Abstract
Because of the rapidly growing older population and increases in longevity, rates of dementia have been rising. Clinical challenges of treating dementia include limited resources and lack of curative therapies. Palliative care approaches improve quality of life and alleviate suffering for dementia patients at the end of life, although implementation may be limited by societal acceptance and feasibility. This review examines the published literature on pain assessments, pain and behavior interventions, tools for advanced care planning, and clinical concerns in dementia patients. Ultimately, modification of the traditional palliative care model may improve outcomes and functioning for dementia patients at all stages of their illness.
Keywords: Dementia, palliative care, hospice, pain, medical management
Introduction
With the growing aging population, there has been a steep increase in incidence of Alzheimer disease and other dementias. Current estimates project the number of people living with dementia to increase from 47 million to over 131 million people by 2050.1 Globally, annual costs of dementia have risen to $818 billion and are anticipated to top a trillion dollars by 2018.2 Despite the overwhelming burden of dementia, there are no effective medical interventions to slow or cure the disease. Thus, dementia may be considered a terminal illness, albeit not in the classic sense, with a slow and progressive decline over the time span of years rather than weeks or months. For example, with Alzheimer dementia most patients who are 65 + years of age survive 4–8 years after their initial diagnosis, with other patients living up to 10 + years.3 However, 40% of the duration of illness is spent in the most severe stage of the disease, with a large proportion of these patients residing in nursing home settings. Also, patients with dementia commonly die from other illnesses (i.e., pneumonia, infections, failure to thrive, injuries) rather than from the dementia itself. Thus, over 60% of dementia patients die in nursing homes compared with 20% of cancer patients and 28% of patients with other diagnoses.4,5 The lack of disease-modifying treatments and slower disease progression makes dementia a unique type of terminal illness, yet palliative approaches are both appropriate and helpful.
Terminal illness tend to follow one of three typical courses, as described by Spathis and Booth:6
Rapid decline (months to weeks) from normal functioning to death, minimal to no ability to provide symptomatic relief. “Fulminate progression” examples include severe cerebrovascular disease, myocardial infarction, prion disease, and certain infections such as Ebola virus.
Moderate decline from normal functioning to death, interspersed with episodes of acute crises that can be temporarily ameliorated. “Moderately modifiable” examples include chronic congestive heart disease, renal disease, chronic obstructive pulmonary disease, and most cancers.
Slow but steady progress from normal functioning to death, where underlying core symptoms of the disease cannot be modified. “Chronically progressive” examples include Alzheimer disease, frontotemporal dementia, and amyotrophic lateral sclerosis.
Illness that follows a chronically progressive model are not well served under the current model of hospice. Because there are no means of intervening in the disease course, the “decision” to stop treatment is really nonexistent. Patients and their families must laboriously struggle through the illness until they reach the “cutoff” point to qualify for hospice care.
Currently, many providers use the Functional Assessment Staging to monitor the progression of Alzheimer disease,7 which outlines seven stages through which patients with Alzheimer disease progress during their illness. Unfortunately, most patients do not qualify for hospice until they reach Stage 7. Criteria for Stage 7 include inability to ambulate, requiring assistance to bathe or dress; urinary and fecal incontinence; or lack of meaningful verbal communication. By the time the illness has progressed to this point, the patient and caregivers have endured much suffering and the patient is incapable of participating in the decision for hospice at that moment in time.
Per the World Health Organization definition, “Palliative care is an approach that improves the quality of life of patients and their families facing the problem associated with life-threatening illness, through the prevention and relief of suffering by means of early identification and impeccable assessment and treatment of pain and other problems, physical, psychosocial and spiritual.” (http://www.who.int/cancer/palliative/definition/en/).8 The traditional model of palliative care (Figure 1A) depicts the involvement of palliative care as increasing while curative therapies wane. Ultimately, hospice assumes care of the patient before death. However, in patients with dementia, there are no curative therapies, and the disease progression differs greatly from other illnesses such as cancer. In the case of dementia, palliative care should become involved at the time of diagnoses, and its role will likely wax and wane with the progression and intensity of symptoms (Figure 1B). Because there is no cure, all therapies for patients with dementia are meant to maintain quality of life for as long as possible, and one may consider these interventions as palliative in nature.
Figure 1.
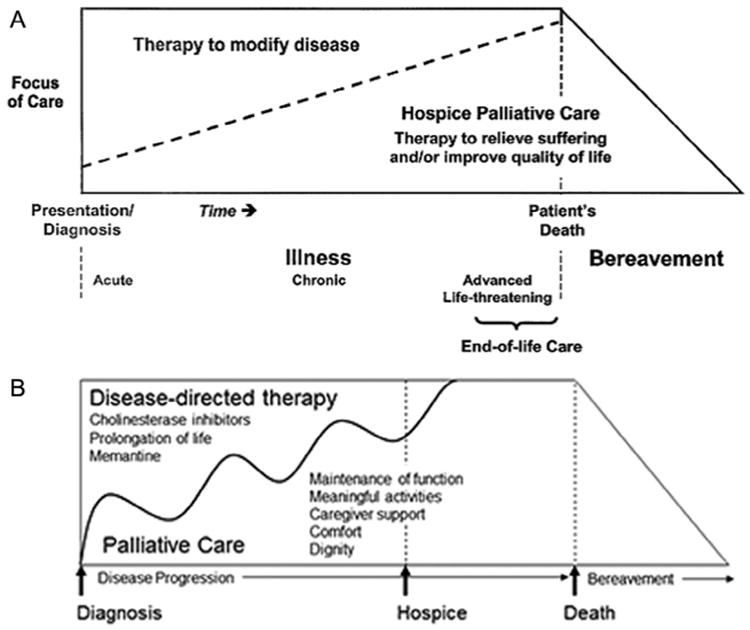
Models of palliative care. [A] Traditional model of palliative care. (Reprinted from Ferris FD, Balfour HM, Bowen K, et al.: A model to guide patient and family care: based on nationally accepted principles and norms of practice. J Pain Sympt Manage 2002; 24:106–123. Copyright 2002, with permission from Elsevier.) [B] Palliative care model modified for dementia. (Modified from Volicer L, Simard J: Palliative care and quality of life for people with dementia: medical and psychosocial interventions. Int Psychogeriatr 2015; 27:1623–1634. Copyright 2015, reproduced with permission from Cambridge University Press.).
