Abstract
In Southeast Asia, envenoming resulting from cobra snakebites is an important public health issue in many regions, and antivenom therapy is the standard treatment for the snakebite. Because these cobras share a close evolutionary history, the amino acid sequences of major venom components in different snakes are very similar. Therefore, either monovalent or polyvalent antivenoms may offer paraspecific protection against envenomation of humans by several different snakes. In Taiwan, a bivalent antivenom—freeze-dried neurotoxic antivenom (FNAV)—against Bungarus multicinctus and Naja atra is available. However, whether this antivenom is also capable of neutralizing the venom of other species of snakes is not known. Here, to expand the clinical application of Taiwanese FNAV, we used an animal model to evaluate the neutralizing ability of FNAV against the venoms of three common snakes in Southeast Asia, including two ‘true’ cobras Naja kaouthia (Thailand) and Naja siamensis (Thailand), and the king cobra Ophiophagus hannah (Indonesia). We further applied mass spectrometry (MS)-based proteomic techniques to characterize venom proteomes and identify FNAV-recognizable antigens in the venoms of these Asian snakes. Neutralization assays in a mouse model showed that FNAV effectively neutralized the lethality of N. kaouthia and N. siamensis venoms, but not O. hannah venom. MS-based venom protein identification results further revealed that FNAV strongly recognized three-finger toxin and phospholipase A2, the major protein components of N. kaouthia and N. siamensis venoms. The characterization of venom proteomes and identification of FNAV-recognizable venom antigens may help researchers to further develop more effective antivenom designed to block the toxicity of dominant toxic proteins, with the ultimate goal of achieving broadly therapeutic effects against these cobra snakebites.
Author summary
Cobra envenomation is a public health issue in Southeast Asia. Currently, antivenom therapy is the standard treatment for snakebite. However, antivenoms are not available in many rural countries and communities or have only limited effectiveness. Taiwan has wealth of experience in producing antivenoms, including the bivalent freeze-dried neurotoxic antivenom (FNAV), which is raised against venom proteins from Bungarus multicinctus and Naja atra. Our results showed that FNAV effectively neutralized the lethality of Naja kaouthia(Thailand) and Naja siamensis (Thailand) venoms, but not Ophiophagus hannah (Indonesia) venom, in an animal model. We further characterized the venom proteome profiles of the four cobras and identified three abundant proteins—neurotoxin, cytotoxin and phospholipase A2—in the venom of N. atra, N. kaouthia and N. siamensisas the major antigens recognized by FNAV. In contrast, we found that β-cardiotoxin and phospholipase A2, common toxin proteins in all king cobra venom samples, are weakly or not recognized by FNAV. Our data provide evidence suggesting the potential use of Taiwan’s FNAV to treat envenomation by other cobra species (N. kaouthia and N. siamensis) in Southeast Asia. Moreover, our findings support the previous recommendation and current experimental approach that major cobra toxins are used as antigens to generate more efficient antivenoms than those currently available.
Introduction
Envenomation through snakebite is an important public health issue in many regions of the world, particularly in tropical countries [1–3]. An estimated 421,000 to 1,841,000 envenomations and 20,000 to 94,000 deaths occur globally each year owing to snakebites. The regions of highest incidence include Southeast Asia, South Asia, Africa, and Latin America [4]. In Southeast Asia, cases involving cobra envenomation are among the most common[5]. There are several clinically significant cobra snakes: Naja atra, Naja kaouthia, Naja siamensis, Naja sputatrix, Naja sumatrana, and Naja philipinensis.
At present, antivenom therapy is the standard treatment for snakebite. To maximize antivenom utility, researchers have applied animal models to evaluate the ability of antivenoms to cross-neutralize the venoms of other snakes in the same genus that represent a public health concern [6, 7]. These approaches, combined with immunological and proteomics techniques, have been successfully used to identify specific venom proteins that can be recognized by antivenom [8–11]. Such information can be used to design a new strategy for improving the immune response of animals against poorly immunogenic antigens or major toxic components so as to further improve the efficacy of antivenoms [12–14].
There are four types of available antivenom against the six most clinically significant snakebites in Taiwan; two are bivalent antivenoms, and the other two are monovalent antivenoms [15, 16]. One of the bivalent antivenoms is freeze-dried neurotoxic antivenom (FNAV), raised against Bungarus multicinctus and N. atra. Previous studies have shown that FNAV exhibits good clinical effects and is well documented to decrease the rate of death caused by bites from these two snakes [17, 18].
The aim of this study was to evaluate whether FNAV has therapeutic potential for envenomations of cobra species outside of Taiwan. In this preclinical study, we analyzed the ability of FNAV to neutralize the venoms of N. kaouthia, N. siamensis and O. hannah. We further investigated the venom proteome of each cobra by liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis and identified FNAV-recognizable components in each. These results not only provide useful information regarding the neutralizing potential of FNAV against heterologous venoms, it also provides valuable clues for improving antivenom efficacy.
Methods
Snake venoms and antivenoms
The lyophilized venom of N. atra was obtained from the Centers for Disease Control, R.O.C (Taiwan). Venoms of two other Southeast Asia Naja species, N. kaouthia and N. siamensis, as well as that of the related king cobra, O. hannah (the sole member of its genus), were purchased from Latoxan (Valence, France). According to Latoxan’s remark, the snakes of N. kaouthia, N. siamensis and O. hannah originate from Thailand, Thailand and Indonesia, respectively. Venoms were collected from several adult specimens, then freeze-dried and stored at -20°C before use. Freeze neurotoxic (FN) antivenoms were purchased as lyophilized powders from the Centers for Disease Control, R.O.C (Taiwan), and stored at 4°C before use.
Animals
Experiments were performed on 7-week-old littermate male mice (C57BL/6Narl strain, 20–25 g). Mice were maintained under specific pathogen-free conditions with a 12:12 hour light-dark cycle at a temperature of 22°C and a humidity level of 60–70%. Animals had ad libitum access to food and water.
Animal ethics statement
Experiments involving the care, bleeding and injection of mice with various venoms were reviewed and approved by the Institutional Animal Care and Use Committee of Chang Gung University (Permit Number: CGU14-024). The protocol of animal study on mice was based on the guidelines given by the Council for International Organizations of Medical Sciences (CIOMS)[19].
Median lethal dose (LD50) assay
Groups of mice (n = 5/group) with a defined weight range (20–25 g) were subcutaneously injected with 0.1 ml of sterile saline solution containing different doses of venom. Six groups of mice were used to conduct this assay per venom. Only one dose was given to each mouse in this experiment. The dosage ranges of N. kaouthia, N. siamensis and O. hannah venom were 0.2–0.45, 0.4–0.7, 0.7–1.2 mg/kg, respectively. LD50 values were determined by recording deaths 24 hours after injection. The LD50 of each venom was calculated using Probit analysis [20] and showed the median with 95% confidence interval.
Median effective dose (ED50) assay
This test involves incubation of a challenge dose, minimal lethal dose (MLD), of venom with different volumes of the antivenom, adjusted to a constant volume with saline solution. The mixtures were incubated for 30 minutes at 37°C, then 0.1-ml aliquots of each mixture were injected subcutaneously into groups of mice (n = 5/group) with a defined weight range (20–25 g). Mice in the control group were injected with a saline solution containing the challenge dose of venom alone, which induces 100% lethality. ED50 values were determined by recording deaths 24 hours after injection. The antivenom was considered ineffective when none of mice, administered with maximum amount of antivenom (0.1 ml), survived. The ED50 of each venom was calculated using Probit analysis [20] and presented as the median with 95% confidence interval. The neutralizing capacity expressed as ED50 and ER50 (median effective ratio), which are defined as the amount of antivenom that gives 50% survival of venom-challenged mice (for ED50) and the ratio of amount of venom to the volume dose of antivenom that keep 50% alive of mice (for ER50). Another term called “potency”, expressed as the amount of venom that is completely neutralized per milliliter of antivenom, was calculated as previously described [21, 22].
Fractionation of venom proteins
Venom proteins of N. kaouthia, N. siamensis, O. hannah and N. atra were respectively separated by reverse-phase high-performance liquid chromatography (RP-HPLC) as previously described [23]. Briefly, crude venom (500 μg protein) was dissolved at 10 mg/mL in aqueous 0.1% trifluoroacetic acid (TFA) and 5% acetonitrile (ACN), and separated by RP-HPLC using a Suppelco Discovery 300 Å C18 (4.6 × 150 mm, 3 μm particle size) column. Flow rate was set to 1 mL/min, and the column was developed with a linear gradient of 0.1% TFA in water (solution A) and 0.1% TFA in ACN (solution B) as follows: isocratic 5% B for 5 minutes, followed by linear gradients of 5−40% B for 95 minutes, 40−70% B for 20 minutes, 70% B for 10 minutes, and re-equilibration with 5% B for 10 minutes. Peaks were detected by monitoring absorbance at 214 nm. Chromatographic fractions were collected manually, dried using a SpeedVac, and then stored at -20°C.
