 4) for evaluation of quality of life.
Hospital Anxiety and Depression Scale (HADS)
This self-administered questionnaire is a screening instrument for anxiety and depression that has been validated in different settings for the general population and for patients with a wide range of medical conditions including breast cancer patients (Abu-Helalah et al., 2014). The scale consists of 14 items and two subscales (anxiety and depression) with seven items in each subscale. Each item is scored from 0 to 3. Total scores for each subscale are calculated by simple summation of individual item responses in the subscales. A higher score indicates more distress. A systematic review of a large number of studies identified a cut-off point of 8/21 for anxiety or depression. For anxiety (HADS-A) this gave a specificity of 0.78 and a sensitivity of 0.9. For depression (HADS-D) this gave a specificity of 0.79 and a sensitivity of 0.83 (Bjelland et al., 2002). Depression and anxiety scores are also classified separately into groups: normal (0-7), mild (8-10), moderate (11-14), and severe (15-21) (Abu-Helalah et al., 2014). Use of the Arabic version of this questionnaire has been previously validated (el-Rufaie and Absood, 1987).
Functional Assessment of Cancer Therapy Breast (FACT-B)
This is a 44-item self-reported instrument designed to measure the multidimensional quality of life (QoL) in patients with breast cancer. The FACT-B consists of the FACT-General (FACT-G) plus the Breast Cancer Subscale (BCS), which complements the general scale with items specific to QoL in breast cancer. FACT-B consists of a number of items divided into several subscales: physical, emotional, social, functional well-being, breast cancer and additional concerns. Each item is rated on a five-point rating scale ranging from 0 (not at all) to 4 (very much). The total score and the subscale scores for the dimension of well-being are calculated with higher scores indicating higher QoL. The FACT-B is appropriate for use in oncology clinical trials, as well as in clinical practice. It demonstrates ease of administration, brevity, reliability, validity, and sensitivity to change (Brady et al., 1997). The Arabic version of the FACT-B questionnaire has been validated as well (Kobeissi et al., 2014).
Statistical Analysis
All collected data were coded, entered and analyzed using the statistical package IBM SPSS software version 24.0 (SPSS Inc., Chicago, IL, USA). Relevant descriptive statistics were computed summarizing the baseline socio-demographic and clinical characteristics of our sample. Means and standard deviations of subscales were evaluated for descriptive purposes. The equality of means across the different categories of independent variables was tested using parametric tests (ANOVA and independent t-test) or non-parametric tests (Kruskall - Wallis and Mann- Whitney tests) of association where appropriate. Additional exploration of the differences among means was determined by post hoc analysis. Multiple linear regressions using stepwise selection method (alpha-to-enter of 0.05, alpha-to-remove of 0.1) were performed to obtain the final model for total HADS, total FACT-B score, and FACT-B subscales relating the scores to their predictors. The final models were chosen depending on R2 and the p-value of the model. A p-value of less than 0.05 was considered significant. Spearman correlation coefficients were used to describe the association between total HADS scores and total FACT-B scores.
Results
Out of 163 patients invited to participate, 150 women fulfilled the study inclusion criteria and completed the interview. The mentioned reasons for not participating were time constraints (n=5), feeling tired (n=3), or unwillingness to share their experience (n=5).
Characteristics of the study sample
Table 1 summarizes the socio-demographic and clinical characteristics of our study sample. The median age of the patients at the time of interview was 53.5 ± 10.4 years with most assessment occurring 3 to 5 years after initial diagnosis (68.7%). The majority of women were of Lebanese nationality (80.7%), married (72.0%), currently or previously employed (66.0%), and had completed a secondary or university level of education (90.7%). Regarding clinical characteristics, the median age at breast cancer diagnosis was 49.0 ± 10.3 years. The percentage of patients diagnosed with stage 0-I, II, III, and IV cancer was 29.3%, 38.7%, 23.3%, and 8.7% respectively. Furthermore, most patients had undergone surgery (97.3%), chemotherapy (79.3%), radiation (80.7%), or hormonal therapy (86.0%) as part of the treatment regimen.
Table 1.