Dementia presents unique challenges in end-of-life care, including difficulty defining the “terminal” phase of the illness, communication challenges, determining the appropriateness of medical and palliative care interventions, integration with existing care delivery systems, and procuring the time and monetary resources necessary for such services.9–11 Suboptimal end-of-life care in dementia (e.g., inadequate pain control, overuse of medical interventions, care that is inconsistent with the patient's wishes) may lead to unnecessary suffering and ethical challenges. The 2015 World Alzheimer Report2 stated that people with dementia “deserve good quality end-of-life care with respect to their dignity and personal wishes” (p. 79) in their Call to Action.
Recommendations for optimal palliative care in dementia were put forth by the European Association for Palliative Care in 2014.12 The 57 recommendations ranged across 11 domains, from the applicability of palliative care in dementia patients to the specific societal and ethical issues. Specifically regarding dementia, the guidelines included shared decision-making for patients with mild dementia and using specific tools and caregiver input to identify sources of discomfort in more severe dementia.12
A few studies have reported that palliative care interventions are helpful for persons with dementia. Inpatient co-management of hospitalized patients with dementia by the palliative care team increased the likelihood of having a care plan, although mortality and admission rates were not significantly different.13 A specialized dementia care unit in the hospital had higher mortality rates but lower rates of observed discomfort, transfers to acute settings, and costs compared with a general medicine unit.14 Last, structured decision aids regarding feeding decisions for surrogate decision-makers reduced decisional conflict.15 Despite compelling reasons to incorporate palliative approaches into dementia care, the research literature has been limited. Similar to the shortage of geriatric mental health specialists, the limited number of palliative specialists to care for the aging population restricts accessibility for patients with dementia who would benefit from palliative interventions for years before death.16,17 In this clinical review we discuss the literature and major clinical issues regarding palliative care approaches in dementia with the goal of educating geriatric mental health providers who will encounter these complex clinical situations.
Literature Review Of Palliative Care Interventions In Dementia
Pain Assessments in Dementia
One focus in the literature was accurate and consistent assessment of pain in patients with dementia. The Preventing Aggression in Veterans with Dementia randomized controlled trial tested the effectiveness of a 6- to 8-week in-home educational intervention for caregivers compared with usual care.18 Although the intervention did not reduce aggression or pain, caregivers found the intervention helpful. Another study compared the consistency of different self-assessments of pain and found verbal reporting to be the easiest to administer and complete, although consistency of ratings depended on the severity of dementia.19 One 2006 review evaluated the evidence for 10 pain assessments designed for use in nonverbal patients with dementia and was unable to recommend any one for broad clinical use, deeming the development and testing to be in early stages.20 Meanwhile, another systematic review of 12 international tools in 2006 found the Pain Assessment Checklist for Seniors with Limited Ability to Communicate and Doloplus2 to be the best scales to use based on validity, sensitivity, and clinical utility.21 A 2014 review of 28 tools was unable to recommend any one pain assessment given the limited evidence on reliability, validity, and clinical utility.22 In clinical practice the Pain Assessment in Advanced Dementia scale is widely used for palliative and hospice patients who have dementia and/or are unable to verbally report pain.23,24 The five-item scale uses observed behaviors of the patient (breathing, negative, vocalization, facial expression, body language, and consolability) to assess pain and guide management. Unfortunately, despite the importance of accurate clinical pain assessments, the literature is mixed regarding which tool is most helpful because of limited research on validating the tools and heterogeneity of dementia populations.
Pain Interventions in Dementia
Evidence of pharmacologic pain interventions in patients with moderate to severe or advanced dementia is also limited and mixed (Table 1). Of the five studies, only two25,26 were randomized and placebo controlled. Three studies included between 20 and 40 subjects,25–27 and four studies focused on nursing home residents.25–28 One study reported on three patients in a community setting using videotapes to assess pain behaviors,29 whereas other studies used a variety of scales to assess discomfort, agitation, or activities. However, the two larger-scale, randomized studies showed improvements in social engagement and agitation with scheduled acetaminophen and medications from a pain protocol.26,28 Despite heterogeneity in these findings, pain is often undertreated in people with dementia, and adequate assessment and intervention is warranted.30,31
Table 1. Pharmacologic Pain Interventions in Moderate to Severe or Advanced Dementia.
