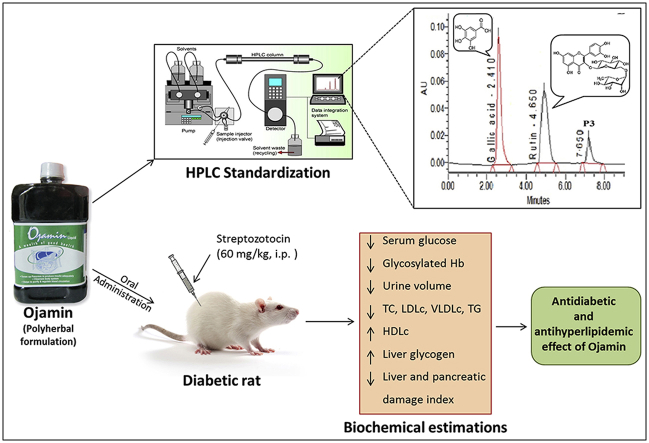
Abstract
Background
Ojamin (OJ), a polyherbal antidiabetic formulation, is extensively used as a food supplement to control diabetes alone or along with synthetic antidiabetic agents. However, it's phytochemical and pharmacological investigations are lacking.
Objective
The present study was undertaken to study antidiabetic and antihyperlipidemic potentials of OJ and its interaction with Metformin in streptozotocin (STZ)-induced diabetic rats.
Materials and methods
Diabetes was induced in Wistar rats by single intraperitoneal (i.p.) injection of streptozotocin (60 mg/kg). Antidiabetic, antihyperlipidemic activities of OJ were evaluated at dose of 0.28 ml/kg by estimating biochemical changes in urine, serum and liver tissue homogenate and histological changes in liver and pancreatic tissues. Metformin (100 mg/kg, p.o.) was used as reference standard drug.
Results
Results indicate that STZ administration caused hyperglycemia, increased serum glycosylated hemoglobin content, altered serum lipid profile, polyuria, decreased liver glycogen content and histological changes in liver and pancreatic tissues. This elevated serum glucose level and urine volume was significantly decreased by OJ. Supplementation with OJ produced significant improvement in serum lipid profile and glycosylated hemoglobin content along with significant increase in the liver glycogen content. OJ treatment also restored histological changes in liver and pancreatic tissue near to the normal. The observed antidiabetic and hypolipidemic effects of OJ were superior to Metformin. Co-treatment of diabetic rats with OJ and Metformin failed to control blood glucose levels.
Conclusion
It is concluded that the OJ possesses significant antidiabetic and antihyperlipidemic activities in rats. However, co-administration of OJ and Metformin is cautioned.
Keywords: Antidiabetic, Antihyperlipidemic, Herb–drug interaction, Metformin, Ojamin, Polyherbal formulation
Graphical abstract
1. Introduction
Diabetes mellitus is an endocrine metabolic disorder characterized by hyperglycemia, altered lipids, carbohydrates, proteins metabolism and it increases the risk of cardiovascular diseases complications [1]. Diabetes mellitus and its associated acute and long-term complications are an important health hazard globally. Its prevalence has now reached pandemic proportions in India [2]. It is reported that the four countries namely China, India, Indonesia and Japan from Asia are center for the global epidemic of diabetes and the number of diabetic patients worldwide has doubled in the past two decades [3]. Herbal drugs have always remained an important source of medicines. As per Indian traditional system of medicine, many medicinal plants have been used for the management of various health disorders including diabetes mellitus. Moreover, some polyherbal formulations, including Ojamin, are recommended as food supplement in diabetes mellitus and these herbal-based formulations are commonly used by the community because they are cost-effective and have fewer side effects than synthetic drugs. Furthermore, there is widespread practice of using such herbal-based drugs with allopathic medicines. Ojamin is Over-the-Counter herbal formulation marketed by M/s Tate Remedies, Pune, India. It contains aqueous extracts of fourteen herbs. The botanical name (family) and quantity of aqueous extract of these plants in 5 ml in formulation are – Aegle marmelos (Rutaceae) 66 mg, Trigonella foenum graecum (Fabaceae) 66 mg, Carum carvi (Umbeliferae) 66 mg, Emblica officinalis (Euphorbiaceae) 400 mg, Terminalia chebula (Combretaceae) 400 mg, Terminalia bellirica (Combretaceae) 400 mg, Swertia chirata (Gentianaceae) 66 mg, Tinospora cordifolia (Menispermaceae) 66 mg, Eugenia jambolana (Myrtaceae) 66 mg, Picrorhiza kurroa (Plantaginaceae) 66 mg, Gymnema sylvestre (Asclepiadaceae) 66 mg, Salacia chinensis Linn (Celastraceae) 76 mg, Curcuma longa (Zingiberaceae) 66 mg and Melia azadirachta (Meliaceae) 66 mg. However, the rationale behind its usefulness as an antidiabetic has not been established yet, through the systematic pharmacological study. With this background, the present research work was undertaken to standardize and evaluate antidiabetic and antihyperlipidemic activities of a polyherbal formulation, Ojamin, using streptozotocin (STZ)-induced diabetic model in rats. Furthermore, the study investigated effect of simultaneous administration of OJ and Metformin in diabetic rats.
2. Materials and methods
2.1. Standardization of OJ by HPLC
The OJ was standardized for the content of marker compounds, gallic acid (GA) and rutin (RU). The HPLC method was developed in the laboratory and validated as per ICH guidelines (data not given). For analysis, 1 ml of OJ formulation was transferred to 10 ml volumetric flask and diluted to mark with the acetonitrile. The solution was sonicated for 20 min, filtered through 0.45μm membrane filter and 10 μl of the solution was injected into the HPLC system (Waters Inc., Milford, MA) consisted of a binary pump (Model: Waters 515 HPLC pump), auto sampler (Model: 717 plus auto-sampler), column heater (Model: CHM, Sr. No. A08CHM 289M) and photodiode array (PDA) detector (Model 2998). Separation was achieved on Kromasil C-18 column (4.6 mm, 5.0μm) maintained at 40°C using the column oven. Isocratic elution was carried out with acetonitrile:water (50:50) as mobile phase (pH: 3.5 adjusted with o-phosphoric acid). Mobile phase flow rate was maintained at 0.6 ml/min and detection was monitored at 310nm, data collection and analysis was performed using Empower-version 2 software (Waters Inc., Milford, MA).
