Abstract
Background
Many studies have measured central and peripheral γ-aminobutyric acid (GABA) levels in patients with depression. We performed a meta-analysis to provide an objective overview of GABA changes in those with unipolar or bipolar depression.
Methods
After a systematic database search, original data were extracted with the help of seminal authors to calculate standardized mean differences. We compared GABA levels between patients with current major depressive episodes and controls, between euthymic patients and controls, and in patients before and after treatment. We performed meta-regressions to explore the influence of demographic and clinical variables on GABA significant mean differences.
Results
For unipolar depression, central and peripheral GABA levels were diminished in currently depressed patients, but normal in euthymic patients, compared with the healthy controls. For bipolar disorder, GABA levels were diminished in medication-free patients, but seemed to be normalized in medicated patients, compared with the healthy controls. We found no significant association with demographic or clinical variables.
Limitations
There was a great heterogeneity across studies, probably because of the substantial variation of clinical characteristics in the included samples. Many subanalyses were performed to assess how the diagnosis, medications, or the type of measurements of peripheral or central GABA levels may affect the main results.
Conclusion
The GABA levels evolved differentially in patients with unipolar and bipolar disorders. Our results suggest that GABA levels could represent a biomarker of symptomatic states in patients with unipolar disorder and would be normalized by mood stabilizers in those with bipolar disorder.
Introduction
Major depression is a highly prevalent disorder with important functional and health consequences.1 It mainly involves abnormal cooperation between emotional and cognitive networks, resulting in impaired emotional and cognitive regulation. Although the neural circuits implicated in the pathophysiology of depression are now better identified, the cellular and molecular changes that cause impairment in these emotional and cognitive networks and their association with depressive symptoms have yet to be determined.
γ-Aminobutyric acid (GABA) is the most important inhibitor of neurotransmitters in the central nervous system. The hypothesis of GABA dysfunction has long be considered in individuals with depression and affective disorders.2 Expression of genes implicated in the function and identification of the GABA components of local cell circuits suggest primary deficits affecting the GABA system, with human postmortem studies reporting a downregulation of gene expression in specific brain regions in depressed patients compared with healthy controls.3–6 Moreover, GABAergic interneurons have been proposed as major players in local and regional brain changes in individuals with depression and have been associated with rumination and increased self-focus in depressed patients.7 However, beyond cellular and gene expression, direction of change in GABA concentration in plasma and brain need to be clearly defined in individuals with mood disorders. The GABA levels may be measured by peripheral dosages in plasma and central dosages in cerebrospinal fluid (CSF) or in some brain regions using proton magnetic resonance spectroscopy (MRS). Results of studies assessing GABA levels in individuals with depression appear highly variable according to the type of measure (plasma, CSF, or MRS) or to clinical characteristics of the included depressed samples. For example, MRS studies have reported GABA diminution,8–10 no GABA change,11–13 or GABA increase in participants with depression compared with healthy participants.14 Furthermore, many authors have described specific implications of GABA neurotransmission according to different clinical characteristics of depression, such as pharmacological resistance,15 anhedonia,16 melancholia9,11 or unipolar or bipolar depression.
To provide a quantitative evaluation of GABA levels according to the type of measurement (plasma, CSF, or MRS) or to the clinical characteristics of depression, we performed a meta-analysis of studies assessing GABA levels in patients with major depression. We further assessed GABA changes according to clinical states (major depressive episode [MDE] and euthymic state) and to the exposure to treatment.
Methods
Data sources and study selection process
We searched the MEDLINE and PsycINFO databases through June 2015, without limits on year of publication, using the keywords “γ-aminobutyric acid” or “GABA” or “inhibitory amino acid” and “magnetic resonance spectroscopy” or “MRS” or “CSF” or “plasma” and “major depressive disorder” or “depressive disorder” or “affective disorder” or “bipolar depression” or “unipolar depression.” Studies were included if they were published in English in a peer-reviewed journal, if they reported GABA levels, if they included patients with a current or a previous diagnosis of unipolar or bipolar depression, and if they compared patients with current MDE or in a euthymic state with healthy controls, or if they compared GABA levels before and after treatment. Studies that did not fulfill all 4 criteria were not included in the analyses. To obtain additional data, we created an email alert after June 2015 in MEDLINE containing the same keywords to identify putative publications of interest. Study selection was performed by one of us (B.R.) and verified by one of us (J.-Y.R.).
Data extraction
For each study, we obtained the means and standard deviations (SDs) for GABA concentrations and for demographic and clinical variables. It was necessary to contact several authors to obtain missing data (see Acknowledgements); however, 1 research team explicitly refused to provide the requested data.17,18 The GABA concentrations were measured in MRS studies in millimoles per kilogram, in millimoles per litre, in parts per million, or via the ratios GABA:creatine or GABA:w, with w being the voxel tissue water resonance.
Regarding demographic and clinical variables, we extracted the percentage of women, the mean age of participants, the mean duration of illness, the mean number of lifetime episodes, the percentage of melancholic episodes and the mean episode duration. Data extraction was performed by one of us (B.R.) and verified by one of us (J.-Y.R.).
Statistical analysis
Data analyses were performed using RevMan version 5.3 (Nordic Cochrane Centre, Cochrane Collaboration). For MRS, plasma, or CSF studies, we calculated standardized mean differences (SMDs) in GABA concentrations in patients with current MDE versus controls, in euthymic patients versus controls, and in patients with current MDE before versus after treatment. The SMDs were defined as the difference between group means divided by the pooled SD. All analyses were performed with a random-effects model, which considered both between-study and within-study variability.19 We considered the SMDs to be significant when the 95% confidence interval (CI) excluded 0 and at p < 0.05.
To assess the putative influence of treatment in GABA changes, we considered studies having reported results in medication-free samples.
For MRS studies, we calculated SMDs for the following regions of interest (ROIs) independently: the occipital cortex,8–12,14,15,20–28 the anterior cingulate cortex (ACC),13,15,16,18,22 the dorsolateral prefrontal cortex (dlPFC),29,30 the ventromedial prefrontal cortex (vmPFC)29,30 and the left inferior frontal gyrus.27
We estimated study heterogeneity using the Q statistic when SMDs were significant. The I2 index, an estimate of the total variation across included studies that was due to heterogeneity rather than chance, was determined by the equation I2 = [(Q − df)÷Q] × 100%.31 In accordance with the Cochrane Handbook for Systematic Reviews of Interventions,31 heterogeneity was considered unimportant for an I2 between 0% and 40%, moderate for an I2 between 30% and 60%, substantial for I2 between 50% and 90%, or considerable for I2 between 75% and 100%. Moreover, to ensure that the overall results were not influenced by a single study, we performed “leave-one-out” sensitivity analyses by repeating the analyses with the consecutive exclusion of each study. We created funnel plots, plotting the standard error of each SMD against the SMD calculated of each included study when at least 5 individual studies contributed to an overall result, and their asymmetry was analyzed to assess the possible influence of publication and location biases.32 Results from leave-one-out sensitivity analyses and funnel plots are included in Appendix 1, available at jpn.ca/160228-a1.
Finally, we conducted meta-regression analyses based on linear regression models for assessing the influence of the clinical heterogeneity of study populations on meta-analysis effect sizes. We performed regression analyses when SMDs or heterogeneity were significant and when a reasonable number of data points were available (≥ 4).
Results
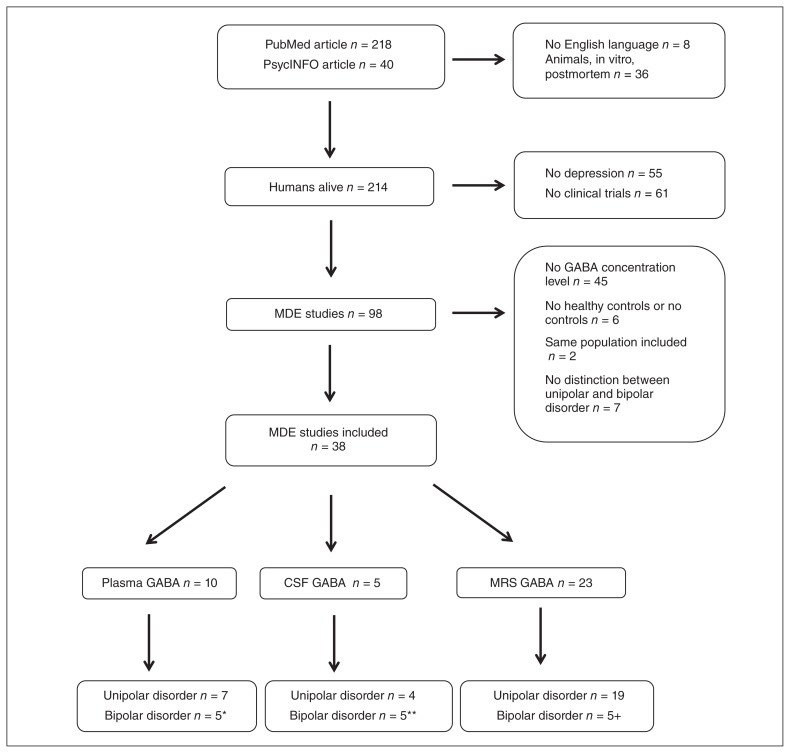
Our search identified 45 studies: 26 studies for the comparison of patients with current MDE versus healthy controls,8–18,30,33–46 12 studies for the comparison of patients with current MDE before treatment versus after treatment,12,20,23,25,26,28,36,43,47–50 and 11 for the comparison of euthymic patients and controls.18,21–23,27,29,38,51–54 Figure 1 depicts the study selection process, and the included plasma, CSF and MRS studies are described in Table 1, Table 2 and Table 3, respectively. We excluded 3 studies assessing plasma GABA levels44,48,55 and 5 studies34,45–47,52 assessing CSF GABA levels from our analyses because clinical samples included patients with unipolar and bipolar depression, and no independent analysis was reported.
Fig. 1.
Study selection process. *The studies by Petty and Schlesser39 and Petty and colleagues41 were included in analyses of unipolar and bipolar disorders. **Studies by Gerner and colleagues,33 Kasa and colleagues,35 Mann and colleagues37 and Post and colleagues50 were incuded in analyses of unipolar and bipolar disorders. †The study by Bhagwagar and colleagues21 was included in analyses of unipolar and bipolar disorders. CSF = cerebrospinal fluid; GABA = γ-aminobutyric acid; MDE = major depressive episode; MRS = magnetic resonance spectroscopy.
Table 1.
Studies included in analyses of plasma GABA level
| Study | Diagnostic status | Disorder state | n | Female sex, % | Mean age, yr | Main results |
|---|---|---|---|---|---|---|
| Petty et al.39 | Unipolar and bipolar | Current MDE | 29 | NA | NA | Diminished GABA in unipolar disorder, no change in bipolar disorder |
| Petty et al.40 | Unipolar | Current MDE | 16 | NA | NA | Diminished GABA |
| Petty et al.41 | Unipolar and bipolar | Current MDE | 113 | NA | NA | Diminished GABA |
| Petty et al.42 | Unipolar | Current MDE | 77 | 0 | 44.7 | Diminished GABA |
| Petty et al.43 | Bipolar | Current MDE | 33 | NA | 41.2 | Diminished GABA |
| Paige et al.38 | Older adults with unipolar MDE | Current MDE | 9 | 44 | 67.4 | Diminished GABA |
| Lu et. al36 | Melancholic unipolar MDE | Current MDE | 27 | 48 | NA | Diminished GABA |
| Berrettini et al.51 | Bipolar | Euthymic | 10 | NA | 35 | Diminished GABA |
| Berrettini et al.52 | Bipolar | Euthymic | 49 | 44 | 35–39 | Diminished GABA in bipolar patients without lithium |
| Palmio et al.49 | Unipolar | Before/after treatment | 10 | 70 | 56 | No GABA level change after ECT |
ECT = electroconvulsive therapy; GABA = γ-aminobutyric acid; MDE = major depressive episode; NA = not available.
Table 2.
Studies included in analyses of cerebrospinal fluid GABA level
| Study | Diagnostic status | Disorder state | n | Female sex, % | Mean age, yr | Main results |
|---|---|---|---|---|---|---|
| Gerner et al.33 | Unipolar and bipolar | Current MDE | 24 | 54 | 42.6 | Diminished GABA |
| Kasa et al.35 | Unipolar and bipolar | Current MDE | 13 | 15 | 42 | Diminished GABA |
| Mann et al.37 | Unipolar and bipolar | Current MDE | 167 | 51 | 36.3 | Diminished GABA |
| Berrettini et al.53 | Bipolar | Euthymic | 25 | 60 | 35 | No GABA level change |
| Post et al.50 | Unipolar and bipolar | Before/after treatment | 7 | 29 | 41 | No GABA level change after carbamazepine |
GABA = γ-aminobutyric acid; MDE = major depressive episode.
Table 3.
Studies included in analyses of magnetic resonance spectroscopy GABA level
| Study | Diagnostic status | Disorder state | n | Female sex, % | Mean age, yr | ROI | Voxels size, mL | Main results |
|---|---|---|---|---|---|---|---|---|
| Sanacora et al.10 | Unipolar | Current MDE | 14 | 43 | 42.9 | OCC | 13.5 | Diminished GABA |
| Kugaya et al.8 | Unipolar | Current MDE | 6 | 0 | 36.2 | OCC | 11.1 | Diminished GABA |
| Sanacora et al.9 | Unipolar | Current MDE | 33 | NA | 41.9 | OCC | 13.5 | Diminished GABA |
| Epperson et al.14 | Postpartum unipolar | Current MDE | 9 | 100 | 30 | OCC | 20.5 | Trend for enhanced GABA |
| Hasler et al.30 | Unipolar | Current MDE | 20 | 65 | 34 | VMPFC & DA/dmPFC | 18 and 30 | Diminished GABA in DA/dmPFC, no change in vmPFC |
| Price et al.15 | Unipolar | Current MDE | 33 | 39 | 42.2 | OCC & ACC | 18 and 18.75 | Diminished GABA in resistant MDE, no change in nonresistant MDE |
| Walter et al.13 | Unipolar | Current MDE | 17 | 58 | 40 | ACC | 17.5 | No GABA level change |
| Murrough et al.17 | Unipolar | Current MDE | 31 | 47.6 | 39.1 | OCC & ACC | 18 and 18.75 | No GABA level change |
| Gabbay et al.16 | Unipolar | Current MDE | 20 | 60 | 16.7 | ACC | 18.75 | Diminished GABA in anhedonic MDE, no change in nonanhedonic MDE |
| Abdallah et al.11 | Unipolar | Current MDE | 23 | 70 | 43 | OCC | 13.5 | No GABA level change |
| Godlewska et al.12 | Unipolar | Current MDE | 33 | 42 | 29.9 | OCC | 10 | No GABA level change |
| Wang et al.18 | Bipolar | Current MDE & Euthymic | 29 | 52 | 34.4 | OCC & mPFC | 18 and 12.5 | No GABA level change |
| Hasler et al.29 | Unipolar | Euthymic | 16 | 75 | 41 | VMPFC & DA/dmPFC | 18 and 30 | No GABA level change |
| Shaw et al.27 | Unipolar | Euthymic | 19 | 100 | 21 | OCC | 27 | No GABA level change |
| Kaufman et al.54 | Bipolar | Euthymic | 13 | 38 | 40 | Basal ganglia | NA | No GABA level change |
| Brady et al.22 | Bipolar | Euthymic | 14 | 43 | 33 | POC & ACC | 16.7 and 16.7 | Enhanced GABA level |
| Godlewska et al.23 | Bipolar | Euthymic | 13 | 54 | 24 | OCC & mPFC | 10 and 6.75 | No GABA level change |
| Sanacora et al.25 | Unipolar | Before/after treatment | 11 | 36 | 39 | OCC | 13.5 | Enhanced GABA after SSRI |
| Sanacora et al.24 | Unipolar | Before/after treatment | 8 | 38 | 46 | OCC | 13.5 | Enhanced GABA after ECT |
| Sanacora et al.26 | Unipolar | Before/after treatment | 8 | NA | NA | OCC | 20.5 | No GABA level change after CBT |
| Valentine et al.28 | Unipolar | Before/after treatment | 10 | 60 | 42 | OCC | 13.5 | No GABA level change after ketamine |
| Abdallah et al.20 | Unipolar | Before/after treatment | 28 | 63 | 42 | OCC | 13.5 | No GABA level change after CBT |
| Godlewska et al.12 | Unipolar | Before/after treatment | 27 | 42 | 30 | OCC | 10 | No GABA level change after SSRI |
ACC = anterior cingulate cortex; CBT = cognitive behavioural therapy; DA/dmPFC = dorsoantero/dorsomedial prefrontal cortex; ECT = electroconvulsive therapy; GABA = γ-aminobutyric acid; MDE = major depressive episode; MRS = magnetic resonance spectroscopy; mPFC = medial prefrontal cortex; NA = not available; OCC = occipital cortex; POC = parieto-occipital cortex; ROI = region of interest; SSRI = selective serotonin reuptake inhibitor; vmPFC = ventromedial prefrontal cortex.
Patients with unipolar depression versus healthy controls
Plasma GABA levels
Althought SMD significantly indicated a decrease in GABA levels in patients with current unipolar MDE compared with healthy controls, heterogeneity was significant across studies (6 studies including a total of 241 patients and 199 healthy controls; test for overall effect: SMD −1.40, 95% CI −2.04 to −0.76, p < 0.001; test for heterogeneity: χ2 = 38.66, p < 0.001, I2 = 87%).36,38–42 Only 1 study included patients without treatment, reporting diminished GABA levels in those with depression;36 its exclusion from analyses did not affect the result.
Plasma GABA levels during euthymic states in patients with unipolar depression were assessed in a single study. Remitted patients (remission defined as Montgomery–Åsberg Depression Rating Scale [MADRS] score < 3) and healthy controls had similar plasma GABA levels (0.99 and 1.15, respectively).38
Treatment-related plasma GABA changes in patients with unipolar depression with current MDE were assessed in 2 studies. In the study by Palmio and colleagues,49 MADRS scores improved from 29 at baseline to 9 at the end of the study. The SMD showed no difference in GABA levels before versus after treatment (2 studies including a total of 18 patients and 18 healthy controls; test for overall effect: SMD −0.07, 95% CI −0.73 to 0.58, p = 0.83; test for heterogeneity: χ2 = 0.04, p = 0.85).36,49
Cerebrospinal fluid GABA levels
The CSF GABA levels were diminished in individuals with current unipolar MDE compared with healthy controls (3 studies including a total of 159 patients and 91 healthy controls; test for overall effect: SMD −0.56, 95% CI −0.96 to −0.17, p = 0.005; test for heterogeneity: χ2 = 3.10, p = 0.21).33,35,37
No study assessed CSF GABA changes in patients with unipolar depression in euthymic states or before versus after treatment.
Magnetic resonance spectroscopy GABA levels
The MRS GABA levels were diminished in patients with unipolar depression compared with healthy controls; however, there was a great heterogeneity across studies (10 studies including a total of 208 patients and 203 healthy controls; test for overall effect: SMD −0.57, 95% CI −0.99 to −0.15, p = 0.007; test for heterogeneity: χ2 = 35.79, p < 0.001, I2 = 75%).8–16,30 This heterogeneity was not explained by medication status, as all studies included unmedicated patients. There was a great heterogeneity among defined ROIs across studies, but subanalyses showed similar findings without changing the level of heterogeneity (e.g., occipital cortex: χ2 = 30.07, p < 0.001, I2 = 80%; prefrontal cortex: χ2 = 6.08, p = 0.05, I2 = 51%). Regarding the heterogeneity among ROIs, results from subanalyses are included in Appendix 1 and should be interpreted cautiously.
Patients with bipolar depression versus healthy controls
Plasma GABA levels
Patients with bipolar depression with current MDE had diminished plasma GABA levels compared with healthy controls (3 studies including a total of 62 patients and 121 healthy controls; test for overall effect: SMD −0.42, 95% CI −0.74 to −0.10, p = 0.001; test for heterogeneity: χ2 = 0.42, p = 0.81).39,41,43
Patients with bipolar disorder in euthymic states had diminished plasma GABA levels compared with healthy controls (3 studies including a total of 65 patients and 87 healthy controls; test for overall effect: SMD −0.64, 95% CI −1.17 to −0.11, p = 0.020; test for heterogeneity: χ2 = 3.54, p = 0.17).51,52 No study assessed plasma GABA changes before and after treatment in patients with bipolar depression.
Cerebrospinal fluid GABA levels
No changes in CSF GABA levels were observed between patients with bipolar disorder and healthy controls (3 studies including a total of 45 patients and 91 healthy controls; test for overall effect: SMD −0.35, 95% CI −1.06 to 0.36, p = 0.34; test for heterogeneity: χ2 = 4.29, p = 0.12).33,35,37
Magnetic resonance spectroscopy GABA levels
Only 2 comparisons between medicated patients with current bipolar depression and healthy controls were identified, and no difference was observed (2 studies including a total of 10 patients and 12 healthy controls; test for overall effect: SMD 0.36, 95% CI −0.51 to 1.23, p = 0.42; test for heterogeneity: χ2 = 0.01, p = 0.93).18
During euthymic states, bipolar disorder was not associated with change in MRS GABA levels (6 studies including a total of 74 patients and 66 healthy controls; test for overall effect: SMD 0.14, 95% CI −0.42 to 0.71, p = 0.62; test for heterogeneity: χ2 = 13.13, p = 0.020, I2 = 62%).18,21–23,54
No study assessing GABA changes before and after treatment was identified.
Patients with unipolar depression versus bipolar depression
Plasma GABA levels
Two studies compared plasma GABA levels between patients with current MDE with unipolar and bipolar disorders.39,41 There was no difference in SMD (2 studies including a total of 112 patients with unipolar depression and 32 with bipolar depression; test for overall effect: SMD −2.17, 95% CI −5.85 to 1.51, p = 0.25), but there was a great heterogeneity across those studies (test for heterogeneity: χ2 = 25.71, p < 0.001, I2 = 96%).
Cerebrospinal fluid GABA levels
The CSF GABA levels were diminished in patients with unipolar depression versus those with bipolar depression (3 studies including a total of 159 patients with unipolar depression and 45 patients with bipolar depression; test for overall effect: SMD −0.34, 95% CI −0.67 to −0.01, p = 0.05; test for heterogeneity: χ2 = 1.53, p = 0.47).33,35,37
Magnetic resonance spectroscopy GABA levels
We did not identify a study comparing MRS GABA levels between patients with unipolar depression and patients with bipolar depression.
Meta-regression
Meta-regression analyses were not significant and did not explain significant SMDs or heterogeneity.
Discussion
The aim of the present meta-analysis was to assess peripheral and brain GABA concentrations in patients with unipolar and bipolar depression and to associate them with patients’ clinical characteristics. The results of our meta-analysis show that central and peripheral GABA levels are diminished in patients with unipolar depression, but tend to reach comparable levels to those of healthy controls in euthymic patients. In patients with bipolar disorder, results were more difficult to synthesize; reduced plasma GABA levels in depressed patients normalized with medication use. Central GABA levels were normal in patients with bipolar depression or during euthymic states. Together, our results suggest that GABA concentration may be associated with clinical states in patients with unipolar disorder, whereas GABA levels should be more closely associated with medication use in those with bipolar disorder; however, this interpretation is based on analyses characterized by high levels of heterogeneity and a small number of included studies.
GABA levels in unipolar depression
We found decreased central and peripheral GABA concentrations in patients with unipolar depression and normal central and peripheral GABA levels in patients in a euthymic state, suggesting that GABA concentration is a state rather than a trait marker of unipolar depression. Diminished GABA levels in these patients are in accordance with the reduction of the expression of inhibitory interneuron markers described in patients with current MDE.7 Indeed, GABAergic interneurons are involved in the regulation of the input and output of excitatory pyramidal neurons. In a recent and coherent model of depression pathophysiology,7 it has been proposed that network dysbalance observed in patients with unipolar depression, thus induced by the dysregulation of glutamatergic and GABAergic innervation, may translate trends to shift in focus from external to internal mental contents associated with unipolar depression, which is a central to depressive symptomatology.56,57 This decrease of GABAergic interneuron activity may thus contribute to enhanced activity of the perigenual ACC and a decrease of dlPFC activity associated with unipolar depression.
No difference in plasma GABA levels was observed before and after treatment in patients with unipolar depression with current MDE, and the possible effects of treatment on central GABA concentrations could not be assessed because of missing data. The absence of treatment-related difference could appear at odds with other results showing different GABA levels in patients with unipolar depression with current MDE and those in a euthymic state. However, the posttreatment-related improvement in depressive symptoms was not necessarily reported in patients in a euthymic state, and many of them may still experience depressive symptoms. For example, Sanacora and colleagues26 reported a mean posttreatment Hamilton Rating Scale for Depression score of 12.3, revealing that patients had clinically significant symptoms. Therefore, this observation supported the idea that plasma GABA levels could mark clinical states and were not affected by antidepressants. Further studies should specifically address this question and assess the time course of GABA levels in parallel with symptomatic evolution.
GABA levels in bipolar depression
For bipolar disorder, we found plasma GABA decreases in medication-free patients and normalized plasma GABA levels in medicated patients, whereas no change was observed for central GABA levels. Antiepileptic drugs, such as valproate, have been shown to enhance GABA levels in preclinical studies,58,59 possibly by the inhibition of some enzymes such as GABA transaminase59,60 or succinic semialdehyde dehydrogenase.60 The enhancement of GABA has also been shown with lithium in a human study.52 This difference further shows the importance of taking into account mood stabilizers, antiepileptic drugs, or lithium when evaluating GABA levels in patients with bipolar disorder. It remains unclear whether the treatment-related normalization of GABA levels in patients with bipolar disorder has clinical relevance. For example, future studies may assess whether GABA levels can predict therapeutic response in patients with bipolar depression, especially at the individual level. Furthermore, although the absence of differences in central GABA levels must take into account the small number of included studies and, therefore, poor statistical power, the apparent discrepancy between peripheral and central GABA levels remains unexplained. Pathophysiological associations between peripheral and central GABA levels in patients with mood disorders have to be clarified.
GABA differences between unipolar and bipolar depression
Direct comparisons between patients with unipolar and bipolar depression with current MDE appeared contradictory. Indeed, plasma GABA levels were similar between patients with unipolar and bipolar depression, whereas CSF GABA levels were decreased in patients with unipolar depression. However, studies measuring plasma GABA levels were all conducted in medicated patients, and leave-one-out analyses for studies assessing CSF GABA levels suggested that medications tended to abolish GABA differences between patients with unipolar and bipolar depression. Future studies should specifically address whether plasma GABA levels may be a biomarker of clinical states in patients with unipolar depression or of therapeutic responses to mood stabilizers in patients with bipolar disorder. Different imbalances of inhibitory and excitatory systems in patients with unipolar and bipolar depression could explain the observed difference between CSF GABA levels in these patients. This idea was also supported by the difference in network dynamics between patients with unipolar and bipolar depression, with a relative specificity for disrupted networks involving the pregenual ACC, regions of the default mode network, or other regions implicated in emotion processing or regulation in patients with unipolar depression61 that are not found in those with bipolar disorder.62
Limitations
Our meta-analysis had several limitations. First, there was a great heterogeneity across studies, probably because of the substantial variation in clinical characteristics of the included samples. However, we performed many subanalyses taking into account the effects associated with the diagnosis, medications, or the type of measurements of peripheral or central GABA levels. Furthermore, we performed meta-regression to assess the possible associations between SMDs and clinical variables. Second, we were not able to correlate central and peripheral dosages of GABA, which could provide important information regarding methodological issues or clinical practice. Further studies should specifically address this question in samples with depression. Third, GABA changes in ROIs, such as the subgenual ACC, a major actor in depression, could not be assessed specifically because of the limited available data. Finally, many secondary analyses were performed with a small number of studies, which has to be taken into account when interpreting the present results.
Conclusion
This meta-analysis provides evidence of the implication of GABA in depression and emphasizes the importance of imbalance of inhibitory and excitatory systems in patients with mood disorders. The GABA levels evolved differentially between patients with unipolar and bipolar depression. Plasma GABA changes in those with unipolar depression were associated with symptomatic states, whereas plasma GABA changes in those with bipolar disorder seemed to be more closely associated with medication use. It remains to be elucidated whether GABA levels could be useful in clinical practice.
Acknowledgements
The authors acknowledge the kind collaboration of Dr. Chadi G. Abdallah (Department of Psychiatry, Yale University School of Medicine), Prof. Gerard Sanacora (Department of Psychiatry, Yale University School of Medicine), Dr. Matthew Mitchell (Clinical Research and Development, Metabolon Inc.), Dr. Gerald Valentine (Department of Psychiatry, Yale University School of Medicine), Dr. Po W. Wang (Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine), Dr. Beata Godlewska (University Department of Psychiatry, Warneford Hospital), Prof. Phillip J. Cowen (University Department of Psychiatry, Warneford Hospital) and Dr. Xinyan Fu (Department of Neurobiology, Zhejiang University School of Medicine), who provided seminal data from their respective studies.
Footnotes
Competing interests: None declared.
Contributors: All authors designed the study. B. Romeo acquired the data, which all authors analyzed. B. Romeo and J.-Y. Rotge wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
References
- 1.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey replication (NCS-R) JAMA. 2003;289:3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.Emrich HM, von Zerssen D, Kissling W, et al. Effect of sodium valproate on mania. The GABA-hypothesis of affective disorders. Arch Für Psychiatr Nervenkrankh. 1980;229:1–16. doi: 10.1007/BF00343800. [DOI] [PubMed] [Google Scholar]
- 3.Tripp A, Kota RS, Lewis DA, et al. Reduced somatostatin in subgenual anterior cingulate cortex in major depression. Neurobiol Dis. 2011;42:116–24. doi: 10.1016/j.nbd.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tripp A, Oh H, Guilloux JP, et al. Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. Am J Psychiatry. 2012;169:1194–202. doi: 10.1176/appi.ajp.2012.12020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sibille E, Morris HM, Kota RS, et al. GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int J Neuropsychopharmacol. 2011;14:721–34. doi: 10.1017/S1461145710001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guilloux JP, Douillard-Guilloux G, Kota R, et al. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry. 2012;17:1130–42. doi: 10.1038/mp.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Northoff G, Sibille E. Why are cortical GABA neurons relevant to internal focus in depression? A cross-level model linking cellular, biochemical and neural network findings. Mol Psychiatry. 2014;19:966–77. doi: 10.1038/mp.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kugaya A, Sanacora G, Verhoeff NPL, et al. Cerebral benzodiazepine receptors in depressed patients measured with [123i]iomazenil SPECT. Biol Psychiatry. 2003;54:792–9. doi: 10.1016/s0006-3223(02)01788-2. [DOI] [PubMed] [Google Scholar]
- 9.Sanacora G, Gueorguieva R, Epperson CN, et al. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry. 2004;61:705–13. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- 10.Sanacora G, Mason GF, Rothman DL, et al. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56:1043–7. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- 11.Abdallah CG, Jiang L, De Feyter HM, et al. Glutamate metabolism in major depressive disorder. Am J Psychiatry. 2014;171:1320–7. doi: 10.1176/appi.ajp.2014.14010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godlewska BR, Near J, Cowen PJ. Neurochemistry of major depression: a study using magnetic resonance spectroscopy. Psychopharmacology (Berl) 2015;232:501–7. doi: 10.1007/s00213-014-3687-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walter M, Henning A, Grimm S, et al. The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch Gen Psychiatry. 2009;66:478–86. doi: 10.1001/archgenpsychiatry.2009.39. [DOI] [PubMed] [Google Scholar]
- 14.Epperson CN, Gueorguieva R, Czarkowski KA, et al. Preliminary evidence of reduced occipital GABA concentrations in puerperal women: a 1H-MRS study. Psychopharmacology (Berl) 2006;186:425–33. doi: 10.1007/s00213-006-0313-7. [DOI] [PubMed] [Google Scholar]
- 15.Price RB, Shungu DC, Mao X, et al. Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: relationship to treatment resistance in major depressive disorder. Biol Psychiatry. 2009;65:792–800. doi: 10.1016/j.biopsych.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gabbay V. Anterior cingulate cortexg-aminobutyric acid in depressed adolescents: relationship to anhedonia. Arch Gen Psychiatry. 2012;69:139. doi: 10.1001/archgenpsychiatry.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murrough JW, Mao X, Collins KA, et al. Increased ventricular lactate in chronic fatigue syndrome measured by 1H MRS imaging at 3.0 T. II: comparison with major depressive disorder. NMR Biomed. 2010;23:643–50. doi: 10.1002/nbm.1512. [DOI] [PubMed] [Google Scholar]
- 18.Wang PW, Sailasuta N, Chandler RA, et al. Magnetic resonance spectroscopic measurement of cerebral gamma-aminobutyric acid concentrations in patients with bipolar disorders. Acta Neuropsychiatr. 2006;18:120–6. doi: 10.1111/j.1601-5215.2006.00132.x. [DOI] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Abdallah CG, Niciu MJ, Fenton LR, et al. Decreased occipital cortical glutamate levels in response to successful cognitive-behavioral therapy and pharmacotherapy for major depressive disorder. Psychother Psychosom. 2014;83:298–307. doi: 10.1159/000361078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhagwagar Z, Wylezinska M, Jezzard P, et al. Reduction in occipital cortex g-aminobutyric acid concentrations in medication-free recovered unipolar depressed and bipolar subjects. Biol Psychiatry. 2007;61:806–12. doi: 10.1016/j.biopsych.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 22.Brady RO, McCarthy JM, Prescot AP, et al. Brain gamma-aminobutyric acid (GABA) abnormalities in bipolar disorder. Bipolar Disord. 2013;15:434–9. doi: 10.1111/bdi.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Godlewska BR, Yip SW, Near J, et al. Cortical glutathione levels in young people with bipolar disorder: a pilot study using magnetic resonance spectroscopy. Psychopharmacology (Berl) 2014;231:327–32. doi: 10.1007/s00213-013-3244-0. [DOI] [PubMed] [Google Scholar]
- 24.Sanacora G, Mason GF, Rothman DL, et al. Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry. 2003;160:577–9. doi: 10.1176/appi.ajp.160.3.577. [DOI] [PubMed] [Google Scholar]
- 25.Sanacora G, Mason GF, Rothman DL, et al. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry. 2002;159:663–5. doi: 10.1176/appi.ajp.159.4.663. [DOI] [PubMed] [Google Scholar]
- 26.Sanacora G, Fenton LR, Fasula MK, et al. Cortical γ-aminobutyric acid concentrations in depressed patients receiving cognitive behavioral therapy. Biol Psychiatry. 2006;59:284–6. doi: 10.1016/j.biopsych.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Shaw A, Brealy J, Richardson H, et al. Marked reductions in visual evoked responses but not g-aminobutyric acid concentrations or g-band measures in remitted depression. Biol Psychiatry. 2013;73:691–8. doi: 10.1016/j.biopsych.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 28.Valentine GW, Mason GF, Gomez R, et al. The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [1H]-MRS. Psychiatry Res Neuroimaging. 2011;191:122–7. doi: 10.1016/j.pscychresns.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasler G, Neumeister A, van der Veen JW, et al. Normal prefrontal gamma-aminobutyric acid levels in remitted depressed subjects determined by proton magnetic resonance spectroscopy. Biol Psychiatry. 2005;58:969–73. doi: 10.1016/j.biopsych.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Hasler G, van der Veen JW, Tumonis T, et al. Reduced prefrontal glutamate/glutamine and g-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- 31.Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. The Cochrane Collaboration; 2011. [Google Scholar]
- 32.Green S, Higgins J. Cochrane handbook for systematic reviews of interventions 426. Chichester. ed: Wiley, UK; 2006. [Google Scholar]
- 33.Gerner RH, Hare TA. CSF GABA in normal subjects and patients with depression, schizophrenia, mania and anorexia nervosa. Am J Psychiatry. 1981;138:1098–101. doi: 10.1176/ajp.138.8.1098. [DOI] [PubMed] [Google Scholar]
- 34.Gerner RH, Fairbanks L, Anderson GM, et al. CSF neurochemistry in depressed, manic, and schizophrenic patients compared with that of normal controls. Am J Psychiatry. 1984;141:1533–40. doi: 10.1176/ajp.141.12.1533. [DOI] [PubMed] [Google Scholar]
- 35.Kasa K, Otsuki S, Yamamoto M, et al. Cerebrospinal fluid GABA and hamovanillic acid in depressive disorders. Biol Psychiatry. 1982;17:877–83. [PubMed] [Google Scholar]
- 36.Lu YR, Fu XY, Shi LG, et al. Decreased plasma neuroactive amino acids and increased nitric oxide levels in melancholic major depressive disorder. BMC Psychiatry. 2014;14:123. doi: 10.1186/1471-244X-14-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mann JJ, Oquendo MA, Watson KT, et al. Anxiety and major depression. Anxiety in major depression and cerebrospinal fluid free gamma-aminobutyric acid. Depress Anxiety. 2014;31:814–21. doi: 10.1002/da.22278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paige LA, Mitchell MW, Krishnan KRR, et al. A preliminary metabolomic analysis of older adults with and without depression. Int J Geriatr Psychiatry. 2007;22:418–23. doi: 10.1002/gps.1690. [DOI] [PubMed] [Google Scholar]
- 39.Petty F, Schlesser MA. Plasma GABA in affective illness. J Affect Disord. 1981;3:339–43. doi: 10.1016/0165-0327(81)90003-3. [DOI] [PubMed] [Google Scholar]
- 40.Petty F, Sherman A. Plasma GABA levels in psychiatric illness. J Affect Disord. 1984;6:131–8. doi: 10.1016/0165-0327(84)90018-1. [DOI] [PubMed] [Google Scholar]
- 41.Petty F, Kramer GL, Dunnan D, et al. Plasma GABA level in mood disorders. Psychopharmacol Bull. 1990;26:157–61. [PubMed] [Google Scholar]
- 42.Petty F, Kramer GL, Guillion CM, et al. Low plasma GABA levels in male patients with depression. Biol Psychiatry. 1992;32:354–63. doi: 10.1016/0006-3223(92)90039-3. [DOI] [PubMed] [Google Scholar]
- 43.Petty F, Kramer GL, Fulton M, et al. Low plasma GABA is a trait-like marker for bipolar illness. Neuropsychopharmacology. 1993;9:125–32. doi: 10.1038/npp.1993.51. [DOI] [PubMed] [Google Scholar]
- 44.Prosser J, Hugues CW, Sheikha S, et al. Plasma GABA in children and adolescents with mood, behavior, and comorbid mood and behavior disorders: a preliminary study. J Child Adolesc Psychopharmacol. 1997;7:181–99. doi: 10.1089/cap.1997.7.181. [DOI] [PubMed] [Google Scholar]
- 45.Roy A, Dejong J, Ferraro T. CSF GABA in depressed patients and normal controls. Psychol Med. 1991;21:613–8. doi: 10.1017/s0033291700022248. [DOI] [PubMed] [Google Scholar]
- 46.Vieira DSS, Naffah-Mazacoratti MG, Zukerman E, et al. Cerebrospinal fluid GABA levels in chronic migraine with and without depression. Brain Res. 2006;1090:197–201. doi: 10.1016/j.brainres.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 47.Carpenter LL, Moreno FA, Kling MA, et al. Effect of vagus nerve stimulation on cerebrospinal fluid monoamine metabolites, norepinephrine, and gamma-aminobutyric acid concentrations in depressed patients. Biol Psychiatry. 2004;56:418–26. doi: 10.1016/j.biopsych.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 48.Devanand D, Shapira B, Petty F, et al. Effects of electroconvulsive therapy on plasma GABA. Convuls Ther. 1995;11:3–13. [PubMed] [Google Scholar]
- 49.Palmio J, Huuhka M, Saransaari P, et al. Changes in plasma amino acids after electroconvulsive therapy of depressed patients. Psychiatry Res. 2005;137:183–90. doi: 10.1016/j.psychres.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 50.Post R, Ballenger JC, Hare T, et al. Lack of effect of carbamazepine on GABA in cerebrospinal fluid. Neurology. 1980;30:1008–11. doi: 10.1212/wnl.30.9.1008. [DOI] [PubMed] [Google Scholar]
- 51.Berrettini W, Nurnberger J, Hare T, et al. Plasma and CSF GABA in affective illness. Br J Psychiatry. 1982;141:483–7. doi: 10.1192/bjp.141.5.483. [DOI] [PubMed] [Google Scholar]
- 52.Berrettini W, Nurnberger J, Hare T, et al. Reduced plasma and CSF GABA in affective illness: effect of lithium carbonate. Biol Psychiatry. 1983;18:85–94. [PubMed] [Google Scholar]
- 53.Berrettini W, Nurnberger J, Hare TA, et al. CSF GABA in euthymic manic-depressive patients and controls. Biol Psychiatry. 1986;21:842–4. doi: 10.1016/0006-3223(86)90251-9. [DOI] [PubMed] [Google Scholar]
- 54.Kaufman RE, Ostacher MJ, Marks EH, et al. Brain GABA levels in patients with bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:427–34. doi: 10.1016/j.pnpbp.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 55.Petty F, Steinberg J, Kramer GL, et al. Desipramine does not alter plasma GABA in patients with major depression. J Affect Disord. 1993b;29:53–6. doi: 10.1016/0165-0327(93)90119-5. [DOI] [PubMed] [Google Scholar]
- 56.Lemogne C, Mayberg H, Bergouignan L, et al. Self-referential processing and the prefrontal cortex over the course of depression: a pilot study. J Affect Disord. 2010;124:196–201. doi: 10.1016/j.jad.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 57.Lemogne C, Delaveau P, Freton M, et al. Medial prefrontal cortex and the self in major depression. J Affect Disord. 2012;136:e1–11. doi: 10.1016/j.jad.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 58.Löscher W. Valproate induced changes in GABA metabolism at the subcellular level. Biochem Pharmacol. 1981;30:1364–6. doi: 10.1016/0006-2952(81)90323-3. [DOI] [PubMed] [Google Scholar]
- 59.Löscher W. Anticonvulsant and biochemical effects of inhibitors of GABA aminotransferase and valproic acid during subchronic treatment in mice. Biochem Pharmacol. 1982;31:837–42. doi: 10.1016/0006-2952(82)90471-3. [DOI] [PubMed] [Google Scholar]
- 60.Piplani S, Verma PK, Kumar A. Neuroinformatics analyses reveal GABAt and SSADH as major proteins involved in anticonvulsant activity of valproic acid. Biomed Pharmacother. 2016;81:402–10. doi: 10.1016/j.biopha.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 61.Sambataro F, Wolf ND, Pennuto M, et al. Revisiting default mode network function in major depression: evidence for disrupted subsystem connectivity. Psychol Med. 2014;44:2041–51. doi: 10.1017/S0033291713002596. [DOI] [PubMed] [Google Scholar]
- 62.Mamah D, Barch DM, Repovš G. Resting state functional connectivity of five neural networks in bipolar disorder and schizophrenia. J Affect Disord. 2013;150:601–9. doi: 10.1016/j.jad.2013.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]