Each fraction was dissolved in sample buffer (125 mM Tris, 25% glycerol, 10% 2-mercaptoethanol, 4% SDS, 0.05% bromophenol blue), and one-half of each sample was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 15% gels. The location of proteins in SDS-PAGE gels was visualized by Coomassie Brilliant Blue staining.
In-gel tryptic digestion
After staining with Coomassie Brilliant Blue dye, the relatively abundant protein bands were excised from the gel and subjected to in-gel tryptic digestion, as described by Lin et al. [24]. Briefly, gel pieces were destained three times with 40% ACN containing 25 mM ammonium bicarbonate for 15 minutes each, reduced by incubating with 5 mM dithiothreitol at 60°C for 30 minutes, and then alkylated by incubating with 15 mM iodoacetamide at room temperature in the dark for 30 minutes. Proteins in the processed gel pieces were digested with freshly prepared trypsin solution containing 20 μg/mL of trypsin (Promega, Madison, WI, USA) in 25 mM ammonium bicarbonate at 37°C for 16 hours, then extracted with 100% ACN containing 1% TFA. Finally, the extracted tryptic peptides were concentrated by SpeedVac and stored at -20°C before use.
LC-MS/MS analysis
Each peptide sample was reconstituted with 0.1%formic acid (FA), and then analyzed on a nano-LC–LTQ-Orbitrap Hybrid Mass Spectrometer (Thermo Fisher, San Jose, CA, USA), as described previously [25]. Briefly, the sample was loaded across a trap column (Zorbax 300SB-C18, 0.3 × 5 mm; Agilent Technologies, Wilmington, DE, USA) at a flow rate of 0.2 μL/min in HPLC buffer (0.1% FA), and separated on a resolving 10-cm analytical C18 column (inner diameter, 75 μm) using a 15-μm tip (New Objective, Woburn, MA, USA). The peptides were eluted using a linear gradient of 0–10% HPLC elution buffer (99.9% ACN containing 0.1% FA) for 3 minutes, 10–30% buffer B for 35 minutes, 30–35% buffer B for 4 minutes, 35–50% buffer B for 1 minute, 50–95% buffer B for 1 minute and 95% buffer B for 8 minute, with a flow rate of 0.25 μL/min across the analytical column. The resolution of the Orbitrap is 30,000, and the ion signal of (Si(CH3)2O)6H+ at 445.120025 (m/z) was used as a lock mass for internal calibration. A procedure that alternated between one MS scan followed by six MS/MS scans for the 10 most abundant precursor ions in the MS scan was applied. The m/z values selected for MS/MS were dynamically excluded for 180 seconds. For MS scans, the m/z value of the scan range was from 400 to 2000 Da. For MS/MS scans, more than 1 × 104 ions were accumulated in the ion trap to generate MS/MS spectra. Both MS and MS/MS spectra were acquired using one scan with maximum fill-times of 1000 and 100 ms for MS and MS/MS analysis, respectively.
Database searching and bioinformatics analysis
Raw MS data files were analyzed using Proteome Discoverer Software (version 1.3.0.339; Thermo Fisher, San Jose, CA, USA) and searched against an in-house–generated Squamata database originated from the UniProt database using the MASCOT search engine (version 2.2; Matrix Science, London, UK). The enzyme specificity parameter was set to “trypsin”, and one missed cleavage was allowed. Carbamidomethylation of cysteines was set as a static modification, and oxidations of methionine, acetyl (protein N-term) and Gln- > pyro-Glu (N-term Q) were set as dynamic modifications. The tolerance of MS was 10 ppm and that of MS/MS was 0.5 Da. The decoy database search approach was assessed for peptide identification, and the criteria of target false discover rate (FDR) was estimated to be <0.01 in this study. Each reported protein ID should have at least two peptide presenting in the sample, and at least one is the unique peptide for the reported protein.
Western blot analysis
HPLC-fractionated samples (totally 200 μg) were resolved by SDS-PAGE, transferred onto polyvinylidene difluoride (PVDF) membranes, and then probed by incubating with 1:5000 (v/v) dilution of antivenom (stock solution, 80 mg/ml) at 4°C for 16 hours. Antivenom-reactive proteins were detected by incubating for 1 hour with alkaline phosphatase-conjugated anti-horse IgG secondary antibodies (Santa Cruz Biotechnology) and visualized using the CDP-Star Western Blot Chemiluminescence Reagent (PerkinElmer, Boston, MA, USA) with fluorescence detection.
Results
Pre-clinical evaluation of the cross-neutralization ability of FNAV
The lethality of the three Southeast Asian cobra venoms, measured as the subcutaneous (s.c.) LD50, was evaluated in a mouse model; the results are shown in Table 1. The LD50 of venom proteins from N. kaouthia, N. siamensis, and O. hannah were determined to be 0.34, 0.56, and 0.98 μg/g, respectively. Neutralization assays performed in mice injected with the minimum lethal dose of venom proteins from each cobra showed that FNAV effectively prevented death of mice induced by venoms of N. kaouthia and N. siamensis; ED50 values were 4.02 μL/mouse (Potency = 0.49 mg/ml) for N. kaouthia and 18.33 μL/mouse (Potency = 0.23 mg/ml) for N. siamensis. However, the lethality of O. hannah venom was not neutralized by FNAV, even at maximum dose of 100 μL/mouse (Table 1).
Table 1. Cross-neutralization ability of FNAV against venoms from N. kaouthia, N. siamensis and O. hannah.
| Venom | Lethality | FNAV | |||
|---|---|---|---|---|---|
| LD50a (μg/g) | MLDb (μg/g) | ED50 (μL/mouse) | ER50 (mg/ml) | Potencyc (mg/ml) | |
| Naja kaouthia | 0.34 (0.22–0.39) | 0.471 ± 0.02 | 4.02 (1.27–5.43) | 2.19 (1.16–6.91) | 0.49 |
| Naja siamensis | 0.56 (0.35–0.62) | 0.756 ± 0.05 | 18.33 (0.59–1.04) | 0.85 (0.65–1.43) | 0.23 |
| Ophiophagus hannah | 0.98 (0.75–1.08) | 1.325 ± 0.08 | NEd | NE | NE |
a LD50: Median Lethal Dose—the dose of venom that induces lethality in 50% of subcutaneously injected mice (20–25 g). The values in parentheses are 95% confidence limits.
b MLD: Minimum Lethal Dose—the lowest dose of venom that induces lethality in 100% of subcutaneously injected mice.
c Potency: defined as the amount of venom in milligram was completely neutralized (100% protection) per milliliter of antivenom.
d NE: Display ineffective.
