Abstract
HIV-1 sequence diversity presents a major challenge for the clinical development of broadly neutralizing antibodies (bNAbs) for both therapy and prevention. Sequence variation in critical bNAb epitopes has been observed in the majority of HIV-1 infected individuals and can lead to viral escape following bNAb monotherapy in humans. In this study, we show that viral sequence diversity can limit both the therapeutic and prophylactic efficacy of bNAbs in rhesus monkeys. We first demonstrate that monotherapy with the V3 glycan-dependent antibody 10-1074, but not PGT121, results in rapid selection of pre-existing viral variants containing N332/S334 escape mutations and loss of therapeutic efficacy in SHIV-SF162P3-infected rhesus monkeys. We then show that the V3 glycan-dependent antibody PGT121 alone and the V2 glycan-dependent antibody PGDM1400 alone both fail to protect against a mixed challenge with SHIV-SF162P3 and SHIV-325C. In contrast, the combination of both bNAbs provides 100% protection against this mixed SHIV challenge. These data reveal that single bNAbs efficiently select resistant viruses from a diverse challenge swarm to establish infection, demonstrating the importance of bNAb cocktails for HIV-1 prevention.
Introduction
Broadly neutralizing antibodies (bNAbs) against HIV-1 Env are currently being developed for both HIV-1 prevention and therapy (1, 2). However, HIV-1 diversity presents a major challenge for the clinical development of bNAbs. Recent studies have demonstrated that bNAb monotherapy in chronically HIV-1-infected individuals rapidly selects for resistant viral variants (3–7), suggesting that cocktails of bNAbs will be required for effective therapeutic strategies. However, the question of whether multiple bNAbs will be required to protect against acquisition of infection has not previously been studied in detail.
Previous studies in non-human primates that have demonstrated bNAb-mediated protection against simian-human immunodeficiency virus (SHIV) challenge have typically used single challenge viruses (8–10). During sexual transmission of HIV-1 across mucosal barriers, however, exposure to a swarm of viral variants is common, even though only a small number of founder viruses typically establish the infection (11, 12). These data suggest that bNAb combinations may also be required for HIV-1 prevention strategies.
In this study, we explored whether single bNAbs would select for resistant SHIV variants in both therapeutic and prophylactic experiments in rhesus monkeys. We also evaluated whether a bNAb cocktail would be required to protect against a mixed SHIV challenge. We selected the V3 glycan-specific antibody PGT121 and the V2 glycan-specific antibody PGDM1400 (13, 14) for this study, as these bNAbs target different epitopes and are both currently being evaluated in clinical trials (NCT02960581, NCT03205917).
Results
Rapid viral escape from single bNAb therapy
It has been hypothesized that certain bNAbs, such as the V3 glycan-dependent antibody PGT121 (15), might have an intrinsically higher bar to escape than other bNAbs targeting this epitope (16) as a result of making multiple glycan contacts on the Env surface, a phenomenon termed “glycan promiscuity” (17). We therefore first compared the therapeutic efficacy of the two related V3 glycan-dependent antibodies, PGT121 and 10-1074 (15, 18), in chronically SHIV-SF162P3-infected rhesus monkeys. Animals were infected with SHIV-SF162P3 approximately 7 months prior to a single i.v. infusion with 10 mg/kg PGT121 (N=4) or 10-1074 (N=2). Both antibodies had comparable neutralizing potency against SHIV-SF162P3 in vitro (IC50 of 0.1 μg/ml for 10-1074, IC50 of 0.1 μg/ml for PGT121) but resulted in markedly different therapeutic efficacy in vivo (Fig. 1). Consistent with our previous findings (16), PGT121 infusion resulted in up to a 3 log reduction of plasma viral loads to undetectable levels, which was followed by viral rebound when PGT121 titers declined to subtherapeutic levels. In contrast, 10-1074 infusion resulted in only a transient 1.5 log decline of plasma viral loads followed by rapid viral rebound to baseline levels by day 14, which is comparable to the reported efficacy of 10-1074 in SHIV-AD8-infected monkeys (19) and HIV-1-infected humans (6).
Figure 1. SHIV-SF162P3 escape from 10-1074 in rhesus monkeys.
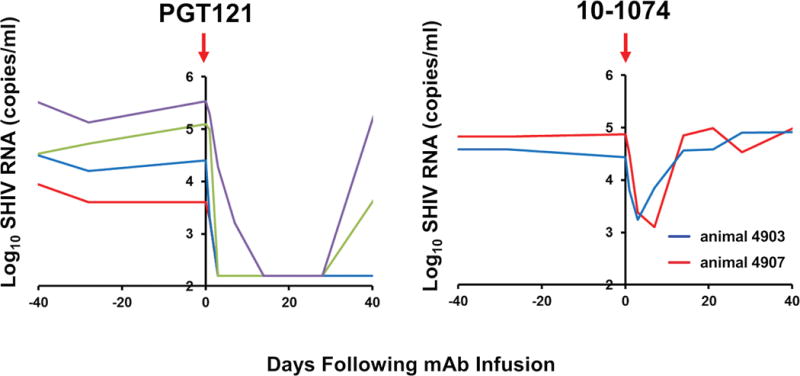
(A) Log plasma viral RNA copies/ml in chronically SHIV-SF162P3-infected rhesus monkeys following infusion with a single dose of 10 mg/kg 10-1074 or PGT121 on day 0. Detection limit is 50 copies/ml.
Viral sequencing by single genome amplification following 10-1074 infusion demonstrated the emergence of Env mutations at critical contact sites (N332D/T/S/I/K, S334N/R), which eliminate the N-linked glycan at position 332 (Fig. 2). These mutations were present at detectable but low levels in the challenge stock and in baseline plasma samples (Fig. 3A), suggesting that 10-1074 selected rare pre-existing viral escape variants. In contrast, no mutations were observed in the PGT121-treated animals following rebound, consistent with our prior observations (16). Moreover, an N332A point mutation in SHIV-SF162P3 completely abrogated 10-1074 neutralization activity in vitro but only partially reduced PGT121 neutralization activity (Fig. 3B). Taken together, these data suggest that PGT121 has a higher bar to escape than 10-1074 for SHIV-SF162P3, presumably as a result of the ability of PGT121 to make multiple glycan contacts on HIV-1 Env (17), and thus a single N332/S334 point mutation in the context of SHIV-SF162P3 may be insufficient to escape from PGT121.
Figure 2. Analysis of SHIV env sequences in 10-1074 treated animals.

Viral sequences by single genome amplification (SGA) of plasma viruses before and after 10-1074 administration. Highlighted in red are amino acid residues that were detected at day 14 and 42.
Figure 3. Frequencies of amino acids at critical PGT121 and 10-1074 contact sites in the SHIV-SF162P3 challenge stock.