| Study | Design | Intervention | Subjects | Outcome |
|---|---|---|---|---|
| Manfredi et al. 200327 | Placebo-controlled crossover trial | All subjects received placebo × 4 weeks, followed by long-acting opioid (oxycodone or morphine) × 4 weeks | 25 nursing home residents with dementia completed both phases | No significant differences in agitation between the two phases by CMAI. |
| Buffum et al. 200425 | Randomized, placebo-controlled, double-blind crossover trial | Crossover design of acetaminophen 650 mg four times a day × 2 weeks compared with placebo × 2 weeks | 39 nursing home residents with severe Alzheimer or vascular dementia | No significant difference in discomfort between the two phases by the MDS. 2,600 mg/day acetaminophen dosing is inadequate for elderly nursing home residents with significant discomfort. |
| Chibnall et al. 200526 | Randomized, placebo-controlled, double-blind crossover trial | Crossover design of acetaminophen 1,000 mg three times a day × 4 weeks compared with placebo × 4 weeks | 25 nursing home residents with Alzheimer, vascular, or unspecified dementia | Significant improvement in social interaction, engagement with media and work-like activity, and spending time outside their rooms when on acetaminophen by DCM |
| Elliott and Horgas 200929 | Within-subjects ABAB withdrawal trial | Alternating treatment phases of acetaminophen four times a day × either 4 or 8 days with nontreatment phases × either 4 or 8 days | 3 community-dwelling patients with dementia | Significant reduction in pain behaviors associated with musculoskeletal pain during treatment phases by videotaped activity-based protocols. |
| Husebo et al. 201128 | Randomized controlled trial | Stepwise pain protocol × 8 weeks, then 4-week follow-up period | 352 nursing home residents with moderate to severe dementia and clinically significant behavioral disturbances | Significant reduction in agitation by CMAI when receiving pain treatment protocol. |
Note: CMAI: Cohen Mansfield Agitation Inventory; DCM: Dementia Care Mapping; MDS: Mean Discomfort Scale; ABAB: alternating baseline (A) and treatment (B) stages to assess the effect of the treatment on the behaviors.
The literature on nonpharmacologic pain and behavior interventions includes a wide range of therapeutic strategies from specialized care teams, cognitive therapy, reflexology, and bathing modifications (Table 2). Similarly, the study samples varied in size and severity of dementia and in experimental design and outcome measures. Overall, all interventions listed in Table 2 reduced pain, agitation, aggressive behaviors, discomfort, or distress. Reviews supported certain interventions, such as psychoeducation and communication skills training for caregivers, pain-monitoring strategies, nonpharmacologic pain interventions (e.g., sensory interventions, music therapy), and engaging the patient in pleasurable activities.32,33 However, other studies have not shown improvements in agitation with aromatherapy, light therapy, caregiver training in cognitive or behavioral therapy, or therapeutic touch techniques.33 Ultimately, given the significant economic and caregiver burden from agitated and aggressive behaviors and sensitivity to medications in the elderly, nonpharmacologic interventions have an important role in alleviating suffering for persons with dementia.
Table 2. Nonpharmacologic Pain and Behavior Interventions.
| Study | Design | Intervention | Subjects | Outcome |
|---|---|---|---|---|
| Chapman and Toseland 200734 | 2 × 2 randomized, partial crossover | Advance illness care team × 8 weeks vs. usual care × 8 weeks | 118 nursing home residents with advanced dementia | Significant reduction in physical nonaggressive behavior by CMAI in the active treatment group. Nonsignificant reduction in pain by the PAINAD scale in the active treatment group. |
| Cipher et al 200735 | Cohort | Multimodal cognitive behavioral therapy (8 sessions over 5 weeks) | 44 long-term care facility residents with mild to moderate dementia, persistent pain, and a psychiatric disorder (depression, anxiety, or severe behavioral disturbances) | Significant reduction in pain by GMPI, behavioral disturbances by GLDS, and depression by GDS post-treatment. |
| Dunn et al. 200236 | Experimental | Thermal bath (modified bed bath) vs. conventional tub bath | 15 continuing care center residents with dementia | Significant reduction in frequency of agitated behaviors with the thermal bath. |
| Hodgson and Andersen 200837 | Experimental, repeated-measures crossover | Reflexology × 4 weeks and friendly visits × 4 weeks | 21 nursing home residents with mild to moderate dementia (randomized to order of interventions) | Significant reduction in in physiologic distress by salivary α-amylase levels and observed pain by CNPI during reflexology. |
| Kovach et al. 199938 | Cohort | Assessment of Discomfort in Dementia Protocol | 104 long-term care residents with end-stage dementia | Significant reduction in discomfort by observed behavioral symptoms and significant increase in use of scheduled analgesics and nonpharmacologic comfort interventions postintervention. |
| Kovach et al. 200639 | Double-blinded, randomized, controlled trial | Serial treatment intervention | 114 nursing home residents with late-stage dementia | Significant reduction in discomfort by Discomfort-DAT and significant increase in use of pharmacologic comfort treatments with active treatment. |
| Sloane et al. 200440 | Randomized, controlled trial with crossover | Person-centered showering and towel bath vs. usual care × 6 weeks | 69 nursing home residents with dementia and agitation or aggression during bathing | Significant reduction in agitation and aggression by CMAI and CAREBA in patient-centered showering and towel-bath groups compared with usual care. |
| Watson et al. 199841 | Randomized, controlled, crossover | Rocking chair use × 6 weeks vs.control | 25 nursing home residents with dementia | Significant reduction in as-needed pain medication usage, depression and anxiety in treatment group. |
Note: CAREBA: Care Recipient Behavior Assessment; CMAI: Cohen Mansfield Agitation Inventory; CNPI: Checklist of Non-verbal Pain Indicators; Discomfort-DAT: Discomfort-Dementia of the Alzheimer's Type scale; GDS: Geriatric Depression Scale; GLDS: Geriatric Level of Dysfunction Scale; GMPI: Geriatric Multidimensional Pain and Illness Inventory; PAINAD: Pain in Advanced Dementia.