Demographic and Clinical Characteristics of the Study Population N=150
| Characteristics | N (%) |
|---|---|
| Age at Interview (Median ± SD) | 53.5 ± 10.4 |
| Age at Diagnosis (Median ± SD) | 49.0 ± 10.3 |
| Nationality | |
| Lebanese | 121 (80.7) |
| Iraqi | 23 (15.3) |
| Other | 6 (4.0) |
| Marital Status | |
| Single | 18 (12.0) |
| Married | 108 (72.0) |
| Divorced or Separated or Widowed | 24 (16.0) |
| Education Level | |
| Primary level or below | 14 (9.3) |
| Secondary level | 62 (41.3) |
| University level | 74 (49.3) |
| Current or Previous Employment | 99(66.0) |
| Household Monthly Income | |
| <1,000$ | 42 (28.0) |
| 1,000$-3,000$ | 54 (36.0) |
| >3,000$ | 35 (23.3) |
| Declined/ Unknown | 19 (12.7) |
| Disease Stage at Diagnosis | |
| Stage 0 or I | 44 (29.3) |
| Stage II | 58 (38.7) |
| Stage III | 35 (23.3) |
| Stage IV | 13 (8.7) |
| Years Since Diagnosis | |
| 0.5 to ≤ 2 years | 20 (13.3) |
| 3 to ≤ 5 years | 103 (68.7) |
| >5 years | 27 (18.0) |
| Treatment Modality Received | |
| Surgery | 146 (97.3) |
| Chemotherapy | 119 (79.3) |
| Radiation | 121 (80.7) |
| Hormonal Therapy | 129 (86.0) |
| Positive Family History of Breast Cancer | 56 (37.3) |
Psychological Well-being Assessment
The median total HADS score (out of 42) was 10.0 ± 8.0. Approximately 41.3% of study participants scored abnormal (scores ≥ 8) on the anxiety subscale and 24.7% on the depression subscale. Severe anxiety and depression were detected among 7.3% and 4.7% of study participants respectively (Figure 1). None of the following variables were found to be statistically significant predictors of total HADS score when accounting for other variables: age at diagnosis, time since diagnosis, marital status, employment status, monthly net income, stage of breast cancer at diagnosis, treatment modalities or family history of breast cancer. The only statistically significant predictors of total HADS score were nationality and level of education (p=0.001, p=0.001 respectively; R2=0.181). In particular, Iraqi patients had significantly higher total HADS scores (18.00 ± 7.32) compared to Lebanese patients (9.00 ± 7.26) p-value=0.001. Patients who had only completed a primary level of education or below had significantly higher total HADS score (21.50 ± 6.41) compared to those who completed secondary (10.00 ± 7.20) or university (9.00 ± 7.93) levels (p=0.001).
Figure 1.
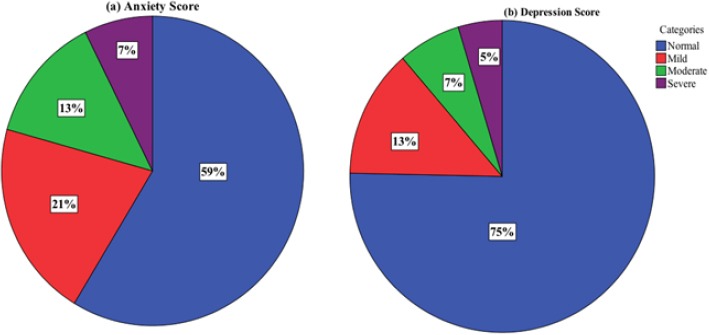
Percentage of Participants Classified as Normal, Mild, Moderate, and Severe According to Their (a) Anxiety Score and (b) Depression Score.
Quality of Life Assessment
Table 2 lists the means and standard deviations for each of the FACT-B subscales as well as the FACT-B TOI (Trial Outcome Index), FACT-G total score, and FACT-B total score (FACT-G plus BCS).
Table 2.
FACT- B Descriptive Statistics
| Mean | SD | |
|---|---|---|
| Physical Well-being Subscale (7-item, score range 0-28) | 22.62 | 4.65 |
| Social Well-being Subscale (7-item, score range 0-28) | 21.71 | 4.79 |
| Emotional Well-being Subscale (6-item, score range 0-24) | 18.92 | 4.08 |
| Functional Well-being Subscale (7-item, score range 0-28) | 21.49 | 4.72 |
| Breast Cancer Subscale (10-item, score range 0-40) | 23.98 | 6.15 |
| Fact-B Trial Outcome Index (Score range 0-96) | 68.09 | 12.82 |
| Fact-G Total Score (Score range 0-108) | 84.74 | 14.32 |
| Fact- B Total Score (Score range 0-148) | 108.72 | 18.77 |
Participants who were Iraqi, had stage IV disease at diagnosis, had a household monthly income below 1,000 USD, or had received chemotherapy exhibited significantly lower total FACT-B scores (Table 3 and Table 4). In addition, Iraqi patients were found to have significantly lower physical well-being, emotional well-being, and breast cancer subscale scores compared to Lebanese patients (Table 3). Patients who underwent chemotherapy or radiotherapy as part of their treatment regimen had lower physical well-being and breast cancer subscale scores compared to their counterparts (Table 4). Participants with a household monthly income greater than 3000$ had significantly higher physical well-being scores (Table 3). With regards to functional well-being, participants who were interviewed more than 5 years since diagnosis had significantly lower scores compared to those interviewed within 2 years from diagnosis. In contrast, patients who underwent surgery had significantly higher functional well-being scores than those who did not (Table 4). Patients diagnosed before the age of 50 had significantly lower breast cancer subscale scores compared to those diagnosed above age 50 (Table 4).