2.2. Antidiabetic and antihyperlipidemic activities of Ojamin
2.2.1. Animals
Male Wistar albino rats weighing between 150 and 250 g supplied by National Institute of Biosciences, Pune, India were used for the study. The animals were acclimatized for ten days under standard conditions in an animal house approved by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India. The animals were given a standard diet supplied by Nutrivet Life Sciences, Pune, India. The study protocol was approved by the Institutional Animal Ethics Committee (Ref. No.: MIP/IAEC/2014-15/M1/Apr/007) of Maharashtra Institute of Pharmacy, Pune, India.
2.2.2. Chemicals and instruments
The Ojamin (OJ) was obtained as a gift sample from M/s Tate Remedies, Pune, India for the study. Gallic acid (Thomas Beaker Chemicals Pvt. Ltd, India), Rutin (Sigma–Aldrich, India) and streptozotocin (Himedia Lab Pvt. Ltd, India) were purchased from the local market. Glucose estimation kit, total cholesterol estimation kit, HDL-cholesterol estimation kit and triglyceride estimation kit manufactured by Benesphera Diagnostics, India were used for the study. All other chemicals and reagents used were of analytical grade and procured from approved vendors. Instruments such as analytical balance (Schimadzu, AUW 220D), UV–visible spectrophotometer (Varian-Cary 100), cold centrifuge (Bioera, BL-165-D), homogenizer (Biolab, B244), ulrasonicator (Ultrasonics, 5.5L-150H), pH meter (Equip-Tronics, EQ-621), UV-semiautoanalyzer (Benesphera, C61) were used for the study.
2.2.3. Selection of OJ dose
Antidiabetic and hypolipidemic activities of OJ were evaluated at the dose of 0.28 ml/kg twice daily which was extrapolated from the adult dose (20 ml, twice daily) commonly used in humans [4].
2.2.4. Induction of diabetes
Diabetes was induced in overnight-fasted rats by administering single intraperitoneal (i.p.) injection of freshly prepared streptozotocin 60 mg/kg bw in 0.1 M citrate buffer (pH 4.5) in a volume of 0.5 ml/kg bw. The induction of diabetes was confirmed by measuring fasting blood glucose level on the fifth day of STZ administration. Rats with fasting blood glucose level of more than 200 mg/dl were considered as diabetics and used for the experiment [5].
2.2.5. Experimental design
Animals were randomly divided into five groups as Groups I, II, III, IV and V containing six animals in each. Group I served as a normal control and was maintained on regular rat food and drinking water ad libitum. All remaining groups i.e. Groups II–V received diabetes induction treatment. Group II served diabetic control group. Group III served as a reference standard treatment group and received Metformin at a dose of 100 mg/kg for 21 days. Group IV served as OJ-treatment group and received OJ at dose of 0.28 ml/kg twice daily for 21 days. Group V served as OJ-Metformin treated group and received OJ (0.28 ml/kg, twice daily) and Metformin (100 mg/kg) for 21 days.
2.2.6. Body weight and serum analysis
All animals were monitored for body weight during the treatment period. Blood was collected on 0th, 7th, 14th and 21st day of the treatment from the retro-orbital sinus under ether anesthesia condition. The serum was separated by centrifugation at 10,000 g for 10 min and analyzed for glucose level by using diagnostic kits. The serum collected on 21st day of the treatment period was also analyzed for total cholesterol (TC), high density lipoprotein cholesterol (HDLc) and triglyceride (TG) levels by using diagnostic kits. The low density lipoprotein cholesterol (LDLc) and very low density lipoprotein-cholesterol (VLDLc) were also calculated by using Friedwald's formula [2].
2.2.7. Urine volume analysis
After blood collection, all animals were kept in individual metabolic cages for the collection of 5 h urine samples and urine volume was measured.
2.2.8. Glycosylated hemoglobin, liver glycogen and histopathological analysis
At the end of treatment period, blood was also collected in EDTA tubes from the retro-orbital sinus under ether anesthesia and analyzed for glycosylated hemoglobin content. After blood collection, animals were sacrificed by cervical dislocation under ether anesthesia; liver and pancreas were isolated and subjected to histopathological analysis. Glycogen content of part of the isolated liver tissue was estimated by the method reported earlier [6].
2.2.9. Statistical analysis
The results were expressed as mean ± standard error of mean (SEM). The statistical significance between diabetic control group and normal control group was calculated by Student's t-test, whereas one-way analysis of variance (ANOVA) followed by Dunnett's comparison test was used to calculate statistical significance between drug-treated groups and diabetic control group. p < 0.05 was considered significant.
3. Results
3.1. HPLC standardization of OJ
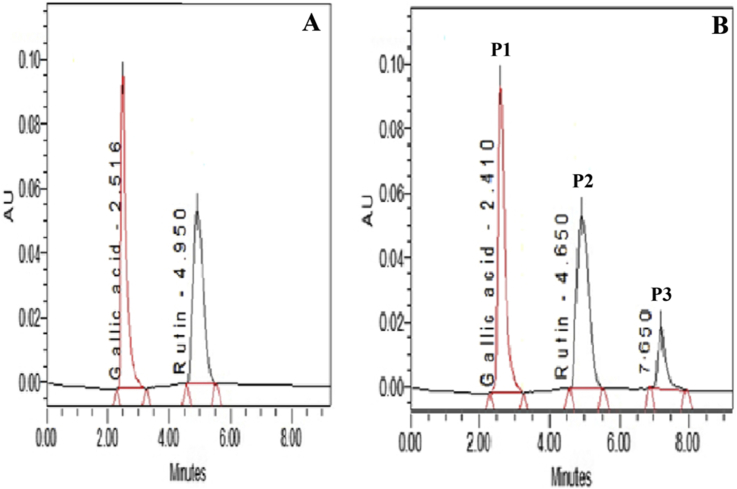
The HPLC chromatogram of OJ showed three major peaks namely P1, P2 and P3 corresponding to retention time 2.41, 4.65 and 7.65 min respectively (Fig. 1). The peak P1 and P2 were identified as gallic acid and rutin, respectively by comparing with HPLC chromatogram of a reference standard of gallic acid and rutin showing peak at 2.51 and 4.95 min respectively. The peak P3 in OJ chromatogram was unidentified peak of some other phytoconstitutent(s) from the formulation. Based on peak area, content of gallic acid and rutin in OJ was found to be 0.0098 and 0.0095% w/v respectively.