Issues In Clinical Practice
Palliative care in advanced dementia is commonly misperceived by the general public as allowing or hastening the passing of loved ones. Common fears include discontinuing or withholding medical treatment, “giving up” by resigning to the notion that the individual with dementia will not be involved in meaningful activities, and a static plan of care once set. Families may benefit from education about the true goals of palliative care in dementia that include the ability to “live, not simply exist.” (p. 1626).42
Advanced Care Planning
Importance
Geriatric psychiatrists can play a special role in the care of these older adults, because the discussion of mental health in the context of cognitive decline is often difficult in busy primary care or other specialty clinics for a variety of practical reasons. Geriatric psychiatrists are in a position to emphasize the importance of advanced care planning (ACP), especially when a patient has first been diagnosed with a cognitive disorder. It is not uncommon for families to avoid ACP, stating “We don't talk about [death].”13 Although there are many tools to help facilitate ACP, a few of which are noted below, simply starting the conversation about what gives one's life meaning can be a powerful way to start the process. Psychiatrists are poised to facilitate these potentially difficult, albeit necessary and often beneficial, discussions during the time when patients retain their ability to make decisions. Psychiatrists are also able to provide education about the natural progression of dementia. Despite major research efforts into development of medications that can prevent or reverse cognitive decline, there is currently no cure. Even basic bodily functions such as walking, swallowing, and speaking are lost at the end stages of cognitive degeneration.
Tools to Aid in ACP
The “Five Wishes” document is an advanced directive written in narrative language in a way that helps people express their wishes in areas that matter most.43 Five Wishes is available in 28 languages and meets legal requirements for an advanced directive in 42 U.S. states. It is available online or in print from the Aging with Dignity Organization.
Go Wish Cards are a set of cards that help a patient put into words what is important to him or her.44 Playing the game with relatives or close friends can help patients to engage in dialogue with their loved ones so they also understand the patient's values.
The Physicians Orders for Life Sustaining Treatments is a single pink-colored page that is transported with patients in the event of critical illness.45 In Section A the patient specifies whether medical teams should or should not attempt cardiopulmonary resuscitation. Section B indicates the patient's preferences regarding intubation, mechanical ventilation, intravenous fluids, and antibiotics versus comfort measures to relieve pain and suffering. Section C addresses the patient's choices regarding trials of long-term artificial nutrition such as feeding tubes. Information for a healthcare agent is also documented. The physician as well as the patient sign. As of December 2016 only four U.S. states (Maryland, Massachusetts, Vermont, and South Dakota) have not conformed to Physicians Orders for Life Sustaining Treatments requirements or have not begun exploring the development of a Physicians Orders for Life Sustaining Treatments program.45
When No Advanced Directive Exists
Unfortunately, emergent situations arise where ACP has not taken place or has not been updated recently. Hospitalist physicians, often new to the patients, are then placed in the position of undertaking emotionally charged family meetings. Goals of care discussions in critical scenarios are an art, and subtle differences in presentation can impact the outcome. In these instances, the following is recommended:
Choose a quiet and private place for discussion.
Begin with a summary of psychiatric and medical treatments thus far.
Inquire what the family knows about the disease process in an open-ended way, being aware of “maladaptive coping” on behalf of the family and/or patient such as idealizing the physician or uncontrollable emotions.
Avoid statements that place the burden of decision on loved ones, such as “Do you want us to do everything possible,” “Would you agree to discontinue care,” “Are you agreeing to refrain from extraordinary measures,” “We should stop aggressive therapy,” or “We will make sure there is no suffering.”
Use statements that provide empathy and comfort without false hope, such as “We will hope for the best, but plan for the worst,” “We wish things were different,” “We're worried that your loved one's life will be shorter than we all hoped for,” or “This does not mean that treatments will be withheld.”
Educate family on the research that shows that morbidity and mortality are high in older patients with dementia on whom invasive life-saving measures are performed. Less than 40% patients older than 70 years of age survive cardiopulmonary resuscitation (18.7% of patients aged 70–79 years, 15.4% of patients aged 80–89 years, and 11.6% of patients age 90 + years), and greater than half of those patients died in the hospital before discharge.46
Us the concept of “palliative paternalism” when maladaptive coping mechanisms are detected. In this model the physician recommends a course of action to relieve the guilt and burden of decision-making.47
Align with other specialists to present a clear picture of clinical outcomes. “A patient is more than the sum of his or her parts.” This often takes significant time to communicate with all providers engaged in the care of the patient to develop a comprehensive and shared assessment of the patient's current status and likely trajectory.
Other Clinical Decision-Making Dilemmas in Advanced Dementia
Medical management of patients with dementia is complicated by several factors: older age, communication difficulties, cognitive impairment, functional decline, and decreased abilities to manage activities of daily living. Dementia patients are more likely to have superimposed age-related chronic diseases that require long-term medications, many of which can adversely affect cognition. Additionally, these illnesses can compound the impact that dementia has on a patient's functioning and ability to manage their medical conditions at home. The challenges of cognitive impairment with resulting deficits in communication, comprehension, and insight into illness and abilities further complicate the clinician's ability to guide the patient's care. This is especially true if the patient does not have capacity to make such decisions and their wishes have not been fully discussed with their surrogate decision-maker or the patient does not recognize that they have an illness that adversely affects their decision making capacity.