Proteomic analysis of venom proteomes of the four Southeast Asian cobras
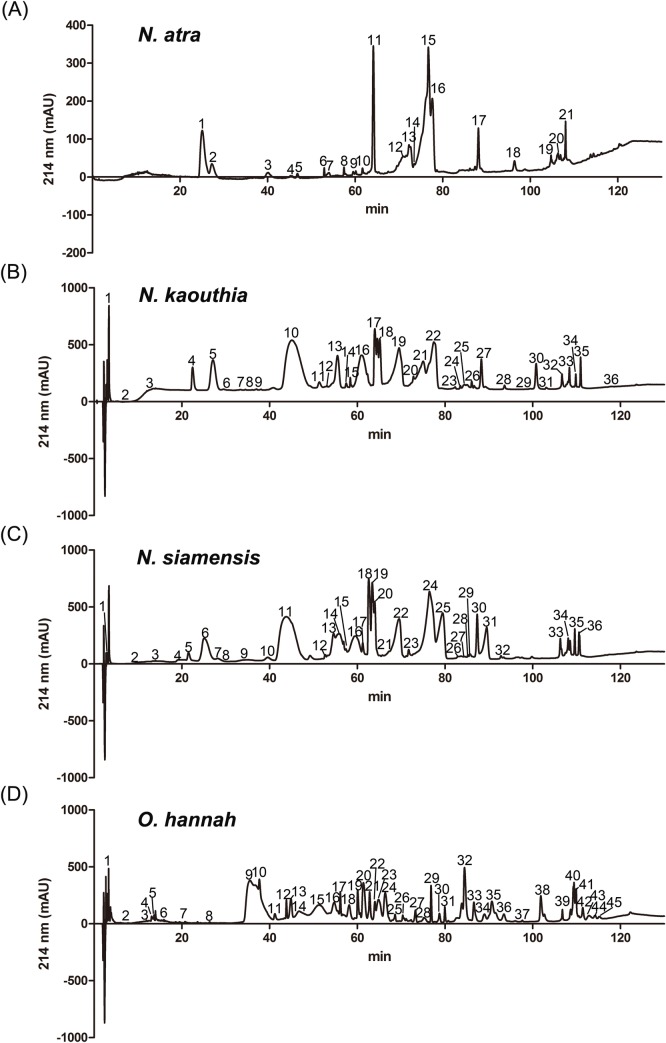
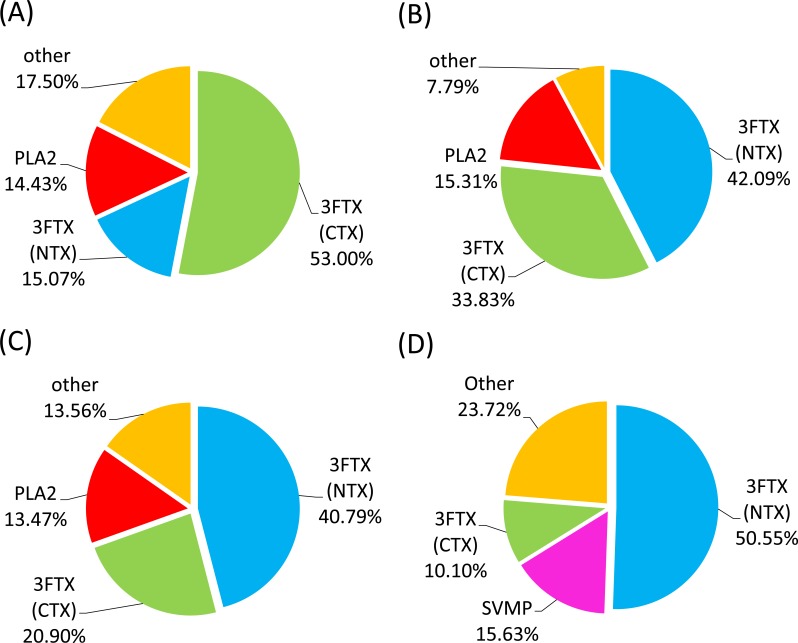
For the venom of N. atra, 21 fractions were collected from HPLC analysis (Fig 1A), and a total of 36 protein bands were assessed by LC-MS/MS for protein identification (Fig 2A and S1 Fig). Protein identification results are summarized in Table 2; additional detailed information is provided in S1 Table. The identified proteins belonged to eight protein families: three-finger toxin (3FTX), phospholipase A2 (PLA2), cysteine-rich secretory protein (CRISP), ohanin/vespryn (O/V), snake venom metalloproteinase (SVMP), venom nerve growth factor (VNGF), 5’ nucleotidase (5NT), and L-amino acid oxidase (LAAO). The 3FTX family proteins could be further categorized into the sub-families, cytotoxin (CTX), neurotoxin (NTX), and muscarinic toxin (MTX). Among these, CTX (53%), NTX (15%) and PLA2 (14%) were the dominant components identified in the N. atra venom proteome (Fig 3A and S2 Table).
Fig 1. RP-HPLC separation of proteins from each cobra venom.
The crude venoms of (A) N. atra, (B) N. kaouthia, (C) N. siamensis, and (D) O. hannah were fractionated on a C18 column; each fraction was collected manually. Shown are the chromatographic patterns for the four HPLC-separated venoms.
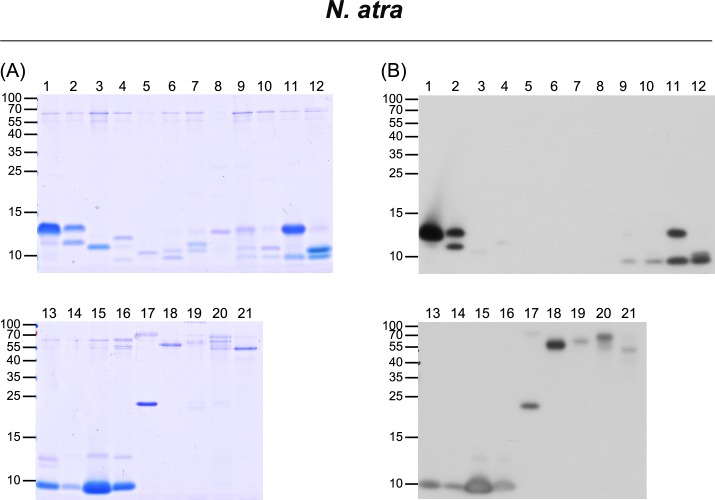
Fig 2. Characterization of HPLC-separated, FNAV-recognizable N. atra venom proteins by LC-MS/MS and Western blot analyses.
HPLC-separated fractions of N. atra venom proteins were analyzed by SDS-PAGE, followed by (A) Coomassie Blue staining and (B) Western blotting using FNAV as a probe.
Table 2. Summary of proteins identified in four venoms by LC-MS/MS analysis.
| N. atra venom | N. kaouthia venom | N. siamensis venom | O. hannah venom | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gel site | Protein Family | Gel site | Protein Family | Gel site | Protein Family | Gel site | Protein Family | Gel site | Protein Family | Gel site | Protein Family | Gel site | Protein Family |
| 1–1 | 3FTX (NTX) | 4–1 | PLA2 | 18–5 | 3FTX (CTX) | 4–1 | LAAO | 28–2 | PLA2 | 9–1 | 3FTX (NTX) | 38–1 | SVMP |
| 1–2 | 3FTX (NTX) | 5–1 | Unknown | 19–1 | Unknown | 5–1 | PLA2 | 29–1 | CRISP | 10–1 | 3FTX (NTX) | 38–2 | SVMP |
| 2–1 | PLA2 | 5–2 | Unknown | 19–2 | Unknown | 6–1 | 3FTX (NTX) | 29–2 | CRISP | 11–1 | 3FTX (NTX) | 40–1 | SVMP |
| 2–2 | 3FTX (NTX) | 5–3 | PLA2 | 19–3 | Unknown | 6–2 | 3FTX (NTX) | 29–3 | PLA2 | 12–1 | 3FTX (NTX) | 40–2 | SVMP |
| 3–1 | 3FTX (NTX) | 5–4 | 3FTX (NTX) | 19–4 | 3FTX (CTX) | 7–1 | Unknown | 29–4 | 3FTX (CTX) | 13–1 | 3FTX (NTX) | 41–1 | SVMP |
| 4–1 | 3FTX (MTX) | 6–1 | PLA2 | 20–1 | Unknown | 7–2 | 3FTX (NTX) | 30–1 | CRISP | 14–1 | 3FTX (NTX) | 42–1 | SVMP |
| 4–2 | Unknown | 8–1 | Unknown | 20–2 | VNGF | 9–1 | 3FTX (NTX) | 30–2 | 3FTX (CTX) | 15–1 | SVMP | 43–1 | SVMP |
| 5–1 | 3FTX (NTX) | 8–2 | Unknown | 20–3 | Unknown | 10–1 | 3FTX (NTX) | 31–1 | CRISP | 15–2 | 3FTX (NTX) | 44–1 | Unknown |
| 6–1 | 3FTX (NTX) | 8–3 | 3FTX (NTX) | 20–4 | 3FTX (CTX) | 11–1 | 3FTX (NTX) | 31–2 | 3FTX (NTX) | 16–1 | 3FTX (NTX) | 45–1 | Unknown |
| 6–2 | 3FTX (NTX) | 8–4 | 3FTX (NTX) | 21–1 | PLA2 | 12–1 | 3FTX (NTX) | 32–1 | Unknown | 16–2 | Kunitz | - | - |
| 7–1 | 3FTX (NTX) | 9–1 | Unknown | 21–2 | 3FTX (CTX) | 13–1 | 3FTX (NTX) | 33–1 | PDE | 17–1 | Unknown | ||
| 7–2 | 3FTX (NTX) | 9–2 | 3FTX (NTX) | 21–3 | 3FTX (CTX) | 14–1 | 3FTX (NTX) | 33–2 | 5NT | 17–2 | Unknown | ||
| 8–1 | VNGF | 10–1 | 3FTX (NTX) | 22–1 | O/V | 15–1 | Unknown | 34–1 | 5NT | 17–3 | 3FTX (NTX) | ||
| 9–1 | PLA2 | 11–1 | 3FTX (NTX) | 22–2 | 3FTX (CTX) | 15–2 | VNGF | 34–2 | CVF | 17–4 | 3FTX (NTX) | ||
| 9–2 | PLA2 | 11–2 | 3FTX (NTX) | 23–1 | 3FTX (CTX) | 15–3 | 3FTX (NTX) | 35–1 | SVMP | 18–1 | 3FTX (NTX) | ||
| 9–3 | 3FTX (CTX) | 12–1 | 3FTX (NTX) | 24–1 | Unknown | 16–1 | PLA2 | 36–1 | SVMP | 18–2 | 3FTX (NTX) | ||
| 10–1 | PLA2 | 12–2 | 3FTX (NTX) | 24–2 | CRISP | 16–2 | 3FTX (NTX) | - | - | 19–1 | Unknown | ||