Table 3.
Socio- Demographic Characteristics of the Study Participants and FACT-B Quality of Life Scores
| Variables | PWB | SWB | EWB | FWB | BCS | TQOL |
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Nationality | ||||||
| Lebanese | 23.18 ± 4.37 | 21.84 ± 4.88 | 19.56 ± 3.46 | 21.86 ± 4.74 | 24.72 ± 5.90 | 111.16 ± 17.86 |
| Iraqi | 19.30 ± 5.11* | 21.58 ± 4.28 | 15.61 ± 5.02* | 20.13 ± 4.42 | 20.49 ± 5.84* | 97.12 ± 18.91* |
| Other | 24.00 ± 3.16 | 19.53 ± 5.02 | 18.67 ± 6.25 | 19.33 ± 4.80 | 22.43 ± 8.28 | 103.95 ± 21.84 |
| Marital Status | ||||||
| Single | 23.06 ± 4.88 | 22.46 ± 4.40 | 18.72 ± 3.34 | 22.61 ± 4.62 | 25.39 ± 5.08 | 112.24 ± 15.53 |
| Married | 22.45 ± 4.70 | 21.50 ± 4.63 | 18.73 ± 4.26 | 21.44 ± 4.74 | 23.12 ± 5.89 | 107.24 ± 18.72 |
| Divorced/Separated/Widowed | 23.04 ± 4.39 | 22.10 ± 5.81 | 19.92 ± 3.76 | 20.88 ± 4.79 | 26.81 ± 7.14 | 112.74 ± 20.88 |
| Educational Status | ||||||
| Primary or below | 20.14 ± 4.82 | 20.43 ± 4.42 | 16.64 ± 4.77 | 18.64 ± 4.63 | 24.37 ± 4.92 | 100.23 ± 17.48 |
| Secondary | 22.81 ± 5.10 | 21.55 ± 4.91 | 19.31 ± 3.93 | 21.44 ± 4.95 | 24.37 ± 6.70 | 109.47 ± 20.12 |
| University | 22.93 ± 4.11 | 22.08 ± 4.77 | 19.03 ± 3.99 | 22.08 ± 4.39 | 23.58 ± 5.91 | 109.70 ± 17.63 |
| Occupation | ||||||
| Currently/previously employed | 22.89 ± 4.65 | 21.69 ± 4.95 | 19.10 ± 3.97 | 21.80 ± 4.71 | 23.58 ± 6.38 | 109.06 ± 19.37 |
| Never employed | 22.10 ± 4.66 | 21.74 ± 4.50 | 18.57 ± 4.32 | 20.90 ± 4.75 | 24.76 ± 5.65 | 108.07 ± 17.71 |
| Monthly Income | ||||||
| <1,000$ | 22.12 ± 4.82§ | 20.91 ± 4.84 | 18.00 ± 4.97 | 20.67 ± 4.48 | 23.35 ± 5.40 | 105.04 ± 17.18§ |
| 1,000$-3,000$ | 21.98 ± 4.82§ | 21.01 ± 5.36 | 18.30 ± 3.75§ | 21.04 ± 5.07 | 23.17 ± 7.16 | 105.49 ± 20.68 |
| >3,000$ | 24.40 ± 3.72 | 22.47 ± 3.68 | 20.46 ± 2.58 | 22.51 ± 4.64 | 25.68 ± 5.48 | 115.51 ± 16.18 |
PWB, Physical Well-Being; SWB, Social Well-Being; EWB, Emotional Well-Being; FWB, Functional Well-Being; BCS, Breast Cancer Subscale; TQOL, Total Quality of Life.
Significantly lower scores compared to Lebanese patients;
Significantly lower scores compared >3000$ cohort
Table 4.