Artificial Nutrition
For a multitude of reasons, such as the loss of ability to coordinate chewing or swallowing or other dental mechanical issues, patients become malnourished. Families often struggle as they see their loved ones “wither away.” The idea of projecting our own experience of hunger onto loved ones with dementia may make these decisions even more challenging. Having open discussions about the change in the feelings of hunger and drive to eat as disease progresses can be helpful to relieve the family's struggle when considering artificial nutrition. It is important in these instances to clarify that tube feeding does not prolong survival. It does not prevent against the aspiration of oral or gastric secretions, which can cause pneumonia even in the absence of food. Tubes are prone to blockage, leaks, or dislodgement and can lead to agitation. In a nationwide study of 36,492 nursing home patients, 5.4% underwent feeding tubes, and there was no difference in survival between those who underwent feeding tubes and those who did not. In another observational study, it was observed that withdrawal of artificial nutrition did not appear to cause an increase in discomfort to patients.48 Oral and hand feeding is recommended by the American Geriatrics Society.49
Antibiotic Use
Patients with advanced dementia are prone to recurrent infections. Forty percent of patients receive antimicrobials in the last few weeks of life often without clinical evidence of infection. This increases the risk of the patient experiencing side effects to the antibiotics, such as abdominal discomfort, nausea, diarrhea, and prevalence of multidrug-resistant bacteria; may predispose to Clostridium difficile infections; increases the likelihood of medication interactions; and increases the need for lab monitoring if administered intravenously. In patients with advanced dementia, those who did not receive antibiotics died during the first 3 days, whereas those who received antibiotics survived only 7 additional days.42 It should not be forgotten that analgesics, antipyretics, and oxygen can provide comfort in the absence of antibiotics.
Withdrawal of Medication
Families may have difficulty understanding why medications are withdrawn as a patient's dementia progresses. However, treatment of conditions that pose long-term problems but are not imminently life threatening (e.g., hypertension, hypercholesterolemia, benign tumors, and diabetes) may cause more harm than good for certain patients. Patients may not reap the pre-ventative benefit of statins, antihypertensive medications, diabetes medications, and chemotherapy depending on their quality of life, functioning, and stage of dementia. Some families may view stopping such preventative medications as hastening or even causing the death of the patient, leading to unnecessary guilt and confusion.
The 2008 Palliative Excellence in Alzheimer Care Efforts (PEACE) consensus panel of experts identified medications that are inappropriate in advanced dementia,50 including preventive medications such as lipid-lowering agents and vitamin supplements, cognitive enhancers, antiplatelet agents, hormones, cytotoxic chemotherapy agents, and leukotriene inhibitors. Always appropriate medications included laxatives, stool softeners, and opioid analgesics. Sometimes appropriate based on symptoms and risk versus benefit justification were antidepressants or antipsychotics.
Emergency Department Visits
Patients with advanced dementia are transported to the hospital on average 1.6 times in the last 3 months of life. A study by Saliba et al.51 showed that 44% of emergency department transfers from nursing homes and 45% of hospital admissions were inappropriate when advanced directives were considered. Hospitalization exposes patients to increased harm, including infectious agents and delirium. Default transfer to hospitals should be used only if consistent with overall goals of care.
Workup of Medical Complaints and Pain
To adequately work up medical complaints in patients with dementia, clinicians must understand the natural progression of the dementia illness that may include loss of function, gait impairment, incontinence, apraxia, anorexia, feeding difficulties, and increased aspiration risk. Pain must be adequately addressed; however, pain may also be an indication of other forms of distress. Additionally, clinicians must consider the consequences of diagnostic testing and how their management of the patient would be impacted by obtaining each test. In certain situations the diagnostic procedure itself could worsen the patient's confusion, disability, or quality of life. Bynum et al.52 reported that a diagnosis of dementia was associated with a threefold increased odds of hospitalization and threefold increased medical expenditures than nondementia patients.
Management of Neuropsychiatric Symptoms
Additionally, neuropsychiatric symptoms such as depression, anxiety, psychosis, and confusion can greatly impact the patient's well-being and quality of life. Patients may withdraw from activities, be uncooperative with personal care, and have impulsive and/or dangerous behaviors that may worsen the burden on their caregivers and lead to institutionalization. In the United States dementia care relies heavily on unpaid caregivers (usually family members), with 15.4 million people providing 18.2 billion hours of unpaid care.53 Also, the caregiver burden in dementia is extended, with 43% of Alzheimer disease caregivers providing care for at least 1–4 years compared with only 33% of nondementia caregivers.
Referral to Hospice
When a dementia patient approaches the end of life, a referral to hospice may be appropriate. Medicare criteria for hospice requires a life expectancy of 6 months or less (as prognosticated by the hospice doctor and primary doctor) and patient acceptance of palliative/comfort care over curative care. As described earlier, dementia patients can enroll in hospice when they reach Functional Assessment Staging Stage 7 and have at least one of the following conditions over the past year: aspiration pneumonia, pyelonephritis, septicemia, multiple stage 3–4 decubitus ulcers, recurrent fever after antibiotics, and inability to maintain fluid and caloric intake, with either 10% weight loss in the past 6 months or a serum albumin < 2.5 g/dL.
Unfortunately, because dementia does not “follow the rules” of a traditional terminal illness, the current framework of hospice may not be adequate for these patients. There may be the perception that a hospice referral is “withholding care” and may hasten death or that patients can only be enrolled in hospice in very dire situations. The criteria may be overly focused on the 6-month survival, which may be more difficult to estimate in dementia patients, particularly those with limited medical comorbidities. Additionally, there are limited resources for caregivers to help them earlier in the disease course, and most involve out-of-pocket expenses.
Patients may benefit from an alternative model of hospice54 that has a gradual and continuous course rather than a dichotomous “yes or no” switchover to hospice care. Additionally, the focus on 6-month life expectancy could be extended or abandoned to ensure that more patients who would benefit from hospice services can qualify for them. Certain hospice services could be offered to patients earlier in their disease course, in a manner that more accurately and appropriately maps the progression of cognitive and functional impairment that occurs in dementia. This could aid in reducing caregiver burden and helping patients while they still have some level of cognitive understanding and can more fully participate in the decision to enroll.
Conclusion
Because of the many challenges of caring for patients with dementia, despite the limited literature, palliative approaches can be very helpful for this patient population. In general, there are several pain assessments available for use with patients with cognitive impairments, although the evidence is mixed regarding which one to use. Such assessments should be chosen based on specific characteristics of the patient (e.g., verbal versus nonverbal communication abilities, level of cognitive impairment) and the care environment (e.g., training and abilities of the caregivers.) Similarly, there are limited data on pharmacologic pain interventions, despite evidence of undertreated pain in patients with dementia. Again, individualized pain protocols may best address the distress of individuals. Nonpharmacologic pain interventions have been studied in a number of heterogeneous and scattered trials with generally positive results and hold promise in reducing the risk of adverse medication effects, improving functioning, and helping caregivers. Ideally, the palliative care approach should be initiated upon diagnosis of dementia illness and include ACP.
In this review, we have presented a number of low-cost/free and practical tools to guide such conversations, educate families, and record patient preferences. Management of acute and chronic medical issues should be guided by palliative care principles to limit over-aggressive and/or futile treatments or testing, preserve quality of life, and avoid the many possible unnecessary adverse effects. When appropriate, patients should be treated for neuropsychiatric symptoms and referred to hospice. Integration of palliative principles and interventions into the care of a patient with dementia can greatly improve the quality of life and disease course.
Highlights.
With limited resources and a lack of curative therapies, clinically treating patients with dementia has many inherent challenges that are compounded by end-of-life issues.
Palliative care approaches can be adapted to improve the quality of life and relieve suffering for patients with dementia and their families.
This clinical review examines the literature regarding pain assessment and management, advance care planning and medical management as applied to dementia care.
Acknowledgments
Supported in part by the National Institute of Mental Health (NIMH T32 Geriatric Mental Health Program MH019934 [Principle Investigator: Dilip V. Jeste, M.D.]) and by the Stein Institute for Research on Aging at the University of California, San Diego.
Footnotes
The authors have no relevant conflicts of interest to report.
References
- 1.Prince M, Comas-Herrera A, Knapp M, et al. World Alzheimer Report 2016: Improving Healthcare for People Living with Dementia. London, UK: Alzheimer's Disease International; 2016. [Google Scholar]
- 2.Prince M, Wimo A, Guerchet M, et al. World Alzheimer Report 2015: The Global Impact of Dementia. London, UK: Alzheimer's Disease International; 2015. [Google Scholar]
- 3.Tom SE, Hubbard RA, Crane PK, et al. Characterization of dementia and Alzheimer's disease in an older population: updated incidence and life expectancy with and without dementia. Am J Public Health. 2015;105:408–413. doi: 10.2105/AJPH.2014.301935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell SL, Teno JM, Miller SC, et al. A national study of the location of death for older persons with dementia. J Am Geriatr Soc. 2005;53:299–305. doi: 10.1111/j.1532-5415.2005.53118.x. [DOI] [PubMed] [Google Scholar]
- 5.Alzheimer's A. 2013 Alzheimer's disease facts and figures. Alzheimers Dement. 2013;9:208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Spathis A, Booth S. End of life care in chronic obstructive pulmonary disease: in search of a good death. Int J Chron Obstruct Pulmon Dis. 2008;3:11–29. doi: 10.2147/copd.s698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sclan SG, Reisberg B. Functional assessment staging (FAST) in Alzheimer's disease: reliability, validity, and ordinality. Int Psychogeriatr. 1992;4(suppl 1):55–69. doi: 10.1017/s1041610292001157. [DOI] [PubMed] [Google Scholar]
- 8.WHO. WHO Definition of Palliative Care. http://www.who.int/cancer/palliative/definition/en/
- 9.Birch D, Draper J. A critical literature review exploring the challenges of delivering effective palliative care to older people with dementia. J Clin Nurs. 2008;17:1144–1163. doi: 10.1111/j.1365-2702.2007.02220.x. [DOI] [PubMed] [Google Scholar]
- 10.Davies N, Maio L, van Riet Paap J, et al. Quality palliative care for cancer and dementia in five European countries: some common challenges. Aging Ment Health. 2014;18:400–410. doi: 10.1080/13607863.2013.843157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Riet Paap J, Mariani E, Chattat R, et al. Identification of the palliative phase in people with dementia: a variety of opinions between healthcare professionals. BMC Palliat Care. 2015;14:56. doi: 10.1186/s12904-015-0053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Steen JT, Radbruch L, Hertogh CM, et al. White paper defining optimal palliative care in older people with dementia: a Delphi study and recommendations from the European Association for Palliative Care. Palliat Med. 2014;28:197–209. doi: 10.1177/0269216313493685. [DOI] [PubMed] [Google Scholar]
- 13.Ahronheim JC, Morrison RS, Baskin SA, et al. Treatment of the dying in the acute care hospital Advanced dementia and metastatic cancer. Arch Intern Med. 1996;156:2094–2100. [PubMed] [Google Scholar]
- 14.Volicer L, Collard A, Hurley A, et al. Impact of special care unit for patients with advanced Alzheimer's disease on patients' discomfort and costs. J Am Geriatr Soc. 1994;42:597–603. doi: 10.1111/j.1532-5415.1994.tb06856.x. [DOI] [PubMed] [Google Scholar]
- 15.Hanson LC, Carey TS, Caprio AJ, et al. Improving decision-making for feeding options in advanced dementia: a randomized, controlled trial. J Am Geriatr Soc. 2011;59:2009–2016. doi: 10.1111/j.1532-5415.2011.03629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zuraw L. As Palliative Care Need Grows, Specialists Are Scarce. http://www.npr.org/sections/health-shots/2013/04/03/176121044/as-palliative-care-need-grows-specialists-are-scarce, NPR, 2013.
- 17.Lupu D. American Academy of Palliative Medicine Workforce Task F: Estimate of current hospice and palliative medicine physician workforce shortage. J Pain Symptom Manage. 2010;40:899–911. doi: 10.1016/j.jpainsymman.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Kunik ME, Snow AL, Wilson N, et al. Teaching caregivers of persons with dementia to address pain. Am J Geriatr Psychiatry. 2017;25:144–154. doi: 10.1016/j.jagp.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Closs SJ, Barr B, Briggs M, et al. A comparison of five pain assessment scales for nursing home residents with varying degrees of cognitive impairment. J Pain Symptom Manage. 2004;27:196–205. doi: 10.1016/j.jpainsymman.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Herr K, Bjoro K, Decker S. Tools for assessment of pain in nonverbal older adults with dementia: a state-of-the-science review. J Pain Symptom Manage. 2006;31:170–192. doi: 10.1016/j.jpainsymman.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Zwakhalen SM, Hamers JP, Abu-Saad HH, et al. Pain in elderly people with severe dementia: a systematic review of behavioural pain assessment tools. BMC Geriatr. 2006;6:3. doi: 10.1186/1471-2318-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lichtner V, Dowding D, Esterhuizen P, et al. Pain assessment for people with dementia: a systematic review of systematic reviews of pain assessment tools. BMC Geriatr. 2014;14:138. doi: 10.1186/1471-2318-14-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warden V, Hurley AC, Volicer L. Development and psychometric evaluation of the Pain Assessment in Advanced Dementia (PAINAD) scale. J Am Med Dir Assoc. 2003;4:9–15. doi: 10.1097/01.JAM.0000043422.31640.F7. [DOI] [PubMed] [Google Scholar]
- 24.Leong IY, Chong MS, Gibson SJ. The use of a self-reported pain measure, a nurse-reported pain measure and the PAINAD in nursing home residents with moderate and severe dementia: a validation study. Age Ageing. 2006;35:252–256. doi: 10.1093/ageing/afj058. [DOI] [PubMed] [Google Scholar]
- 25.Buffum MD, Sands L, Miaskowski C, et al. A clinical trial of the effectiveness of regularly scheduled versus as-needed administration of acetaminophen in the management of discomfort in older adults with dementia. J Am Geriatr Soc. 2004;52:1093–1097. doi: 10.1111/j.1532-5415.2004.52305.x. [DOI] [PubMed] [Google Scholar]
- 26.Chibnall JT, Tait RC, Harman B, et al. Effect of acetaminophen on behavior, well-being, and psychotropic medication use in nursing home residents with moderate-to-severe dementia. J Am Geriatr Soc. 2005;53:1921–1929. doi: 10.1111/j.1532-5415.2005.53572.x. [DOI] [PubMed] [Google Scholar]
- 27.Manfredi PL, Breuer B, Wallenstein S, et al. Opioid treatment for agitation in patients with advanced dementia. Int J Geriatr Psychiatry. 2003;18:700–705. doi: 10.1002/gps.906. [DOI] [PubMed] [Google Scholar]
- 28.Husebo BS, Ballard C, Sandvik R, et al. Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: cluster randomised clinical trial. BMJ. 2011;343:d4065. doi: 10.1136/bmj.d4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elliott AF, Horgas AL. Effects of an analgesic trial in reducing pain behaviors in community-dwelling older adults with dementia. Nurs Res. 2009;58:140–145. doi: 10.1097/NNR.0b013e318199b599. [DOI] [PubMed] [Google Scholar]
- 30.Tan EC, Jokanovic N, Koponen MP, et al. Prevalence of analgesic use and pain in people with and without dementia or cognitive impairment in aged care facilities: a systematic review and meta-analysis. Curr Clin Pharmacol. 2015;10:194–203. doi: 10.2174/157488471003150820144958. [DOI] [PubMed] [Google Scholar]
- 31.Evers MM, Purohit D, Perl D, et al. Palliative and aggressive end-of-life care for patients with dementia. Psychiatr Serv. 2002;53:609–613. doi: 10.1176/appi.ps.53.5.609. [DOI] [PubMed] [Google Scholar]
- 32.Bradford A, Shrestha S, Snow AL, et al. Managing pain to prevent aggression in people with dementia: a nonpharmacologic intervention. Am J Alzheimers Dis Other Demen. 2012;27:41–47. doi: 10.1177/1533317512439795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livingston G, Kelly L, Lewis-Holmes E, et al. A systematic review of the clinical effectiveness and cost-effectiveness of sensory, psychological and behavioural interventions for managing agitation in older adults with dementia. Health Technol Assess. 2014;18:1–226, v-vi. doi: 10.3310/hta18390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chapman DG, Toseland RW. Effectiveness of advanced illness care teams for nursing home residents with dementia. Soc Work. 2007;52:321–329. doi: 10.1093/sw/52.4.321. [DOI] [PubMed] [Google Scholar]