| 10–2 | 3FTX (MTX) | 13–1 | SVMP | 24–3 | O/V | 17–1 | PLA2 | 19–2 | PLA2 | ||||
| 10–3 | 3FTX (CTX) | 13–2 | PLA2 | 24–4 | 3FTX (NTX) | 17–2 | 3FTX (MTX) | 19–3 | 3FTX (CTX) | ||||
| 11–1 | PLA2 | 13–3 | 3FTX (NTX) | 25–1 | Unknown | 18–1 | Unknown | 20–1 | 3FTX (CTX) | ||||
| 11–2 | 3FTX (CTX) | 14–1 | PLA2 | 25–2 | CRISP | 18–2 | PLA2 | 21–1 | PLA2 | ||||
| 12–1 | 3FTX (CTX) | 14–2 | 3FTX (NTX) | 25–3 | PLA2 | 18–3 | 3FTX (CTX) | 21–2 | 3FTX (CTX) | ||||
| 12–2 | 3FTX (CTX) | 14–3 | 3FTX (NTX) | 25–4 | 3FTX (NTX) | 19–1 | PLA2 | 22–1 | 3FTX (CTX) | ||||
| 13–1 | VNGF | 14–4 | 3FTX (MTX) | 26–1 | CRISP | 19–2 | 3FTX (CTX) | 23–1 | 3FTX (CTX) | ||||
| 13–2 | VNGF | 15–1 | VNGF | 26–2 | 3FTX (NTX) | 20–1 | Unknown | 24–1 | 3FTX (NTX) | ||||
| 13–3 | 3FTX (CTX) | 15–2 | 3FTX (NTX) | 27–1 | SVMP | 20–2 | PLA2 | 25–1 | 3FTX (NTX) | ||||
| 14–1 | 3FTX (CTX) | 16–1 | Unknown | 27–2 | CRISP | 20–3 | 3FTX (CTX) | 26–1 | Unknown | ||||
| 15–1 | O/V | 16–2 | Unknown | 28–1 | CRISP | 21–1 | 3FTX (CTX) | 27–1 | PLA2 | ||||
| 15–2 | 3FTX (CTX) | 16–3 | PLA2 | 29–1 | SVMP | 22–1 | PLA2 | 28–1 | 3FTX (NTX) | ||||
| 16–1 | O/V | 16–4 | PLA2 | 30–1 | SVMP | 22–2 | 3FTX (CTX) | 29–1 | 3FTX (NTX) | ||||
| 16–2 | 3FTX (CTX) | 16–5 | 3FTX (NTX) | 31–1 | SVMP | 23–1 | VNGF | 30–1 | Unknown | ||||
| 17–1 | CRISP | 16–6 | 3FTX (NTX) | 32–1 | PDE | 23–2 | 3FTX (CTX) | 31–1 | O/V | ||||
| 18–1 | SVMP | 17–1 | Unknown | 32–2 | 5NT | 24–1 | O/V | 32–1 | CRISP | ||||
| 19–1 | 5NT | 17–2 | PLA2 | 32–3 | SVMP | 24–2 | 3FTX (CTX) | 33–1 | SVMP | ||||
| 20–1 | LAAO | 17–3 | 3FTX (NTX) | 32–4 | GPX | 25–1 | 3FTX (CTX) | 34–1 | Unknown | ||||
| 21–1 | SVMP | 17–4 | 3FTX (NTX) | 33–1 | LAAO | 26–1 | Unknown | 35–1 | Unknown | ||||
| - | - | 18–1 | Unknown | 33–2 | CVF | 26–2 | 3FTX (CTX) | 35–2 | SVMP | ||||
| 18–2 | Unknown | 33–3 | GPX | 27–1 | CRISP | 35–3 | O/V | ||||||
| 18–3 | PLA2 | 34–1 | SVMP | 27–2 | 3FTX (CTX) | 35–4 | CRISP | ||||||
| 18–4 | Unknown | 35–1 | SVMP | 28–1 | CRISP | 36–1 | SVMP | ||||||
Fig 3. Pie charts showing the relative abundance of cobra venom protein families.
The composition of venom proteins from (A) N. naja, (B) N. kaouthia, (C) N. siamensis, and (D) O. hannah. Results are based on venom protein identification and HPLC peak intensity, and show the major protein components, expressed as a percentage.
The venom of N. kaouthia yielded 36 protein fractions in HPLC analyses (Fig 1B). A total of 79 protein bands were analyzed by LC-MS/MS analysis for protein identification (Fig 4A and S2 Fig). Eleven different protein families were identified (Table 2): 3FTX (NTX), 3FTX (CTX), PLA2, SVMP, CRISP, O/V, LAAO, VNGF, glutathione peroxidase (GPX), 3FTX (MTX), cobra venom factor (CVF), 5NT, and phosphodiesterase (PDE). Detailed information is shown in S3 Table. The top three major protein components were similar to those of the venom proteome of N. atra; however, the most abundant protein family was NTX (40%) rather than CTX (Fig 3B and S2 Table).
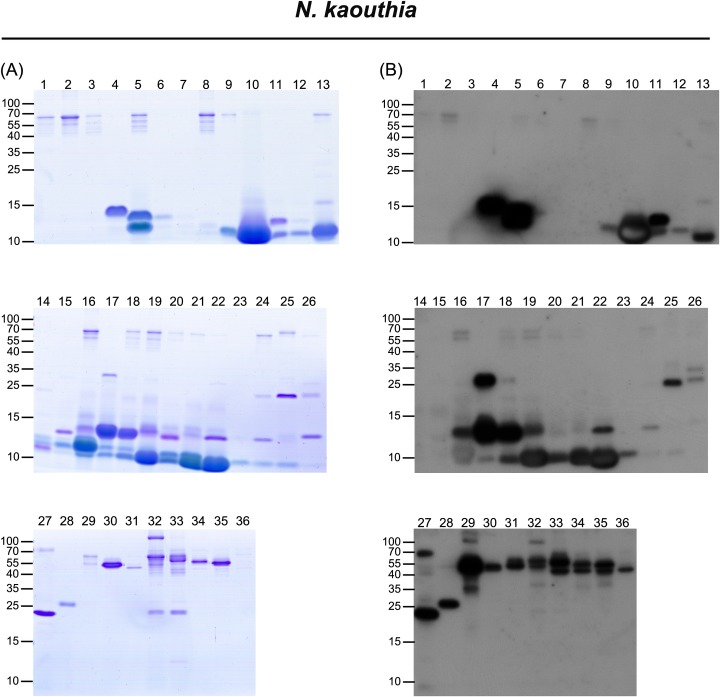
Fig 4. Characterization of HPLC-separated, FNAV-recognizable N. kaouthia venom proteins by LC-MS/MS and Western blot analyses.
HPLC-separated fractions of N. kaouthia venom proteins were analyzed by SDS-PAGE, followed by (A) Coomassie Blue staining and (B) Western blotting using FNAV as a probe.
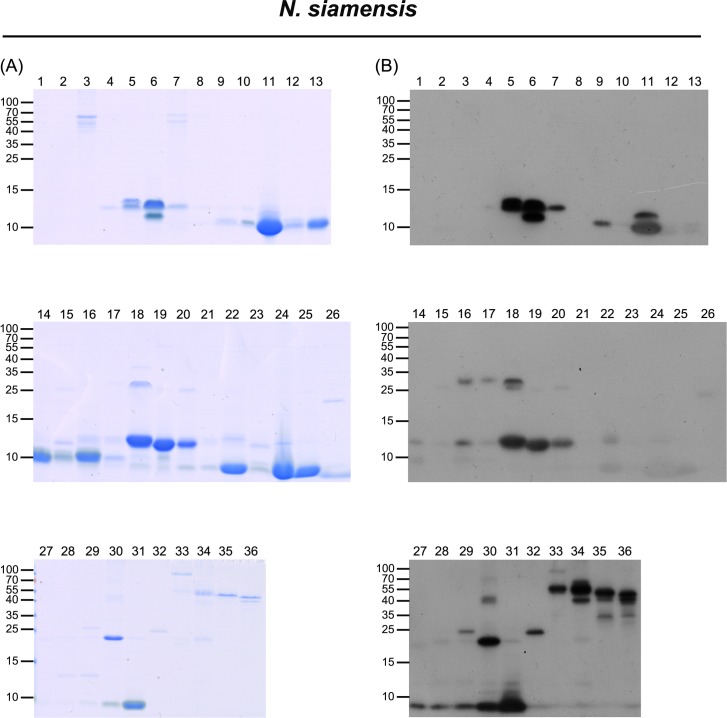
Thirty-six fractions were obtained from HPLC separation of N. siamensis venom (Fig 1C), and 56 protein bands were selected for LC-MS/MS analysis (Fig 5A and S3 Fig). The identified proteins could be categorized into nine protein families: 3FTX (NTX), 3FTX (CTX), PLA2, CRISP, SVMP, 5NT, 3FTX (MTX), VNGF, LAAO, O/V and CVF (Table 2). Detailed information is shown in S4 Table. The relative abundances of protein components in this venom were very similar to those of N. kaouthia; the three major protein families were NTX (42%), CTX (33%) and PLA2 (15%), which collectively accounted for approximately 90% of N. siamensis venom proteins (Fig 3C and S2 Table).