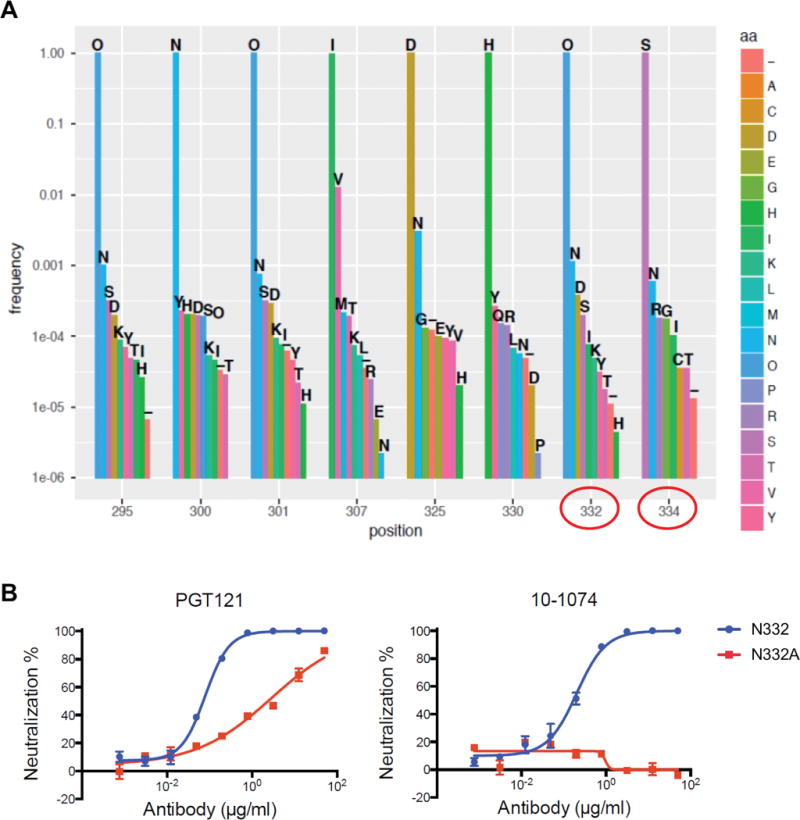
(A) “O” indicates an Asn that is part of a potential N-linked glycosylation site (PNGS) (positions 295, 301, 332). The critical N332 and S334 residues are circled in red. (B) PGT121 and 10-1074 neutralization potency of SHIV-SF162P3 (wild-type) and SHIV-SF162P3 containing an N332A mutation.
Complementary HIV-1 coverage by PGT121 and PGDM1400
PGT121 covers approximately 60–70% of global viruses (15), and thus we explored which bNAbs would complement PGT121 to improve global virus coverage (20). PGDM1400 is a V2 glycan-dependent bNAb with exceptional neutralizing potency and breadth (13). We evaluated the neutralizing activity of PGT121, PGDM1400, and the combination of PGT121+PGDM1400 against a panel of 118 multiclade pseudoviruses in TZM-bl assays. PGDM1400 covered 77% of viruses in this panel with a median IC80 of 0.57 μg/ml, and PGT121 covered 60% of viruses in this panel with a median IC80 of 3.26 μg/ml (Fig. 4A). The combination of both bNAbs neutralized 97% of pseudoviruses with a median IC80 of 0.03 μg/ml, indicating that the combination was substantially more potent than either bNAb alone (P=1.0×10−6 and P=4.2×10−11 comparing IC80 titers for the combination versus PGDM1400 alone and PGT121 alone, respectively, Wilcoxon rank-sum tests) and also had greater breadth than either bNAb alone (P=2.2×10−6 and P=2.2×10−13 comparing breadth for the combination versus PGDM1400 alone and PGT121 alone, respectively, Fisher’s exact tests) (Fig. 4B). These data suggest a remarkable complementarity between PGT121 and PGDM1400, as viruses resistant to one of these antibodies are largely susceptible to the other antibody. The combination of PGT121+PGDM1400 covered 97–100% of viruses from clades A, B, C, and CRF01 in this global panel (Fig. 4C). Moreover, the combination led to complete (>95%) neutralization of 86% of viruses in this panel, as compared with 59% for PGDM1400 and 53% for PGT121 (P=9.3×10−6 and P=5.1 × 10−8, respectively, Fisher’s exact tests) (Fig. S1).
Figure 4. Complementarity of neutralization profiles of PGDM1400 and PGT121 against HIV-1.
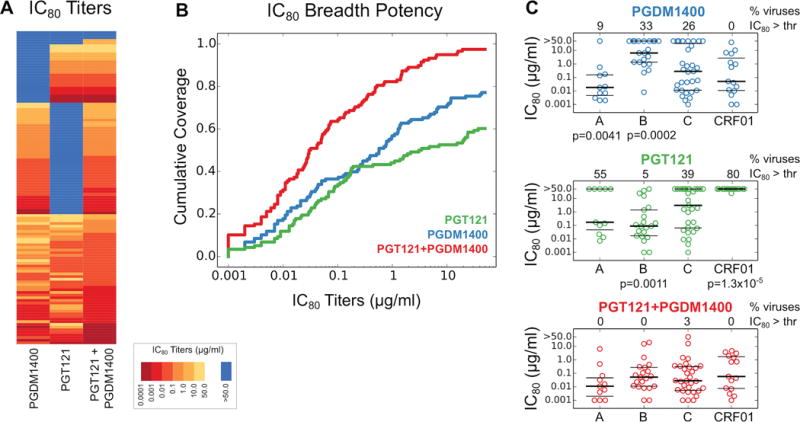
(A) IC80 titers for PGDM1400, PGT121, and the combination of PGT121+PGDM1400 against a panel of 118 multiclade viruses. IC80 titers are shown as a heatmap with viruses represented in rows. Dark red indicates more potent neutralization and light yellow indicates less potent neutralization. Blue indicates IC80 neutralization titers >50 μg/ml. The IC80 titers in the combination reflect the concentration of each bNAb. (B) IC80 breadth-potency plots for PGDM1400, PGT121, and the combination of PGT121+PGDM1400. (C) IC80 titers for PGDM1400, PGT121, and the combination of PGT121+PGDM1400 for pseudoviruses from clades A, B, C, and CRF01. Bold horizontal lines represent medians, thin horizontal lines are are 25th and 75th percentiles. The percent of viruses with IC80 titers >50 μg/ml are shown on the top of each panel. P values reflect Fisher’s exact tests.
In vivo protection against mixed SHIV challenge
The complementarity of PGT121 and PGDM1400 in vitro suggest the combination of these two bNAbs as an HIV-1 prevention strategy. We therefore evaluated the protective efficacy of PGDM1400 alone, PGT121 alone, or the combination of PGT121+PGDM1400 in rhesus monkeys against a mixed SHIV challenge with the clade B SHIV-SF162P3 (21) and the clade C SHIV-325C (22). SHIV-SF162P3 was sensitive in vitro to PGT121 but resistant to PGDM1400, whereas SHIV-325C was sensitive in vitro to PGDM1400 but resistant to PGT121 (Table 1). 20 rhesus monkeys (N=5/group) were infused i.v. with 10 mg/kg of PGDM1400, 10 mg/kg of PGT121, the combination of 5 mg/kg PGT121 + 5 mg/kg PGDM1400, or phosphate-buffered saline as control. Animals were challenged the following day by the i.r. route with 500 TCID50 of SHIV-SF162P3 + 500 TCID50 of SHIV-325C. Pharmacokinetics of the two bNAbs were similar by both idiotype-specific ELISAs and virus-specific NAb assays (Figs. S2, S3, S4).
Table 1.