Clinical Characteristics of the Study Participants and FACT-B Quality of Life Scores
| Variables | PWB | SWB | EWB | FWB | BCS | TQOL |
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Age at Diagnosis | ||||||
| ≤50 | 22.58 ± 4.58 | 21.64 ± 5.00 | 18.98 ± 3.82 | 21.49 ± 4.98 | 23.01 ± 6.06* | 107.70 ± 18.44 |
| >50 | 22.67 ± 4.77 | 21.79 ± 4.55 | 18.85 ± 4.42 | 21.49 ± 4.42 | 25.18 ± 6.08 | 109.99 ± 19.23 |
| Years Since Diagnosis | ||||||
| ≤ 2 years | 24.10 ± 4.00 | 22.73 ± 4.02 | 19.55 ± 3.71 | 23.00 ± 4.08 | 24.13 ± 4.68 | 113.51 ± 14.08 |
| 3 to ≤ 5 years | 22.51 ± 4.78 | 21.51 ± 4.88 | 18.76 ± 4.18 | 21.60 ± 4.63 | 24.12 ± 6.14 | 108.50 ± 19.33 |
| >5 years | 21.93 ± 4.50 | 21.71 ± 5.01 | 19.07 ± 4.08 | 19.96 ± 5.23Ŧ | 23.34 ± 7.23 | 106.01 ± 19.54 |
| Stage | ||||||
| 0 or I or II | 23.11 ± 4.18 | 21.69 ± 4.97 | 19.26 ± 3.72 | 21.54 ± 4.82 | 24.29 ± 5.73 | 109.89 ± 17.83 |
| III | 22.37 ± 5.29 | 22.43 ± 4.02 | 18.74 ± 4.77 | 22.23 ± 4.83 | 23.43 ± 7.39 | 109.20 ± 21.88 |
| IV | 19.46 ± 5.39 | 19.88 ± 5.08 | 16.69 ± 4.42 | 19.15 ± 2.79 | 23.05 ± 5.92 | 98.24 ± 14.53* |
| Surgery | ||||||
| Yes | 22.72 ± 4.57 | 21.81 ± 4.76 | 19.03 ± 4.03 | 21.62 ± 4.71 | 24.01 ± 6.17 | 109.18 ± 18.73 |
| No | 19.00 ± 6.93 | 17.83 ± 4.93 | 15.00 ± 4.83 | 17.00 ± 2.94* | 23.06 ± 5.93 | 91.89 ± 12.02 |
| Chemotherapy | ||||||
| Yes | 21.94 ± 4.76* | 21.53 ± 4.85 | 18.66 ± 4.20 | 21.31 ± 4.85 | 23.20 ± 6.05* | 106.65 ± 19.05* |
| No | 25.23 ± 3.04 | 22.38 ± 4.57 | 19.90 ± 3.51 | 22.19 ± 4.20 | 26.99 ± 5.65 | 116.69 ± 15.46 |
| Radiotherapy | ||||||
| Yes | 22.20 ± 4.87* | 21.79 ± 4.94 | 18.78 ± 4.21 | 21.31 ± 4.67 | 23.51 ± 6.33* | 107.58 ± 19.48 |
| No | 24.38 ± 3.08 | 21.37 ± 4.18 | 19.52 ± 3.50 | 22.28 ± 4.97 | 25.94 ± 4.93 | 113.48 ± 14.81 |
| Hormonal Therapy | ||||||
| Yes | 22.76 ± 4.39 | 21.72 ± 4.88 | 19.05 ± 3.79 | 21.50 ± 4.79 | 24.04 ± 5.99 | 109.06 ± 18.04 |
| No | 21.76 ± 6.07 | 21.63 ± 4.26 | 18.14 ± 5.61 | 21.48 ± 4.39 | 23.63 ± 7.22 | 106.64 ± 23.13 |
| Family History of Breast Cancer | ||||||
| Yes | 22.70 ± 4.16 | 21.70 ± 4.79 | 19.21 ± 3.32 | 21.66 ± 4.25 | 23.91 ± 6.04 | 109.19 ± 15.79 |
| No | 22.57 ± 4.94 | 21.71 ± 4.81 | 18.74 ± 4.48 | 21.39 ± 5.01 | 24.02 ± 6.24 | 108.44 ± 20.41 |
PWB, Physical Well-Being; SWB, Social Well-Being; EWB, Emotional Well-Being; Functional Well-Being; BCS, Breast Cancer Subscale; TQOL, Total Quality of Life.
Significantly lower scores compared to all other cohorts within each demographic category for each score;
Significantly lower FWB scores compared to ≤ 2 years post-diagnosis cohort.
Predictors of Quality of Life
When accounting for all possible confounders, the most significant predictors of: total FACT-B scores were nationality and chemotherapy (p=0.023, p=0.034 respectively; R2= 0.080); physical well-being score was chemotherapy (p=0.001, R2= 0.082); social well-being score was income (p=0.021, R2= 0.036); emotional well-being score were nationality and surgery (p=0.002, p=0.046 respectively; R2= 0.090); functional well-being score were time since diagnosis and education (p=0.021, p=0.022 respectively; R2= 0.067); and breast cancer subscale score were chemotherapy, nationality, and age at diagnosis (p= 0.023, 0.027, 0.043 respectively; R2= 0.113).