Fig. 1.
HPLC of (A) reference standard gallic acid and rutin at RT = 2.516 and 4.950 min respectively and (B) OJ standardized to gallic acid (P1) at RT = 2.410 and rutin (P2) at RT = 4.650 min.
3.2. Effect of OJ on body weight
Basal body weight of animals was considered to be statistically equivalent (Table 1). Induction of diabetes caused significant alteration in body weight of rats during experimental period when compared against normal control rats. At the end of treatment period, mean body weight of normal control rats was 335.00 ± 20.50 g, and was significantly (p < 0.01) decreased to 227.00 ± 10.20 g in diabetic control rats. This decreased body weight was significantly improved by treatment with OJ and Metformin. However, administration of OJ-Metformin produced significant (p < 0.001) decrease in body weight as compared with normal control rats. This decrease in body weight by OJ-Metformin was more than that observed in diabetic control rats (Table 1).
Table 1.
Effect of OJ on body weight, urine volume and serum glucose level in streptozotocin induced diabetic rats.
| Day | Group-I (Normal control) | Group-II (Diabetic control) | Group-III (Metformin-treated) | Group-IV (OJ-treated) | Group-V (OJ-Metformin treated) |
|---|---|---|---|---|---|
| Body weight (g) | |||||
| 0 | 210.00 ± 8.94 | 208.01 ± 7.92 | 208.00 ± 6.01 | 207.00 ± 6.15 | 217.00 ± 6.67 |
| 7 | 320.00 ± 15.70 | 292.00 ± 6.01 | 323.00 ± 23.50 | 277.00 ± 30.00 | 234.13 ± 13.10 |
| 14 | 332.00 ± 18.30 | 253.00 ± 8.82† | 350.00 ± 28.80∗∗ | 287.00 ± 23.60 | 193.00 ± 11.20 |
| 21 | 335.00 ± 20.50 | 227.00 ± 10.20†† | 355.00 ± 25.40∗∗∗ | 285.00 ± 26.30 | 198.00 ± 12.20 |
| Urine volume (ml/5 h) | |||||
| 0 | 1.20 ± 0.22 | 2.03 ± 0.37 | 1.48 ± 0.31 | 1.08 ± 0.18 | 1.60 ± 0.42 |
| 7 | 1.73 ± 0.32 | 8.65 ± 1.10††† | 4.73 ± 0.82∗∗ | 3.12 ± 0.55∗∗∗ | 2.67 ± 0.76∗∗∗ |
| 14 | 1.87 ± 0.32 | 9.42 ± 0.98††† | 6.12 ± 0.53∗∗ | 2.23 ± 0.42∗∗∗ | 1.93 ± 0.36∗∗∗ |
| 21 | 1.85 ± 0.32 | 10.50 ± 2.03††† | 5.47 ± 1.09∗ | 2.37 ± 0.38∗∗∗ | 1.77 ± 0.42∗∗∗ |
| Serum glucose (mg/dl) | |||||
| 0 | 86.00 ± 1.24 | 83.60 ± 2.04 | 101.00 ± 4.21 | 87.00 ± 7.21 | 83.40 ± 4.42 |
| 7 | 112.00 ± 7.91 | 361.00 ± 7.72††† | 243.00 ± 34.30∗ | 203.00 ± 29.70∗∗∗ | 356.00 ± 9.45 |
| 14 | 125.00 ± 13.20 | 354.00 ± 17.60††† | 241.00 ± 24.80∗ | 211.00 ± 22.70∗∗ | 229.00 ± 37.10∗ |
| 21 | 78.40 ± 5.14 | 348.00 ± 6.05††† | 170.00 ± 45.00∗∗∗ | 143.00 ± 13.10∗∗∗ | 237.00 ± 17.30∗ |
Values are expressed as mean ± SEM, n = 6, †p < 0.05; ††p < 0.01; †††p < 0.001 vs. normal control, ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 vs. diabetic control. Values of diabetic control group were compared with normal control and those of drug-treated groups with diabetic control.
3.3. Effect of OJ on urine volume
Basal urine volume of rats was considered to be statistically similar (Table 1). Induction of diabetes caused significant increase in urine volume during the experimental period as compared to normal control rats. At the end of treatment period, mean urine volume of normal control rats was 1.85 ± 0.32 ml/5 h, which was significantly (p < 0.001) increased to 10.50 ± 2.03 ml/5 h in diabetic control rats. The urine volume was significantly decreased by treatment with OJ, Metformin and OJ-Metformin. OJ-Metformin treatment was found to be equipotent to OJ treatment alone but more effective than Metformin alone (Table 1).
3.4. Effect of OJ on serum glucose level
Basal serum glucose level of rats was considered to be statistically equal (Table 1). There was significant increase in serum glucose level of diabetic control rats during the experimental period. At the end of experimental period, serum glucose levels of diabetic control rats was significantly (p < 0.001) increased from 78.40 ± 5.14 mg/dl to 348.00 ± 6.05 mg/dl as compared to normal control rats. This increased serum glucose level was significantly decreased by treatment with OJ, Metformin and OJ-Metformin. OJ-Metformin treatment was found to be less effective than OJ and Metformin alone in this regard.