IC50 and IC80 neutralization titers (in μg/ml) of PGT121 and PGDM1400 against SHIV-SF162P3 and SHIV-325C.
| Antibody | SHIV-SF162P3 | SHIV-325C | ||
|---|---|---|---|---|
| IC50 | IC80 | IC50 | IC80 | |
| PGDM1400 | >50 | >50 | 0.015 | 0.061 |
| PGT121 | 0.085 | 0.17 | >50 | >50 |
Following challenge, all sham control animals became infected with peak plasma viral loads of 6.60–7.73 log RNA copies/ml on day 14 (Fig. 5). Similarly, 100% (5 of 5) of animals that received PGDM1400 alone became infected with peak plasma viral loads of 5.46–7.74 log RNA copies/ml, and 100% (5 of 5) of animals that received PGT121 alone also became infected but with lower peak plasma viral loads of 3.97–5.26 log RNA copies/ml. In contrast, 0% (0 of 5) of animals that received the combination of PGT121+PGDM1400 exhibited detectable plasma viral loads (>50 RNA copies/ml) at any point in time (Fig. 5), indicating that only the PGT121+PGDM1400 combination protected against this mixed SHIV challenge (P=0.008 comparing the combination group with either the PGDM1400 alone group or the PGT121 alone group, Fisher’s exact tests).
Figure 5. Protective efficacy of the combination of PGT121+PGDM1400 against a mixed SHIV challenge in rhesus monkeys.
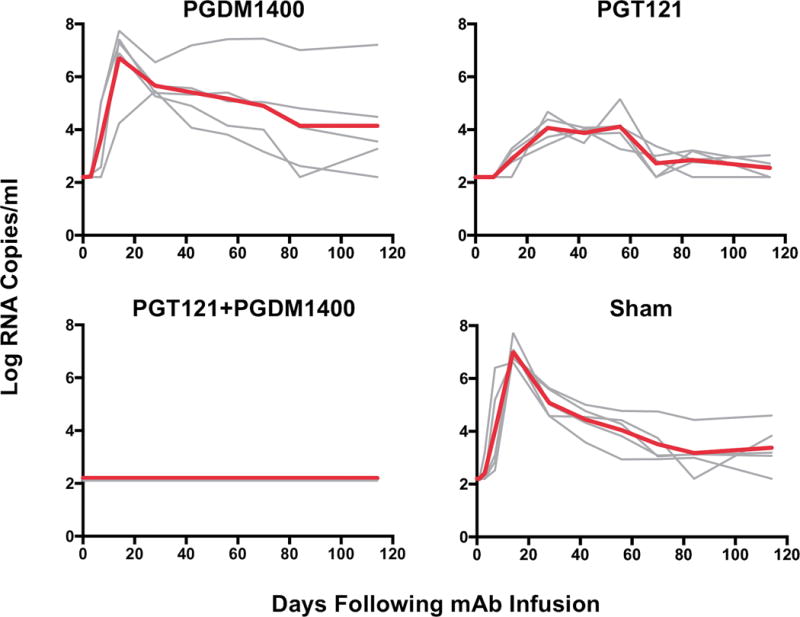
5 animals per group received either an i.v. single dose of PGDM1400, PGT121, the combination of PGT121+PGDM1400, or saline (Sham) before being rectally challenged with a high dose of both SHIV-SF162P3 and SHIV-325c. Log plasma viral RNA copies/ml following mixed challenge with SHIV-SF162P3 and SHIV-325C. Red line indicates median values. Detection limit is 50 copies/ml.
Viral sequencing by single genome amplification at weeks 2-6 following challenge indicated that 100% of clones in the PGT121-treated animals were SHIV-325C, whereas 100% of clones in the PGDM1400-treated animals were SHIV-SF162P3 (Fig. 6, Table 2), consistent with the resistance patterns of these viruses (Table 1). The sham controls were detectably infected with only SHIV-SF162P3 (Fig. 6, Table 2), which has greater replication capacity than SHIV-325C. These findings were confirmed by strain-specific RT-PCR assays (Figs. S5, S6), demonstrating that each bNAb alone efficiently selected resistant variants from the challenge swarm for the establishment of primary infection.
Figure 6. Analysis of SHIV env sequences in breakthrough infections.
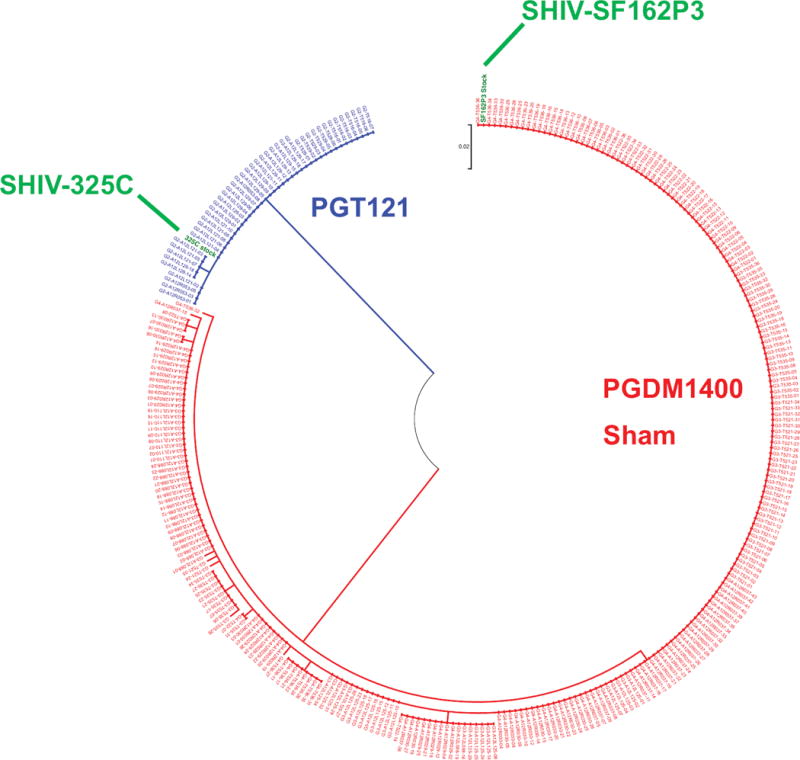
Maximum likelihood tree depicting SHIV env sequences by single genome amplification (SGA) of plasma viruses at weeks 2-6 following challenge. Highlighted in green are the sequences of the challenge stock SHIVs.
Table 2.
Number of SHV-strain-specific single genome sequences in the animals that experienced break-through infection.
| Total SGA sequences | |||
|---|---|---|---|
| Group | Monkey | SHIV-SF162P3 | SHIV-325c |
| PGT121 alone | A12R053 | 0 | 8 |
| A12L121 | 0 | 11 | |
| A12L129 | 0 | 15 | |
| T516 | 0 | 2 | |
| T529 | 0 | 6 | |
| PGDM1400 alone | A12L110 | 18 | 0 |
| A12L125 | 33 | 0 | |
| A12L088 | 24 | 0 | |
| T521 | 35 | 0 | |
| T535 | 36 | 0 | |
| Sham | A12R029 | 25 | 0 |
| A12R030 | 27 | 0 | |
| A12R037 | 43 | 0 | |
| T522 | 36 | 0 | |
| T536 | 36 | 0 | |
Diversity of HIV-1 sequences in target bNAb epitopes
We next evaluated the variation in key PGDM1400 and PGT121 contact and signature positions in HIV-1-infected humans. Signature residues were resolved using a phylogenetically corrected strategy using large panels of M group or C clade viruses (B. Korber et al., submitted), and contact sites were found by structural modeling using structures of PGT145 and PGT122 complexed with HIV-1 Env and of PGDM1400 and PGT121 Fabs (13, 23–25) (Fig. S7). The variation of key signature residues at contact sites for PGDM1400 and PGT121 binding to HIV-1 Env from multiple clades was then defined (Figs. S8, S9). In these Logo plots, “O” represents an Asn that occurs in a potential N-linked glycosylation site (PNGS).