Fig 5. Characterization of HPLC-separated, FNAV-recognizable N. siamensis venom proteins by LC-MS/MS and Western blot analyses.
HPLC-separated fractions of N. siamensis venom proteins were analyzed by SDS-PAGE, followed by (A) Coomassie blue staining and (B) Western blotting using FNAV as a probe.
The venom of O. hannah was initially separated into 45 fractions by HPLC analysis (Fig 1D), and further resolved into 49 protein bands for LC-MS/MS analysis (Fig 6A and S4 Fig). Only six protein families were identified (Table 2): 3FTX (NTX), SVMP, 3FTX (CTX), CRISP, PLA2, O/V and Kunitz-type protease inhibitor (Kunitz). The results of these analyses are shown in S5 Table. Unlike the case for the other three cobras, the three predominant protein components in O. hannah venom were NTX (50%), SVMP (15%) and CTX (10%), with PLA2 accounting for only 3–4% of the venom components (Fig 3D and S2 Table).
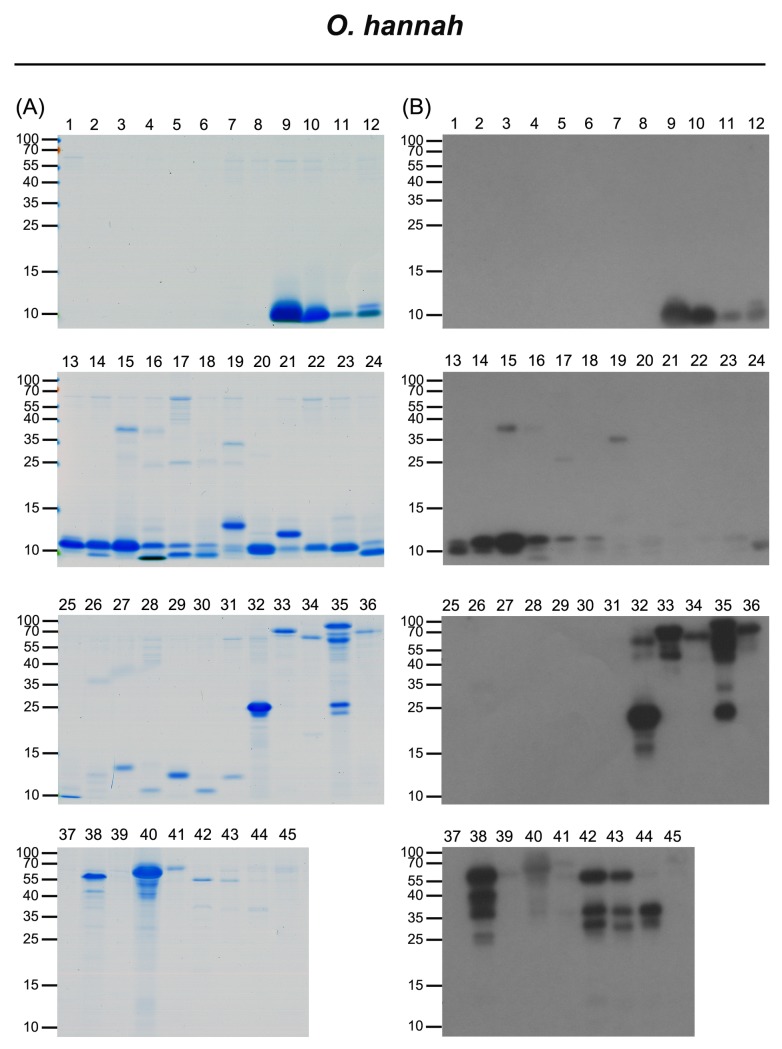
Fig 6. Characterization of HPLC-separated, FNAV-recognizable O. hannah venom proteins by LC-MS/MS and western blot analyses.
HPLC-separated fractions of O. hannah venom proteins were analyzed by SDS-PAGE, followed by (A) Coomassie Blue staining and (B) Western blotting using FNAV as a probe.
Immunological profile of FNAV against venom proteins of the four cobras
Using Western blotting to investigate the immunoreactivity of FNAV towards the isolated protein fractions of the four snake venoms, we found that most of the components of N. atra venom were well recognized by FNAV (Fig 2B), including CTX (factions 11–16), NTX (fractions 1, 2), PLA2 (fraction 11), CRISP (fraction 17), and SVMP (fraction 18). Minor protein components present in fractions 3–8 did not, or weakly react with FNAV. The proteins identified in these fractions were categorized as belonging to NTX, MTX, and VNGF protein families (Table 2). Similarly, most of the major components of N. kaouthia and N. siamensis venoms were also reactive to FNAV. These included NTX (factions 10, 16), CTX (factions 19–22) and PLA2 (factions 4, 5, 17, 18) of N. kaouthia (Fig 4B), and NTX (factions 6, 11, 31), PLA2 (fractions 5, 18–20) and CTX (factions 27–30) of N. siamensis (Fig 5B). However, protein components in fractions 14 and 15 of N. kaouthia venom (Fig 4B and Table 2) and fractions 21, 22 (band 2), 23, 24, 25 and 26 (band 2) of N. siamensis venom (Fig 5B and Table 2) were weakly recognized by FNAV in Western blots, suggesting that these proteins may not be neutralized by FNAV, even though FNAV was capable of blocking the lethality of these two venoms in our animal model. Protein identification results (Table 2) showed that the major proteins in these fractions belonged to members of the CTX family. In addition to CTX, some NTX proteins detected in fractions 14–16 of N. siamensis venom also displayed very weak signals in Western blot analyses. For HPLC-fractionated O. hannah venom, NTX (fractions 9–18), CRISP (fraction 32, 35) and SVMP (fraction 33, 35, 38, 40, 42–44) immunoreacted strongly toward FNAV, whereas those in fractions 19 (band 3), 20, 21 (band 2), 22, 23, 28 and 29 were weakly detected, or not detected, by Western blotting (Fig 6and Table 2). The major protein in these fractions with lower immunereactivity was identified as β-cardiotoxin, a member of the CTX protein family (S5 Table). Moreover, the major proteins in fractions 19, 21 and 27, identified as PLA2 family proteins, also showed poor immunoreactivity to FNAV.
Discussion
It should not come as a great surprise that species within the same genus would have evolved similar venom components. Thus, is conceivable that FNAV against the venom of N. atra could neutralize the snakebite of other Naja species. However, no dependable report has been provided to confirm this. In this study, we evaluated the therapeutic potential of FNAV against the venoms of three Southeast Asian venomous snakes and verified that FNAV neutralizes the lethal effects of N. kaouthia and N. siamensis venom in a mouse model.
Taiwan has more than four decades of experience in antivenom manufacture and refinement. Taiwan’s antivenoms are recognized for their quality and are known to be among the best antivenoms in the world. According to a previous clinical survey, most Taiwanese patients are successfully treated by administration of 1 vial of antivenom and are typically discharged without complications [18]. From the clinical perspectives, N. atra causes severe local necrosis but little flaccid paralysis in envenomed humans, however, the venom from cobra species (N. simensis and N. kauothia) used in this study predominately cause flaccid paralysis and severe local necrosis in envenomed subjects. Other relevant in-vivo tests such as minimum necrotic dose [26] and in-vitro nerve-muscle preparations [27, 28] should be performed before we can claim the clinical effectiveness of FNAV against N. siamensis and N. kaouthia snakebites [29]. For example, the neutralization of neurotoxicity by N. kaouthia monovalent antivenom (NKMAV) against three N. kaouthia from different regions has been evaluated by using the chick biventer cervicis nerve-muscle preparation system [30]. The alpha-neurotoxin-induced twitch depression could be prevented by pre-incubation of tissue with NKMAV, however, it didn’t fully restore the nerve-muscle contraction when the NKMAV added after the twitch depression onset. Therefore, this observation indicates that even though the FNAV has the ability to neutralize the lethality, or even the neurotoxicity in this neutralization assay, whether it can be used in real clinical setting (i.e. addition of antivenom after the symptoms onset) should be further confirmed. In addition, few studies have evaluated the immunoreactivity of antivenom against cobra venoms, and the results indicated the relatively lower neutralization ability toward NTX and CTX and PLA2 [31, 32].Further suggestions have been put forward to solve the low potency of cobra antivenom by preparing a purified venom-mixture containing only NTX, CTX and PLA2 as immunogens for antivenom preparation [31, 33]. Our current study on the immunoreactivity of FNAV also revealed that the NTX, CTX and PLA2 are the major immunological toxins in N. kaouthia and N. siamensis venom as well. The "cross-neutralization" phenomenon of FNAV against N. kaouthia and N. siamensis is potentially useful for further research into common antigenicity and perhaps the merging of a broader scale polyspecific antivenom, but more works need to be done to elucidate the immunological properties related to each major toxins involved in the pathophysiology and cross-neutralization.