Correlation between Anxiety, Depression and Quality of Life
Total HADS scores were highly negatively correlated with QoL FACT-B total scores (rs= -0.73, p=0.001). In addition, total anxiety (rs= -0.66, p=0.001) and total depression (rs= -0.69, p=0.001) scores were highly correlated with impaired QoL.
Discussion
Both, anxiety and depression are common and significant morbidities in the studied breast cancer population. The total HADS score as well as the percentages of patients suffering from anxiety and depression are comparable to regional and international figures. Numerous studies have demonstrated that approximately one quarter to one third of breast cancer patients suffer from distress, anxiety, and depression following diagnosis and treatment of breast cancer (Burgess et al., 2005). A study conducted on female breast cancer patients in Jordan, noted a mean total HADS score of 18.0 ± 9.0 with 53% of participants scoring abnormal on the anxiety subscale and 45% on the depression scale. Around 14% of patients suffered severe anxiety and 8% had severe depression (Abu-Helalah et al., 2014). A possible explanation for why these figures are higher than those reported in our study, is that the majority of patients were assessed 1-3 years after diagnosis as opposed to 3-5 years in our study. This is consistent with findings from Burgess et al., (2005) that showed these conditions were more likely to occur early on in the disease course. It is therefore expected that levels of anxiety and depression improve with time since diagnosis.
However, it is still concerning that a good proportion of patients have clinically undiagnosed anxiety and depression. The level of psychological distress present is still greater than that noted in the general Arab population. A review of the epidemiology of anxiety disorders in the Arab world revealed 10-30% prevalence of “any anxiety disorder”, with lifetime prevalence of 16.7% in the Lebanese population (Tanios et al., 2009). Similarly, up to 7% of the Middle East/North Africa population suffer from major depression (Ferrari et al., 2013). Our results suggest significant levels of psychological distress that might be attributed to poor psychological screening and counseling during clinic visits.
When accounting for possible confounders, Iraqi patients were found to have significantly higher total HADS scores compared to their Lebanese counterparts. This is likely due to the stress associated with having to travel to another country in order to seek appropriate medical care, lack of adequate follow-up and support, in addition to the stressful living conditions in Iraq.
Level of education was also found to be a statistically significant predictor of total HADS score with worse scores in those who have only completed a primary level of education or below. This is consistent with some studies that highlighted a positive association between low educational level and socioeconomic status with worsening depressive symptoms and anxiety (Simpson et al., 2002; Al-Zaben et al., 2015).
However, many of the socio-demographic and clinical characteristics formerly identified as statistically significant predictors of total HADS score were not demonstrated in our study. For instance, patients with advanced metastatic disease were at higher risk for anxiety and depression compared to those with local disease (Jacob et al., 2016). This could be partly explained by the relatively low number of patients with metastatic breast cancer included in this study.
The majority of our study participants reported good to high overall quality of life with relatively similar mean total FACT-B scores obtained in Western countries (Hamer et al., 2017). Our results suggested that participants who were Iraqi, had stage IV at disease diagnosis, had a household monthly income below 1,000 USD, or had received chemotherapy exhibited significantly lower overall quality of life scores. In addition, Iraqi patients were found to have significantly lower physical well-being, emotional well-being, and breast cancer subscale scores compared to Lebanese patients. Iraqi patients are expected to have lower overall and subscale scores due to lack of adequate assessment of treatment side effects, symptom follow-up, and emotional support as noted above. Disease stage and presence of metastasis were also significant predictors of global health and QoL among breast cancer patients and reported extensively in the literature (Jassim and Whitford, 2013). As far as financial status is concerned, women with low family monthly income had on average lower total QoL scores compared to higher income women (Al-Naggar et al., 2011). Lower income may lead to a limited accessibility to primary preventive measures for detection of breast cancer at an early disease stage (Merkin et al., 2002), as well as affect the adequacy of treatment and tangible support available to the patient, leading to worse QoL (Kornblith et al., 2007; So et al., 2009; Jassim and Whitford, 2013). Common side effects of adjuvant chemotherapy such as nausea, fatigue, alopecia, sleep difficulties and premature menopause, can have a great impact on QoL (Janz et al., 2007). In fact, the number and severity of adverse events has been negatively correlated with appraisal of QoL (Longman et al., 1999). This can explain the lower physical well-being, breast cancer subscale, and total FACT-B scores in patients who underwent chemotherapy in our sample. However, a significant body of evidence has revealed that the impairment in quality of life due to such therapy is minor and limited to short- term rather than long-term quality of life (Kornblith et al., 2003).