- 35.Cipher DJ, Clifford PA, Roper KD. The effectiveness of geropsychological treatment in improving pain, depression, behavioral disturbances, functional disability, and health care utilization in long-term care. Clin Gerontol. 2007;30:23–40. [Google Scholar]
- 36.Dunn JC, Thiru-Chelvam B, Beck CH. Bathing Pleasure or pain? J Gerontol Nurs. 2002;28:6–13. doi: 10.3928/0098-9134-20021101-05. [DOI] [PubMed] [Google Scholar]
- 37.Hodgson NA, Andersen S. The clinical efficacy of reflexology in nursing home residents with dementia. J Altern Complement Med. 2008;14:269–275. doi: 10.1089/acm.2007.0577. [DOI] [PubMed] [Google Scholar]
- 38.Kovach CR, Weissman DE, Griffie J, et al. Assessment and treatment of discomfort for people with late-stage dementia. J Pain Symptom Manage. 1999;18:412–419. doi: 10.1016/s0885-3924(99)00094-9. [DOI] [PubMed] [Google Scholar]
- 39.Kovach CR, Logan BR, Noonan PE, et al. Effects of the Serial Trial Intervention on discomfort and behavior of nursing home residents with dementia. Am J Alzheimers Dis Other Demen. 2006;21:147–155. doi: 10.1177/1533317506288949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sloane PD, Hoeffer B, Mitchell CM, et al. Effect of person-centered showering and the towel bath on bathing-associated aggression, agitation, and discomfort in nursing home residents with dementia: a randomized, controlled trial. J Am Geriatr Soc. 2004;52:1795–1804. doi: 10.1111/j.1532-5415.2004.52501.x. [DOI] [PubMed] [Google Scholar]
- 41.Watson NM, Wells TJ, Cox C. Rocking chair therapy for dementia patients: Its effect on psychosocial well-being and balance. Am J Alzheimer's Dis. 1998;13:296–308. [Google Scholar]
- 42.Palliative care and the quality of life for people with dementia: medical and psychosocial interventions. Int Psychogeriatr. 2015;27:1623–1634. doi: 10.1017/S1041610214002713. [DOI] [PubMed] [Google Scholar]
- 43.Five Wishes. changing the way we talk about and plan for care at the end of life. Aging with Dignity. 2015 https://www.agingwithdignity.org/five-wishes.
- 44.The Go Wish Game, CODA Alliance. 2005 http://www.gowish.org/
- 45.National POLST Paradigm, Tides Center. 2017 http://polst.org/ [Google Scholar]
- 46.The chance of survival and the functional outcome after in hospital cardiopulmonary resuscitation. Age Aging. 2014;43:456–463. doi: 10.1093/ageing/afu035. [DOI] [PubMed] [Google Scholar]
- 47.Roeland E, Cain J, Onderdonk C, et al. When open-ended questions don't work: the role of palliative paternalism in difficult medical decisions. J Palliat Med. 2014;17:415–420. doi: 10.1089/jpm.2013.0408. [DOI] [PubMed] [Google Scholar]
- 48.Mitchell S. Palliative Care of Patients with Advanced Dementia. 2017 https://www.uptodate.com/contents/palliative-care-of-patients-with-advanced-dementia.
- 49.American Geriatrics Society Ethics C., Clinical P., Models of Care C. American Geriatrics Society feeding tubes in advanced dementia position statement. J Am Geriatr Soc. 2014;62:1590–1593. doi: 10.1111/jgs.12924. [DOI] [PubMed] [Google Scholar]
- 50.Holmes HM, Sachs GA, Shega JW, et al. Integrating palliative medicine into the care of persons with advanced dementia: identifying appropriate medication use. J Am Geriatr Soc. 2008;56:1306–1311. doi: 10.1111/j.1532-5415.2008.01741.x. [DOI] [PubMed] [Google Scholar]
- 51.Saliba D, Kington R, Buchanan J, et al. Appropriateness of the decision to transfer nursing facility residents to the hospital. J Am Geriatr Soc. 2000;48:154–163. doi: 10.1111/j.1532-5415.2000.tb03906.x. [DOI] [PubMed] [Google Scholar]
- 52.Bynum JP, Rabins PV, Weller W, et al. The relationship between a dementia diagnosis, chronic illness, medicare expenditures, and hospital use. J Am Geriatr Soc. 2004;52:187–194. doi: 10.1111/j.1532-5415.2004.52054.x. [DOI] [PubMed] [Google Scholar]
- 53.Alzheimer's A. 2016 Alzheimer's disease facts and figures. Alzheimers Dement. 2016;12:459–509. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 54.Sachs GA, Shega JW, Cox-Hayley D. Barriers to excellent end-of-life care for patients with dementia. J Gen Intern Med. 2004;19:1057–1063. doi: 10.1111/j.1525-1497.2004.30329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