Recently, three polyvalent antivenoms and one monovalent antivenom have been used to evaluate the neutralization potency against venoms of N. kaouthia and N. siamensis [7, 26]. The potency of Vin polyvalent antivenom (VPAV) and Bharat polyvalent antivenom, both raised against Indian Naja naja, Bungarus caeruleus, Daboia russelli and Echis carinatus, were determined to be 0.28 mg/ml and 0.37 mg/ml for N. kaouthia venom, respectively. To neutralize the venom of N. siamensis, the potency of the two antivenoms were reported to be 0.52 mg/ml and 0.14 mg/ml, respectively [26]. The neuro polyvalent antivenom (NPAV) was obtained from horse hyperimmunized with venoms of N. kaouthia, O. hannah, Bungarus candidus and Bungarus fasciatus. It has been demonstrated that both NPAV and the NKMAV have the same potency to neutralize the venoms of N. Kaouthia (0.94 mg/ml) and N. siamensis (1.15 mg/ml) [7]. Thus, these polyvalent antivenoms including FNAV reported here have the therapeutic potential for N. kaouthia and N. siamensis envenoming, and may serve as backup materials for snakebite treatment. Further analyses of these polyvalent antivenoms are needed to evaluate and compare their potential for clinical usage, such as the IgG content per antivenom, thermal stability, adverse effect and microbial contamination.
During our exploration of the FNAV-recognizable venom proteome of N. atra, we surprisingly found that protein components present in HPLC fractions 3–8 of N. atra venom (Fig 2) were weakly recognized by FNAV, considerably lower than others, despite the fact that FNAV was generated in horses hyperimmunized with N. atra venom. These proteins were identified by MS-based analysis as long neurotoxin homolog proteins (S1 Table). According to previous studies [34, 35], even though it has a characteristic of five disulfide bridges which can be classified as long neurotoxin, it exhibited the ability to inhibit acetylcholine-induced muscle contraction as cobrotoxin, a short neurotoxin; however, the degree of inhibition was less than half that of cobrotoxin. On the other hand, FNAV could react strongly toward fractions 1 and 2 from N. atra venom, which were identified as NTX and constituted ~15% of the whole venom. The short neurotoxin subtypes eluted in the early phase of RP-HPLC have been previously evaluated in mice models as the most lethal components in cobra venoms [13, 36]. These observations collectively suggest that the effectiveness of FNAV toward N. atra venom in mice models could be mainly contributed by the recognition of NTXs.
Our data showed that N. kaouthia and N. siamensis venoms could be cross-neutralized by FNAV. The classification of these species is still a matter of dispute, with some databases, such as Uniprot (http://www.uniprot.org/taxonomy/8649), considering N. siamensis to be the same as N. kaouthia. The data from our present study indicate the high similarity between the N. kaouthia and N. siamensis venoms; the LD50 of N. kaouthia and N. siamensis venoms differ only marginally (0.34 μg/g v.s. 0.56 μg/g, with overlapped 95% C.I. 0.22–0.39 v.s. 0.35–0.62, see Table 1) and their composition patterns in term of the major components (~40% neurotoxins, 15% PLA2 in particular, see Fig 3) are almost comparable (based on chromatogram and proteomes, see Figs 1, 4 and 5). In spite of these similarities, we also observed differences between their venom proteome. For example, the protein “hemorrhagic metalloproteinase-disintegrin-like kaouthiagin (P82942)” identified in fraction 30 from N. kaouthia venom was not detected N. siamensis venom. Additionally, N. siamensis venom contained a fewer amount of cytotoxin proteins as compared to N. kaouthia venom. These venom antigen variations probably led to the difference in the neutralization of FNAV tested in mice.
N. kaouthia is primarily distributed to Malaysia, Thailand and Vietnam, and its venom proteomes and toxicity in these countries have been previously reported [7, 37]. The lethality of N. kaouthia venom in these different regions is reportedly different, with that from Thailand being more venomous than Malaysian or Vietnamese venom. Furthermore, the Thai N. kaouthia venom contains higher amounts of long neurotoxins, while the Malaysian and Vietnamese specimens are particularly rich in cytotoxins. This geographical proteomic variation supported the observation that N. kaouthia venom from Thailand has higher neuromuscular depressant activity than that from Malaysia or Vietnam [30]. In the present study, we only tested the ability of FNAV to neutralize the venom of N. kaouthia from Thailand. Thus, if there are future hopes of using FNAV to treat N. kaouthia envenomation in Malaysia and Vietnam, the ability of FNAV to cross-neutralize N. kaouthia venom from Malaysia and Vietnam should be re-evaluated, notwithstanding the fact that these are the same species as N. kaouthia from Thailand. In addition, the neutralization of the foundation toxicity from these venoms by FNAV should be tested in the future as well.
O. hannah venom represents the only venom that could not be neutralized by FNAV in current study, although FNAV did cross-reacted intensely with the major lethal toxins (neurotoxins, which formed 50% of proteome based on our venom proteome and immunoprofiling analyses as shown in Figs 3 and 6and S5 Table). It was found that β-cardiotoxin, identified in the HPLC fractions 20–23, is one of venom proteins weakly recognized or even non-recognized by FNAV. Previous studies have reported that β-cardiotoxin is a natural exogenous β-blocker [38] that can bind to β1- and β2-adrenergic receptors, causing a dose-dependent decrease in heart rate. Intraperitoneal injection of this protein into mice induces labored breathing, impaired locomotion, and death within 30 minutes; however, the lethal dose of β-cardiotoxin is higher than 10 μg/g, suggesting that β-cardiotoxin might not be the major toxins of O. hannah venom. In addition, PLA2 in O. hannah venom also showed poor immunoreactivity to FNAV. It is well known that there are numerous PLA2 isoforms with different physiological/pathological functions in the snake venoms. Although these PLA2s show very high similarity in their three dimensional folding, their primary structures (amino acid sequences) can be varied significantly [39]. Theoretically, these sequence variations may confer distinct immunological properties for different PLA2s. We aligned and compared the sequences between PLA2s identified from different snakes, O. hannah, N. atra, N. kaouthia and B. multicinctus(S5 Fig). This analysis revealed that the sequence similarity is quite low between O. Hannah PLA2 and N. atra PLA2 (64%) or between O. Hannah PLA2 and B. multicinctus PLA2 (59%). However, the sequence similarity between N. atra PLA2 and N. kaouthia PLA2 is much higher (up to 95%). Therefore, this alignment analysis together with our immunological profiling data suggest that O. Hannah PLA2 might have distinct antigenic site(s) as compared with PLA2 from venoms of N. atra, N. kaouthia and B. multicinctus. Although we observed that the immunorecognition of FNAV toward β-cardiotoxin and PLA2 is weakly, the pharmacological activities of both β-cardiotoxin and PLA2 seem not to correlate with the major symptom (neurotoxicity) caused by O. hannah venom. Hence, the conflicting finding that FNAV could react strongly to the major neurotoxins yet failed to neutralize the lethality at the challenge dose used remains unresolved here and warrants further study. Furthermore, O. hannah is the only venomous snake whose whole genome has been sequenced [40]. Its venom transcriptome and proteome have been studied as well [23, 40–43]. These studies reported the presence of different amounts of LAAO family proteins in the O. hannah venom, which may be due to geographical variation of the venom. However, there were not any LAAO proteins identified in our O. hannah venom proteome. The exact reason(s) for this discrepancy is (are) currently unknown. One of the possible reasons is that the amount of LAAO protein in our O. hannah venom might be too low to be detected after the whole process for venom sample preparation and fractionation. Another possibility is that LAAO protein might have been degraded in the venom after long-term storage and thus could not be detected.
