Abstract
Intracellular Cl− homeostasis is regulated by anion-permeable channels and transporters and contributes to excitability of many cell types including smooth muscle and interstitial cells of Cajal (ICC). Our aims were to investigate the effects on electrical activity in mouse jejunal muscle strips, of substituting extracellular Cl− (Cl−o) with the impermeant anions, gluconate and isethionate. On reducing Cl−o, effects were observed on electrical slow waves with small effects on smooth muscle membrane voltage (Em). Restoration of Cl− hyperpolarized smooth muscle Em proportional to the change in Cl−o concentration. Replacement of 90% of Cl−o with gluconate reversibly abolished slow waves in 5/9 preparations. Slow waves were maintained in isethionate. Gluconate and isethionate substitution had similar concentration dependent effects on peak amplitude, frequency, width at half peak amplitude, rise time and decay time of residual slow waves. Gluconate reduced free ionized Ca2+ in Krebs solutions to 0.13 mM. In Krebs solutions containing normal Cl− and 0.13 mM free Ca2+, slow wave frequency was lower, width at half peak amplitude was smaller and decay time was faster. The transient hyperpolarization following restoration of Cl−o was not observed in W/Wv mice, which lack pacemaker ICC in the small intestine. We conclude that in smooth muscle cells, the resting Cl− conductance is low, whereas transmembrane Cl− movement in ICC plays a major role in generation or propagation of slow waves. Furthermore, these data support a role for ICC in setting smooth muscle Em and that altering Cl− homeostasis in ICC can alter smooth muscle Em.
Keywords: Gastrointestinal Motility, Pacemaker Potentials, Chloride transport
INTRODUCTION
It has been established for more than 40 years that Cl− is not passively distributed in gastrointestinal smooth muscle (Casteels, 1971; El-Sharkaway & Daniel, 1975; Aickin & Brading, 1983; Barajas-Lopez et al., 1989) and that there is a complex relationship between the distribution and transport of Cl− and the distribution and transport of other ions including Na+, K+ and Ca2+, as well as responses to changes in pH and osmolality. The full complement of Cl− ion transporters and Cl− selective channels has not been determined for the cell types in gastrointestinal muscularis externa of any species. However, examination of the transcriptional profiles recently published for mouse small intestinal cell types (Chen et al., 2007; Lee et al., 2015; Lee et al., 2017) reveals that RNA for CLCN voltage-gated Cl− channels, two Cl− transporting, Slc4a anion exchangers (AE2 and AE3) and the KCC Slc12a cation coupled Cl− transporters as well as several members of the anoctamin/TMEM16 family of Ca2+ activated Cl− channels are expressed in smooth muscle cells (Lee et al., 2015). Interstitial cells of Cajal (ICC) also express many of these same molecules (Chen et al., 2007; Lee et al., 2015) with selective enrichment of anoctamin 1 (Ano1, TMEM16A), Slc12a2 (NKCC1), Slc12a5 (KCC2), Slc12a7 (KCC4) and Slc4a3 (AE3) in ICC relative to other cells in the muscularis externa (Chen et al., 2007). Furthermore, the expression data from ICC have been confirmed for the Ca2+ activated Cl− channel, Ano1 and the Na+,K+, two Cl− co-transporter (NKCC1) in functional studies and those studies have revealed important roles in pacemaker generation by ICC in gastrointestinal smooth muscle (Wouters et al., 2006; Gomez-Pinilla et al., 2009, Hwang et al., 2009; Singh et al., 2014).
ICC are cells that generate the spontaneous electrical activity or pacemaker potential that drives the electrical ‘slow waves’ (Szurszewski, 1987; Ward et al., 1994; Huizinga et al., 1995), set membrane potential gradients to neighboring smooth muscle cells (Sha et al., 2007), mediate neuromuscular signaling (Ward et al., 2000; Ward et al., 2006) and act as mechanosensors (Strege et al., 2003; Won et al., 2005). ICC express the Kit receptor tyrosine kinase and mutant mice hypomorphic for Kit expression such as the W/Wv mutant lack ICC in the myenteric region of the small intestine and do not have slow wave activity (Maeda et al., 1992; Ward et al., 1994; Huizinga et al., 1995). El-Sharkaway & Daniel (1975) reported evidence that Cl− was involved in the generation of the electrical slow wave prior to the identification of the ICC as the source of what they described as “control potentials”. Subsequent studies have provided evidence that the secondary plateau component of the electrical slow wave is dependent on the opening of Ca2+-activated Cl− channels (Hirst & Edwards, 2001; Kito et al., 2002; Kito & Suzuki, 2003) and this observation was supported by data showing that slow waves are absent in Ano1 knockout mice (Hwang et al., 2009; Singh et al., 2014) and are altered in Ano1 conditional knockout mice (Malysz et al., 2017). Slow waves are also altered in NKCC1 knockout mice as well as following treatment of wild type tissues with the NKCC1 inhibitor bumetanide (Wouters et al., 2006). Functionally, the properties of electrical slow waves alter gastrointestinal smooth muscle contractile patterns in various ways depending on whether the peak of the slow wave depolarization is large enough and the smooth muscle cells are sufficiently excitable to initiate a contractile event. The duration of the slow wave, which is altered by the rise and decay times of the events, contributes to the duration of each contractile event and the frequency of the slow waves determine the patterns of contractility that develop and propagate across each region of the gastrointestinal tract (Sanders et al., 2014). Changes to electrical slow wave activity and ICC network density are associated with several gastrointestinal motility disorders including gastroparesis, chronic unexplained nausea and vomiting, and slow transit constipation (Lyford et al., 2002; Grover et al., 2011; O’Grady et al., 2012; Angeli et al., 2015).
The resting membrane potential in the smooth muscle sets the excitability of the tissue and ICC also set the membrane potential gradients in the smooth muscle, as identified in a series of experiments that found that carbon monoxide generated by heme oxygenase-2 in ICC of the myenteric region of the small intestine was the agent responsible for the gradient (Sha et al., 2007; Farrugia et al., 2003; Bauer & Sanders, 1986). In the small intestine, the outer circular smooth muscle adjacent to the myenteric ICC is more hyperpolarized than the inner circular smooth muscle layer and this has been proposed to represent a physiological rheostat that regulates how far across the circular muscle layer a contraction is initiated in response to each electrical slow wave (Sha et al., 2007). K+ selective ion channels are considered to be the conductances that contribute most significantly to the resting membrane potential of ICC and smooth muscle (Flynn et al., 1999; Farrugia et al., 1993) but the presence of Cl− -selective transporters and ion channels in ICC was not taken into account in these studies and they also have the capacity to contribute to the membrane potentials of ICC and smooth muscle.
Unlike vascular smooth muscle cells (Chipperfield & Harper, 2000), Cl− channel activity in smooth muscle cells does not appear to play a major role in the excitability of gastrointestinal smooth muscle. There are few reports of Cl− channels in gastrointestinal smooth muscle cells and one report that directly states that Cl− currents could not be detected in smooth muscle cells from the mouse small intestine (Tokutomi et al., 1995). In the light of an increased understanding of the activities and the distribution of Cl− channels and transporters in gastrointestinal smooth muscle, we tested the effects of two anionic substitutes for Cl−, gluconate and isethionate, on the electrical activity of mouse small intestine. These ions are not transported by Cl− ion exchangers (Owen & Prastein, 1985; Koncz & Daugirdas, 1994) and permeate poorly through Cl− channels (Greenwood & Large, 1999; Pezier et al., 2010; Reyes et al., 2014). The objectives of these studies were to determine the effects on membrane potential and electrical activity in the small intestinal muscularis externa of substituting extracellular Cl− with the impermeant anions gluconate and isethionate.
METHODS
Ethical Approval
The mice used in this study were maintained and the experiments were performed with approval from the Institutional Animal Care and Use Committee of the Mayo Clinic (IACUC, Rochester, MN, USA) under protocol numbers A00002343-16 and A70813-14. The mice had free access to food and water at all times. Food and water were checked and changed every day. Animals were killed by CO2 inhalation.
Tissue preparation
Adult (4 weeks or older) wild-type mice of either sex (WT, C57BL/6J) and W/Wv mutant mice were used. W/WV mutant mice were obtained from The Jackson Laboratory, Bar Harbor, Maine, USA. Jejunum segments (about 10 mm long) were removed and opened along the mesenteric border in Krebs solution. Muscle strips with intact myenteric plexus were prepared and pinned on Sylgard elastomer (Dow Corning Corp., Midland MI) coated custom made recording chambers (60 mm × 15 mm) with the longitudinal muscle facing upward. Muscles were pinned using fine wire pins (California Fine Wire Company, Grover Beach, CA, USA). Glass capillary microelectrodes (borosilicate glass tube, 1.2 mm OD, 0.6 mm ID, 75 mm length, FHC Inc., Bowdoin, ME, USA) were pulled using a P-97 micropipette puller (Sutter Instruments, Novato, CA, USA). Microelectrodes filled with 3 M KCl had tip resistances ranging between 70 to 90 MΩ. Transmembrane potentials were measured using an Axoclamp 2B amplifier (Axon Instruments/Molecular Devices Corp., Sunnyvale, CA, USA) or Duo 773 amplifier (World Precision Instruments, Sarasota FL) and a Digidata 1440A acquisition system, and stored on a computer running Axoscope 10.0 software (Axon). Signals were recorded at a sampling rate 2 kHz (interval of 500 μs). The bath electrode was dipped in an agarose salt bridge made with 3 M KCl to eliminate any junction potential following a change in the ionic composition. Muscle recordings of W/Wv mutant animals were confirmed by the presence of evoked inhibitory junction potentials (IJPs). Electrical field stimulation was applied using two platinum wires placed perpendicular to the longitudinal axis of the preparation.
Solutions and drugs
The electrophysiological recording chamber was constantly perfused with an oxygenated Krebs solution aerated with 97% O2-3% CO2, and the pH of the solution was maintained at 7.35–7.45 at 37°C (±1°C). The Krebs solution had the following ionic composition (in mM): Na+, 137; K+, 5.9; HCO3−, 15.5; H2PO4−, 1.2; Ca2+, 2.5; Mg2+, 1.2; Cl−, 134; glucose, 11.5. This solution contains a normal concentration of 134 mM extracellular Cl−. Krebs solutions containing low extracellular chloride concentrations i.e., [Cl−]o = 13.3 mM, 39.9 mM and 119.8 mM were prepared by equimolar replacement of NaCl with either the sodium salts of gluconate or isethionate as required. These concentrations were chosen by cutting the levels of Cl− to 90%, then 30% then 10% of normal levels. The Ca2+-adjusted sodium gluconate solution had the same composition as regular Krebs solution except NaCl was omitted and replaced with Na-gluconate (120 mM) and CaCl2 was 12.5 mM; the final [Cl−] was 33.3 mM. In bicarbonate-free Krebs solution, 15.5 mM HEPES was used instead of NaHCO3 and pH adjusted to 7.4 using NaOH (adding additional 7.5 – 10 mM Na+); the bicarbonate free solution was gassed with 100% O2. The extracellular concentration of ionized, free Ca2+ in different Krebs solutions was measured using a Ca2+ ion-selective electrode (Vernier, Beaverton, OR, USA). Solutions containing low [Ca2+]o were prepared by omitting CaCl2 from the Krebs solution and were balanced for chloride with NaCl. Muscles were allowed to equilibrate for 1–2 hrs before experiments were started. Electrical recordings were carried out in the presence of 2–5 μM nicardipine which was dissolved in ethanol at the stock concentration of 10 mM (Sigma-Aldrich, St Louis, MO, USA) to minimize muscle contractility and to attain stable impalements. The membrane potential was also studied from the small intestine of W/WV mutant mice in the presence of tetrodotoxin (TTx; EMD Chemicals, Inc., San Diego, CA, USA) at 0.5 μM concentration to block neurotransmission.
Data Analysis for Electrophysiology
Data were analyzed off-line using Clampfit 10.3.1.5 software (Axon Instruments) or MiniAnalysis version 6 (Decatur, IL, USA) for the following slow wave parameters: (i) the resting membrane potential (RMP, mV), (ii) instantaneous frequency defined as the reciprocal of the inter-event interval (peak to peak) at the time point indicated (Hz), (iii) peak amplitude (mV), (iv) half-width (ms), (v) rise time 10%–90% (ms) and (vi) decay time 90%–10% (ms). All the raw slow wave recordings were low-pass filtered using Butterworth (8-pole) in Clampfit with a cut-off of 60 Hz without altering the shape of the slow wave. In low [Cl−]o Krebs solution, spontaneous electrical activity was accepted for analysis if the slow wave peak amplitude of the events was greater than 3 mV. Hence, some data (in Table 1) in the present study are underrepresented wherein no slow wave was observed and/or the peak amplitude of the events was less than 3 mV.
Table 1.
Effects of low [Cl−]o gluconate Krebs solution on slow wave
| SW properties | Perfusion Period | [Cl−]o: 13.3 mM N=8 |
[Cl−]o: 39.9 mM N=9 |
[Cl−]o: 119.8 mM N=9 |
|---|---|---|---|---|
| Membrane Potential (Em, mV) | Ctl | −59.67 ± 4.96 | −60.49 ± 5.07 | −59.77 ± 5.51 |
| 20–40 s | −63.07 ± 4.58* | −62.19 ± 4.85* | −59.70 ± 5.51 | |
| 4–5 min | −61.97 ± 4.45 | −60.50 ± 6.05 | −60.14 ± 5.40 | |
| Wash 5–10 min | −61.01 ± 4.91 | −59.93 ± 5.23 | −60.38 ± 6.31 | |
| Membrane Potential (ΔEm, mV) | ||||
| 20–40 s | −3.41 ± 1.09* | −1.70 ± 0.78* | −0.07 ± 0.36 | |
| 4–5 min | −2.31 ± 2.94 | −0.01 ± 3.39 | −0.36 ± 1.34 | |
| Wash 5–10 min | −1.35 ± 3.21 | −0.56 ± 3.07 | −0.60 ± 2.39 | |
| Peak Amplitude (mV) | Ctl | 18.83 ± 7.15 | 19.51 ± 7.21 | 19.88 ± 6.43 |
| 20–40 s | 19.52 ± 7.52 | 19.11 ± 6.92 | 19.40 ± 5.90 | |
| 4–5 min | 6.84 ± 7.90* | 11.43 ± 8.82* | 20.36 ± 5.69 | |
| Wash 5–10 min | 21.87 ± 5.70* | 21.31 ± 5.71 | 22.15 ± 5.48* | |
| Half-width (ms)ǂ | Ctl | 679.5 ± 66.5 (N=4) | 709.6 ± 110 (N=8) | 728.7 ± 178 |
| 20–40 s | 572.2 ± 76.5* (N=4) | 632.1 ± 107* (N=8) | 733.2 ± 195 | |
| 4–5 min | 383.0 ± 41.9* (N=4) | 423.5 ± 123* (N=8) | 732.6 ± 174 | |
| Wash 5–10 min | 676.3 ± 45.9 (N=4) | 690.4 ± 122 (N=8) | 704.0 ± 138 | |
| Rise Time 10%–90% (ms)ǂ | Ctl | 107.5 ± 35.3 (N=4) | 137.6 ± 42.5 (N=8) | 140.6 ± 59.4 |
| 20–40 s | 87.44 ± 24.4 (N=4) | 135.1 ± 62.0 (N=8) | 142.4 ± 80.3 | |
| 4–5 min | 216.8 ± 85.2 (N=4) | 297.6 ± 138.4* (N=8) | 153.4 ± 72.7 | |
| Wash 5–10 min | 96.12 ± 17.2 (N=4) | 131.6 ± 59.7 (N=8) | 144.0 ± 57.3 | |
| Decay Time 90%–10% (ms)ǂ | Ctl | 765.0 ± 70.8 (N=4) | 770.5 ± 149 (N=8) | 770.6 ± 162 |
| 20–40 s | 620.5 ± 48.2* (N=4) | 660.8 ± 117* (N=8) | 750.9 ± 148 | |
| 4–5 min | 361.6 ± 108* (N=4) | 456.8 ± 89.7* (N=8) | 740.0 ± 163 | |
| Wash 5–10 min | 764.0 ± 52.5 (N=4) | 744.6 ± 120 (N=8) | 748.8 ± 190 | |
| Instantaneous Frequency (Hz)ǂ | Ctl | 0.57 ± 0.06 (N=4) | 0.56 ± 0.08 (N=8) | 0.54 ± 0.07 |
| 20–40 s | 0.64 ± 0.03 (N=4) | 0.58 ± 0.08 (N=8) | 0.55 ± 0.07 | |
| 4–5 min | 0.40 ± 0.07* (N=4) | 0.41 ± 0.06* (N=8) | 0.54 ± 0.07 | |
| Wash 5–10 min | 0.59 ± 0.05 (N=4) | 0.56 ± 0.06 (N=8) | 0.54 ± 0.06 |
Values are presented as mean ± STDEV; N=number of animals;
P < 0.05 vs. Control (Ctl); ANOVA with Bonferroni post test
data for the SW properties in the above table are underrepresented where in no slow wave was observed (see text in the section Results)
Statistics
Data are expressed as means ± standard deviation (STDEV). Statistical significance was determined by Graphpad Prism using the appropriate statistical tests as indicated in the text. P values of less than 0.05 were considered as a statistically significant difference. The ‘N’ values refer to the number of animals.
RESULTS
Effects of reduction of [Cl−]o on slow waves recorded from smooth muscle cells by substituting with gluconate−
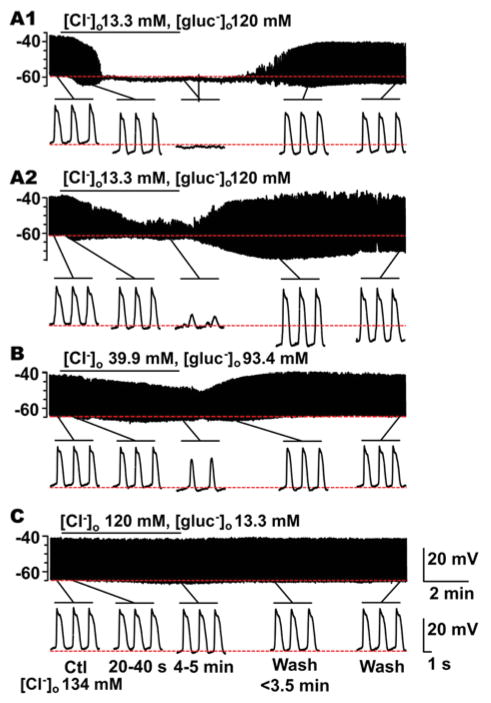
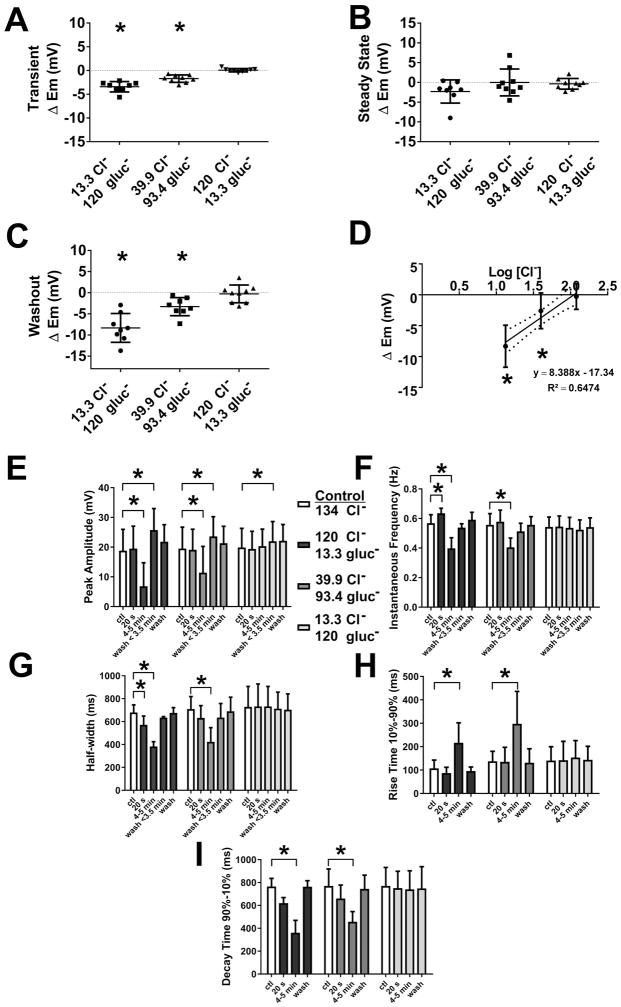
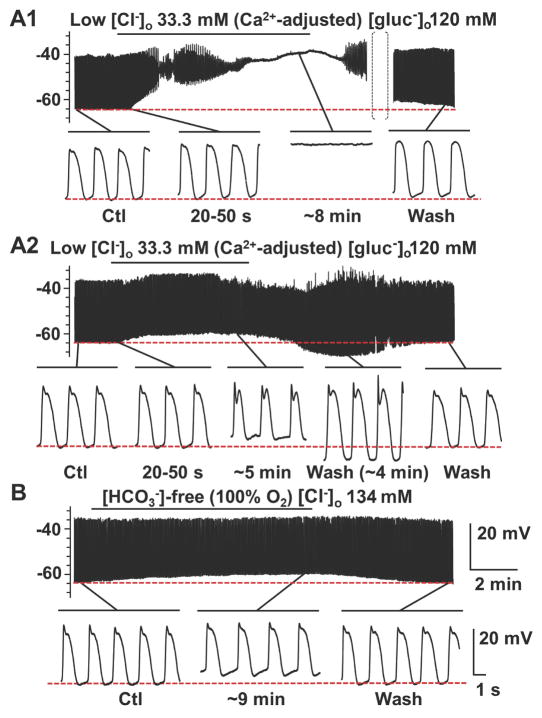
The effects of changing extracellular chloride concentration ([Cl−]o) on the electrical properties of smooth muscle were investigated by replacing 90%, 70%, and 11% of [Cl−]o with the less permeant anion gluconate− resulting in the following [Cl−]o: 13.3 mM, 39.9 mM and 119.8 mM, respectively. Application of gluconate Krebs solution containing 13.3 mM [Cl−]o resulted in a transient hyperpolarization of the membrane potential (Em, mV) in the first 40 s period after the solution change (Fig 1A1, A2 and Fig 2A; Table 1, ΔEm). This effect was concentration dependent as shown in Fig 1B,C and Fig 2A (Table 1, ΔEm). The transient hyperpolarization in response to gluconate Krebs solution containing 13.3 mM [Cl−]o was accompanied by changes to the electrical slow wave. The instantaneous frequency (Hz) of the slow wave was increased by 9.4% (Table 1; Fig 2F); and the events were shorter in duration as indicated by a decrease in half-width (ms) (Table 1; Fig 2G). These transient effects were not sustained on prolonged perfusion of low [Cl−]o with a gluconate Krebs solution. Longer perfusion with low [Cl−]o, gluconate-containing Krebs solution also produced concentration dependent effects on the electrical properties of mouse jejunal circular smooth muscle (Fig 1A1, A2-C). At 13.3 mM [Cl−]o, gluconate-containing Krebs solution, the electrical slow wave was abolished in 4 out of 8 experiments after the solution change (Fig 1A1). In 39.9 mM [Cl−]o slow wave was abolished in 1 out of 9 experiments; and in 119.8 mM [Cl−]o, gluconate-containing Krebs, the slow wave was not abolished in any experiments (N=9) even after up to 11–15 min perfusion. However, the peak amplitude of the electrical slow wave was significantly reduced after 4–5 min perfusion in 39.9 mM [Cl−]o, gluconate-containing Krebs in a manner consistent with the concentration dependence of the slow wave amplitude on [Cl−]o (Fig 2E; Table 1). Complete solution change in our system takes 5 min. The membrane potentials recorded when the electrical properties had reached a steady state after application of low [Cl−]o, gluconate-containing Krebs solution (4–5 min) were not different from the membrane potentials prior to solution change for any Cl− concentration (Fig 2B).
Fig. 1.
The effect of replacing extracellular chloride, [Cl−]o with gluconate on slow wave activity recorded from mouse jejunal smooth muscle layer. Replacement of [Cl−]o by gluconate significantly altered slow wave properties in a concentration dependent manner (see Fig. 2). A1, A2-C, Representative traces of intracellular recording made in [Cl−]o/[gluconate−]o: 13.3/120 mM (A1, A2); 39.9/93.4 mM (B); and 120/13.3 mM (C), respectively. Control [Cl−]o was 134 mM. In 5 out of 9 experiments, slow waves were abolished (A1). In the remaining 4 experiments, small amplitude residual slow wave activity was observed (A2). The horizontal bar over each trace indicates the perfusion period of low [Cl−]o solution. Expanded time scales are shown at the bottom of A1, A2, B, and C with different time points before and after the low [Cl−]o perfusion.
Fig. 2.
Measurement of electrical slow wave properties upon replacing extracellular chloride, [Cl−]o with gluconate. A–H, Summarized data from N=4–9 experiments illustrating the effects of reduced [Cl−]o on electrical properties: A transient (< 1min) concentration-dependent hyperpolarization in membrane potential (Em) was observed in the solution change (A, ΔEm). At steady state (after 4–5 min of perfusion), no change was observed in Em (B, ΔEm). On returning to normal Krebs solution, a transient, concentration-dependent hyperpolarization in Em (C, ΔEm) was observed. (D) shows the concentration dependence on a logarithmic scale. Slow waves that remained at steady state (after 4–5 min of perfusion) were analyzed, concentration-dependent reduction in slow wave amplitude (E); decreases in the instantaneous frequency (F); shortening of slow wave width (G, half-width); slower rise times of 10%–90% of peak amplitude (H); and reduction in the decay times of 90%–10% of peak amplitude (I) were seen (see text for details). These effects were reversible on washout. Values are mean ± STDEV (N=4–9, *P < 0.05, ANOVA with Bonferroni’s correction for multiple comparisons).
In experiments where a slow wave remained (Fig 1A2) after 5 min perfusion with low [Cl−]o, gluconate-containing Krebs solution (N = 4 and 8 with 13.3 and 39.9 mM Cl− respectively), significant, concentration-dependent effects were observed on instantaneous frequency, half width, rise time and decay time of the residual slow wave activity (Fig 2F–I). The frequency of the slow waves was significantly slowed by 29.8 and 26.8% in 13.3 mM and 39.9 mM [Cl−]o, gluconate-containing Krebs respectively (Fig 2F; Table 1). Extracellular Cl− concentrations of 13.3 mM and 39.9 mM, gluconate-containing Krebs also reduced the duration of the slow waves, as indicated by the width at half of the peak amplitude (Fig 2G; Table 1); slowed the rise times between 10 and 90% of peak amplitude (Fig 2G; Table 1); and reduced the decay times from 90 to 10% of peak amplitude (Fig 2I; Table 1).
On returning to normal Krebs solution, a transient hyperpolarization was observed (avg. of < 3.5 min after the solution change) that was dependent on the concentration of Cl− substituted during the treatment (Fig 1A, B and Fig 2C; ΔEm, 13.3 mM [Cl−]o: −8.32 ± 3.41 mV; 39.9 mM [Cl−]o: −2.60 ± 2.89 mV; N=8–9; P < 0.05 vs. baseline; paired t test); and the peak amplitude of the electrical slow wave was significantly increased (Fig 2E; Control: 18.50 ± 7.41 mV; 13.3 mM [Cl−]o: 25.75 ± 7.71 mV; Control: 19.84 ± 7.41 mV; 39.9 mM [Cl−]o: 23.54 ± 6.23 mV; N=8–9; P < 0.05 for both concentration; paired t test). The membrane potential and properties of the slow wave returned to control values after complete washout of the low [Cl−]o gluconate Krebs solution.
Effects of removal of [Cl−]o using isethionate− as an alternative anion substitute
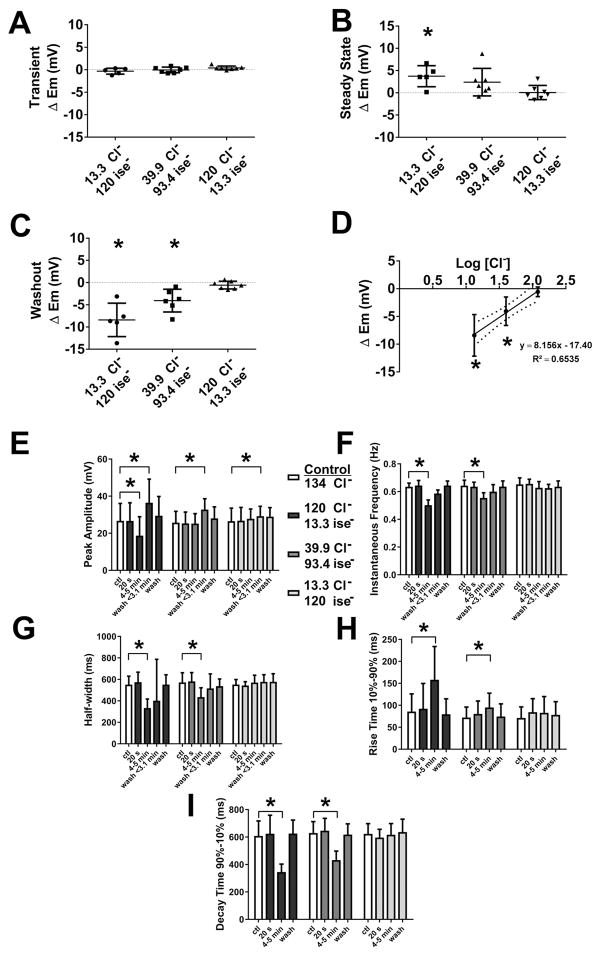
Gluconate is reported to have biological activities that are not related to its function as an anion substitute (Christoffersen & Skibsted, 1975). Therefore, we investigated the effect on electrical activity in mouse jejunal circular smooth muscle of replacing extracellular Cl− with isethionate (Fig 3A–C), a different anion with low permeability through Cl− channels. Replacement of [Cl−]o with isethionate affected both membrane potential and slow wave properties. Application of 13.3 mM [Cl−]o, isethionate Krebs solution did not cause the transient effect on membrane potential in the first 40 s period (Fig 4A) after the solution change that was observed with the gluconate Krebs solution (Fig 2A). However, unlike gluconate, replacement of extracellular Cl− with isethionate did cause a significant depolarization of the membrane potential after the solution change (4–5 min) (Fig 3A and 4B; Table 2, ΔEm). Perfusion with 39.9 mM and 119.8 mM [Cl−]o isethionate Krebs had no significant effect on the membrane potential (Fig 4B; Table 2, ΔEm). The effects of low [Cl−]o isethionate Krebs solution on slow waves were similar to those observed when using gluconate as a substitute except that slow wave activity remained after complete solution exchange in 13.3 mM [Cl−]o, isethionate Krebs in 5 out of 5 experiments. However, the peak amplitude was reduced by an average of 30% following complete solution exchange with 13.3 mM [Cl−]o, isethionate Krebs (Fig 3A and Fig 4E; Table 2). No significant effects on the instantaneous frequency of the slow wave and duration of events were observed following perfusion with the other two extracellular Cl− concentrations of isethionate Krebs (Fig 3B, C, and 4F,G; Table 2). The effects of isethionate substitution on the other slow wave parameters were concentration dependent and resulted in smaller electrical slow waves at a lower frequency (Fig 3A–C). In 13.3 and 39.9 mM [Cl−]o, isethionate Krebs the instantaneous frequency was reduced by 21.9 and 14.1% respectively (Fig 4F; Table 2). Low [Cl−]o, isethionate Krebs also significantly reduced the duration of the slow waves as indicated by the width of the events at half the peak amplitude (Fig 4G; Table 2); slowed the rise times between 10 and 90% of peak amplitude (Fig 4H; Table 2); and decreased the decay times from 90 to 10% of peak amplitude (Fig 4I, Table 2). Upon washout (avg. of < 3.5 min after the solution change) the effects of low [Cl−]o isethionate Krebs solution on slow wave activity were similar to the effects of low [Cl−]o gluconate Krebs in that a transient hyperpolarization in membrane potential (Fig 3A, B and Fig 4C,D; ΔEm, 13.3 mM [Cl−]o: −8.40 ± 3.76 mV; 39.9 mM [Cl−]o: −4.06 ± 2.56 mV; N=5–6; P < 0.05 vs. baseline; paired t test); and the peak amplitude of the electrical slow wave was significantly increased (Fig 4E; Control: 24.92 ± 10.60 mV; 13.3 mM [Cl−]o: 36.49 ± 12.71 mV; Control: 25.17 ± 7.04 mV; 39.9 mM [Cl−]o: 32.76 ± 5.83 mV; N=5–6; P < 0.05 for both concentration; paired t test). The electrical activity fully returned to control values after complete washout of the low [Cl−]o isethionate Krebs solution.
Fig. 3.
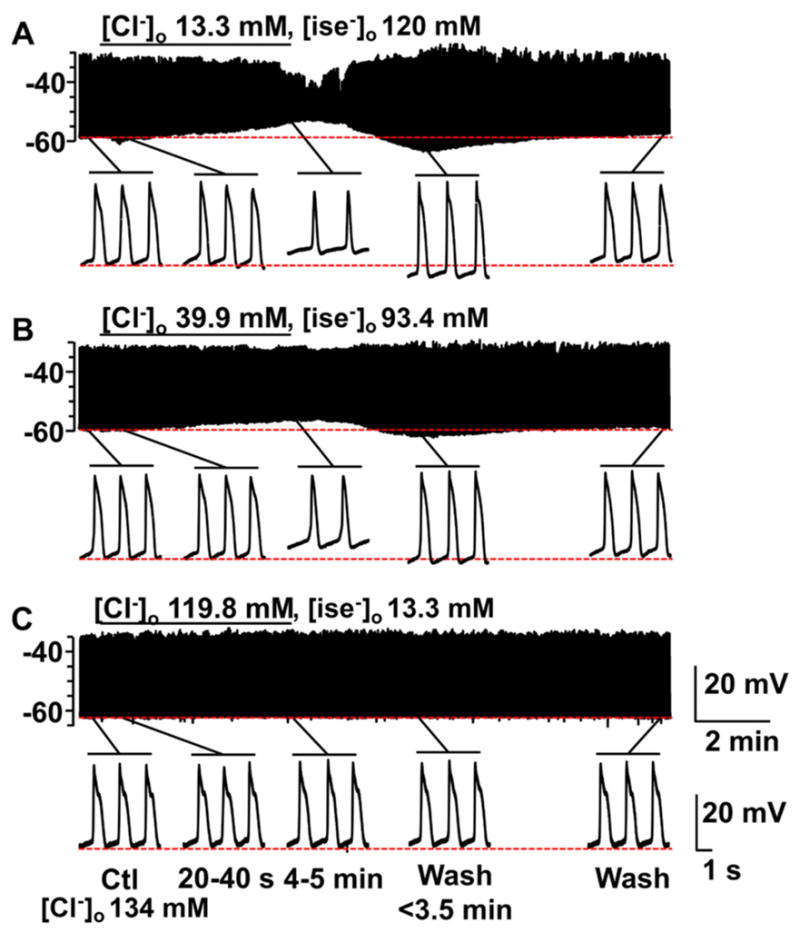
The effect of replacing extracellular chloride, [Cl−]o with isethionate on slow wave activity recorded from mouse jejunal smooth muscle layer. Replacement of [Cl−]o by isethionate had different effects from gluconate on slow wave activity (see Fig. 1). A–C, Representative traces of intracellular recording made in [Cl−]o/[isethionate−]o: 13.3/120 mM (A); 39.9/93.4 mM (B); and 119.8/13.3 mM (C), respectively. Control [Cl−]o was 134 mM. The horizontal bar over each trace indicates the perfusion period of low [Cl−]o solution. Expanded time scales are shown at the bottom of A, B and C with different time points before and after the low [Cl−]o perfusion. A significant concentration dependent alteration in slow wave properties was seen (see Fig. 4).
Fig. 4.
Measurement of electrical slow wave properties upon replacing extracellular chloride, [Cl−]o with isethionate. A–H, Summarized data from N=5–7 experiments illustrating the effects of reduced [Cl−]o on electrical properties: Replacement of [Cl−]o with isethionate had no transient effects in membrane potential, Em (A, ΔEm). At steady state (after 4–5 min of perfusion), a concentration-dependent depolarization in Em was observed (B, ΔEm). Upon returning to normal Krebs solution, a transient, concentration-dependent hyperpolarization in Em (C, ΔEm) was observed. (D) shows the concentration dependence on a logarithmic scale. Slow waves that remained at steady state (after 4–5 min of perfusion) were analyzed, concentration-dependent reduction in slow wave amplitude (E); decreases in the instantaneous frequency (F); shortening of slow wave width (G, half-width); slower rise times at 10%–90% of peak amplitude (H); and reduction in the decay times at 90%–10% of peak amplitude (I) were seen (see text for details). These effects were reversible on washout. Values are mean ± STDEV (N=5–7, *P < 0.05, ANOVA with Bonferroni’s correction for multiple comparisons).
Table 2.
Effects of low [Cl−]o isethionate Krebs solution on slow wave
| SW properties | Perfusion Period | [Cl−]o: 13.3 mM N=5 |
[Cl−]o: 39.9 mM N=7 |
[Cl−]o: 119.8 mM N=7 |
|---|---|---|---|---|
| Membrane Potential (Em, mV) | Ctl | −60.85 ± 3.96 | −63.47 ± 5.46 | −62.82 ± 4.08 |
| 20–40 s | −61.18 ± 4.18 | −63.52 ± 5.63 | −62.43 ± 4.20 | |
| 4–5 min | −57.12 ± 3.42* | −61.08 ± 4.65 | −62.76 ± 3.63 | |
| Wash 5–10 min | −62.75 ± 4.39 | −63.76 ± 4.09 | −63.88 ± 3.65 | |
| Membrane Potential (ΔEm, mV) | ||||
| 20–40 s | −0.33 ± 0.66 | −0.05 ± 0.62 | 0.40 ± 0.42 | |
| 4–5 min | 3.73 ± 2.34* | 2.4 ± 3.10 | 0.06 ± 1.59 | |
| Wash 5–10 min | −1.90 ± 1.32 | −0.29 ± 3.25 | −1.05 ± 1.85 | |
| Peak Amplitude (mV) | Ctl | 26.71 ± 9.38 | 25.72 ± 6.10 | 26.54 ± 7.00 |
| 20–40 s | 26.68 ± 9.73 | 25.31 ± 6.09 | 26.80 ± 7.12 | |
| 4–5 min | 18.70 ± 10.25* | 25.22 ± 5.36 | 27.82 ± 5.27 | |
| Wash 5–10 min | 29.49 ± 10.32 | 28.00 ± 6.21 | 28.97 ± 4.87 | |
| Half-width (ms)ǂ | Ctl | 551.1 ± 78.87 | 572.0 ± 90.33 | 552.3 ± 46.23 |
| 20–40 s | 573.8 ± 93.62 | 581.5 ± 82.20 | 543.2 ± 35.61 | |
| 4–5 min | 333.7 ± 83.04* | 435.3 ± 86.88* | 570.2 ± 68.97 | |
| Wash 5–10 min | 552.1 ± 90.50 | 537.2 ± 66.77 | 578.0 ± 75.19 | |
| Rise Time 10%–90% (ms)ǂ | Ctl | 85.69 ± 40.24 | 72.18 ± 23.81 | 71.10 ± 25.20 |
| 20–40 s | 92.10 ± 30.82 | 80.24 ± 29.47 | 84.30 ± 30.82* | |
| 4–5 min | 158.2 ± 75.68 | 95.21 ± 32.39 | 82.71 ± 37.15 | |
| Wash 5–10 min | 79.66 ± 35.00 | 74.56 ± 28.73 | 78.20 ± 29.97 | |
| Decay Time 90%–10% (ms)ǂ | Ctl | 608.2 ± 109.4 | 629.4 ± 82.86 | 623.3 ± 74.34 |
| 20–40 s | 624.1 ± 135.0 | 646.6 ± 90.00 | 596.3 ± 60.24* | |
| 4–5 min | 345.1 ± 57.91* | 432.6 ± 65.13* | 617.2 ± 81.31 | |
| Wash 5–10 min | 625.8 ± 97.82 | 618.1 ± 78.35 | 636.3 ± 94.00 | |
| Instantaneous Frequency (Hz)ǂ | Ctl | 0.64 ± 0.03 | 0.64 ± 0.04 | 0.65 ± 0.05 |
| 20–40 s | 0.65 ± 0.04 | 0.64 ± 0.03 | 0.66 ± 0.03 | |
| 4–5 min | 0.50 ± 0.04* | 0.55 ± 0.04* | 0.63 ± 0.05 | |
| Wash 5–10 min | 0.64 ± 0.03 | 0.64 ± 0.04 | 0.64 ± 0.04 |
Values are presented as mean ± STDEV; N=number of animals;
P < 0.05 vs. Control (Ctl); ANOVA with Bonferroni post test
Concentration of free Ca2+ in low [Cl−]o gluconate vs. isethionate Krebs solution
It has been reported that free Ca2+ concentrations in solution are lowered by substitution of Cl− with gluconate or isethionate (Neufeld & Wright, 1995). Therefore, we measured the free Ca2+ concentration in Krebs solutions containing gluconate and isethionate at pH 7.4 after equilibration with CO2 using a Ca2+-ion selective electrode. Replacement of NaCl by equimolar Na-gluconate in Krebs solution reduced the free [Ca2+] in a concentration dependent manner with a maximal reduction of approximately 85% in free Ca2+ in normal Krebs (Fig 5; Control, 134 mM Cl−: 0.86 ± 0.20 mM Ca2+; 120.3 mM gluconate, 13.3 mM Cl−: 0.13 ± 0.04 mM Ca2+; 94.1 mM gluconate, 39.9 mM Cl−: 0.17 ± 0.05 mM Ca2+; 14.2 mM gluconate, 119.8 mM Cl−: 0.53 ± 0.18 mM Ca2+; N=5; all P < 0.05; unpaired t test). Isethionate substitution had a lesser effect than gluconate substitution on free Ca2+ levels in solution with a maximal reduction in free Ca2+ of 30% at 120.3 mM Na-isethionate, 13.3 mM NaCl (Fig 5; Control, 134 mM Cl−: 0.86 ± 0.20 mM Ca2+; 120.3 mM isethionate, 13.3 mM Cl−: 0.54 ± 0.12 mM Ca2+; 94.1 mM isethionate, 39.9 mM Cl−: 0.56 ± 0.15 mM Ca2+; 14.2 mM isethionate, 119.8 mM Cl−: 0.57 ± 0.19 mM Ca2+; N=5; all P < 0.05; unpaired t test). Thus, it appears possible that the chelation of free Ca2+ by gluconate could mediate some of the effects of gluconate or isethionate on membrane potential and/or slow wave properties.
Fig. 5.
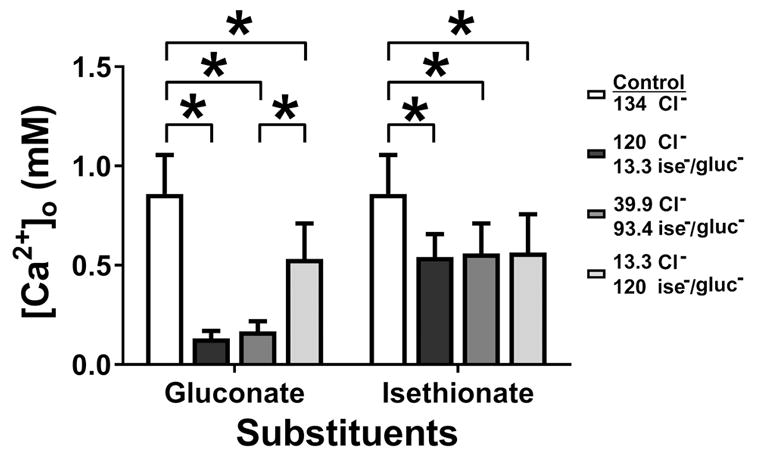
The calcium binding capacity of the sodium salts of gluconate (Na-gluc) and isethionate (Na-ise) in low chloride Krebs solutions as measured using Ca2+ ion-selective electrode. Gluconate and isethionate reduced free Ca2+ in Krebs solution. Histogram data showing a significant decrease in free Ca2+ (see text for details). Values are mean ± SEM (N=5, *P < 0.05, ANOVA with Bonferroni’s correction for multiple comparisons).
Effects of reducing [Ca2+]o
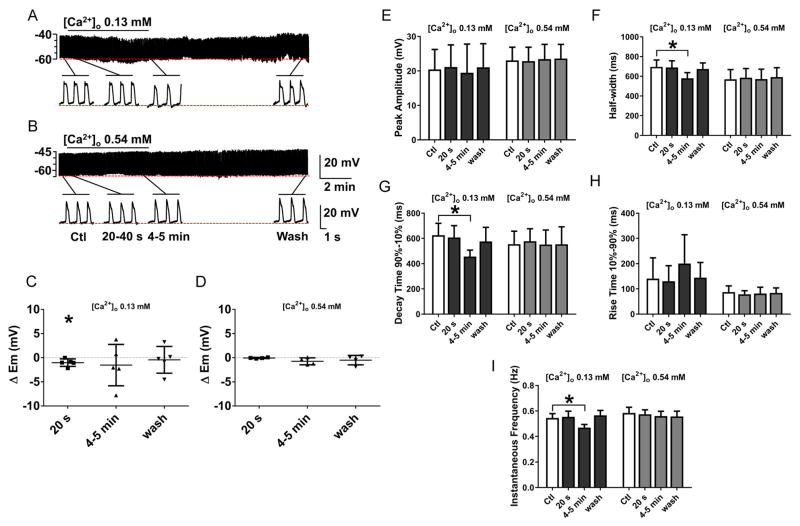
To test the effects of free Ca2+ concentration on electrical activity in mouse jejunal smooth muscle, we reduced the added CaCl2 in the Krebs solution to give free Ca2+ concentrations that were determined using the Ca2+ sensitive electrode to be similar to those measured in gluconate and isethionate Krebs solutions. Lowering the extracellular Ca2+ concentrations to the levels found in 13.3 mM Cl− gluconate (Fig 6A; 0.13 mM Ca2+) or 13.3 mM Cl− isethionate (Fig 6B; 0.54 mM Ca2+) Krebs had no significant effects on membrane potential (Fig 6C, D). Slow wave properties were slightly altered at a free Ca2+ concentration of 0.13 mM, which corresponds to the concentration of Ca2+ in 13.3 mM Cl− gluconate Krebs but not affected at a free Ca2+ concentration of 0.54 mM (Fig 6E–I). In the presence of 0.13mM free Ca2+ Krebs solution, the peak amplitude of the slow wave did not change (Fig 6E; Table 3) but significant changes were observed in the instantaneous frequency, width at half peak amplitude and decay time from 90–10% of the peak of the slow waves. Reducing the extracellular Ca2+ concentration to 0.13 mM decreased the instantaneous frequency by 14.6% (Fig 6A, F; Table 3); decreased the width at half peak amplitude by 16.7% (Fig 6A, G; Table 3); increased the rise time from 10%–90% of peak by 42.3% (Fig 6A, H; Table 3), and decreased the decay time from 90%–10% of peak amplitude by 27.1% (Fig 4A, I; Table 3). These effects on slow wave activity were reversible on washout and not accompanied by any transient hyperpolarization after washout. These results indicate that the effect of lowering [Cl−]o with either gluconate or isethionate Krebs solution are not similar to the effect of lowering [Ca2+]o on the electrical activity of mouse small intestine. Therefore, the predominant effects observed with gluconate and isethionate Krebs solution are not due to calcium chelation.
Fig. 6.
The effect on slow wave activity of reducing [Ca2+]o in Krebs to the level of [Ca2+]o obtained by adding gluconate (A) and isethionate (B) (see Fig. 5). Lowering [Ca2+]o did not replicate the effect of replacing [Cl−]o with gluconate or isethionate on electrical activity in mouse jejunal smooth muscle layer. A–B, Representative traces of intracellular recordings upon perfusion with 0.13 mM and 0.54 mM [Ca2+]o. The horizontal bar over each trace indicates the perfusion period of low [Ca2+]o solution. Expanded time scales are shown below the traces. C–I, Summarized data illustrating the effects of reduced [Ca2+]o on slow wave properties: At 0.13 mM [Ca2+]o, (equivalent to the level found in 13.3 mM Cl−, 120.3 mM gluconate Krebs) decreases in the instantaneous frequency (F), shortening of slow wave width (G) and a decrease in the decay times from 90 to 10% of peak amplitude (I) were observed. These effects were reversible on washout. No change was observed in membrane potential (C) and the rest of the slow wave properties (E, H). No effects were observed at 0.54 mM [Ca2+]o (equivalent to 13.3 mM Cl−, 120.3 mM isethionate Krebs; panel D–I). Values are mean ± SEM (N=4–5, *P < 0.05, ANOVA with Bonferroni’s correction for multiple comparisons).
Table 3.
Effects of low [Ca2+]o Krebs solution on slow wave properties
| SW properties | Perfusion Period | [Ca2+]o: 0.13 mM N=5 | [Ca2+]o: 0.54 mM N=4 |
|---|---|---|---|
| Membrane Potential (Em, mV) | Ctl | −62.89 ± 3.88 | −63.66 ± 2.90 |
| 20–40 s | −63.91 ± 4.03* | −63.71 ± 3.00 | |
| 4–5 min | −64.41 ± 5.47 | −64.39 ± 2.79 | |
| Wash 5–10 min | −63.33 ± 4.78 | −64.16 ± 2.18 | |
| Change in Membrane Potential (ΔEm, mV) | |||
| 20–40 s | −1.02 ± 0.77* | −0.06 ± 0.11 | |
| 4–5 min | −1.52 ± 4.28 | −0.74 ± 0.72 | |
| Wash 5–10 min | −0.43 ± 2.77 | −0.50 ± 0.98 | |
| Peak Amplitude (mV) | Ctl | 20.43 ± 5.81 | 23.06 ± 3.84 |
| 20–40 s | 21.14 ± 6.43 | 22.84 ± 4.04 | |
| 4–5 min | 19.45 ± 8.34 | 23.44 ± 4.28 | |
| Wash 5–10 min | 21.07 ± 6.84 | 23.60 ± 4.11 | |
| Half-width (ms) | Ctl | 695.5 ± 69.96 | 569.5 ± 98.06 |
| 20–40 s | 689.3 ± 67.46 | 585.5 ± 93.70 | |
| 4–5 min | 579.6 ± 57.53* | 571.4 ± 100.7 | |
| Wash 5–10 min | 673.9 ± 61.35 | 592.4 ± 94.75 | |
| Rise Time 10%–90% (ms) | Ctl | 140.6 ± 82.21 | 87.02 ± 24.77 |
| 20–40 s | 130.3 ± 61.37 | 78.42 ± 14.31 | |
| 4–5 min | 200.0 ± 114.7 | 81.38 ± 25.03 | |
| Wash 5–10 min | 144.0 ± 60.47 | 83.92 ± 19.78 | |
| Decay Time 90%–10% (ms) | Ctl | 624.9 ± 95.26 | 553.9 ± 103.8 |
| 20–40 s | 606.8 ± 94.67 | 577.8 ± 99.34 | |
| 4–5 min | 455.8 ± 51.27* | 550.8 ± 116.4 | |
| Wash 5–10 min | 575.6 ± 112.9 | 553.3 ± 139.1 | |
| Instantaneous Frequency (Hz) | Ctl | 0.55 ± 0.03 | 0.58 ± 0.04 |
| 20–40 s | 0.55 ± 0.04 | 0.57 ± 0.04 | |
| 4–5 min | 0.47 ± 0.02* | 0.56 ± 0.04 | |
| Wash 5–10 min | 0.57 ± 0.04 | 0.55 ± 0.04 |
Values are presented as mean ± STDEV; N=number of animals;
P < 0.05 vs. Control (Ctl) or baseline; ANOVA with Bonferroni post test
Effect of reduced [Cl−]o on W/Wv mouse small intestine
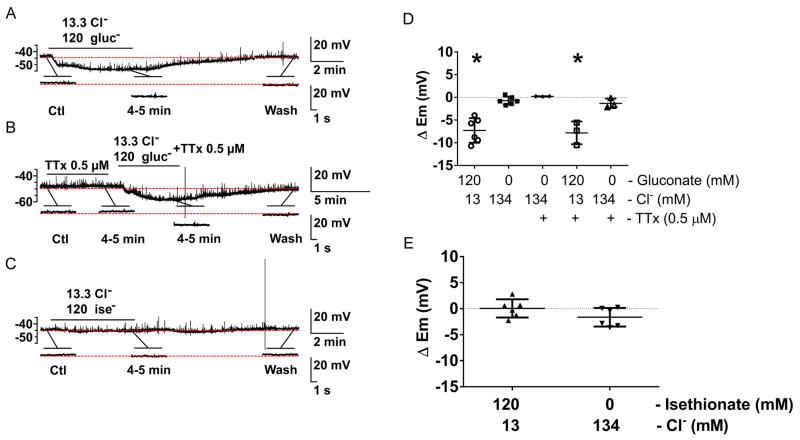
The effects of [Cl−]o on the membrane potential were also studied in smooth muscle of the small intestine of W/WV mutant mice (Fig 7A–C). No slow waves were recorded in any of the six mutant mice, which lack the small intestinal myenteric ICCs (ICC-MY) (Ward et al., 1994). Application of 13.3 mM Cl− gluconate Krebs solution had a rapid hyperpolarizing effect on membrane potential (Em), which was sustained (Fig 7D, ΔEm, 13.3 mM Cl−: −7.27 ± 2.71 mV; N=6; P < 0.05 vs. baseline; paired t test) and was insensitive to tetrodotoxin (0.5 μM) (Fig 7D, ΔEm, 13.3 mM Cl− +TTx: −7.81 ± 2.51 mV; N=3; P < 0.05 vs. baseline + TTx; paired t test) indicating that the hyperpolarization was independent of neuronal activity. The effect of low Cl− gluconate Krebs solution on the membrane potential was reversible upon washout. Low [Cl−]o isethionate Krebs solution had no significant effect on the membrane potential (Fig 7E, ΔEm,13.3 mM Cl−: 0.05 ± 1.75 mV; N=6; P > 0.05 vs. baseline; paired t test) suggesting the gluconate effects were related to reduced extracellular Ca2+. These combined data indicate that reduced extracellular Ca2+, but not reduced extracellular Cl−, causes hyperpolarization of the membrane potential in the W/Wv mouse and support the evidence that extracellular Cl− plays a minor role in setting the membrane potential in mouse jejunal smooth muscle and that ICC play a role in setting the smooth muscle membrane potential.
Fig. 7.
The effect of a 90% reduction in [Cl−]o on the spontaneous electrical activity recorded from the mutant W/Wv, an ICC deficient mouse. Gluconate but not isethionate caused a rapid, tetrodotoxin insensitive, hyperpolarizing effect on membrane potential (Em) in W/Wv mice. A–C, Representative traces with sodium salts of gluconate (A), gluconate in the presence of TTx (B), and isethionate (C). The horizontal bar over each trace indicates the perfusion period of low [Cl−]o solution with and without TTx. Expanded time scales are shown below the traces. D, E, Summarized data illustrating the effect of reduced [Cl−]o on Em. Replacement of Cl− with gluconate (4–5 min) caused a significant hyperpolarization in Em that was reversed on washout and not different after TTx (0.5 μM, D). Isethionate did not cause the same effect (E). Data for individual experiments are shown, whiskers represent the means ± STDEV (N=3–6, *P < 0.05, ANOVA with Bonferroni’s correction for multiple comparisons).
Effect of compensating for the Ca2+ chelating effect of gluconate
In order to overcome the sequestration of free Ca2+ by gluconate, we supplemented the Krebs solutions containing gluconate with sufficient CaCl2 to bring the free Ca2+ concentration up to 1mM as confirmed using the Ca2+-ion selective electrode. The resulting [Cl−]o in this solution was 33.3 mM and the effect of the solution was to cause similar effects to those observed in 33.9 mM Cl−, 94.1 mM isethionate Krebs solution (Fig 8A1,A2). There was no transient hyperpolarization after solution change and after 4–5 min of incubation the membrane potential was significantly depolarized by 5.61 ± 1.5 mV (Table 4; N = 6, P < 0.05; ANOVA with Bonferroni post-test). The peak amplitude of the slow wave was reduced by 12% at this time point and the durations and decay times for the slow waves were significantly reduced by 24% and 26% respectively (Table 4, N = 6, P < 0.05; ANOVA with Bonferroni post-test). In recordings from 1 out of 8 cells, the slow wave was abolished after prolonged incubation (Fig 8A1) and in 3 out 7 tissues it was not possible to hold the recording throughout washout due to development of visible contractile activity accompanied by action potential spiking (see expanded traces Fig 8A2 – wash) in the continued presence of L-type Ca2+ channel blocker. We ascribe the effect to either the ability of high divalent cation concentrations to displace dihydropyridines like nicardipine from L-type Ca2+ channels or diminished block of the channels when the cells hyperpolarize on washout (Lee & Tsien, 1983). The contractile activity did not allow us to reliable measure and quantify the transient hyperpolarization immediately after washout in these experiments. The effects of the high Ca2+, 33.9 mM Cl−, 120.7 mM gluconate Krebs solution further clarify that substitution of Cl−o with both gluconate and isethionate has similar effects when the effects of Ca2+ chelation are taken into account.
Fig. 8.
The effect of low [Cl−]o gluconate Krebs solution corrected for the effects of Ca2+ chelation and the effect of replacement of HCO3− with HEPES. Replacement of [Cl−]o by gluconate after correcting the free Ca2+ concentration to 1mM significantly altered slow wave properties (see Table 4). Representative traces of intracellular recording made in [Cl−]o: 33.3 mM, 119.8 gluconate (A1, A2). In 1 out of 9 experiments, slow waves were abolished (A1). In the remaining 8 experiments, residual slow wave activity was observed (A2). The horizontal bar over each trace indicates the perfusion period of low [Cl−]o solution. Expanded time scales are shown at the bottom of A1, A2. Replacement of HCO3− with HEPES in Krebs solution gassed with 100% O2 resulted in small but significant changes in the slow wave properties and a small depolarization in the membrane potential (see Table 4). The horizontal bar over each trace indicates the perfusion period with HCO3− -free solution. Expanded time scales are shown below.
Table 4.
Effects of low [Cl−]o, Ca2+-supplemented gluconate Krebs solution, and bicarbonate-free Krebs solution on slow wave
| SW Properties | Perfusion Period | Na-gluconate Krebs Free Ca2+ ≈1 mM, N = 7ǂ |
HEPES-buffered Krebs N = 6 |
|---|---|---|---|
| Membrane Potential (Em, mV) | Ctl | −65.11 ± 4.03 | −64.42 ± 2.05 |
| 20–50 s | −64.62 ± 3.62 | - | |
| 4–10 min | −59.50 ± 4.44* | −58.08 ± 3.10* | |
| Wash (5–10 min) | −61.02 ± 2.49 (N=4) | −64.52± 4.00 | |
| Membrane Potential Change (ΔEm, mV) | |||
| 20–50 s | 0.48 ± 1.08 | ||
| 4–10 min | 5.61 ± 1.50* | 6.34 ± 3.23* | |
| Wash (5–10 min) | 2.69 ± 1.37 (N=4) | −0.09 ± 3.66 | |
| Peak Amplitude (mV) | Ctl | 26.49 ± 2.81 | 24.16 ± 3.26 |
| 20–50 s | 26.65 ± 2.74 | - | |
| 4–10 min | 23.39 ± 2.81* | 17.87 ± 5.97* | |
| Wash (5–10 min) | 23.71 ± 2.40 (N=4) | 23.40 ± 6.21 | |
| Half-width duration (ms) | Ctl | 772.30 ± 124.63 | 823.45 ± 88.11 |
| 20–50 s | 812.57 ± 128.21 | ||
| 4–10 min | 593.02 ± 137.72* | 1020.46 ± 248.62 | |
| Wash (5–10 min) | 700.25 ± 202.18 (N=4) | 925.78 ± 160.64 | |
| Rise Time 10–90% (ms) | Ctl | 105.83 ± 18.33 | 132.50 ± 44.48 |
| 20–50 s | 111.68 ± 34.69 | - | |
| 4–10 min | 110.80 ± 33.36 | 275.51 ± 266.71 | |
| Wash (5–10 min) | 126.36 ± 46.29 (N=4) | 168.20 ± 152.65 | |
| Decay Time 100–10% (ms) | Ctl | 911.86 ± 138.49 | 923.54 ± 77.65 |
| 20–50 s | 957.66 ± 135.68 | - | |
| 4–10 min | 724.97 ± 149.25* | 1006.32 ± 96.85 | |
| Wash (5–10 min) | 901.06 ± 208.70 (N=4) | 1026.53 ± 143.98 | |
| Instantaneous Frequency (Hz) | Ctl | 0.55 ± 0.04 | 0.53 ± 0.05 |
| 20–50 s | 0.55 ± 0.06 | - | |
| 4–10 min | 0.54 ± 0.06 | 0.43 ± 0.06* | |
| Wash (5–10 min) | 0.59 ± 0.10 (N=4) | 0.49 ± 0.08 |
Values are presented as mean ± STDEV; N=number of animals;
P < 0.05 vs. Control (Ctl); ANOVA with Bonferroni post test
data for washout are underrepresented due to increase in contractile activity after of gluconate.
Effect of removing HCO3− from the extracellular solution
Cl− transport is coupled to HCO3− and could change intracellular pH via the anion exchangers, Slc4a2/AE2 (in smooth muscle) and Slc4a3/AE3 (in ICC and smooth muscle) (Alper, 2009) and thereby alter slow wave activity and membrane potential. Therefore we investigated the effect of removing HCO3−, using HEPES to buffer the extracellular solutions and gassing the solutions with 100% O2. A significant, reversible slow depolarization of membrane potential was observed in HCO3− -free solutions that was accompanied by a 26% decrease in the amplitude and a 20% decrease in the frequency of the electrical slow wave (Fig 8B, Table 4). However no change was detected in the rise times, decay times or width of the slow wave events. These data indicate that changes in intracellular pH may play a role in the setting of the smooth muscle membrane potential and peak amplitude of the electrical slow wave but that Cl− movement, independent of HCO3− ions, determines the duration of the plateau component of the electrical slow wave.
DISCUSSION
In this study, we observed that substituting Cl− with less permeant anions had significant concentration-dependent effects on the smooth muscle electrical slow wave activity. Effects on smooth muscle membrane potential in mouse jejunal smooth muscle were small in magnitude and were consistent when using different anion substitutes. These observations indicate that the resting Cl− conductance in smooth muscle cells is small, whereas Cl− movement plays a major role in the generation or propagation of the electrical slow wave. Restoration of normal extracellular Cl− levels was associated with a robust, reproducible smooth muscle hyperpolarization, which was not observed in W/Wv mice when taking into account extracellular Ca2+, which represents further direct evidence for the role of ICC in setting the smooth muscle membrane potential and the importance of Cl− homeostasis in ICC function. Tomita and colleagues observed similar effects in recordings from the muscle layers of the guinea pig gastric antrum (Tomita & Hata, 2000; Tomita et al., 2000) confirming the presence of conserved mechanisms by which Cl− contributes to gastrointestinal electrical activity between species and from the stomach to the small intestine. Although not fully understood, more recent studies on specific Cl− channels and transporters have identified some of these conserved mechanisms and our data are consistent with a complex interaction between Cl− homeostasis and electrical activity in gastrointestinal smooth muscle. The significance of these observations is in the demonstration that changes in Cl− channel activity, or in the electrochemical equilibrium of Cl− across the plasma membrane of ICC, will significantly alter slow wave duration and the size and pattern of contractile responses in smooth muscle cells as well as the membrane potential and excitability of small intestinal smooth muscle. Previous studies have shown that splice variants of the Ca2+ activated Cl− channel, Ano1, that alter the Cl− currents generated by this gene, are differentially expressed in the gastric muscle layers of patients with diabetic gastroparesis (Mazzone et al., 2011). Physiological and pathological regulation of expression of NKCC1 and KCC2, Cl− transporters in dorsal root and central neurons (Fiumelli & Woodin, 2007) alter the excitability of these neurons and therefore slow wave activity in ICC may be under similar regulatory control via these molecules. These observations indicate that changes to the activity and/or expression of Cl− channels and transporters in ICC can alter motility patterns in health and disease as well as represent a therapeutic opportunity.
We focused on ionic substitution and did not test pharmacological regulators of Cl− transport in this study due to the poor selectivity and low potency of the currently available compounds. All studies were done in either 97% O2 or 100% O2, which while hyperoxic, is typical for studies on gastrointestinal smooth muscle preparations.
Adjustment of the homeostatic set-points for Cl− distribution by Cl− transport in the ICC are the likely explanation for the robust and reproducible concentration dependent hyperpolarization in wild type but not W/Wv mice following washout of either gluconate or isethionate. The magnitude of these effects and the slope of the relationship between changes in membrane voltage and changes in extracellular Cl− concentration were also similar for the two substitutes. Aickin (Aickin & Brading, 1990) reported robust Cl− transport in taenia from the guinea pig caecum on restoration of normal extracellular Cl− concentrations following depletion by glucuronate and gluconate and that Cl− transport in this tissue was mediated by bumetanide-sensitive NKCC transport as well as DIDS-sensitive HCO3−/Cl− anion exchange. It is known that ICC regulate smooth muscle membrane potential and contribute to the membrane potential gradient across the circular smooth muscle (Liu & Huizinga, 1993; Sha et al., 2007). Thus we can predict that after equilibration in low extracellular Cl−, intracellular Cl− will become depleted, then following the return to physiological, extracellular Cl− concentrations, ICC become hyperpolarized until the activity of NKCC1 and possibly other Cl− transporters returns the trans-membrane Cl− distribution and membrane potential to the normal set point. During the period of re-equilibration, the sizes of the electrical slow waves are larger and the frequency and width at half peak amplitudes were reduced for both gluconate and isethionate substitution but the membrane potential at the peak of slow wave does not exceed the level observed in the control period. There was no change in the rise or decay times of the slow wave events. These observations further support the conclusion that the dominant role of Cl− channels in mouse intestinal smooth muscle are to regulate the secondary, plateau component of the electrical slow waves with smaller contributions to the initiation and primary rise to peak of the events. Cl− transport by NKCC1 and possibly HCO3−/Cl− anion exchange sets the Cl− concentration at levels where channel activity is effective in contributing to the normal slow wave activity. However, even though HCO3−/Cl− anion exchangers are expressed on both ICC and smooth muscle cells, the effects of HCO3− substitution was modestand limited to effects on membrane potential (thus implying that electrogenic anion exchangers such as the Na+/HCO3− transporters are functional in these cells) and slow wave frequency, consistent with a role for intracellular pH in these electrical properties and confirming that Cl− movement, independent of intracellular pH, appears to determine the duration of plateau component of the electrical slow wave.
When using either gluconate or isethionate as Cl− substitutes, consistent concentration dependent effects were observed on the electrical slow wave, which were reversible on washout. The amplitudes, frequencies and widths at half peak amplitude of the slow waves were reduced with substitution by gluconate and isethionate, with gluconate having a slightly larger effect than the same concentration of isethionate. From a physiological perspective, these observations indicate that normal Cl− concentration gradients are required to generate, large, sustained depolarization of intestinal smooth muscle cells that can result in organized contractile patterns and normal gastrointestinal motility.
Our observations that substitution of Cl− with gluconate and isethionate have differing and modest effects on smooth muscle membrane potential prior to washout are consistent with previous reports (El-Sharkaway & Daniel, 1975; Tomita & Hata, 2000; Tomita et al., 2000) but are not what might be predicted for a simple system, since reduced extracellular Cl− should cause a depolarization when there is significant permeability to Cl− in the cells under study, due to ECl becoming more positive when the extracellular Cl− concentration is lowered. For gastrointestinal smooth muscle cells, intracellular Cl− is generally accepted as being in the range determined for guinea pig taenia coli at 60–73 mM with ECl at around −40 mV (Casteels, 1971; Aickin & Brading, 1983), these values indicate that intracellular Cl− is not in Donnan equilibrium with the resting membrane potential and dependent on the active transport of Cl−. In wild type mice, the electrical activity recorded from smooth muscle is recorded from a relatively intact system and is the consequence of regulation of ionic permeability, transport and channel activity in ICC, smooth muscle cells and neurons. Therefore complex responses might be expected. However in recordings from W/Wv mice, following incubation with tetrodotoxin to block neuronal activity, the signal is derived from only the smooth muscle cells, as myenteric ICCs are absent from the W/Wv mice (Maeda et al., 1992; Ward et al., 1994; Huizinga et al., 1995). In the preparations from the small intestine of W/Wv mice, isethionate had no effect and gluconate caused a small hyperpolarization in membrane potential. Since gluconate but not isethionate reduced free Ca2+ concentrations in bicarbonate Krebs, it is likely that this small hyperpolarization was due to an unknown effect of the reduced extracellular Ca2+. Thus Cl− distribution appears to have little intrinsic effect on the excitability of mouse small intestinal smooth cells at the resting membrane potential, which is consistent with a dearth of reports of Cl− currents in isolated gastrointestinal smooth muscle and in contrast with studies on vascular smooth muscle where Cl− channels have been identified and where altering Cl− channel activity directly alters vascular smooth muscle excitability and tone (Greenwood, 1999; Chipperfield & Harper, 2000).
The effect of high gluconate concentrations on free Ca2+ in solution is well established and is due to the incomplete ionization of Ca-gluconate and the comparatively high solubility of the un-ionized salt at pH 7.4 in bicarbonate buffered Krebs solution (Skibsted & Kilde, 1972). Thus, a small part of the decrease in the frequency and the half width of the slow wave in gluconate containing Krebs solutions can be accounted for by the reduction of free extracellular Ca2+ concentrations to 0.14 mM as was previously reported for slow waves in the canine gastric antrum (Ward et al., 2004). The effect of Ca2+ associating with gluconate can be overcome by proportionately raising the total CaCl2 added to the solution (Tomita et al., 2000) as we did here, if it is necessary to use gluconate instead of isethionate, the more permeant, but less avid chelator of Ca2+, as a substitute for Cl−. Our studies clearly identify the impact of not only gluconate and isethionate but also bicarbonate on the free Ca2+ in physiological saline and the utility of obtaining direct, accurate measurements of the available Ca2+ in these solutions.
The larger effects of Cl− substitution in wild type mouse small intestine on electrical slow waves are likely due to alterations in Cl− exchange and distribution in the myenteric ICC that are coupled to the smooth muscle cells. Reductions in frequency, amplitude and the half width of the slow wave at steady state following replacement of Cl− are consistent with the important role in the generation of the electrical slow wave of a Ca2+-activated Cl− channel, Ano1, which is expressed on ICC (Gomez-Pinilla et al., 2009; Hwang et al., 2009). Activation of a Cl− conductance has long been linked to the basic mechanism of the electrical slow wave in that replacement of NaCl with either sucrose or Na-isethionate reduced the frequency or abolished the electrical activity in the rabbit small intestine and the dog colon (El-Sharkaway & Daniel, 1975; Barajas-Lopez et al., 1989). There is some disagreement as to the mechanism for this effect. Shortening of the half width of the slow wave indicates that a Cl− conductance is important for the secondary, plateau component of the slow wave, an effect that was previously reported in rabbit jejunum smooth muscle (El-Sharkaway & Daniel, 1975), guinea pig gastric antrum (Kito et al., 2002), myenteric ICC from the mouse small intestine (Kito & Suzuki, 2003) and myenteric ICC from the rabbit small intestine (Kito et al., 2014). However, constitutive knockout of Ano1 leads to complete loss of the electrical slow wave in mouse small intestine (Hwang et al., 2009; Singh et al., 2014) and it has been proposed that Ano1 contributes to the upstroke of the pacemaker potential as well as the plateau (Blair et al., 2014). We observed a decrease in the peak amplitude and a reduced frequency of the slow waves when Cl− is replaced, consistent with this, but others have found that shortening of the slow wave and a reduction in the plateau component can be separated from the effects on the initial peak (Tomita et al., 2000, Kito et al, 2014; Kito & Suzuki, 2003). In the study by Tomita et al. (2000) on guinea pig gastric antrum, replacement of Cl− shortened the width of the slow wave, but did not result in a reduced amplitude or reduced frequency of the electric slow wave when extracellular K+ was removed from the solutions. This implicated a role for NKCC transport in the effects of Cl− substitution on slow wave frequency, which is mediated by NKCC1 in ICC and was demonstrated in studies that showed that the NKCC inhibitor, bumetanide reduces slow wave frequency and amplitude (Wouters et al., 2006).
Overall, our study is a systematic examination of the concentration dependent effects of Cl− substitution on the electrical slow wave activity in mouse jejunal smooth muscle. We conclude that the identification of the roles of each of the transporters and ion channels that regulate Cl− homeostasis should be pursued so that the activity of each component can be integrated with the activities of other ion transporters to gain a better understanding of how Cl− homeostasis alters motility of gastrointestinal smooth muscle. From our observations, we can exclude a major role for Cl− transport in the smooth muscle cells, but Cl− is clearly important and tightly regulated in ICC to modulate electrical activity of the mouse small intestine.
NEW FINDINGS.
What is the central question of this study?
The central question was to investigate the roles of extracellular chloride in electrical slow waves and resting membrane potential of mouse jejunal smooth muscle by substituting chloride, with the impermeant anions, gluconate and isethionate.
What is the main finding and its importance?
The main finding was that in smooth muscle cells, the resting Cl− conductance is low, whereas transmembrane Cl− movement in interstitial cells of Cajal (ICC) is a major contributor to the shape of electrical slow waves. Furthermore, the data confirm that ICC set the smooth muscle membrane potential and that altering Cl− homeostasis in ICC can alter the smooth muscle membrane potential.
Acknowledgments
FUNDING
The work was supported by NIH RO1, DK57061.
We thank Mr. Seth Eisenman, Gary Stoltz and Mrs. Kristy Zodrow for their assistance.
Footnotes
COMPETING INTERESTS
The authors declare that they have no competing interests.
- The experiments were performed in the laboratory of Dr Gianrico Farrugia and the Mayo Clinic, Rochester, MN USA.
- Conception or design of the work: SSA, SJG, JM, JHS and GF
- Acquisition, analysis, or interpretation of data for the work: SSA, SJG, JM, SL, DRL, JHS, GF
- Drafting the work or revising it critically for important intellectual content: All Authors
- All authors approved the final version of the manuscript
- All of the authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
- All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
References
- Aickin CC, Brading AF. Towards an estimate of chloride permeability in the smooth muscle of guinea-pig vas deferens. J Physiol. 1983;336:179–197. doi: 10.1113/jphysiol.1983.sp014575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin CC, Brading AF. The effect of loop diuretics on Cl− transport in smooth muscle of the guinea-pig vas deferens and taenia from the caecum. J Physiol. 1990;421:33–53. doi: 10.1113/jphysiol.1990.sp017932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper SL. Molecular physiology and genetics of Na+-independent SLC4 anion exchangers. J Exp Biol. 2009;212:1672–1683. doi: 10.1242/jeb.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeli TR, Cheng LK, Du P, Wang TH, Bernard CE, Vannucchi MG, Faussone-Pellegrini MS, Lahr C, Vather R, Windsor JA, Farrugia G, Abell TL, O’Grady G. Loss of Interstitial Cells of Cajal and Patterns of Gastric Dysrhythmia in Patients With Chronic Unexplained Nausea and Vomiting. Gastroenterology. 2015;149:56–66. e55. doi: 10.1053/j.gastro.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barajas-Lopez C, Den Hertog A, Huizinga JD. Ionic basis of pacemaker generation in dog colonic smooth muscle. J Physiol. 1989;416:385–402. doi: 10.1113/jphysiol.1989.sp017767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer AJ, Sanders KM. Passive and active membrane properties of canine gastric antral circular muscles. Am J Physiol. 1986;251:C268–273. doi: 10.1152/ajpcell.1986.251.2.C268. [DOI] [PubMed] [Google Scholar]
- Blair PJ, Rhee PL, Sanders KM, Ward SM. The significance of interstitial cells in neurogastroenterology. J Neurogastroenterol Motil. 2014;20:294–317. doi: 10.5056/jnm14060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R. The distribution of chloride ions in the smooth muscle cells of the guinea-pig’s taenia coli. J Physiol. 1971;214:225–243. doi: 10.1113/jphysiol.1971.sp009429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Ordog T, Chen J, Young DL, Bardsley MR, Redelman D, Ward SM, Sanders KM. Differential gene expression in functional classes of interstitial cells of Cajal in murine small intestine. Physiol Genomics. 2007;31:492–509. doi: 10.1152/physiolgenomics.00113.2007. [DOI] [PubMed] [Google Scholar]
- Chipperfield AR, Harper AA. Chloride in smooth muscle. Prog Biophys Mol Biol. 2000;74:175–221. doi: 10.1016/s0079-6107(00)00024-9. [DOI] [PubMed] [Google Scholar]
- Christoffersen CR, Skibsted LH. Calcium ion activity in physiological salt solutions: influence of anions substituted for chloride. Comp Biochem Physiol A Comp Physiol. 1975;52:317–322. doi: 10.1016/s0300-9629(75)80094-6. [DOI] [PubMed] [Google Scholar]
- El-Sharkaway TY, Daniel EE. Ionic mechanisms of intestinal electrical control activity. Am J Physiol. 1975;229:1287–1298. doi: 10.1152/ajplegacy.1975.229.5.1287. [DOI] [PubMed] [Google Scholar]
- Farrugia G, Irons WA, Rae JL, Sarr MG, Szurszewski JH. Activation of whole cell currents in isolated human jejunal circular smooth muscle cells by carbon monoxide. Am J Physiol. 1993;264:G1184–1189. doi: 10.1152/ajpgi.1993.264.6.G1184. [DOI] [PubMed] [Google Scholar]
- Farrugia G, Lei S, Lin X, Miller SM, Nath KA, Ferris CD, Levitt M, Szurszewski JH. A major role for carbon monoxide as an endogenous hyperpolarizing factor in the gastrointestinal tract. Proc Natl Acad Sci U S A. 2003;100:8567–8570. doi: 10.1073/pnas.1431233100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiumelli H, Woodin MA. Role of activity-dependent regulation of neuronal chloride homeostasis in development. Curr Opin Neurobiol. 2007;17:81–86. doi: 10.1016/j.conb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Flynn ER, McManus CA, Bradley KK, Koh SD, Hegarty TM, Horowitz B, Sanders KM. Inward rectifier potassium conductance regulates membrane potential of canine colonic smooth muscle. J Physiol. 1999;518:247–256. doi: 10.1111/j.1469-7793.1999.0247r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR, Lorincz A, Pozo MJ, Pasricha PJ, Van de Rijn M, West RB, Sarr MG, Kendrick ML, Cima RR, Dozois EJ, Larson DW, Ordog T, Farrugia G. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1370–1381. doi: 10.1152/ajpgi.00074.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood IA, Large WA. Modulation of the decay of Ca2+-activated Cl− currents in rabbit portal vein smooth muscle cells by external anions. J Physiol. 1999;516(Pt 2):365–376. doi: 10.1111/j.1469-7793.1999.0365v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood IA, Large WA. Properties and role of chloride channels in smooth muscle. In: Kozlowski R, editor. Chloride Channels. ISIS Medical Media Ltd; Oxford: 1999. pp. 121–137. [Google Scholar]
- Grover M, Farrugia G, Lurken MS, Bernard CE, Faussone-Pellegrini MS, Smyrk TC, Parkman HP, Abell TL, Snape WJ, Hasler WL, Unalp-Arida A, Nguyen L, Koch KL, Calles J, Lee L, