Abstract
Background
Pashanabheda is used as antiurolithiatic in Ayurveda. In the present study, Aerva lanata (L) Juss. ex. Schult (Amaranthaceae) from Western Ghats of India was selected for isolation of active constituents and screening for antiurolithiatic potentials.
Objective
Screening of compounds isolated from A. lanata for antiurolithiatic potentials.
Materials and methods
Ethylene glycol (0.75% v/v) induced urolithiasis model was used to study the antiurolithiatic activity in male Wistar albino rats. The animals were divided into five groups containing six each. Based on the LD50 of the plant extract (2000 mg/kg b.w) equivalent dose was calculated from their yield. Two isolated compounds (quercetin and betulin) of A. lanata were screened for antiurolithiatic potentials in calculi induced (ethylene glycol 0.75% v/v) male Wistar albino rats by administering 2 mg/kg b.w/day orally as test dose for 28 days.
Results
The urine volume was found to be significantly increased from 12.76 ± 0.10 ml to 21.35 ± 0.20 ml in the rats treated by quercetin and 21.50 ± 0.21 ml in rats treated by betulin. Urine microscopy revealed significant reduction (p < 0.001) in the size of calculi and significantly enhanced (p < 0.001) excretion of calcium, oxalate, phosphate, whereas the level of magnesium was increased. SEM of kidney sections has revealed reduction in the calculi in treated animals. Serum analysis has revealed significant reduction in the level of BUN and creatinine in treated rats.
Conclusion
The isolated quercetin and betulin from A. lanata have shown mild diuretic effect as well as antiurolithiatic effect by significantly reducing the size of calculi in the kidneys and enhancing the excretion of calcium, phosphate, oxalate while maintaining the level of magnesium, which is reported to be one of the calculi inhibiting factors.
Keywords: Urolithiasis, Ayurveda, Quercetin, Betulin, Pashanabheda, Kidney stones
Graphical abstract
1. Introduction
Pashanabheda (stone breaking) plants are a group of medicinal plants which are used in Indian traditional medicinal system by Ayurvedic practitioners as antiurolithiatic drugs. Traditionally Aerva lanata (L) also known as Pashanabheda, belonging to the family Amaranthaceae, used for various medicinal uses including both antiurolithiatic and diuretic [1], [2], [3], [4].
The traditional medicine system of India is a rich source of valuable medicinal plants but there is no scientific data reported to establish the activity of these plants. Hence these plants need to be evaluated, based on their biological efficacy and chemical constituents for the drug development [5]. So we have selected Aerva lanata whole plant, cultivated in Western Ghats region of India for the present study. The whole plant was subjected to bioactivity guided isolation and screening for antiurolithiatic activity in order to investigate and justify the traditional claim.
It is reported that flavanoids, triterpenoids and saponins such as α-amyrin, β-amyrin, lupeol from different plants showed antiurolithiatic and diuretic activity. Many plant extracts and different fractions possessing these active constituents have been screened for antiurolithiatic activity [6], [7]. It is reported that flavanoids are found to be present in stem bark and triterpenoids are present in root bark portions of some plants such as Crataeva nurvala, Crataeva magna (Capparaceae) which acts as antiurolithiatic [8]. Hence the whole plant is selected for the study as different parts of the plant Aerva lanata are reported to be potent [10], [11], [12].
The reported phytochemical constituents present in Aerva lanata are responsible for various biological activities. These constituents include alkaloids (ervine, methylervine, ervoside, aervine, methylaervine, aervoside, ervolanine, and aervolanine), flavanoids (kaempferol, quercetin, isorhamnetin, persinol, persinosides A and B), methyl grevillate, lupeol, lupeol acetate benzoic acid, β-sitosteryl acetate and tannic acid [9].
Although Aerva lanata is used traditionally by Ayurvedic physicians, however, there is no report on the antiurolithiatic potentials by any of the active constituents isolated from Aerva lanata (L) cultivated in Western Ghats of Khanapur region from Belagavi district of Karnataka. Hence this plant was selected for isolating and developing new lead molecules for urolithiasis.
2. Materials and methods
2.1. Collection of plant
The whole plant Aerva lanata was collected from Western Ghats region of Khanapur (Belagavi Dist, Karnataka) in March 2011. The plant was authenticated by taxonomist Dr. Harsha Hedge, Scientist B, RMRC, Belagavi (Specimen No-RMRC-507).
2.2. Extraction and fractionation
The plant material was shade dried and then it was ground to coarse powder. The powdered dry material was used for the extraction using hydroalcoholic (80-20%) and followed by fractionation with different organic solvents such as dichloromethane (fraction I), ethyl acetate (fraction II), n-butanol (fraction III) to separate different groups of polar compounds like flavanoids, triterpenoids and saponins following the technique of liquid–liquid separation. As polar compounds would come in polar solvents, these two fractions (ethyl acetate fraction and n-butanol fraction) which were found to be rich with flavanoids and triterpenoids were analyzed by phytochemical study and spectral analysis. Based on these study results, ethyl acetate and n-butanol fractions which were found to contain flavanoids and triterpenoids were subjected to in vivo analysis for antiurolithiatic potential on ethylene glycol (0.75% v/v) induced urolithiatic model of Wistar albino male rats [13], [14].
2.3. Isolation and characterization
Based on the in vivo bioactivity study results of fractions, (ethyl acetate and n-butanol) the fractions were found to be potent, may be due to the presence of polar compounds. Hence these two fractions were subjected to isolation of active constituents using column chromatography technique followed by purification of the isolated constituents by preparative Thin Layer Chromatography (TLC). The fraction (II) and fraction (III) were loaded separately in the glass column by dissolving in chloroform (5 g in 500 ml each fraction) and separation was carried out. The isolated compound was identified with preparative TLC. This isolation technique has yielded two compounds AEF 1(isolated compound 1) and AEF 2.3 (isolated compound 2) from ethyl acetate and n-butanol fractions respectively and these two compounds were characterized as quercetin and betulin using Infra-Red, Nuclear Magnetic Resonance and Liquid Chromatography Mass Spectroscopy [15].
2.4. Animals
Male Wistar albino rats weighing 150–200 g were purchased from Sri Venkateshwara traders, Bengaluru. They were housed in acryl fiber cages at 23 ± 2 °C, humidity 50 ± 1% and were kept on a 12 h light/dark cycle. They were fed with standard chow feed (Amrut laboratories, Sangali) and water ad libitum and acclimatized for 15 days before the study. Experimental protocol reported in this study was approved by the Institutional Animal Ethical Committee of CPCSEA, Govt. of India (IAEC-Resolution No-13, 31-07-2010) and carried out in accordance with OECD guidelines.
2.5. Chemicals and drugs
Ethylene glycol (0.75% v/v) and sodium carboxy methyl cellulose were purchased from Himedia Lab (Mumbai) and Loba Chemie (Mumbai) respectively. Reference drug Cystone was purchased from Himalaya Herbal Healthcare, Bangalore. Demineralized water and analytical grade chemicals/solvents were procured from local market.
Electron microscope–Inverted microscope, Lobamade, model no-TCM-400 was used for urine microscopy study.
2.6. Drug administration
The reference drug and isolated compounds (quercetin and betulin) were administered orally through stainless steel oral feeding tube. Sodium CMC 1% of the weight was added to the isolated compounds for preparing the test doses.
2.7. Acute toxicity assay
Acute toxicity assay was conducted for hydroalcoholic extract as per OECD guideline 423 (limit test-standard protocol). Six male Wistar albino rats (three animals in each step) and total 12 animals were randomly selected. The extract was found to be safe up to 2000 mg/kg b.w. No separate toxicity study for the test compound was done since the hydroalcoholic extract was found to be safe as per IAEC [16].
2.8. Evaluation of antiurolithiatic activity of isolated compounds on ethylene glycol (0.75% v/v) induced albino rats
Ethylene glycol (0.75% v/v) induced urolithiasis model was used to study the antiurolithiatic activity in male Wistar albino rats. The animals were divided into five groups containing six each. The group I served as control and fed with normal rat food and water ad libitum. Group II to V received ethylene glycol (0.75% v/v) orally in drinking water from day 1 to day 28 for the induction of renal calculi (day 1 to day 14-induction period). Group II served as disease induced group. Group III received reference drug Cystone (750 mg/kg b.w) from 14th day to 28th day (treatment period). Group IV received quercetin-2 mg/kg b.w. from 14th day to 28th day (equivalent dose), group V received betulin-2 mg/kg b.w (equivalent dose) from 14th day to 28th day (treatment period) [17], [18], [19]. This equivalent dose was calculated based on the yield of the isolated compound from the corresponding fraction/s (as per IAEC).
Urine and serum analysis was carried out at the end of the study. The blood sample (1 ml) was collected on 28th day from each animal through retro-orbital plexus under anesthetic conditions. Urine samples were collected from all the animals on 14th and 28th day. Biochemical investigations of calcium, oxalate, magnesium, phosphate from urine sample and BUN and creatinine from blood serum sample was carried out. Both the kidneys were isolated from all the animals of different groups and subjected to histopathological study for the detection of calculus in kidneys [20].
Physical changes in experimental animals like weight of the animals and changes in urine volume were also monitored from day 1 to day 28. Urine volume was measured on day 14 and day 28.
2.9. Histopathology of harvested kidney section and staining method
Histopathology of the harvested kidney section was carried out to study the effect of drug on the calculi in kidney and also to know its effect on the internal structures of the kidney which shall give information about efficacy of the drug as well as its toxicity. This was carried out as per standard protocols. Staining of the kidney section was carried out as per the standard techniques of histology.
2.10. Statistical analysis
The biochemical results were expressed as mean ± SEM. Statistical significance of the observations from urine analysis (calcium, oxalate, phosphate, magnesium), serum creatinine and BUN from blood serum analysis was calculated using one way analysis of variance test (ANOVA), followed by Dunnett's t-test, value less than p < 0.001 were considered as statistically significant. Histopathological results were analyzed by semi-qualitative analysis.
3. Results
Both the isolated compounds were subjected to in vivo screening of antiurolithiatic potentials on male Wistar albino rats for 28 days based on their in silico docking study results of quercetin and betulin with the enzyme Oxalate oxidase (ETE 2) downloaded from Protein Data Bank (PDB) which is reportedly responsible for urolithiasis in animals [15].
3.1. Histopathological study
Kidneys of all animals harvested after 28 days were subjected to histopathological studies. The sections of kidneys of treated rats with ethylene glycol has shown deposition of micro crystals of calcium oxalate in cortex region whereas in the kidney section of the treated groups, the crystal deposition was significantly less (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5).
Fig. 1.
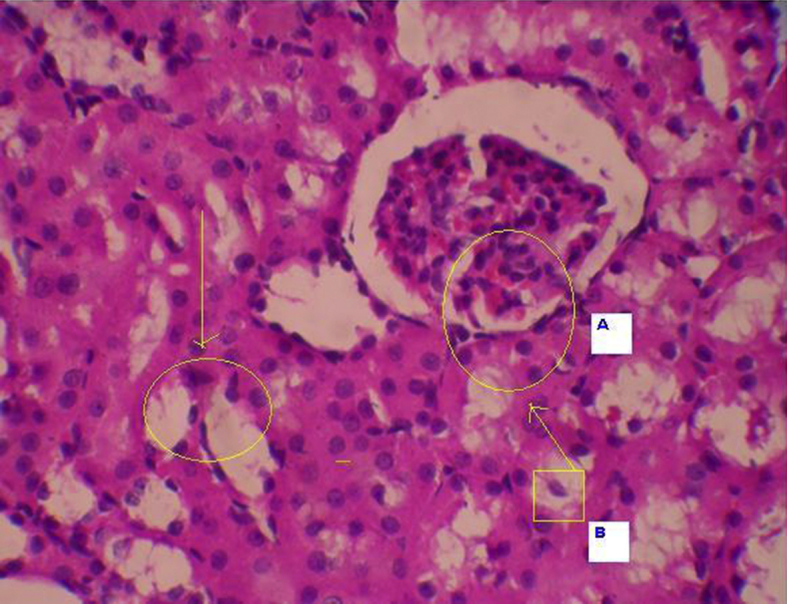
Induced animals group-A–Glomerular congestion, B–Tubular degeneration.