We have characterized the venom proteomes and FNAV-recognizable venom proteins of these four Southeast Asian snakes, N. atra, N. kaouthia, N. siamensis and O. hannah, allowing us to identify the major venom components, both FNAV-reactive and -unreactive. This information should advance our understanding of venom immunogenicity and facilitate further improvement of antivenom design, which allow us to predict the cross neutralization to the level of cobra specific toxins [12]. For venoms from three Naja species—N. atra, N. kaouthia and N. siamensis—the three major venom components were identified as CTX, NTX and PLA2, which also represent the dominant targets recognized by FNAV. The sequences of these three components are highly similar between each Naja species, and the major functions of them are responsible for the toxic effects, necrosis, and neurotoxicity observed in cobra-envenomed patients [44–47]. Our study further strengthens the previous report that CTX, NTX and PLA2 are the most abundant and medically-relevant toxin components in the venom of cobra species [13, 14, 31]. To extend the use of FNAV for treating life-threatening snake envenomations in areas with antivenom shortages, it would be ideal to determine the ability of FNAV to neutralize the venom from all Naja species. However, because the venoms from several countries are unavailable, we were only able to obtain venoms from three Naja species for the present study. Three other Naja species—Naja naja, Naja nivea and Naja haje—are important targets for further studies to evaluate the FNAV cross-neutralization ability. N. naja is mainly distributed in India, where a large proportion of global snakebites occur [4]. Snakebite mortality remains high in modern India, with approximately 40,000 deaths per year [48, 49]. On the other hand, N. nivea and N. haje live in Africa, where few antivenoms are available and antivenom is in short supply [50]. These two areas may urgently need new antivenoms to solve their local snakebite crises.
Supporting information
(TIF)
(TIF)
(TIF)
(TIF)
(A) N. atra versus O. hannah, (B) B. multicinctus versus O. hannah, and (C) N. atra versus N. kaouthia.
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
We wish to acknowledge Dr. Kun-Yi Chien and Dr. Chi-Der Chen and the Chang Gung University Proteomics Core Laboratory for their excellent assistance generating the proteomics data presented in this investigation.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Ministry of Education, Taiwan (grant EMRPD1F0031 and EMRPD1G0021 to Chang Gung University), by the Ministry of Science and Technology, Taiwan (grants MOST 104-2325-B-182-003 and 105-2325-B-182-001 to JSY); and by Chang Gung Memorial Hospital, Taiwan (grants CMRPG3F0241-242 to CCLin, CIRPD3B0013, CRRPD1F0051, CMRPD190364, CLRPD190016 and BMRP208 to JSY) The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chippaux JP. Snake-bites: appraisal of the global situation. Bull World Health Organ. 1998;76(5):515–24. [PMC free article] [PubMed] [Google Scholar]
- 2.Harrison RA, Hargreaves A, Wagstaff SC, Faragher B, Lalloo DG. Snake envenoming: a disease of poverty. PLoS Negl Trop Dis. 2009;3(12):e569 doi: 10.1371/journal.pntd.0000569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swaroop S, Grab B. Snakebite mortality in the world. Bull World Health Organ. 1954;10(1):35–76. [PMC free article] [PubMed] [Google Scholar]
- 4.Kasturiratne A, Wickremasinghe AR, de Silva N, Gunawardena NK, Pathmeswaran A, Premaratna R, et al. The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008;5(11):e218 doi: 10.1371/journal.pmed.0050218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reid HA. Cobra-Bites. Br Med J. 1964;2(5408):540–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casewell NR, Cook DA, Wagstaff SC, Nasidi A, Durfa N, Wuster W, et al. Pre-clinical assays predict pan-African Echis viper efficacy for a species-specific antivenom. PLoS Negl Trop Dis. 2010;4(10):e851 doi: 10.1371/journal.pntd.0000851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leong PK, Sim SM, Fung SY, Sumana K, Sitprija V, Tan NH. Cross neutralization of Afro-Asian cobra and Asian krait venoms by a Thai polyvalent snake antivenom (Neuro Polyvalent Snake Antivenom). PLoS Negl Trop Dis. 2012;6(6):e1672 doi: 10.1371/journal.pntd.0001672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calvete JJ, Sanz L, Perez A, Borges A, Vargas AM, Lomonte B, et al. Snake population venomics and antivenomics of Bothrops atrox: Paedomorphism along its transamazonian dispersal and implications of geographic venom variability on snakebite management. J Proteomics. 2011;74(4):510–27. doi: 10.1016/j.jprot.2011.01.003 [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez JM, Solano G, Pla D, Herrera M, Segura A, Villalta M, et al. Assessing the preclinical efficacy of antivenoms: from the lethality neutralization assay to antivenomics. Toxicon. 2013;69:168–79. doi: 10.1016/j.toxicon.2012.11.016 [DOI] [PubMed] [Google Scholar]
- 10.Lomonte B, Escolano J, Fernandez J, Sanz L, Angulo Y, Gutierrez JM, et al. Snake venomics and antivenomics of the arboreal neotropical pitvipers Bothriechis lateralis and Bothriechis schlegelii. J Proteome Res. 2008;7(6):2445–57. doi: 10.1021/pr8000139 [DOI] [PubMed] [Google Scholar]
- 11.Pla D, Gutierrez JM, Calvete JJ. Second generation snake antivenomics: comparing immunoaffinity and immunodepletion protocols. Toxicon. 2012;60(4):688–99. doi: 10.1016/j.toxicon.2012.04.342 [DOI] [PubMed] [Google Scholar]
- 12.Calvete JJ, Sanz L, Pla D, Lomonte B, Gutierrez JM. Omics meets biology: application to the design and preclinical assessment of antivenoms. Toxins (Basel). 2014;6(12):3388–405. Epub 2014/12/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laustsen AH, Gutierrez JM, Lohse B, Rasmussen AR, Fernandez J, Milbo C, et al. Snake venomics of monocled cobra (Naja kaouthia) and investigation of human IgG response against venom toxins. Toxicon. 2015;99:23–35. Epub 2015/03/17. doi: 10.1016/j.toxicon.2015.03.001 [DOI] [PubMed] [Google Scholar]
- 14.Tan NH, Wong KY, Tan CH. Venomics of Naja sputatrix, the Javan spitting cobra: A short neurotoxin-driven venom needing improved antivenom neutralization. J Proteomics. 2017;157:18–32. Epub 2017/02/06. doi: 10.1016/j.jprot.2017.01.018 [DOI] [PubMed] [Google Scholar]
- 15.Liau MY, Huang RJ. Toxoids and antivenoms of venomous snakes in Taiwan. J Toxicol-Toxin Rev. 1997;16(3):163–75. [Google Scholar]
- 16.Chieh-Fan C, Tzeng-Jih L, Wen-Chi H, Hua-Wei Y. Appropriate Antivenom Doses for Six Types of Envenomations Caused by Snakes in Taiwan. J Venom Anim Toxins. 2009;15(3):479–90. [Google Scholar]
- 17.Hung DZ. Taiwan's venomous snakebite: epidemiological, evolution and geographic differences. Trans R Soc Trop Med Hyg. 2004;98(2):96–101. [DOI] [PubMed] [Google Scholar]
- 18.Lin CC, Chaou CH, Tseng CY. An investigation of snakebite antivenom usage in Taiwan. J Formos Med Assoc. 2016;115(8):672–7. doi: 10.1016/j.jfma.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 19.Howard-Jones N. A CIOMS ethical code for animal experimentation. WHO Chron. 1985;39(2):51–6. [PubMed] [Google Scholar]
- 20.Finney DJ. Probit analysis: a statistical treatment of the sigmoid response curve 2d ed. Cambridge Eng.: University Press; 1952. xiv, 318 p. p. [Google Scholar]
- 21.Morais V, Ifran S, Berasain P, Massaldi H. Antivenoms: potency or median effective dose, which to use? J Venom Anim Toxins. 2010;16(2):191–3. [Google Scholar]
- 22.Araujo HP, Bourguignon SC, Boller MA, Dias AA, Lucas EP, Santos IC, et al. Potency evaluation of antivenoms in Brazil: the national control laboratory experience between 2000 and 2006. Toxicon. 2008;51(4):502–14. Epub 2007/12/25. doi: 10.1016/j.toxicon.2007.11.002 [DOI] [PubMed] [Google Scholar]