Patients who underwent radiotherapy as part of their treatment regimen had lower physical well-being and breast cancer subscale scores compared to their counterparts, likely due to side effects of treatment. On the other hand, a study in Yemen showed that women who received radiotherapy had on average better total QoL scores compared to those who didn’t (Al-Naggar et al., 2011).
Our results showed that participants who were interviewed more than 5 years since diagnosis had significantly lower functional well-being scores compared to those interviewed within 2 years from diagnosis. A similar finding was reported by Al-Naggar et al., (2011), whereby women assessed within 1-2 years since diagnosis had significantly higher functional and social well-being scores than those with more than 5 years since diagnosis. Holzner et al., (2001) also reported that women in the group 2-5 years after initial diagnosis had improved QoL compared to women in the group >5 years. This interesting finding contrasts with reports from other studies that have suggested better ability of women to overcome with time the physical and psychological burden imposed by their cancer and its treatment (Cohen et al., 2000).
Patients diagnosed before the age of 50 had significantly lower breast cancer subscale scores compared to those diagnosed above age 50. This is consistent with a study performed in Canada using the FACT-B questionnaire, that showed young women < 50 years old with breast cancer were more likely to have a decreased QoL and increased symptom burden than older women (Hamer et al., 2017). This suggests younger patients could be more vulnerable to the physical and psychological impact of breast cancer and have more specific concerns regarding sexual attractiveness, hair loss, and change in weight for example. As such, there is a greater need for supportive care services provided to this younger group. Other studies that did not show an association between age and quality of life scores may have had different age groupings and utilized different questionnaires that did not address specific breast cancer concerns (Jassim and Whitford, 2013).
Although the majority of quality of life studies conducted on breast cancer patients have utilized the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) and Quality of Life Breast Cancer Specific Version (QLQ-BR23) validated questionnaires instead of FACT-B, many of the symptom scales were also correlated with total HADS score. In fact, patients who were more anxious and more depressed (with higher HADS scores) reported more symptoms and were less functional (Alawadi and Ohaeri, 2009). Another study from Iran showed statistically significant correlation between HADS score with global health scores and emotional functioning (Montazeri et al., 2003). Similarly, our results showed impaired quality of life (lower FACT-B scores) in patients with worse psychological well-being (higher HADS scores).
This study has several strengths including the use of standardized measures for the assessment of anxiety, depression and quality of life, high response rate, and clinically meaningful analysis. However, it is not without limitations. Among the major limitations of this study are: lack of a disease-free control group for comparison of psychological well-being scores, failure to collect information regarding extent of disease and progression or recurrence of breast cancer since baseline, and lack of a well-representative sample due to small sample size. In addition, this study did not compare quality of life of the same individuals at several time points but rather compared different participants with various time elapsed since diagnosis. Being a cross-sectional study might introduce some selection bias into the study population. However, every effort was made to include as many patients being seen in our clinics as possible during the recruitment period. We believe we were able to capture the vast majority of them.
In conclusion, this study has many implications for practice and policy as well as future research considerations. There is a vital need for the development of individualized interventions for the management of disease and treatment-related symptoms, in addition to community-based psychosocial support programs that tailor to the physical and psychological well-being of breast cancer patients in the Levant region. Greater attention should be given to cultural differences among breast cancer patients.
Abbreviations
AUBMC: American University of Beirut Medical Center
HADS: Hospital Anxiety and Depression Scale
HADS-A: Hospital Anxiety and Depression Scale- Anxiety subscale
HADS-D: Hospital Anxiety and Depression Scale- Depression subscale
FACT-B: Functional Assessment of Cancer Therapy for Breast Cancer
QoL: Quality of Life
PWB: Physical Well-Being
SWB: Social Well-Being
EWB: Emotional Well-Being
FWB: Functional Well-Being
BCS: Breast Cancer Subscale
FACT- B TOI: FACT-B Trial Outcome Index
FACT-G: Functional Assessment of Cancer Therapy- General
TQOL: Total Quality of Life
EORTC QLQ-C30: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire
QLQ-BR23: Quality of Life Breast Cancer Specific Version
ANOVA: Analysis of Variance
SPSS: Statistical Package for Social Sciences
Declarations
Ethics approval and consent to participate: The study was reviewed and approved by the American University of Beirut Institutional Review Board. The patients provided informed written consent prior to study enrollment.
Competing Interests
The authors declare that they have no competing interests.