3.5. Effect of OJ on serum total cholesterol (TC) level
The mean serum TC levels of normal control rats was 90.71 ± 3.10 mg/dl, which was significantly (p < 0.001) increased to 159.00 ± 7.56 mg/dl in the diabetic control rats (Table 2). This increased serum TC level was significantly decreased by treatment with OJ, Metformin and OJ-Metformin. The reference standard Metformin was found to be most effective in this regard. OJ-Metformin treatment was found to be less effective than OJ and Metformin alone in reducing serum TC level (Table 2).
Table 2.
Effect of OJ on serum lipid profile in streptozotocin-induced diabetic rats at the end of treatment period.
| Parameters | Group-I (Normal control) | Group-II (Diabetic control) | Group-III (Metformin-treated) | Group-IV (OJ-treated) | Group-V (OJ-Metformin treated) |
|---|---|---|---|---|---|
| TC (mg/dl) | 90.71 ± 3.10 | 159.00 ± 7.56††† | 89.80 ± 3.15∗∗∗ | 105.01 ± 4.30∗∗ | 126.00 ± 7.70∗ |
| HDLc (mg/dl) | 40.90 ± 8.98 | 19.20 ± 3.89††† | 39.90 ± 7.74∗∗∗ | 41.70 ± 5.41∗∗∗ | 31.70 ± 4.56∗∗ |
| LDLc (mg/dl) | 30.04 ± 3.50 | 102.0 ± 6.34††† | 20.40 ± 4.59∗∗∗ | 44.70 ± 6.80∗∗ | 72.80 ± 5.60∗ |
| VLDLc (mg/dl) | 19.80 ± 2.50 | 37.80 ± 4.20††† | 29.50 ± 1.89∗∗ | 19.20 ± 2.05∗∗∗ | 21.50 ± 3.70∗∗∗ |
| TG (mg/dl) | 99.20 ± 12.50 | 189.00 ± 10.10††† | 148.00 ± 9.31∗∗ | 97.20 ± 10.20∗∗∗ | 108.00 ± 8.50∗∗∗ |
| Liver glycogen (mg/g) | 0.05 ± 0.001 | 0.02 ± 0.001††† | 0.04 ± 0.001∗∗∗ | 0.05 ± 0.002∗∗∗ | 0.06 ± 0.004∗∗∗ |
| Glycosylated hemoglobin (%) | 2.66 ± 0.40 | 8.36 ± 0.79††† | 3.56 ± 0.47∗∗∗ | 3.71 ± 0.55∗∗∗ | 3.96 ± 0.39∗∗∗ |
Values are expressed as mean ± SEM, n = 6, TC: total cholesterol; HDLc: high density lipoprotein cholesterol; LDLc: low density lipoprotein cholesterol; VLDLc: very low density lipoprotein-cholesterol; TG: triglycerides, †††p < 0.001 vs. normal control, ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 vs. diabetic control. Values of diabetic control group were compared with normal control and those of drug-treated groups with diabetic control.
3.6. Effect of OJ on serum HDL-cholesterol level
Induction of diabetes caused significant (p < 0.001) decrease in serum HDL-cholesterol levels of 40.90 ± 8.98 mg/dl to 19.20 ± 3.89 mg/dl when compared against normal control rats (Table 2). Treatment with OJ, Metformin and OJ-Metformin produced significant increase in the serum HDL-cholesterol levels as compared to diabetic control rats. The reference standard Metformin was found to be equipotent to OJ, whereas OJ-Metformin treatment was less effective than OJ and Metformin alone in increasing serum HDL-cholesterol level (Table 2).
3.7. Effect of OJ on serum LDL-cholesterol level
The mean serum LDLc levels of normal control rats was 30.04 ± 3.50 mg/dl, which significantly (p < 0.001) increased to 102.0 ± 6.34 mg/dl in the diabetic control rats (Table 2). This increased serum LDLc level significantly decreased by treatment with OJ, Metformin and OJ-Metformin. The reference standard Metformin was found to be more potent than OJ in this regard. OJ-Metformin treatment was less effective than OJ and Metformin alone in decreasing serum LDLc level (Table 2).
3.8. Effect of OJ on serum VLDLc level
Induction of diabetes caused significant (p < 0.001) increase in serum VLDLc levels from 19.80 ± 2.50 mg/dl to 37.80 ± 4.20 mg/dl when compared against normal control rats (Table 2). This increased serum VLDLc level significantly decreased by treatment with OJ, Metformin and OJ-Metformin. OJ and OJ-Metformin were found to be more effective than Metformin alone in this regard (Table 2).
3.9. Effect of OJ on serum triglycerides level
The mean serum triglyceride level of normal control rats was 99.20 ± 12.50 mg/dl, which significantly (p < 0.001) increased to 189.00 ± 10.10 mg/dl in the diabetic control rats (Table 2). This increased serum triglyceride level significantly decreased by treatment with OJ, Metformin and OJ-Metformin. OJ and OJ-Metformin were found to be equipotent and more effective than Metformin alone in decreasing serum triglyceride level (Table 2).
3.10. Effect of OJ on liver glycogen content
The mean liver glycogen content of normal control rats was found 0.05 ± 0.001 mg/g, which significantly (p < 0.001) decreased to 0.02 ± 0.001 mg/g in the diabetic control rats (Table 2). This decreased liver glycogen content significantly increased by treatment with OJ, Metformin and OJ-Metformin. The OJ, Metformin and OJ-Metformin were found to be equipotent in this regard (Table 2).
3.11. Effect of OJ on serum glycosylated hemoglobin
Induction of diabetes caused significant (p < 0.001) increase in serum glycosylated hemoglobin content from 2.66 ± 0.40% to 8.36 ± 0.79 as compared to normal control rats (Table 2). This increased glycosylated hemoglobin content significantly decreased by treatment with OJ, Metformin and OJ-Metformin. The OJ, Metformin and OJ-Metformin were found to be equipotent in decreasing serum glycosylated hemoglobin content (Table 2).