- 23.Petras D, Heiss P, Sussmuth RD, Calvete JJ. Venom Proteomics of Indonesian King Cobra, Ophiophagus hannah: Integrating Top-Down and Bottom-Up Approaches. J Proteome Res. 2015;14(6):2539–56. doi: 10.1021/acs.jproteome.5b00305 [DOI] [PubMed] [Google Scholar]
- 24.Lin SJ, Chang KP, Hsu CW, Chi LM, Chien KY, Liang Y, et al. Low-molecular-mass secretome profiling identifies C-C motif chemokine 5 as a potential plasma biomarker and therapeutic target for nasopharyngeal carcinoma. J Proteomics. 2013;94:186–201. doi: 10.1016/j.jprot.2013.09.013 [DOI] [PubMed] [Google Scholar]
- 25.Wu CC, Hsu CW, Chen CD, Yu CJ, Chang KP, Tai DI, et al. Candidate serological biomarkers for cancer identified from the secretomes of 23 cancer cell lines and the human protein atlas. Mol Cell Proteomics. 2010;9(6):1100–17. doi: 10.1074/mcp.M900398-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan CH, Leong PK, Fung SY, Sim SM, Ponnudurai G, Ariaratnam C, et al. Cross neutralization of Hypnale hypnale (hump-nosed pit viper) venom by polyvalent and monovalent Malayan pit viper antivenoms in vitro and in a rodent model. Acta Trop. 2011;117(2):119–24. doi: 10.1016/j.actatropica.2010.11.001 [DOI] [PubMed] [Google Scholar]
- 27.Silva A, Hodgson WC, Isbister GK. Cross-Neutralisation of In Vitro Neurotoxicity of Asian and Australian Snake Neurotoxins and Venoms by Different Antivenoms. Toxins (Basel). 2016;8(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silva A, Kuruppu S, Othman I, Goode RJ, Hodgson WC, Isbister GK. Neurotoxicity in Sri Lankan Russell's Viper (Daboia russelii) Envenoming is Primarily due to U1-viperitoxin-Dr1a, a Pre-Synaptic Neurotoxin. Neurotox Res. 2017;31(1):11–9. doi: 10.1007/s12640-016-9650-4 [DOI] [PubMed] [Google Scholar]
- 29.Silva A, Hodgson WC, Isbister GK. Antivenom for Neuromuscular Paralysis Resulting From Snake Envenoming. Toxins (Basel). 2017;9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan KY, Tan CH, Sim SM, Fung SY, Tan NH. Geographical venom variations of the Southeast Asian monocled cobra (Naja kaouthia): venom-induced neuromuscular depression and antivenom neutralization. Comp Biochem Physiol C Toxicol Pharmacol. 2016;185–186:77–86. doi: 10.1016/j.cbpc.2016.03.005 [DOI] [PubMed] [Google Scholar]
- 31.Huang HW, Liu BS, Chien KY, Chiang LC, Huang SY, Sung WC, et al. Cobra venom proteome and glycome determined from individual snakes of Naja atra reveal medically important dynamic range and systematic geographic variation. J Proteomics. 2015;128:92–104. doi: 10.1016/j.jprot.2015.07.015 [DOI] [PubMed] [Google Scholar]
- 32.Leong PK, Fung SY, Tan CH, Sim SM, Tan NH. Immunological cross-reactivity and neutralization of the principal toxins of Naja sumatrana and related cobra venoms by a Thai polyvalent antivenom (Neuro Polyvalent Snake Antivenom). Acta Trop. 2015;149:86–93. doi: 10.1016/j.actatropica.2015.05.020 [DOI] [PubMed] [Google Scholar]
- 33.Ratanabanangkoon K, Tan KY, Eursakun S, Tan CH, Simsiriwong P, Pamornsakda T, et al. A Simple and Novel Strategy for the Production of a Pan-specific Antiserum against Elapid Snakes of Asia. PLoS Negl Trop Dis. 2016;10(4):e0004565 doi: 10.1371/journal.pntd.0004565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin SR, Huang HB, Wu BN, Chang LS. Characterization and cloning of long neurotoxin homolog from Naja naja atra. Biochem Mol Biol Int. 1998;46(6):1211–7. [DOI] [PubMed] [Google Scholar]
- 35.Chang L, Lin S, Wang J, Hu WP, Wu B, Huang H. Structure-function studies on Taiwan cobra long neurotoxin homolog. Biochim Biophys Acta. 2000;1480(1–2):293–301. [DOI] [PubMed] [Google Scholar]
- 36.Wong KY, Tan CH, Tan NH. Venom and Purified Toxins of the Spectacled Cobra (Naja naja) from Pakistan: Insights into Toxicity and Antivenom Neutralization. Am J Trop Med Hyg. 2016;94(6):1392–9. Epub 2016/03/30. doi: 10.4269/ajtmh.15-0871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan KY, Tan CH, Fung SY, Tan NH. Venomics, lethality and neutralization of Naja kaouthia (monocled cobra) venoms from three different geographical regions of Southeast Asia. J Proteomics. 2015;120:105–25. doi: 10.1016/j.jprot.2015.02.012 [DOI] [PubMed] [Google Scholar]
- 38.Rajagopalan N, Pung YF, Zhu YZ, Wong PT, Kumar PP, Kini RM. Beta-cardiotoxin: a new three-finger toxin from Ophiophagus hannah (king cobra) venom with beta-blocker activity. FASEB J. 2007;21(13):3685–95. doi: 10.1096/fj.07-8658com [DOI] [PubMed] [Google Scholar]
- 39.Kini RM. Excitement ahead: structure, function and mechanism of snake venom phospholipase A2 enzymes. Toxicon. 2003;42(8):827–40. doi: 10.1016/j.toxicon.2003.11.002 [DOI] [PubMed] [Google Scholar]
- 40.Vonk FJ, Casewell NR, Henkel CV, Heimberg AM, Jansen HJ, McCleary RJ, et al. The king cobra genome reveals dynamic gene evolution and adaptation in the snake venom system. Proc Natl Acad Sci U S A. 2013;110(51):20651–6. doi: 10.1073/pnas.1314702110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang HC, Tsai TS, Tsai IH. Functional proteomic approach to discover geographic variations of king cobra venoms from Southeast Asia and China. J Proteomics. 2013;89:141–53. Epub 2013/06/26. doi: 10.1016/j.jprot.2013.06.012 [DOI] [PubMed] [Google Scholar]
- 42.Danpaiboon W, Reamtong O, Sookrung N, Seesuay W, Sakolvaree Y, Thanongsaksrikul J, et al. Ophiophagus hannah venom: proteome, components bound by Naja kaouthia antivenin and neutralization by N. kaouthia neurotoxin-specific human ScFv. Toxins (Basel). 2014;6(5):1526–58. Epub 2014/05/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan CH, Tan KY, Fung SY, Tan NH. Venom-gland transcriptome and venom proteome of the Malaysian king cobra (Ophiophagus hannah). BMC Genomics. 2015;16:687 Epub 2015/09/12. doi: 10.1186/s12864-015-1828-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osipov AV, Kasheverov IE, Makarova YV, Starkov VG, Vorontsova OV, Ziganshin R, et al. Naturally occurring disulfide-bound dimers of three-fingered toxins: a paradigm for biological activity diversification. J Biol Chem. 2008;283(21):14571–80. doi: 10.1074/jbc.M802085200 [DOI] [PubMed] [Google Scholar]
- 45.Chen KC, Kao PH, Lin SR, Chang LS. The mechanism of cytotoxicity by Naja naja atra cardiotoxin 3 is physically distant from its membrane-damaging effect. Toxicon. 2007;50(6):816–24. doi: 10.1016/j.toxicon.2007.06.011 [DOI] [PubMed] [Google Scholar]
- 46.Huang LF, Zheng JB, Xu Y, Song HT, Yu CX. A snake venom phospholipase A2 with high affinity for muscarinic acetylcholine receptors acts on guinea pig ileum. Toxicon. 2008;51(6):1008–16. doi: 10.1016/j.toxicon.2008.01.006 [DOI] [PubMed] [Google Scholar]
- 47.Faiz MA, Ahsan MF, Ghose A, Rahman MR, Amin R, Hossain M, et al. Bites by the Monocled Cobra, Naja kaouthia, in Chittagong Division, Bangladesh: Epidemiology, Clinical Features of Envenoming and Management of 70 Identified Cases. Am J Trop Med Hyg. 2017;96(4):876–84. Epub 2017/02/01. doi: 10.4269/ajtmh.16-0842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alirol E, Sharma SK, Bawaskar HS, Kuch U, Chappuis F. Snake bite in South Asia: a review. PLoS Negl Trop Dis. 2010;4(1):e603 doi: 10.1371/journal.pntd.0000603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohapatra B, Warrell DA, Suraweera W, Bhatia P, Dhingra N, Jotkar RM, et al. Snakebite mortality in India: a nationally representative mortality survey. PLoS Negl Trop Dis. 2011;5(4):e1018 doi: 10.1371/journal.pntd.0001018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chippaux JP, Massougbodji A, Diouf A, Balde CM, Boyer LV. Snake bites and antivenom shortage in Africa. Lancet. 2015;386(10010):2252–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(TIF)
(TIF)
(A) N. atra versus O. hannah, (B) B. multicinctus versus O. hannah, and (C) N. atra versus N. kaouthia.
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.