Funding
No funding was provided for this study.
Authors’ Contributions
RA contributed to the collection of data, analysis and interpretation of data, and drafting the manuscript. HED participated in the design of the study and data collection. BA and RR participated in data collection. HA, DM, and ST were involved in revising the manuscript critically for important intellectual content. AT participated in the conception and design of the study, revised and helped to draft the manuscript. All authors read and approved the final manuscript. The authors have agreed to be accountable for all aspects of the work.
References
- Abu-Helalah M, Al-Hanaqta M, Alshraideh H, et al. Quality of life and psychological well-being of breast cancer survivors in Jordan. Asian Pac J Cancer Prev. 2014;15:5927–36. doi: 10.7314/apjcp.2014.15.14.5927. [DOI] [PubMed] [Google Scholar]
- Al-Naggar RA, Nagi NM, Ali MM, et al. Quality of life among breast cancer patients in Yemen. Asian Pac J Cancer Prev. 2011;12:2335–41. [PubMed] [Google Scholar]
- Al-Zaben FN, Sehlo MG, Koenig HG. A cross-sectional study of anxiety and marital quality among women with breast cancer at a university clinic in western Saudi Arabia. Saudi Med J. 2015;36:1168–75. doi: 10.15537/smj.2015.10.12254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alawadi SA, Ohaeri JU. Health - related quality of life of Kuwaiti women with breast cancer: a comparative study using the EORTC Quality of Life Questionnaire. BMC Cancer. 2009;9:222. doi: 10.1186/1471-2407-9-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjelland I, Dahl AA, Haug TT, et al. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the functional assessment of cancer therapy-breast quality-of-life instrument. JClin Oncol. 1997;15:974–86. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- Burgess C, Cornelius V, Love S, et al. Depression and anxiety in women with early breast cancer: five year observational cohort study. BMJ. 2005;330:702. doi: 10.1136/bmj.38343.670868.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Hack TF, de Moor C, et al. The effects of type of surgery and time on psychological adjustment in women after breast cancer treatment. Ann Surg Oncol. 2000;7:427–34. doi: 10.1007/s10434-000-0427-9. [DOI] [PubMed] [Google Scholar]
- El Saghir NS, Khalil MK, Eid T, et al. Trends in epidemiology and management of breast cancer in developing Arab countries: a literature and registry analysis. Int J Surg. 2007;5:225–33. doi: 10.1016/j.ijsu.2006.06.015. [DOI] [PubMed] [Google Scholar]
- El Saghir NS, Shamseddine AI, Geara F, et al. Age distribution of breast cancer in Lebanon: increased percentages and age adjusted incidence rates of younger-aged groups at presentation. J Med Liban. 2002;50:3–9. [PubMed] [Google Scholar]
- el-Rufaie OE, Absood G. Validity study of the hospital anxiety and depression scale among a group of Saudi patients. Br J Psychiatry. 1987;151:687–8. doi: 10.1192/bjp.151.5.687. [DOI] [PubMed] [Google Scholar]
- Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10:e1001547. doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick E, Tyroller M, Panzer M. Anxiety, depression and quality of life of cancer patients undergoing radiation therapy: a cross-sectional study in a community hospital outpatient centre. Eur J Cancer Care (Engl) 2007;16:130–6. doi: 10.1111/j.1365-2354.2006.00720.x. [DOI] [PubMed] [Google Scholar]
- Hamer J, McDonald R, Zhang L, et al. Quality of life (QOL) and symptom burden (SB) in patients with breast cancer. Support Care Cancer. 2017;25:409–19. doi: 10.1007/s00520-016-3417-6. [DOI] [PubMed] [Google Scholar]
- Holzner B, Kemmler G, Kopp M, et al. Quality of life in breast cancer patients--not enough attention for long-term survivors? Psychosomatics. 2001;42:117–23. doi: 10.1176/appi.psy.42.2.117. [DOI] [PubMed] [Google Scholar]
- Jacob L, Bleicher L, Kostev K, et al. Prevalence of depression, anxiety and their risk factors in German women with breast cancer in general and gynecological practices. J Cancer Res Clin Oncol. 2016;142:447–52. doi: 10.1007/s00432-015-2048-5. [DOI] [PubMed] [Google Scholar]
- Janz NK, Mujahid M, Chung LK, et al. Symptom experience and quality of life of women following breast cancer treatment. J Womens Health (Larchmt) 2007;16:1348–61. doi: 10.1089/jwh.2006.0255. [DOI] [PubMed] [Google Scholar]
- Jassim GA, Whitford DL. Quality of life of Bahraini women with breast cancer: a cross sectional study. BMC Cancer. 2013;13:212. doi: 10.1186/1471-2407-13-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SM, LaCroix AZ, Li W, et al. Depression and quality of life before and after breast cancer diagnosis in older women from the Women's Health Initiative. JCancer Surviv. 2015;9:620–9. doi: 10.1007/s11764-015-0438-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobeissi L, Saad MA, Doumit M, et al. Face validity of the functional assessment of cancer therapy-breast symptom index (FACT- B) into formal arabic. Middle East J Cancer. 2014;5:151–65. [Google Scholar]
- Kornblith AB, Herndon JE, Weiss RB, et al. Long-term adjustment of survivors of early-stage breast carcinoma, 20 years after adjuvant chemotherapy. Cancer. 2003;98:679–89. doi: 10.1002/cncr.11531. [DOI] [PubMed] [Google Scholar]
- Kornblith AB, Powell M, Regan MM, et al. Long-term psychosocial adjustment of older vs younger survivors of breast and endometrial cancer. Psychooncology. 2007;16:895–903. doi: 10.1002/pon.1146. [DOI] [PubMed] [Google Scholar]
- Kroenke CH, Kubzansky LD, Schernhammer ES, et al. Social networks, social support, and survival after breast cancer diagnosis. J Clin Oncol. 2006;24:1105–11. doi: 10.1200/JCO.2005.04.2846. [DOI] [PubMed] [Google Scholar]
- Longman AJ, Braden CJ, Mishel MH. Side-effects burden, psychological adjustment, and life quality in women with breast cancer: pattern of association over time. Oncol Nurs Forum. 1999;26:909–15. [PubMed] [Google Scholar]
- Matsuda A, Yamaoka K, Tango T, et al. Effectiveness of psychoeducational support on quality of life in early-stage breast cancer patients: a systematic review and meta-analysis of randomized controlled trials. Qual Life Res. 2014;23:21–30. doi: 10.1007/s11136-013-0460-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkin SS, Stevenson L, Powe N. Geographic socioeconomic status, race, and advanced-stage breast cancer in New York City. Am J Public Health. 2002;92:64–70. doi: 10.2105/ajph.92.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montazeri A, Vahdaninia M, Ebrahimi M, et al. The hospital anxiety and depression scale (HADS): translation and validation study of the Iranian version. Health Qual Life Outcomes. 2003;1:14. doi: 10.1186/1477-7525-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahou BH, El Rhazi K, Ouasmani F, et al. Quality of life in Arab women with breast cancer: a review of the literature. Health Qual Life Outcomes. 2016:14. doi: 10.1186/s12955-016-0468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamseddine A, Saleh A, Charafeddine M, et al. Cancer trends in Lebanon: a review of incidence rates for the period of 2003-2008 and projections until 2018. Popul Health Metr. 2014:12. doi: 10.1186/1478-7954-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamseddine AI, Musallam KM. Cancer epidemiology in Lebanon. Middle East J Cancer. 2010;1:41–4. [Google Scholar]
- Simpson JS, Carlson LE, Beck CA, et al. Effects of a brief intervention on social support and psychiatric morbidity in breast cancer patients. Psychooncology. 2002;11:282–94. doi: 10.1002/pon.565. [DOI] [PubMed] [Google Scholar]
- So WK, Marsh G, Ling WM, et al. The symptom cluster of fatigue, pain, anxiety, and depression and the effect on the quality of life of women receiving treatment for breast cancer: a multicenter study. Oncol Nurs Forum. 2009;36:205–14. doi: 10.1188/09.ONF.E205-E214. [DOI] [PubMed] [Google Scholar]
- Society AC. Cancer facts and figures 2015 [Online] Atlanta: American Cancer Society; 2015. [[Accessed May 27 2017]]. [Google Scholar]
- Stafford L, Judd F, Gibson P, et al. Anxiety and depression symptoms in the 2 years following diagnosis of breast or gynaecologic cancer: prevalence, course and determinants of outcome. Support Care Cancer. 2015;23:2215–24. doi: 10.1007/s00520-014-2571-y. [DOI] [PubMed] [Google Scholar]
- Tanios CY, Abou-Saleh MT, Karam AN, et al. The epidemiology of anxiety disorders in the Arab world: a review. J Anxiety Disord. 2009;23:409–19. doi: 10.1016/j.janxdis.2008.10.009. [DOI] [PubMed] [Google Scholar]
- WHO. World Health Organization [Online] World Health Organization; 2017. [[Accessed May 27 2017]]. Breast cancer: prevention and control. Available: http://www.who.int/cancer/detection/breastcancer/en/index1.html . [Google Scholar]