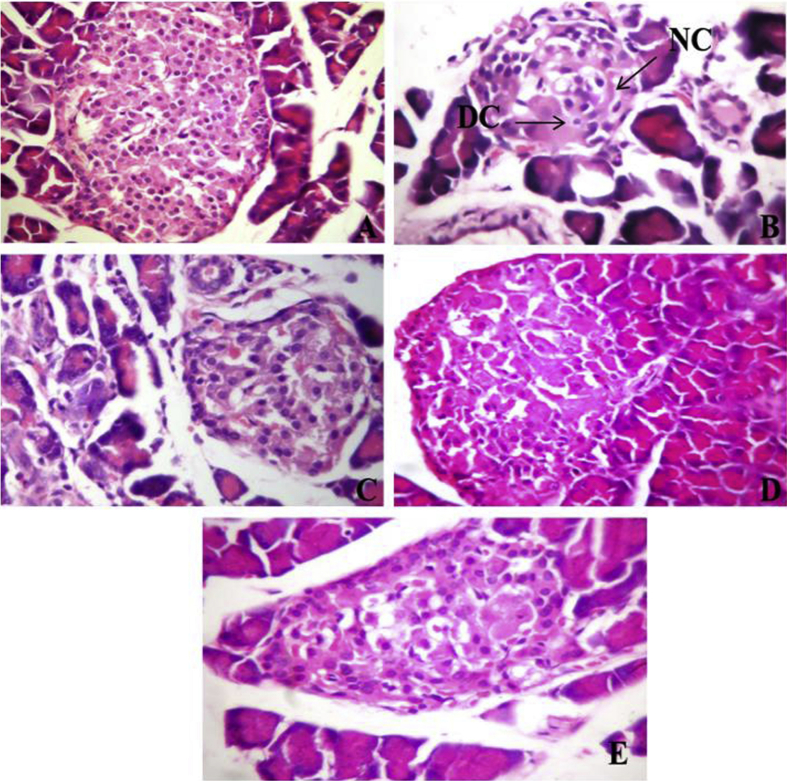
3.12. Effect of OJ on histology of the pancreas
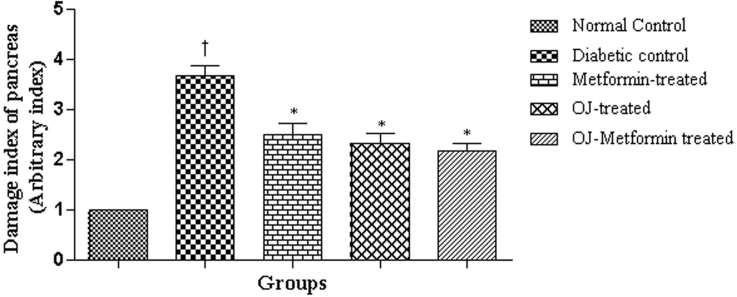
Pancreatic sections of normal control rats showed intact histological features of endocrine and exocrine components (Fig. 2). Diabetes induction by streptozotocin administration caused moderate histological changes such as necrotic and degenerative changes in the islets of Langerhans with decreased number of β-cells (Fig. 2). This resulted in increase in the damage index (Nos.) of pancreatic tissue from 0.00 to 3.62 ± 0.18 in the diabetic control rats when compared against normal control rats. Treatment with OJ, Metformin and OJ-Metformin prevented these histological changes (Fig. 2) and thereby caused a significant decrease in the damage index of pancreatic tissue (Fig. 3).
Fig. 2.
Microscopic images of pancreatic sections from (A) normal control animals showing patches of abundant β-cell, (B) diabetic control animals showing moderate histological changes such as necrotic changes (marked as ‘NC’) and degenerative changes (marked as ‘DC’) in islets with loss of β-cells, (C) diabetic animals treated with Metformin (100 mg/kg) showing minimal to mild changes, (D) diabetic animals treated Ojamin (0.28 ml/kg, twice daily) showing minimal to mild changes and (E) diabetic animals treated simultaneously with Ojamin (0.28 ml/kg, twice daily) and Metformin (100 mg/kg) showing minimal changes (H and E ×100).
Fig. 3.
Damage index in pancreatic section of control and treatment groups. †p < 0.001 vs. normal control, ∗p < 0.001 vs. diabetic control. Values of diabetic control group were compared with normal control and those of drug-treated groups with diabetic control.
3.13. Effect of OJ on liver histology
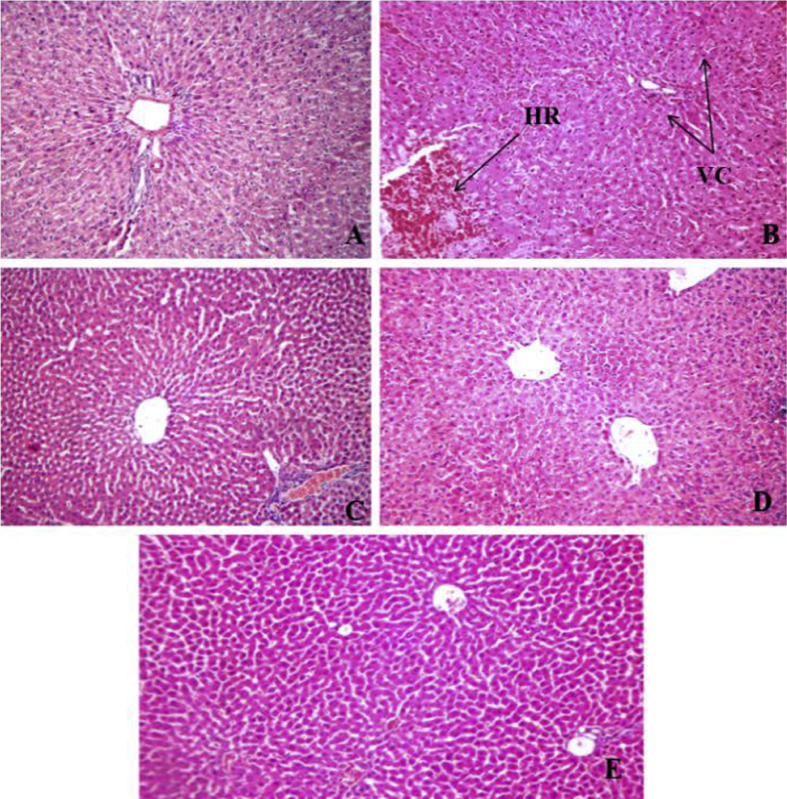
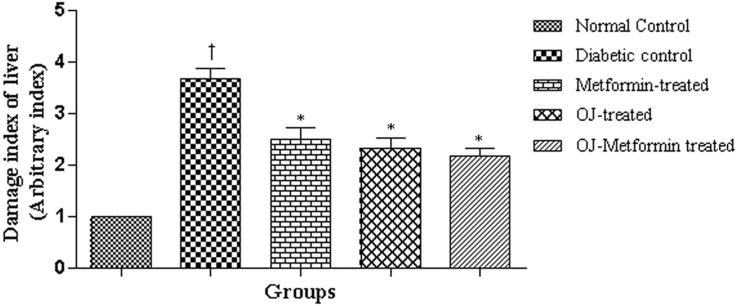
The microscopic examination of liver tissue of normal control rats showed normal histological features (Fig. 4). Induction of diabetes caused marked pathological changes in liver parenchyma such as cellular swelling with degenerative changes, vacuolar changes and foci of hemorrhages. This resulted in increase in the damage index (Nos.) of liver tissue from 0.00 to 3.75 ± 0.20 in the diabetic control rats when compared against normal control. Treatment with OJ, Metformin and OJ-Metformin prevented these histological changes (Fig. 4) and thereby caused a significant decrease in the damage index of liver tissue (Fig. 5).
Fig. 4.
Microscopic images of liver sections from (A) normal control animals, (B) diabetic control animals showing moderate pathological changes such as hemorrhage (marked as ‘HR’) and vascular changes (marked as ‘VC’) of hepatocytes, (C) diabetic animals treated with Metformin (100 mg/kg) showing mild changes, (D) diabetic animals treated Ojamin (0.28 ml/kg, twice daily) showing minimal to mild changes and (E) diabetic animals treated simultaneously with Ojamin (0.28 ml/kg, twice daily) and Metformin (100 mg/kg) showing mild to minimal changes (H and E ×100).
Fig. 5.
Damage index in liver section of control and treatment groups. †p < 0.001 vs. normal control, ∗p < 0.001 vs. diabetic control. Values of diabetic control group were compared with normal control and those of drug-treated groups with diabetic control.
4. Discussion
A number of animal models using rats have been used to induce diabetes mellitus. Out of these models, most reliable and hence commonly employed method is intraperitoneal administration of streptozotocin to rats. Therefore, we evaluated the antidiabetic and antihyperlipidemic activity of Ojamin using this model. It is reported that intraperitoneal injection of 60 mg/kg doses of streptozotocin in adult Wistar rats induces experimental diabetes mellitus in the 2–4 days [7]. It is reported that the streptozotocin induces diabetes by impairing glucose oxidation and reduction of insulin biosynthesis and secretion [8], [9].
The mechanism of streptozotocin-induced diabetes in rats mainly involves DNA alkylation-induced generation of reactive oxygen species (ROS) and enhanced formation of nitric oxide (NO) in the β-cell of pancreas [10]. Streptozotocin enters the β-cell and forms methyl nitrosourea moiety that causes alkylation of DNA. The O6 position of guanine residues in DNA is especially susceptible for the alkylation. This alkylation-induced DNA damage causes activation of poly ADP-ribosylation that leads to depletion of cellular NAD+ and ATP. This results in enhanced ATP dephosphorylation. The augmented ATP dephosphorylation increases the supply of substrate for xanthine oxidase resulting in the formation of superoxide radicals. Consequently, hydrogen peroxide and hydroxyl radicals are also generated [10]. Furthermore, streptozotocin liberates toxic amounts of NO that inhibits aconitase activity and participates in DNA damage. As a result, β-cells undergo the destruction by necrosis [10]. The synergistic action of both NO and ROS may also contribute to DNA fragmentation and other deleterious changes caused by streptozotocin [11].
Consistent with previous reports, diabetic control rats showed a reduction in body weight [12]. Uncontrolled diabetes is associated with severe muscle wasting [13]. The decrease in body weight in diabetes was due to the impairment of insulin action in the conversion of glucose into glycogen and catabolism of fats, inhibition of lipolysis [14]. However, treatment with OJ and Metformin prevented the rate of decrease in the body weight of rats. This effect in diabetic rats may be due to its preventive action on pancreatic beta cells destruction by STZ which improves insulin levels, stabilization of blood glucose in diabetic rats and thereby inhibition of muscle wasting and increase in the serum protein level were obtained. It is also found that treatment with OJ-Metformin combination caused decrease in body weight as compared to normal control rats. This indicates that co-administration of OJ and Metformin interacts with each other and inhibits their beneficial effect of body weight of diabetic rats. This interaction requires further investigation so that OJ can be used effectively along with Metformin in the diabetic patients.
In the present study, increased urine output was observed in diabetic control animals which are in line with previously published report [15]. Treatment with OJ, Metformin and OJ-Metformin combination caused significant reduction in urine output. This may be due to decreased blood glucose level which abolishes the hyperglycemia-induced osmotic diuresis.
Induction of diabetes caused significant increase in blood sugar level. However, treatment with OJ, Metformin and OJ-Metformin combination prevented this elevation in blood sugar level. It is reported that some drugs produces hypoglycemic action by potentiating the insulin effect, either by increasing the pancreatic secretion of insulin from the β-cells or its release from bound insulin, whereas others act through extra-pancreatic mechanism by inhibition of hepatic glucose production or correction of insulin resistance [16], [17]. The OJ may have acted through one of the above mechanisms. In the present study, it was also noted that OJ-Metformin combination has antihyperglycaemic effect which is less effective than OJ and Metformin alone. This indicates that the simultaneous administration of OJ and Metformin to diabetic rats control elevated blood sugar level less effectively and this might be due to interaction of some constituents from OJ formulation and Metformin in the body.
In the present study, OJ produced significant beneficial effects on the lipid profile in diabetic rats. Treatment of diabetic rats with OJ caused a significant decrease in the serum cholesterol, LDL-cholesterol, VLDL-cholesterol and triglycerides levels. Furthermore, OJ increased serum HDL-cholesterol level in diabetic rats. This effect might be due to increased secretion of insulin from β-cells of the pancreas that further stimulate fatty acid synthesis and also incorporation of fatty acids into triglycerides in the liver and adipose tissue.
Glycosylated hemoglobin was produced through glycosylation of hemoglobin. It is formed progressively and irreversibly over a period of time and is stable till the life of the RBC and is unaffected by diet, insulin or exercise on the day of testing. Therefore, glycosylated hemoglobin is commonly used as an excellent marker of overall glycemic control and predicts risks for the development and/or progression of diabetic complications [18], [19]. It is reported that 10% stable reduction in glycosylated hemoglobin determines a 35% risk reduction for retinopathy, a 25%–44% risk reduction for nephropathy and a 30% risk reduction for neuropathy [20]. In the present study, the significant increase in glycosylated hemoglobin level was observed in diabetic control animals. This elevated glycosylated hemoglobin was significantly decreased by OJ, Metformin and OJ-Metformin combination treatment. This indicates that the overall blood glucose level is controlled by the OJ treatment which might be due to improvement in insulin secretion and this action represents that OJ has an ability to prevent the development of diabetes associated complications.
Insulin is a stimulator of glycogenesis and inhibitor of glycogenolysis. Therefore, glycogen content of liver and skeletal muscle markedly decreases due to lack of insulin in the diabetic state [21]. In the present study, liver glycogen content was significantly reduced in diabetic control rats. However, treatment with OJ, Metformin and OJ-Metformin combination prevented this glycogen depletion in the liver tissue. This effect might possibly be due to stimulation of insulin release or by improvement of glycogenesis in muscle and liver.
It is a known fact that inadequate glucose control in diabetes leads to various acute or chronic complications including atherosclerosis. Alteration in the serum lipid profile was known to occur in diabetes and this is such as to increase the risk for coronary heart disease. Diabetic-induced hyperlipidemia was attributed to excess mobilization of fat from the adipose tissue owing to the under-utilization of glucose [22]. In diabetic condition, increased levels of TC, TG and reduced level of HDL along with altered composition of LDL particles were commonly reported [23]. A reduction in serum lipids, particularly of the LDL, VLDL and triglycerides is considered as beneficial for the long-term prognosis of diabetic patients [24]. Lowering of blood glucose and plasma lipid levels through dietary modification and drug therapy appears to be associated with a decrease in the risk of vascular disease. In the present study, OJ treatment produced beneficial effects on the serum total cholesterol, LDL-cholesterol, VLDL-cholesterol and triglycerides level in diabetic rats. This action of OJ is considered as the most fruitful outcome because OJ provides not only symptomatic action but also useful to control acute as well as chronic complications of diabetes.
Studies on diabetes mellitus showed that the occurrence of oxidative stress arises as a result of increase in the level of production of free radicals and diminishes cells antioxidant capabilities, which all together can cause oxidative stress and tissue damage in diabetic patients [25], [26]. It has been also expressed that most of the diabetic complications are mediated through oxidative stress [27], [28]. Studies also suggest involvement of free radicals in pancreatic cell destruction [29]. In the present study, induction of diabetes caused severe pathological changes in the pancreas and liver tissues and thereby produced increase in the damage index. However, treatment with OJ, Metformin and OJ-Metformin prevented these pathological changes and restored damage index near to normal. This indicates the ability of OJ to lower the oxidative stress and its potential to avoid diabetic complications.
Physical and behavioral observations indicated heavy hair loss, reduced alertness and decreased locomotor activity in diabetic control and Metformin-treated animals. However, OJ-treated animals were active without any sign of hair-loss. Locomotor activity and extent of hair-loss in OJ-Metformin treated animals were intermediate to the animals treated with OJ and Metformin alone. However, further scientific study is required to explore these beneficial effects of OJ over Metformin.
5. Conclusion
It was possible to conclude that the administration of OJ exerts significant antidiabetic and antihyperlipidemic effect in streptozotocin-induced diabetic rats. Therefore, OJ is valuable for therapeutic application for diabetes. It also appears that the simultaneous administration of OJ and Metformin control diabetic condition less effectively as compared to OJ and Metformin alone. Therefore, simultaneous administration of OJ and Metformin should be undertaken only under direct supervision of medical practitioners. Further studies are needed to elucidate the exact mechanism of antidiabetic and antihyperlipidemic actions of OJ so as to develop it as a potent antidiabetic formulation.
Source of support
Tate Remedies, Pune.
Conflict of interest
None.
Acknowledgement
The authors wish to express their gratitude to the Management and Principal of MAEER's, Maharashtra Institute of Pharmacy, Pune for providing facilities and necessary support.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Davis S. Insulin, oral hypoglycemic agents and the pharmacology of the endocrine pancreas. In: Brunton L., Lazo J., Parker K., editors. Goodman and Gilman's the pharmacological basis of therapeutics. McGraw Hill; New York: 2006. pp. 1613–1646. [Google Scholar]
- 2.Datta A., Bagchi C., Das S., Mitra A., Pati A.D., Tripathi S.K. Antidiabetic and antihyperlipidemic activity of hydroalcoholic extract of Withania coagulans Dunal dried fruit in experimental rat models. J Ayurveda Integr Med. 2013;4:99–106. doi: 10.4103/0975-9476.113880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimmet P.Z., Magliano D.J., Herman W.H., Shaw J.E. Diabetes: a 21st century challenge. Lancet Diabetes Endocrinol. 2014;2:56–64. doi: 10.1016/S2213-8587(13)70112-8. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh M.N. Hilton & Company; Calcutta: 1984. Fundamental of experimental pharmacology; p. 192. [Google Scholar]
- 5.Nagmoti D.M., Kothavade P.S., Bulani V.D., Gawali N.B., Juvekar A.R. Antidiabetic and antihyperlipidemic activity of Pithecellobium dulce (Roxb.) Benth seeds extract in streptozotocin-induced diabetic rats. Eur J Integr Med. 2015;7:263–273. [Google Scholar]
- 6.Van der Vies J. Two methods for the determination of glycogen in liver. Biochem J. 1954;57:410. doi: 10.1042/bj0570410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akbarzadeh A., Norouzian D., Mehrabi M.R., Jamshidi S.H., Farhangi A., Allah Verdi A. Induction of diabetes by streptozotocin in rats. Indian J Clin Biochem. 2007;22:60–64. doi: 10.1007/BF02913315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolaffi J.L., Nagamastu S., Harris J., Grodsky G.M. Protection by thymidine, an inhibitor of polyadenosine diphosphate ribosylation of streptozotocin inhibition of insulin secretion. Endocrinology. 1987;20:2117–2122. doi: 10.1210/endo-120-5-2117. [DOI] [PubMed] [Google Scholar]
- 9.West E., Simon O.R., Morrison E.Y. Streptozotocin alters pancreatic beta cells responsiveness to glucose within six hours of injection in rats. West Indian Med J. 1996;45:60–62. [PubMed] [Google Scholar]
- 10.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50:537–546. [PubMed] [Google Scholar]
- 11.Lenzen S. Oxidative stress: the vulnerable beta-cell. Biochem Soc Trans. 2008;36:343–347. doi: 10.1042/BST0360343. [DOI] [PubMed] [Google Scholar]
- 12.Kumar S., Kumar V., Prakash O.M. Antidiabetic and hypolipidemic activities of Kigelia pinnata flowers extract in streptozotocin induced diabetic rats. Asian Pac J Trop Biomed. 2012;2:543–546. doi: 10.1016/S2221-1691(12)60093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castaneda C. Muscle wasting and protein metabolism. J Anim Sci. 2002;80:E98–E105. [Google Scholar]
- 14.Gillespie K.M. Type 1 diabetes: pathogenesis and prevention. CMAJ. 2006;2:165–170. doi: 10.1503/cmaj.060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Onwuli D.O., Brown H., Ozoani H.A. Antihyperglycemic effect of Tetracarpidium conophorum nuts in alloxan-induced diabetic female rats. ISRN Endocrinol. 2014:124974. doi: 10.1155/2014/124974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eddouks M., Jouad H., Maghrani M., Lemhadri A., Burcelin A. Inhibition of endogenous glucose production accounts for hypoglycemic effect of Spergularia purpurea in streptozotocin mice. Phytomedicine. 2003;10:594–599. doi: 10.1078/094471103322331890. [DOI] [PubMed] [Google Scholar]
- 17.Pari L., Satheesh M.A. Antidiabetic activity of Boerhaavia diffusa L on hepatic key enzymes in experimental diabetes. J Ethnopharmacol. 2004;91:109–113. doi: 10.1016/j.jep.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Kameswararao B., Kesavulu M.M., Apparao C.H. Evaluation of antidiabetic effect of Momordica cymbalaria fruit in alloxan-induced diabetic rats. Fitorapia. 2003;74:7–13. doi: 10.1016/s0367-326x(02)00297-6. [DOI] [PubMed] [Google Scholar]
- 19.Tembhurne S.V., Sakarkar D.M. Protective effect of Murraya koenigii (L) leaves extract in streptozotocin induced diabetics rats involving possible antioxidant mechanism. J Med Plant Res. 2010;4:2418–2423. [Google Scholar]
- 20.Calisti L., Tognetti S. Measure of glycosylated hemoglobin. Acta Biomed. 2005;76:59–62. [PubMed] [Google Scholar]
- 21.Prabhu D., Nappinnai M., Ponnudurai K., Thiruganasambantham A., Srinivasan S., Ramvikas M. Effects of Turnera umlifolia (Linn.) on blood glucose level of normal and alloxan-induced diabetic rats. Iran J Pharmacol Ther. 2009;8:77–81. [Google Scholar]
- 22.Okokon J.E., Nwaper P.A., Okokon P.J., Umoh E.E., Udobang J.A. Antidiabetic and hypolipidemic activities of ethanolic root extract of Croton zambesicus on alloxan-induced diabetic rats. Asian J Pharm Biol Res. 2011;1:493–499. [Google Scholar]
- 23.Howard B.V., Robbins D.C., Sievers M.L., Lee E.T., Rhoades D., Devereux R.B. LDL cholesterol as a strong predictor of coronary heart disease in diabetic individuals with insulin resistance and low LDL: the Strong Heart Study. Arterioscler Thromb Vasc Biol. 2000;20:830–835. doi: 10.1161/01.atv.20.3.830. [DOI] [PubMed] [Google Scholar]
- 24.Chattopadhyay R.R., Bandyopadhyay M. Effect of Azadirachta indica leaf extract on serum lipid profile changes in normal and streptozotocin induced diabetic rats. Afr J Biomed Res. 2005;8:101–104. [Google Scholar]
- 25.Descorbeth M., Anand-Srivastava M.B. Role of oxidative stress in high glucose and diabetes induced increased expression of Gq/11 alpha proteins and associated signaling in vascular smooth muscle cells. Free Radic Biol Med. 2010;49:1395–1405. doi: 10.1016/j.freeradbiomed.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 26.Chang Y.C., Chuang L.M. The role of oxidative stress in the pathogenesis of type 2 diabetes: from molecular mechanism to clinical implication. Am J Transl Res. 2010;2:316–331. [PMC free article] [PubMed] [Google Scholar]
- 27.Matkovics B., Kotorman M., Varga I.S., Hai D.Q., Varga C. Oxidative stress in experimental diabetes induced by streptozotocin. Acta Physiol Hung. 1997;85:29–38. [PubMed] [Google Scholar]
- 28.Kavalali G., Tuncel H., Goksel S., Hatemi H.H. Hypoglycemic activity of Urtica pilulifera in streptozotocin-diabetic rats. J Ethnopharmacol. 2003;84:241–245. doi: 10.1016/s0378-8741(02)00315-x. [DOI] [PubMed] [Google Scholar]
- 29.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51:216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]