We assessed sequence variability in these key PGDM1400 and PGT121 signature positions in 10 HIV-1-infected individuals that had been sampled longitudinally and for whom there were >150 full-length published env sequences in the Los Alamos Sequence Database. The key N332 PNGS for PGT121 exhibited variability in 8/10 individuals, including in those with primarily sensitive virus, and the D325 site showed variability in 7/10 individuals (Fig. 8; Table S1). D325 is a key contact residue, and a D325A substitution can reduce neutralization sensitivity 9-fold and can completely abrogate sensitivity in conjunction with the loss of the N332 glycosylation site (26). The key N160 PNGS for PGDM1400 was variable in only 3/10 individuals, but a critical R166 signature was either variable or lost in 5/10 individuals, and the key K/R169 positive charge was either variable or lost in 10/10 individuals (Fig. 8; Table S1) (23). These findings in a random sample of HIV-1 infected individuals suggest that diverse resistance profiles evolve over time in HIV-1-infected humans.
Discussion
Our data demonstrate that bNAbs can rapidly and specifically select for resistant viral variants within a diverse challenge swarm, resulting in efficient breakthrough infection with resistant SHIV strains. Both PGDM1400 alone and PGT121 alone failed to protect against challenge with a mixture of SHIV-SF162P3 and SHIV-325C in rhesus monkeys, whereas the combination of both bNAbs protected with 100% efficacy. These findings suggest that a combination of bNAbs will be required to provide optimal protection against acquisition of HIV-1 infection.
All the sham controls exhibited only SHIV-SF162P3 replication by both single-genome amplification as well as subtype-specific RT-PCR assays. The absence of detectable SHIV-325C replication in the sham controls suggests that SHIV-SF162P3 has higher replicative capacity than SHIV-325C in vivo, although we cannot exclude the possibility that a low level of SHIV-325C replication occurred in these animals below the level of detection (<50 copies/ml). In the presence of PGT121 selection pressure, however, robust infection with SHIV-325C occurred in 100% of animals. These findings are consistent with the observation that during primary HIV-1 infection transmitted founder viruses with higher replication rates are selected from a mixed swarm (27). Whether bNAb-mediated selection of resistant viral variants occurs entirely at the mucosal site of inoculation or at distal sites of early infection (28, 29), however, remains to be determined.
Multiple previous studies have shown that single bNAbs and NAb combinations robustly protect against SHIV challenges in rhesus monkeys (8–10), presumably as a result of relatively limited viral sequence diversity in most SHIV challenge stocks. Our findings suggest that the increased viral diversity observed in HIV-1 swarms may compromise the efficacy of single bNAbs for prevention of acquisition of HIV-1 infection, as a result of a greater frequency of resistant viral variants and the remarkable efficiency of bNAbs to select resistant viruses within a swarm. Indeed, in the plasma viruses of the 10 randomly selected individuals that we analyzed, variations in key PGDM1400 and PGT121 contact and signature positions were frequent. Moreover, bNAbs rapidly select minor resistant variants in chronically infected hosts, as shown here for 10-1074 as well as in prior studies in SHIV-infected rhesus monkeys (19) and HIV-1-infected humans (3–7), although certain bNAbs such as PGT121 may have a higher bar to escape than 10-1074 against a subset of viruses. PGT121 and PGDM1400, both alone and in combination, are currently being explored in clinical trials (NCT02960581, NCT03205917).
A limitation of this study are that even two SHIVs might not be representative of a diverse HIV-1 swarm. In addition, this study assessed only one bNAb combination, and thus testing additional bNAb combinations against mixed SHIV challenges will be required to further define the generalizability of our findings.
Taken together, these data demonstrate the importance of developing bNAb cocktails for both prevention and therapy of HIV-1 infection to cover viral diversity as well as resistant variants within diverse viral populations.
Materials and Methods
Animals and study design
The overall objective of this study was to investigate the protective efficacy of PGDM1400 and PGT121, alone or in combination, against a challenge with two SHIVs in rhesus monkeys. One additional study was performed to test the therapeutic activity of the antibodies PGT121 and 10-1074 in chronically SHIV-SF162P3 infected macaques. Animals were randomly allocated to groups, and virologic data was generated blinded. Placebo controls (saline) were used for the protection study. 5 animals per group was used similar to previous studies (10, 30, 31). The number of animals analyzed is stated in the figure legends. Primary data can be found in the Figures. All animal studies were approved by the appropriate Institutional Animal Care and Use Committee (IACUC). Monkeys were housed at Bioqual, Rockville, MD and AlphaGenesis, Yemasee, SC.
Single SHIV treatment study
6 outbred, Indian-origin male and female rhesus monkeys (Macaca mulatta) were genotyped and selected as negative for the protective MHC class I alleles Mamu-A*01, Mamu-B*08, and Mamu-B*17. TRIM5 polymorphisms were balanced equally among groups. Animals were chronically infected with SHIV-SF162P3 and received 10 mg/kg of PGT121 or 10-1074 by the i.v. route.
Double SHIV challenge study
20 outbred, Indian-origin male and female rhesus monkeys (Macaca mulatta) were genotyped and selected as negative for the protective MHC class I alleles Mamu-A*01, Mamu-B*08, and Mamu-B*17. TRIM5 polymorphisms were balanced equally among groups. Animals received 5-10 mg/kg of PGT121, PGDM1400 or both by the i.v. route and were challenged i.r. with 500 TCID50 of SHIV-SF162P3 + 500 TCID50 of SHIV-325C. Generation of these SHIV stocks has been previously described (21, 22, 32).
Antibodies
10-1074, PGT121 and PGDM1400 were produced as previously described (15) and purified by Protein A affinity matrix (GE Healthcare). All the monoclonal antibody preparations were endotoxin free.
ELISAs
PGDM1400 and PGT121 antibody titers were measured by a quantitative ELISA as previously described (15) using anti-idiotypic antibody coated microtiter plates to capture the monoclonal antibodies followed by detection using a HRP-conjugated anti-human IgG antibody.
TZM-bl neutralization assays
Neutralization of the two SHIV challenge stocks was evaluated in vitro by using TZM-bl target cells and a luciferase reporter assay as described (33).
Viral RNA assays
Plasma SHIV RNA levels were measured using a gag-targeted quantitative real-time RT-PCR assay, and tissue levels of SHIV RNA and DNA were measured using gag-targeted, nested quantitative hybrid real time/digital RT-PCR and PCR assays, as previously described (16).
Strain-specific RT-PCR assays
Viral RNA was reverse transcribed using the universal SHIV primer vl-EnvR, 5’-CAATAATTGTCTGGCCTGTACCGTC. Probes and primers were designed to target the specific regions of either SHIV-SF162P3 or SHIV-325C. Primers including SF162P3 vl-EnvF, 5’-TTGGAGAATGCTACTAATACCAC, vl-EnvR, 5’-CCTATGCTTGTGGTGACGTT and probe, 5’-ATGAACAGAGGAGAAATA linked to FAM and BHQ (Biosearch Technology) were used for SHIV-SF162P3-specific viral load assay; primers including 325C vl-EnvF, 5’-CGGGAAACATAACATGTAGATCAAA, vl-EnvR, 5’-TTCTCCCTTTTCTCCTCCATCA and probe, 5’-ACAGGACTACTGGTGACAC linked to Cy5 (Integrated DNA Technologies) were used for SHIV-325C-specific viral load assay.