Fig. 2.
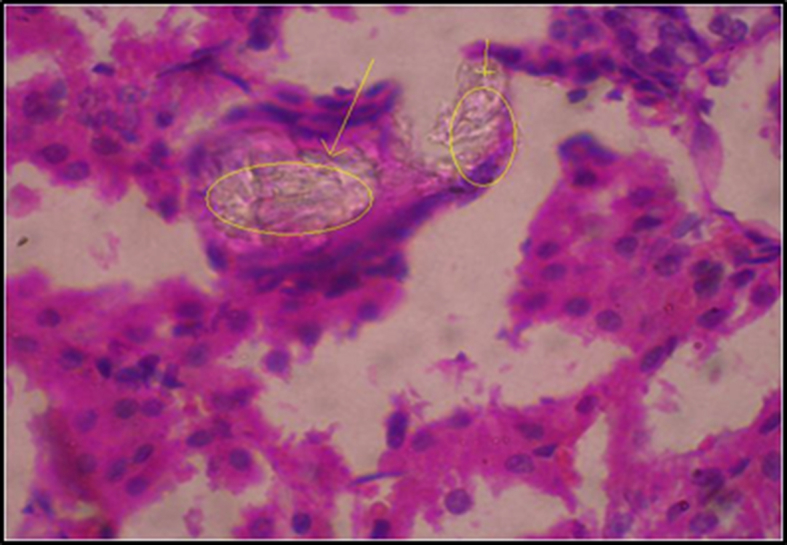
Microcrystals-Induced animals group.
Fig. 3.
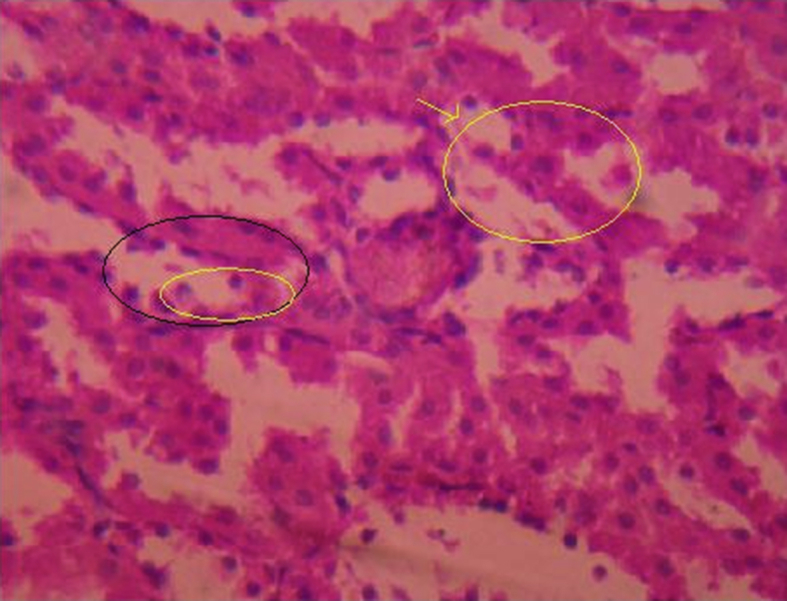
Induced animals group-Oedema.
Fig. 4.
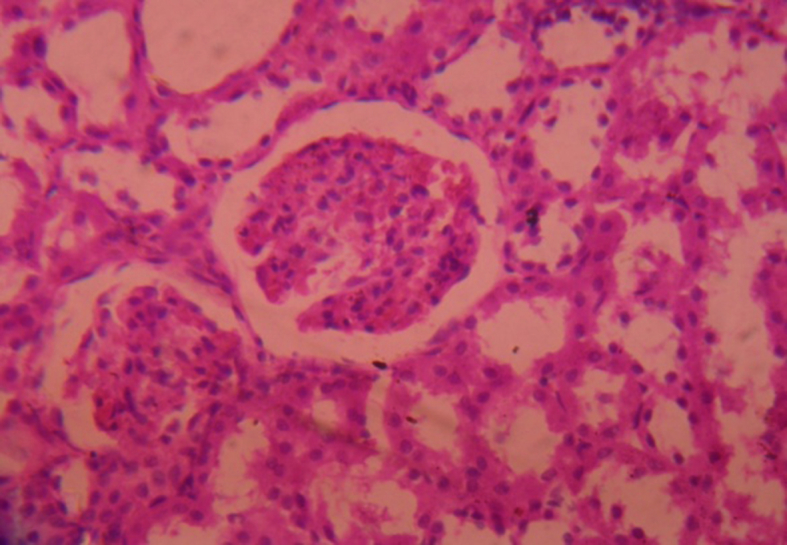
Histo of Quercetin treated group.
Fig. 5.
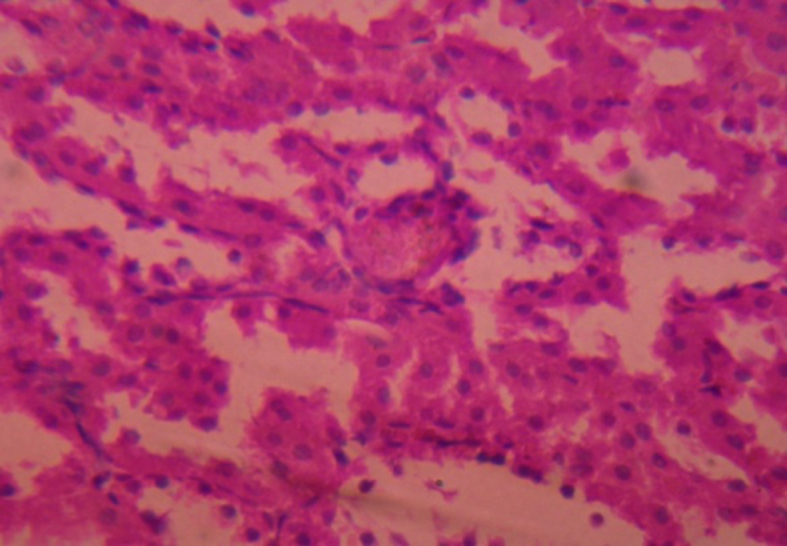
Histo of Betulin treated group.
There was no significant tubular damage, hemorrhage, disrupted brush border and tubular congestion in the kidney sections (cortex) of the rats treated with quercetin and botulin compared with the kidney sections of disease induced animals.
3.2. Urine analysis
Based on the measurement of urine, it was found that the volume of urine in normal rats was found to be 9.47 ± 0.08 ml on 14th day and 9.38 ± 0.09 ml on 28th day. The urine volume was found to be 12.65 ± 0.11 ml on 14th day and 12.76 ± 0.10 ml on 28th day in the rats treated with ethylene glycol. This volume of urine significantly increased in rats treated by betulin on 28th day. The weight of the animals (day 1 to day 28) was also monitored and it was observed that there was an increase in the weight of disease induced animals and there was a reduction in the weight of animals treated with the isolated compounds. Urine analysis under the electron microscope (Inverted microscope, Lobamade, model no-TCM-400) has shown reduction in the size and shape of the kidney stones in treated rats as compared to disease induced rats (Fig. 6, Fig. 7, Fig. 8, Fig. 9).
Fig. 6.
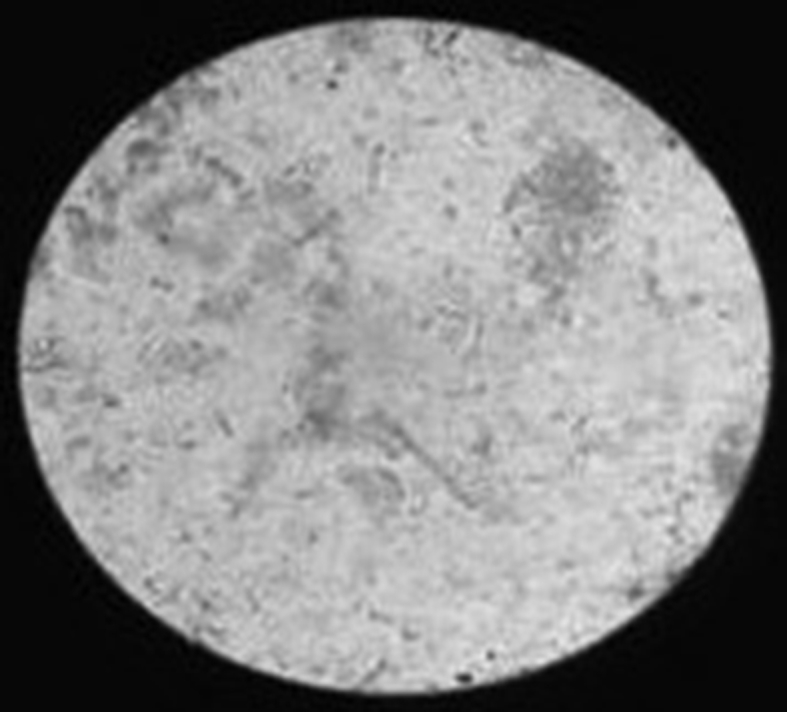
Normal urine sample-no stones.
Fig. 7.
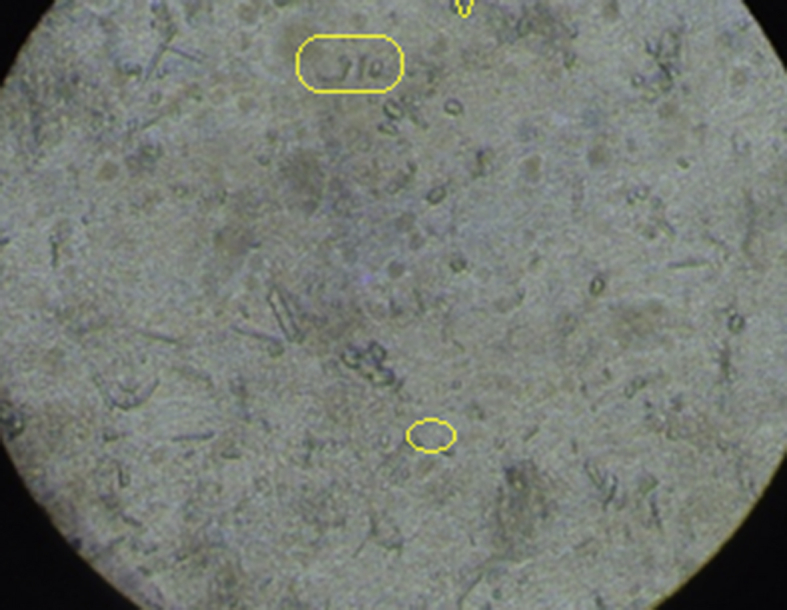
Disease induced group urine-stones formation.
Fig. 8.
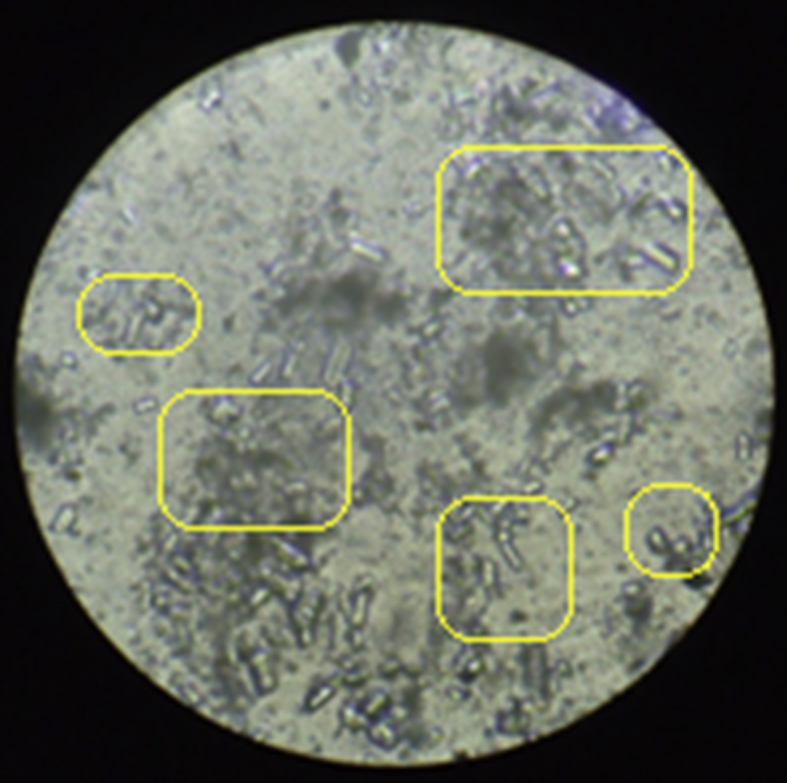
Quercetin treated animal's urine.
Fig. 9.
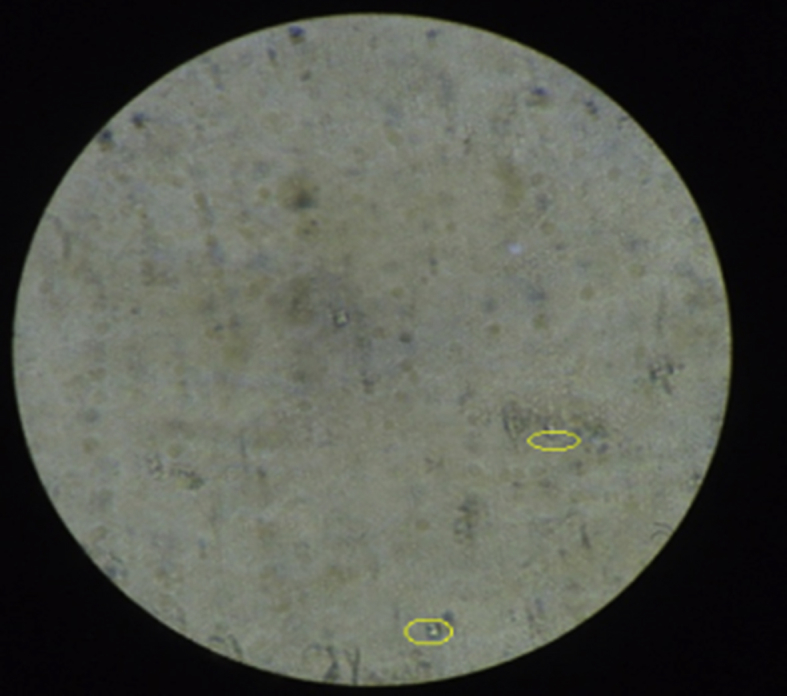
Betulin treated animal's urine.
3.3. Urine biochemistry
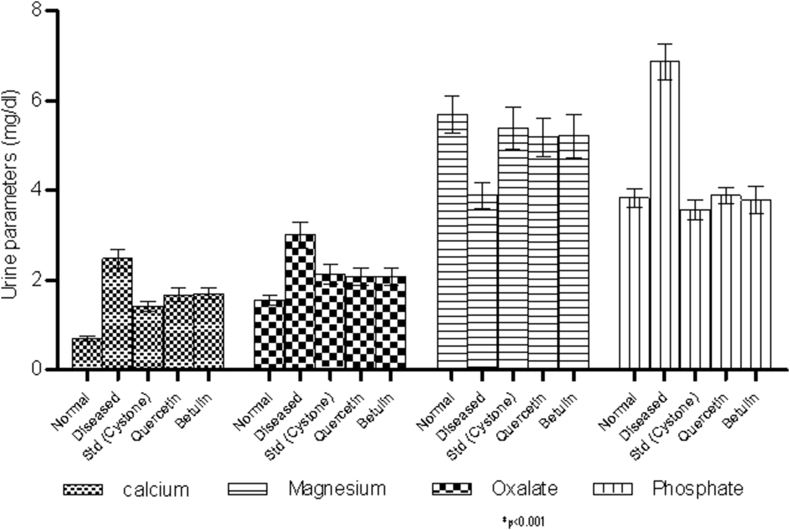
From the urine analysis it was found that the levels of calcium, phosphate, oxalate were high in the disease induced animals, whereas the levels of these ions were found to be significantly less (p < 0.001) in the urine samples of the animals treated with the quercetin and betulin (Fig. 10), however the level of magnesium was significantly increased in treated rats. The pH of urine in treated animals with isolated compounds was found to be slightly alkaline (pH 6).
Fig. 10.
Urine biochemistry.
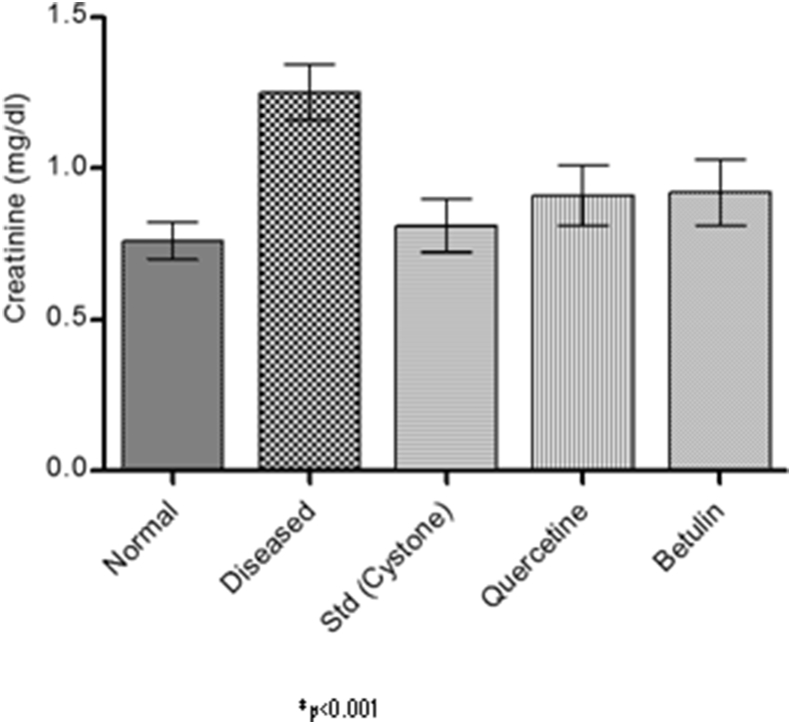
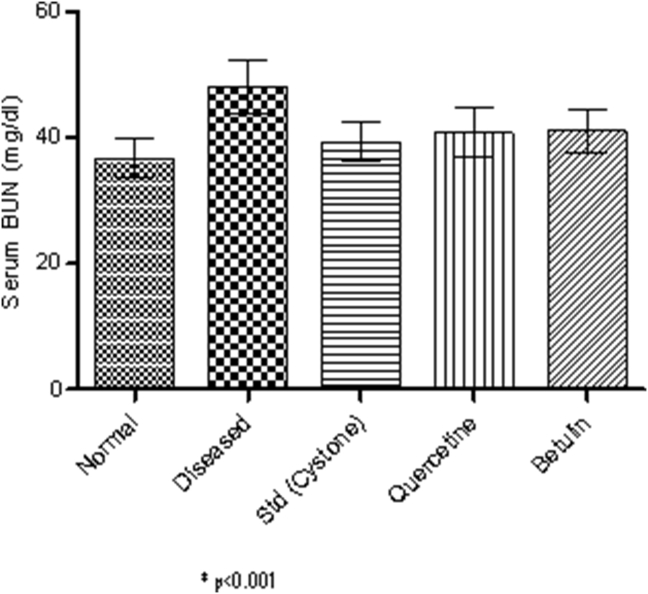
Serum analysis after 28th day was carried out to know the levels of creatinine and BUN. It was found that creatinine and BUN values were more in case of disease induced animals than the normal rats and were significantly reduced in treated animals (Fig. 11, Fig. 12).
Fig. 11.
Serum creatinine.
Fig. 12.
Serum BUN.
4. Discussion
Kidney stones are reportedly affecting mankind since long time and have been one of the causes for renal failure [18]. As there is no single effective drug available for urolithiasis today, surgery is considered to be the best option especially when other alternatives fail. However it is expensive and not affordable for common man. Hence the natural drugs are considered to be next alternative. Pashanabheda plants are a group of medicinal plants which are used in Indian traditional medicinal system by Ayurveda practitioners as antiurolithiatic drugs and A. lanata (L) is used conventionally as both antiurolithiatic and diuretic.
This study examined the antiurolithiatic efficacy of the two isolated constituents of A. lanata. Based on the bioactivity guided fractionation followed by isolation, these active constituents were characterized as quercetin and betulin [15]. These two active constituents were studied by in silico method by docking into a protein ETE 2 of the enzyme Oxalate oxidase downloaded from PDB [15]. The in silico results gave an insight into the binding of the enzyme which reportedly participates in crystal formation and based on these results they were subjected to in vivo studies on calculi induced male Wistar albino rats by ethylene glycol (0.75% v/v) for 28 days [17], [18], [19], [20], [21].
As it was found from research reports that urinary super saturation is responsible for calculi formation, the urinary concentration of oxalate is found to be increased in ethylene glycol induced animals. This may be due to increased urinary retention and excretion of oxalate [16]. In the present study it was found that urinary oxalate was increased in ethylene glycol induced urolithiasis rats whereas the excretion of oxalate was decreased in rats treated with quercetin and betulin which may be due to the inhibition of formation of oxalate by the isolated plant constituents. This may also be due to the inhibition of the activity of Oxalate oxidase enzyme which is reportedly responsible for the stone formation.
Normal urine contains many calculi inhibitors and magnesium is one such inhibitor. Low levels of magnesium are observed in stone forming rats. The magnesium level returns to normal level after the drug treatment. Magnesium forms complex with oxalate and reduces the super saturation of calcium oxalate and as a consequence reduces the growth and nucleation rate of calcium oxalate crystals. Magnesium inhibits oxalate absorption and excretion and thus prevents its supersaturation [22], [23], [24], [25], [26], [27]. Both the isolated compounds quercetin and betulin significantly increased the urinary magnesium level and thus reduce the risk of calcium oxalate stone formation.
In urolithiasis, the calculi deposition in urinary tract obstructs glomerular filtration rate (GFR) and consequently urine outflow decreases. Due to this, the waste products particularly nitrogenous substances like creatinine and BUN may get accumulated in blood. The treatment by quercetin and betulin has significantly (p < 0.001) lowered the elevated serum levels of BUN and creatinine. This has reduced the risk of obstruction to the urine flow by the waste materials in the urinary tract. The elevated level of nitrogenous substances in serum also indicates possibility of damage to the kidneys which is significantly minimized in the rats treated with isolated compounds [28], [29], [30].
The urine microscopy also revealed that concentration of various ions was altered drastically in urine after the treatment with the isolated compounds. The urine sample of normal group of rats showed absence of microcrystals; the urine sample of disease induced rats showed the presence of microcrystals (pentagonal) whereas the urine sample of treated rats showed the presence of small fragments. This creates a favorable environment for the nucleation and crystal formation in animals whereas a significant decrease in the level of these ions observed in the urine sample of the animals treated with the isolated compounds has resulted in the reduction of crystal formation [31].
The urine samples of normal and treated animals were collected on 14th and 28th day and a comparative analysis was carried out for weight of the animals, weight of kidneys and volume of urine which were monitored. This analysis has revealed that there was significant increase in the volume of urine in the animals treated with quercetin and betulin. This signifies the diuretic effect of the isolated compounds. The comparative analysis of the weight of animals has revealed that there was an increase in the weight of treated animals than the normal animals which may be mainly due to urine and stone deposition in the animals. The weights of harvested kidneys of treated animals has revealed that the weight was more in disease induced animals due to stone deposition whereas the weights were reduced in animals treated with quercetin and betulin significantly. This reduction in the weights may be due to reduction in the stone deposition as well as the excretion of the stones by isolated compounds. However, the diuretic effect of the isolated compounds in treated animals needs to be studied systematically to establish the diuretic activity [32], [33].
The urine microscopy has also revealed that the urine samples of experimental animals treated with quercetin and betulin has shown significant reduction in quantity of stones compared to disease induced urine samples. This shows a significant effect of the isolated compounds in the reduction of stones in treated animals (Fig. 6, Fig. 7, Fig. 8, Fig. 9).
Scanning Electron Microscopic (SEM) examination of cortex region of kidney showed significant changes in the tubular epithelial cells of the test groups, compared to the control group (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5). The glomeruli in kidneys of the groups treated with reference drug and isolated compounds did not show any major structural changes. The cell organelles were also found intact in the epithelial cells. Microscopic study of kidney sections have helped us to understand the deposition of microcrystals in tubules which may be responsible for the inflammation in disease induced rats and this has been significantly restored to normal by isolated compounds. Deposition of calculi may affect the reabsorption of solutes from the tubular lumen leading to a reduction in the passive reabsorption of water at the level of PCT. However quercetin and betulin were found equally effective in reducing the amount of calculi and their removal from kidneys by their diuretic activity. Both the isolated compounds have produced alkalinizing effect in urine.
The serum analysis revealed an increase in creatinine and BUN levels in disease induced animals than the normal animals whereas these values were significantly reduced in case of animals treated with quercetin and betulin (Fig. 11, Fig. 12). This indicated that creatinine and BUN were secreted more in disease induced animals due to calculi formation and this indicates deposition of nitrogenous substance and possibility of renal damage [28]. The isolated compounds have significantly reduced the excretion of creatinine and BUN by inhibiting stone formation and reducing the stone size.
5. Conclusion
Two compounds were isolated from Aerva lanata and characterized as quercetin and betulin by modern analytical techniques. Based on in silico study by docking into Oxalate oxidase (ETE 2), these two compounds were screened for in vivo antiurolithiatic activity on calculi induced by ethylene glycol (0.75% v/v) in male Wistar albino rats.
It was found that both quercetin and betulin were equally potent and their antiurolithiatic activity was found to be associated with the diuretic activity. However both quercetin and betulin are good substances for urolithiasis.
Conflict of interests
None.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Kirtikar K., Basu B. 2nd ed. vol. II. International Book Distributors; Dehradun: 2005. (Indian medicinal plants). [Google Scholar]
- 2.Veronika B., Saeed R.K. Herbal medicines in the management of urolithiasis: alternative or complementary? Planta Medica. 2012;7:1095–1103. doi: 10.1055/s-0029-1185719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramachandran S., Vijayakumar T.M., Saisandeep V., Ramsai K., Dhanaraju M.D. Antilithiatic activity of polyherbal extract on ethylene glycol-induced lithiasis in rats. Eur J Biomed Sci. 2011;3:36–39. [Google Scholar]
- 4.Nadkarni K.M. vol. I. Popular Prakashan; Bombay: 1999. pp. 49–50. (Indian materia medica). [Google Scholar]
- 5.Suman Herbs: an alternative approach in nephroprotection. Res J Pharnacognosy Phytochem. 2013;5:15–21. [Google Scholar]
- 6.Nagal A., Singla R.K. Herbal resources with antiurolithiatic effects: a review. Indo Glob J Pharm Sci. 2013;3:6–14. [Google Scholar]
- 7.Patel P.K., Patel M.A., Saralai M.G., Gandhi T.R. Antiurolithiatic activity of saponin rich fraction from the fruits of Solanum xanthocarpum Schrad. & Wendl. (Solanaceae) against ethylene glycol induced urolithiasis in rats. J Ethnopharmacol. 2012;144:160–170. doi: 10.1016/j.jep.2012.08.043. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharjee A. Phytochemical and ethno-pharmacological profile of Crataeva nurvala Buch-Hum (Varuna): a review. Asian Pac J Trop Biomed. 2012;2:1162–1168. [Google Scholar]
- 9.Aggarwal S., Tandon C., Forouzandeh M., Singla S., Kiran R., Jethi R. Role of a protein inhibitor isolated from human renal stone matrix in urolithiasis. Indian J Biochem Biophy. 2005;1:113–117. [PubMed] [Google Scholar]
- 10.Ramachandra TV, Suja A. Sahyadri Western ghats-Biodiversity Information System [Internet][2012–2013, Cited 2014]Available from: http://ces.iisc.ernet.in/biodiversity.
- 11.Deshmukh B.S. Ex-situ conservation studies on ethno-medicinal rare, endemic plant species from Western Ghats of Maharashtra. Int J Pharm Bio Sci. 2010;1:1–5. [Google Scholar]
- 12.Peringattulli N.K., Decruse S.W. Conservation of medicinal plants of Western Ghats, India and its sustainable utilization through in vitro technology. Vitro Cell Dev Biol Plant. 2011;47:110–122. [Google Scholar]
- 13.Adepu A., Narala S., Ganji A., Sapnil C. A review on natural plant: Aerva lanata. Int J Pharma Sci. 2013;3:398–402. [Google Scholar]
- 14.Hadjzadeh M., Khoei A., Hadjzadeh Z., Parizady M. Ethanolic extract of Nigella sativa L. seeds on ethylene glycol-induced kidney calculi in rats. Urol J. 2007;4:86–90. [PubMed] [Google Scholar]
- 15.Dinnimath B.M., Jalalpure S.S. In silico antiurolithiatic screening of Aerva lanata (L) isolated constituents. IJPER. 2015;49:126–133. [Google Scholar]
- 16.Sanjib B., Pallab K.H. Acute and sub-chronic toxicity study of Trichosanthes dioica root in mice. Am Eurasian J Toxicol Sci. 2013;5:30–35. [Google Scholar]
- 17.Atmani F., Slimani Y., Mimouni M., Hacht B. Prophylaxis of calcium oxalate stones by Herniaria hirsuta on experimentally induced nephrolithiasis in rats. BJU Int. 2003;92:137–140. doi: 10.1046/j.1464-410x.2003.04289.x. [DOI] [PubMed] [Google Scholar]
- 18.Parmar N.S., Prakash Shiv. Narosa Publishing House; New Delhi: 2006. Screening methodology in pharmacology. [Google Scholar]
- 19.Karadi R.V., Gadge N.B., Alagawadi K.R., Savadi R.V. Effect Moringa oleifera Lam. root-wood on ethylene glycol induced urolithiasis in rats. J Ethnopharmacol. 2006;105:306–311. doi: 10.1016/j.jep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Gunatilake M. Aerva lanata (Polpala): its effects on the structure and function of the urinary tract. Phcog Res. 2012;4:181–188. doi: 10.4103/0974-8490.102259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butterweck V., Saeed R.K. Herbal medicines in the management of urolithiasis: alternative or complementary? Planta Med. 2013;75:1095–1103. doi: 10.1055/s-0029-1185719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Velikovic D.T., Nikolova M.T., Ivancheva S.V., Stojanovic J.B., Veljkovic V.B. Extraction of flavonoids from garden (Salvia officinalis L.) and glutinous (Salvia glutinosa L.) sage by ultrasonic and classical maceration. J Serb Chem Soc. 2007;72:73–80. [Google Scholar]
- 23.Kumar R., Kumar T., Kamboj V., Chander H. Pharmacological evaluation of ethanolic extract of Kigelia pinnata fruit against ethylene glycol induced urolithiasis in rats. Asian J Plant Sci Res. 2012;2:63–72. [Google Scholar]
- 24.Gadge N.B., Jalalpure S.S. Curative treatment with extracts of Bombax ceiba L. (Bombacaceae) fruit reduces risk of calcium oxalate urolithiasis in rats. Pharm Biol. 2012;50:338–343. doi: 10.3109/13880209.2011.604332. [DOI] [PubMed] [Google Scholar]
- 25.Mohammed T., Amine L., Khadija E., Farouk L., Ibtissam Z., Younes E. Lemon juice has protective activity in a rat urolithiasis model. BMC Urol. 2007;7:18. doi: 10.1186/1471-2490-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siener R., Jahnen A., Hesse A. Influence of a mineral water rich in calcium, magnesium and bicarbonate on urine composition and the risk of calcium oxalate crystallization. Eur J Clin Nutr. 2004;58:270–276. doi: 10.1038/sj.ejcn.1601778. [DOI] [PubMed] [Google Scholar]
- 27.Soundarajan P., Mahesh R., Ramesh T., Hazeena Begum V. Effect of Aerva lanata on calcium oxalate urolithiasis in rats. Indian J Exp Biol. 2006;44:981–986. [PubMed] [Google Scholar]
- 28.Doddametikurke R.B., Biyani C.S., Browning A.J., Cartledge J.J. The role of urinary kidney stone inhibitors and promoters in the pathogenesis of calcium containing renal stones. EAU-EBU Update Ser. 2007;5:126–136. www.europeanurology.com [cited 2012] available from: [Google Scholar]
- 29.Jawalekar S.L., Surve V.T., Bhutey A. Effect of dietary habbit & fluid intake in patients with Urolithiasis. Ann Biol Res. 2013;4:246–251. [Google Scholar]
- 30.Kumar R., Kapoor R., Mittal B., Kumar A., Mittal R.D. Evaluation of urinary abnormalities in urolithiasis patients: a study from north India. Indian J Clin Biochem. 2003;18:209–215. doi: 10.1007/BF02867389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gotsman I., Zwas D., Planer D., Admon D., Lotan C., Keren A. The significance of serum urea and renal function in patients with heart failure. Medicine (Baltimore) 2010;89:197–203. doi: 10.1097/MD.0b013e3181e893ee. [DOI] [PubMed] [Google Scholar]
- 32.Thangarathinam N., Jayshree N., Mehta A.V., Ramanathan L. Effect of polyherbal formulation on ethylene glycol induced urolithiasis. Int J Pharm Pharm Sci. 2013;5:994–997. [Google Scholar]
- 33.Geetha K., Venkappayya D., Manavalan R. Beneficial role of b-Amyrin from toothbrush tree Salvadora persica in experimental hyperoxaluria. Asian J Chem. 2010;22:6547–6552. doi: 10.1358/mf.2010.32.9.1549114. [DOI] [PubMed] [Google Scholar]