SHIV-SF162P3 deep sequencing
Env amplicon deep sequencing was performed using pairs of primers designed to target V3 and V2 regions of SHIV-SF162P3 Env. SHIV-SF162P3 RNA was isolated and reverse transcribed by SF162P3 NGS-V3R, 5’-TTCTGGGTCCCCTCCTGAGG. Primers including NGS-V3F, 5’-GTACAGCTGAAGGAATCTGTAG, and NGS-V3R were used for V3 amplification; primers including NGS V2F, 5’- GTAATTGGAAAGAGATGAACAGAGGAG, and NGS V2R, 5’- GGGCACAATAATGTATGGGAATTGG were used for V2 amplification. NEBNext DNA Library Prep Kit for Illumina (New England Biolabs) was used for V3 and V2 amplicon library preparation based on manufacturer’s protocols. Illumina MiSeq Sequencing 150bp Paired End (PE150) was performed by the Dana-Farber Cancer Institute Molecular Biology Core Facility.
Sequence analysis
Adaptor sequences and low-quality bases (quality scores < 30) were removed from Illumina sequences using bbduk from the BBTools suite (http://jgi.doe.gov/data-and-tools/bbtools). The following adaptor sequences were used: the NEBnext ‘universal’ primer, 5′-AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCTCTTCCGATCT-3′ and its complement; two index primers, 5′-CAAGCAGAAGACGGCATACGAGAT-CACTGT-GTGACTGGAGTTCAGACGTGTGCTCTTCCGATC-3′ (for the V2 sequences), and 5′-CAAGCAGAAGACGGCATACGAGAT-TCAAGT-GTGACTGGAGTTCAGACGTGTGCTCTTCCGATC-3′ (for the V3 sequences), and their reverse complements (dash characters delimit the complements of the sequencing bar codes). Adaptor trimming used the parameters “ktrim=r k=27 mink=4 hdist=2 tpe tbo”; quality trimming used “qtrim=rl trimq=30”. Paired-end sequences were aligned against SF162P3 Env using Bowtie2 (http://bowtie-bio.sourceforge.net/bowtie2/index.shtml; version 2.1.0; parameters “–very-sensitive-local –reorder”), sorted and indexed using samtools, processed into paired-end fasta files and translated into amino-acids using custom Perl code (wfischer@lanl.gov). PCR-primer derived sequences were removed by visual inspection of translated sequences (V3-F: VQLKESV; V3-R: SGGDPE; V2-F: NWKEMNRG; V2-R: PIPIHYCA) using Aliview (34). Sequences were removed from the data set if they were not complete throughout the regions of interest, or if they contained stop-codons. Potential N-linked glycosylation sites (PNGS) were computed by matches to the recognition sequence N-x-[ST], where x represents any amino-acid other than proline, and represented in alignments by the letter ‘O’. Finally, columns matching signature positions were extracted from the alignment based on HXB2 numbering (https://www.hiv.lanl.gov/content/sequence/LOCATE/locate.html, and the PDB entry 5FYJ, chain G).
Single genome amplification (SGA)
SGA assays were performed essentially as described (11) except that the primers using here were designed to target conserved region of SHIV SF162P3 and 325C. Briefly, viral RNA was isolated and reverse transcribed to viral cDNA using SHIV SGA EnvR1, 5′-CAATAATTGTCTGGCCTGTACCGTC. First- round PCR was carried out with Q5 High-Fidelity 2X Master Mix (NEB) together with primer SHIV SGA EnvF1, 5’-TATGGGGTACCTGTGTGGAA and SHIV SGA EnvR1. PCR conditions were programmed as follows, 1 cycle of 98°C for 30 s, 35 cycles of 98°C for 15s, 55°C for 15s and 72°C for 55s, followed by a final extension of 72°C for 2 min. 1 μL of first-round PCR product was add to Q5 Master Mix with primer SHIV SGA EnvF2, 5’-GCCTGTGTACCCACAGAC and EnvR2, 5’- ATAGTGCTTCCTGCTGC. PCR conditions were programmed as above but increased to 45 cycles for the second step. Amplicons from cDNA dilutions resulting in less than 30% positive were considered to result from amplification of a single cDNA amplification and were processed for sequencing. For each sample, 15-30 sequences were analyzed.
Env contact sites for PGDM1400
Since no structures exist for PGDM1400 in complex with Env trimers, a cryo-EM structure of the related antibody PGT145 in complex with Env trimer (23) and structural modeling was used to find Env contact sites for PGDM1400. The HCDR3 from the crystal structure of the PGDM1400 Fab (13) to the PGT145 Fab, was aligned using positions 88-108 and the ‘align’ function from Pymol. All Env amino acids and glycans that had any heavy atoms within 8.5Å of PGDM1400 heavy atoms were isolated. The list below shows PGDM1400 HXB2 positions of contact sites (including glycans). Most of the contact sites are shared between PGDM1400 and PGT145, with 119,129,130 and 158 in contact with PGT145 alone, and 200 and 314 in contact PGDM1400 alone. When the PGDM1400 contact sites contained amino acids that were significantly associated with sensitivity or resistance with a q-value of < 0.2 (Korber et al., submitted), they are shown below in bold, and these are the positions that were tracked globally and within subjects using published longitudinal sequencing data (Fig. 4). Positions 160-162 comprise a glycosylation site with the motif Nx[ST] that is essential an Env to be sensitive to PGDM1400 neutralization. A positive charge at position 169 was also particularly highly significantly associated with PGDM1400 sensitivity (Korber et al, submitted). The other sites (161, 165, 166, and 167) had significant but more modest associations.
PGDM1400 contact sites: 120 121 122 123 124 125 126 127 128 159 160 161 162 163 164 165 166 167 168 169 170 171 200 309 312 313 314 315.
Env contact sites for PGT121
Since no structures exist for PGT121 in complex with Env trimers, a cryo-EM structure of the related antibody PGT122 in complex with Env trimer (24) was used and the contact surface of PGT121 on the SOSIP trimer was modeled. Specifically, HCDR3 from the crystal structure of the PGT121 Fab crystal structure (35) to the PGT122 Fab was aligned using positions 88-101 and the ‘align’ function from Pymol. Then all Env amino acids and glycans that had any heavy atoms within 8.5Å of PGT121/PGT122 Fab heavy atoms were isolated. These contact sites are listed below. Env sequences in hypervariable region of V1 cannot be aligned, so standard signature analysis on this region was not performed, and these positions are shown italicized. The contact sites with significant PGT121 resistance signatures (q < 0.2, Korber et al, submitted), are shown below in bold.
PGT121 contact sites: 133 134 135 136 137 138 138A 138B 138C 138D 138E 151 152 154 175 296 297 298 299 300 301 302 322 322A 323 324 325 326 327 328 329 330 331 332 385 414 415 416 417 418 419.
PGT121 glycan sites: 156 301 332 392 413 442.
Several strong signatures that were not directly in the contact surface were also included. The potential N-linked glycosylation site (PNGS) at 295 is a favored signature for PGT121, but it is not present in this particular structure. The potential N-linked glycosylation site (PNGS) at 334, which is often gained when the key signature site when the PNGS at N332 is lost, was also included. The signature sites were then resolved using a phylogenetically corrected strategy (36, 37) to identify amino acid in key positions that are associated with either resistance or sensitivity to PGT121 to large panels of 207 M group viruses or 200 C clade viruses (Korber et al., submitted).
Statistical analyses
Analyses of independent data were performed by Wilcoxon rank sum tests, and comparisons of categorical variables were performed using Fisher’s exact tests. P values less than 0.05 were considered significant. Combination IC80 titers were calculated using the Bliss-Hill model, which has been shown to provide accurate predictions of combination neutralization properties using those of individual bNAbs (20). Statistical analyses were performed using GraphPad Prism or the Stats module in Scipy (http://www.scipy.org2015). MEGA6 was used to do the single genome sequence alignments and create the maximum likelihood tree.
Supplementary Material
Fig S1. Complementarity of complete neutralization profiles of PGDM1400 and PGT121 against HIV-1.
Fig S2. Serum concentration of PGDM1400 and PGT121 in antibody treated animals by ELISA.
Fig S3. Serum concentration of PGDM1400 and PGT121 in antibody treated animals by TZM-bl neutralizing antibody assays.
Fig. S4. Comparison of ELISA and TZM-bl neutralization assays for evaluating. pharmacokinetics of PGDM1400 and PGT121.
Fig. S5. Protective efficacy as measured by SHIV-SF162P3-specific viral loads.
Fig. S6. Protective efficacy as measured by SHIV-325C-specific viral loads.
Fig. S7. Structural modeling of PGT121 and PGDM1400 contact sites.
Fig. S8. Clade diversity of key signature residues for PGDM1400.
Fig. S9. Clade diversity of key signature residues for PGT121.
Table S1. Intrapatient variation in key contact signatures for PGDM140 and PGT121.
Figure 7. Intrapatient variation in key contact signatures for PGDM1400 and PGT121.
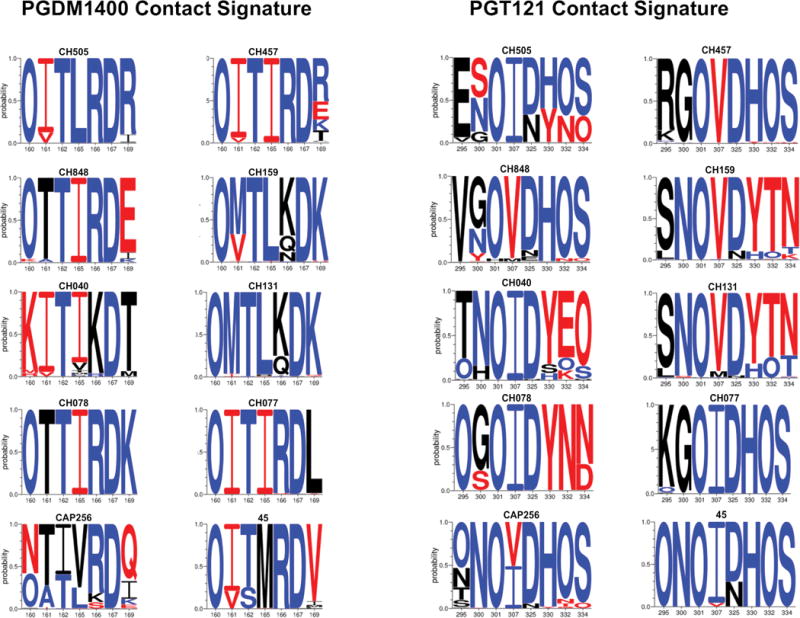
LOGO plots of viral sequence diversity in the key PGDM1400 and PGT121 contact sites (Figs. S6-S8) in 10 HIV-1-infected individuals for whom >150 full-length env sequences were available in the Los Alamos Sequence Database. The subject ID is indicated above each plot. The x-axis indicates the amino-acid position based on HxB2 numbering. The y-axis indicates the probability of an amino-acid at this location. “O” indicates an Asn that is part of a potential N-linked glycosylation site (PNGS). Blue reflects sensitivity signatures, red reflects resistance signatures, and black reflects no statistically significant associated with antibody sensitivity.
One sentence summary.
A combination of HIV-1-specific broadly neutralizing antibodies are necessary to protect rhesus monkeys against a mixed SHIV challenge.
Acknowledgments
We thank S. Mojta, P. Gandhi, B. Keele, W. Rinaldi, M. Ferguson, M. Lewis, and J. Misamore for generous advice, assistance, and reagents.
Funding: We acknowledge support from the American Foundation for AIDS Research (109219), the National Institutes of Health (AI096040, AI100663, AI106408, AI124377, AI126603), and the Ragon Institute of MGH, MIT, and Harvard.
Footnotes
Author Contributions: B.J., P.T.L., B.T.K., and D.H.B. designed the studies and interpreted the data. P.T.L., P.A., N.B.M., J.B.W., and D.R.B. led the virologic assays. K.W., W.M.F., and B.T.K. led the sequence analyses, model development, and statistical analyses. J.P.N., K.M., E.N.B., S.K., M.K., J.A.L., and M.S.S. led the immunologic assays. D.H.B., L.J.T., and J.R.M. led the analysis of the challenge stocks. B.J., P.T.L., B.T.K., and D.H.B. wrote the paper with all co-authors.
Competing interests: The authors declare that they have no competing financial interests.
Data and materials availability: The data presented in this paper are tabulated in the main paper and in the supplementary materials. Materials are available with an appropriate MTA and requests for materials should be addressed to D.H.B. (dbarouch@bidmc.harvard.edu).
References and Notes
- 1.Pegu A, Hessell AJ, Mascola JR, Haigwood NL. Use of broadly neutralizing antibodies for HIV-1 prevention. Immunol Rev. 2017;275:296–312. doi: 10.1111/imr.12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Julg B, Barouch DH. Novel immunological strategies for HIV-1 eradication. J Virus Erad. 2015;1:232–236. doi: 10.1016/S2055-6640(20)30931-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bar KJ, Sneller MC, Harrison LJ, Justement JS, Overton ET, Petrone ME, Salantes DB, Seamon CA, Scheinfeld B, Kwan RW, Learn GH, Proschan MA, Kreider EF, Blazkova J, Bardsley M, Refsland EW, Messer M, Clarridge KE, Tustin NB, Madden PJ, Oden K, O’Dell SJ, Jarocki B, Shiakolas AR, Tressler RL, Doria-Rose NA, Bailer RT, Ledgerwood JE, Capparelli EV, Lynch RM, Graham BS, Moir S, Koup RA, Mascola JR, Hoxie JA, Fauci AS, Tebas P, Chun TW. Effect of HIV Antibody VRC01 on Viral Rebound after Treatment Interruption. The New England journal of medicine. 2016;375:2037–2050. doi: 10.1056/NEJMoa1608243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheid JF, Horwitz JA, Bar-On Y, Kreider EF, Lu CL, Lorenzi JC, Feldmann A, Braunschweig M, Nogueira L, Oliveira T, Shimeliovich I, Patel R, Burke L, Cohen YZ, Hadrigan S, Settler A, Witmer-Pack M, West AP, Jr, Juelg B, Keler T, Hawthorne T, Zingman B, Gulick RM, Pfeifer N, Learn GH, Seaman MS, Bjorkman PJ, Klein F, Schlesinger SJ, Walker BD, Hahn BH, Nussenzweig MC. HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature. 2016;535:556–560. doi: 10.1038/nature18929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caskey M, Klein F, Lorenzi JC, Seaman MS, West AP, Jr, Buckley N, Kremer G, Nogueira L, Braunschweig M, Scheid JF, Horwitz JA, Shimeliovich I, Ben-Avraham S, Witmer-Pack M, Platten M, Lehmann C, Burke LA, Hawthorne T, Gorelick RJ, Walker BD, Keler T, Gulick RM, Fatkenheuer G, Schlesinger SJ, Nussenzweig MC. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522:487–491. doi: 10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caskey M, Schoofs T, Gruell H, Settler A, Karagounis T, Kreider EF, Murrell B, Pfeifer N, Nogueira L, Oliveira TY, Learn GH, Cohen YZ, Lehmann C, Gillor D, Shimeliovich I, Unson-O’Brien C, Weiland D, Robles A, Kummerle T, Wyen C, Levin R, Witmer-Pack M, Eren K, Ignacio C, Kiss S, West AP, Jr, Mouquet H, Zingman BS, Gulick RM, Keler T, Bjorkman PJ, Seaman MS, Hahn BH, Fatkenheuer G, Schlesinger SJ, Nussenzweig MC, Klein F. Antibody 10-1074 suppresses viremia in HIV-1-infected individuals. Nature medicine. 2017;23:185–191. doi: 10.1038/nm.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lynch RM, Boritz E, Coates EE, DeZure A, Madden P, Costner P, Enama ME, Plummer S, Holman L, Hendel CS, Gordon I, Casazza J, Conan-Cibotti M, Migueles SA, Tressler R, Bailer RT, McDermott A, Narpala S, SO’Dell Wolf G, Lifson JD, Freemire BA, Gorelick RJ, Pandey JP, Mohan S, Chomont N, Fromentin R, Chun TW, Fauci AS, Schwartz RM, Koup RA, Douek DC, Hu Z, Capparelli E, Graham BS, Mascola JR, Ledgerwood JE, V. R. C. S. Team Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Science translational medicine. 2015;7:319ra206. doi: 10.1126/scitranslmed.aad5752. [DOI] [PubMed] [Google Scholar]
- 8.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 9.Moldt B, Rakasz EG, Schultz N, Chan-Hui PY, Swiderek K, Weisgrau KL, Piaskowski SM, Bergman Z, Watkins DI, Poignard P, Burton DR. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:18921–18925. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pegu A, Yang ZY, Boyington JC, Wu L, Ko SY, Schmidt SD, McKee K, Kong WP, Shi W, Chen X, Todd JP, Letvin NL, Huang J, Nason MC, Hoxie JA, Kwong PD, Connors M, Rao SS, Mascola JR, Nabel GJ. Neutralizing antibodies to HIV-1 envelope protect more effectively in vivo than those to the CD4 receptor. Science translational medicine. 2014;6:243ra288. doi: 10.1126/scitranslmed.3008992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, Wei X, Jiang C, Kirchherr JL, Gao F, Anderson JA, Ping LH, Swanstrom R, Tomaras GD, Blattner WA, Goepfert PA, Kilby JM, Saag MS, Delwart EL, Busch MP, Cohen MS, Montefiori DC, Haynes BF, Gaschen B, Athreya GS, Lee HY, Wood N, Seoighe C, Perelson AS, Bhattacharya T, Korber BT, Hahn BH, Shaw GM. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilen CB, Parrish NF, Pfaff JM, Decker JM, Henning EA, Haim H, Petersen JE, Wojcechowskyj JA, Sodroski J, Haynes BF, Montefiori DC, Tilton JC, Shaw GM, Hahn BH, Doms RW. Phenotypic and immunologic comparison of clade B transmitted/founder and chronic HIV-1 envelope glycoproteins. Journal of virology. 2011;85:8514–8527. doi: 10.1128/JVI.00736-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sok D, van Gils MJ, Pauthner M, Julien JP, Saye-Francisco KL, Hsueh J, Briney B, Lee JH, Le KM, Lee PS, Hua Y, Seaman MS, Moore JP, Ward AB, Wilson IA, Sanders RW, Burton DR. Recombinant HIV envelope trimer selects for quaternary-dependent antibodies targeting the trimer apex. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:17624–17629. doi: 10.1073/pnas.1415789111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong CH, Phogat S, Wrin T, Simek MD, Protocol GPI, Koff WC, Wilson IA, Burton DR, Poignard P. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong CH, Phogat S, Wrin T, Simek MD, Koff WC, Wilson IA, Burton DR, Poignard P. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barouch DH, Whitney JB, Moldt B, Klein F, Oliveira TY, Liu J, Stephenson KE, Chang HW, Shekhar K, Gupta S, Nkolola JP, Seaman MS, Smith KM, Borducchi EN, Cabral C, Smith JY, Blackmore S, Sanisetty S, Perry JR, Beck M, Lewis MG, Rinaldi W, Chakraborty AK, Poignard P, Nussenzweig MC, Burton DR. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013;503:224–228. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sok D, Doores KJ, Briney B, Le KM, Saye-Francisco KL, Ramos A, Kulp DW, Julien JP, Menis S, Wickramasinghe L, Seaman MS, Schief WR, Wilson IA, Poignard P, Burton DR. Promiscuous glycan site recognition by antibodies to the high-mannose patch of gp120 broadens neutralization of HIV. Science translational medicine. 2014;6:236ra263. doi: 10.1126/scitranslmed.3008104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mouquet H, Scharf L, Euler Z, Liu Y, Eden C, Scheid JF, Halper-Stromberg A, Gnanapragasam PN, Spencer DI, Seaman MS, Schuitemaker H, Feizi T, Nussenzweig MC, Bjorkman PJ. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E3268–3277. doi: 10.1073/pnas.1217207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shingai M, Nishimura Y, Klein F, Mouquet H, Donau OK, Plishka R, Buckler-White A, Seaman M, Piatak M, Jr, Lifson JD, Dimitrov DS, Nussenzweig MC, Martin MA. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature. 2013;503:277–280. doi: 10.1038/nature12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagh K, Bhattacharya T, Williamson C, Robles A, Bayne M, Garrity J, Rist M, Rademeyer C, Yoon H, Lapedes A, Gao H, Greene K, Louder MK, Kong R, Karim SA, Burton DR, Barouch DH, Nussenzweig MC, Mascola JR, Morris L, Montefiori DC, Korber B, Seaman MS. Optimal Combinations of Broadly Neutralizing Antibodies for Prevention and Treatment of HIV-1 Clade C Infection. PLoS pathogens. 2016;12:e1005520. doi: 10.1371/journal.ppat.1005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barouch DH, Stephenson KE, Borducchi EN, Smith K, Stanley K, McNally AG, Liu J, Abbink P, Maxfield LF, Seaman MS, Dugast AS, Alter G, Ferguson M, Li W, Earl PL, Moss B, Giorgi EE, Szinger JJ, Eller LA, Billings EA, Rao M, Tovanabutra S, Sanders-Buell E, Weijtens M, Pau MG, Schuitemaker H, Robb ML, Kim JH, Korber BT, Michael NL. Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell. 2013;155:531–539. doi: 10.1016/j.cell.2013.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Julg B, Keele BF, Wagh K, Pegu A, Sok D, Abbink P, Schmidt SD, Wang K, Chen X, Joyce M Gordon, Georgiev IS, Choe M, Kwong PD, Doria-Rose NA, Le K, Louder MK, Bailer RT, Moore PL, Korber B, Seaman MS, Karim SS Abdool, Morris L, Koup RA, Mascola JR, Burton DR, Barouch DH. Broadly neutralizing antibodies targeting the HIV-1 envelope V2 apex confer protection against a clade C SHIV challenge. Science translational medicine. 2017 doi: 10.1126/scitranslmed.aal1321. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JH, Andrabi R, Su CY, Yasmeen A, Julien JP, Kong L, Wu NC, McBride R, Sok D, Pauthner M, Cottrell CA, Nieusma T, Blattner C, Paulson JC, Klasse PJ, Wilson IA, Burton DR, Ward AB. A Broadly Neutralizing Antibody Targets the Dynamic HIV Envelope Trimer Apex via a Long, Rigidified, and Anionic beta-Hairpin Structure. Immunity. 2017;46:690–702. doi: 10.1016/j.immuni.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stewart-Jones GB, Soto C, Lemmin T, Chuang GY, Druz A, Kong R, Thomas PV, Wagh K, Zhou T, Behrens AJ, Bylund T, Choi CW, Davison JR, Georgiev IS, Joyce MG, Kwon YD, Pancera M, Taft J, Yang Y, Zhang B, Shivatare SS, Shivatare VS, Lee CC, Wu CY, Bewley CA, Burton DR, Koff WC, Connors M, Crispin M, Baxa U, Korber BT, Wong CH, Mascola JR, Kwong PD. Trimeric HIV-1-Env Structures Define Glycan Shields from Clades A, B, and G. Cell. 2016;165:813–826. doi: 10.1016/j.cell.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Julien JP, Sok D, Khayat R, Lee JH, Doores KJ, Walker LM, Ramos A, Diwanji DC, Pejchal R, Cupo A, Katpally U, Depetris RS, Stanfield RL, McBride R, Marozsan AJ, Paulson JC, Sanders RW, Moore JP, Burton DR, Poignard P, Ward AB, Wilson IA. Broadly neutralizing antibody PGT121 allosterically modulates CD4 binding via recognition of the HIV-1 gp120 V3 base and multiple surrounding glycans. PLoS Pathog. 2013;9:e1003342. doi: 10.1371/journal.ppat.1003342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sok D, Pauthner M, Briney B, Lee JH, Saye-Francisco KL, Hsueh J, Ramos A, Le KM, Jones M, Jardine JG, Bastidas R, Sarkar A, Liang CH, Shivatare SS, Wu CY, Schief WR, Wong CH, Wilson IA, Ward AB, Zhu J, Poignard P, Burton DR. A Prominent Site of Antibody Vulnerability on HIV Envelope Incorporates a Motif Associated with CCR5 Binding and Its Camouflaging Glycans. Immunity. 2016;45:31–45. doi: 10.1016/j.immuni.2016.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joseph SB, Swanstrom R, Kashuba AD, Cohen MS. Bottlenecks in HIV-1 transmission: insights from the study of founder viruses. Nat Rev Microbiol. 2015;13:414–425. doi: 10.1038/nrmicro3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Ghneim K, Sok D, Bosche WJ, Li Y, Chipriano E, Berkemeier B, Oswald K, Borducchi E, Cabral C, Peter L, Brinkman A, Shetty M, Jimenez J, Mondesir J, Lee B, Giglio P, Chandrashekar A, Abbink P, Colantonio A, Gittens C, Baker C, Wagner W, Lewis MG, Li W, Sekaly RP, Lifson JD, Burton DR, Barouch DH. Antibody-mediated protection against SHIV challenge includes systemic clearance of distal virus. Science. 2016 doi: 10.1126/science.aag0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hessell AJ, Jaworski JP, Epson E, Matsuda K, Pandey S, Kahl C, Reed J, Sutton WF, Hammond KB, Cheever TA, Barnette PT, Legasse AW, Planer S, Stanton JJ, Pegu A, Chen X, Wang K, Siess D, Burke D, Park BS, Axthelm MK, Lewis A, Hirsch VM, Graham BS, Mascola JR, Sacha JB, Haigwood NL. Early short-term treatment with neutralizing human monoclonal antibodies halts SHIV infection in infant macaques. Nature medicine. 2016;22:362–368. doi: 10.1038/nm.4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parren PW, Marx PA, Hessell AJ, Luckay A, Harouse J, Cheng-Mayer C, Moore JP, Burton DR. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. Journal of virology. 2001;75:8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shingai M, Donau OK, Plishka RJ, Buckler-White A, Mascola JR, Nabel GJ, Nason MC, Montefiori D, Moldt B, Poignard P, Diskin R, Bjorkman PJ, Eckhaus MA, Klein F, Mouquet H, Lorenzi JC Cetrulo, Gazumyan A, Burton DR, Nussenzweig MC, Martin MA, Nishimura Y. Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques. The Journal of experimental medicine. 2014;211:2061–2074. doi: 10.1084/jem.20132494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang HW, Tartaglia LJ, Whitney JB, Lim SY, Sanisetty S, Lavine CL, Seaman MS, Rademeyer C, Williamson C, Ellingson-Strouss K, Stamatatos L, Kublin J, Barouch DH. Generation and evaluation of clade C simian-human immunodeficiency virus challenge stocks. J Virol. 2015;89:1965–1974. doi: 10.1128/JVI.03279-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montefiori DC. Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol Biol. 2009;485:395–405. doi: 10.1007/978-1-59745-170-3_26. [DOI] [PubMed] [Google Scholar]
- 34.Larsson A. AliView: a fast and lightweight alignment viewer and editor for large datasets. Bioinformatics. 2014;30:3276–3278. doi: 10.1093/bioinformatics/btu531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Julien JP, Sok D, Khayat R, Lee JH, Doores KJ, Walker LM, Ramos A, Diwanji DC, Pejchal R, Cupo A, Katpally U, Depetris RS, Stanfield RL, McBride R, Marozsan AJ, Paulson JC, Sanders RW, Moore JP, Burton DR, Poignard P, Ward AB, Wilson IA. Broadly neutralizing antibody PGT121 allosterically modulates CD4 binding via recognition of the HIV-1 gp120 V3 base and multiple surrounding glycans. PLoS pathogens. 2013;9:e1003342. doi: 10.1371/journal.ppat.1003342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhattacharya T, Daniels M, Heckerman D, Foley B, Frahm N, Kadie C, Carlson J, Yusim K, McMahon B, Gaschen B, Mallal S, Mullins JI, Nickle DC, Herbeck J, Rousseau C, Learn GH, Miura T, Brander C, Walker B, Korber B. Founder effects in the assessment of HIV polymorphisms and HLA allele associations. Science. 2007;315:1583–1586. doi: 10.1126/science.1131528. [DOI] [PubMed] [Google Scholar]
- 37.Gnanakaran S, Daniels MG, Bhattacharya T, Lapedes AS, Sethi A, Li M, Tang H, Greene K, Gao H, Haynes BF, Cohen MS, Shaw GM, Seaman MS, Kumar A, Gao F, Montefiori DC, Korber B. Genetic signatures in the envelope glycoproteins of HIV-1 that associate with broadly neutralizing antibodies. PLoS Comput Biol. 2010;6:e1000955. doi: 10.1371/journal.pcbi.1000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Complementarity of complete neutralization profiles of PGDM1400 and PGT121 against HIV-1.
Fig S2. Serum concentration of PGDM1400 and PGT121 in antibody treated animals by ELISA.
Fig S3. Serum concentration of PGDM1400 and PGT121 in antibody treated animals by TZM-bl neutralizing antibody assays.
Fig. S4. Comparison of ELISA and TZM-bl neutralization assays for evaluating. pharmacokinetics of PGDM1400 and PGT121.
Fig. S5. Protective efficacy as measured by SHIV-SF162P3-specific viral loads.
Fig. S6. Protective efficacy as measured by SHIV-325C-specific viral loads.
Fig. S7. Structural modeling of PGT121 and PGDM1400 contact sites.
Fig. S8. Clade diversity of key signature residues for PGDM1400.
Fig. S9. Clade diversity of key signature residues for PGT121.
Table S1. Intrapatient variation in key contact signatures for PGDM140 and PGT121.


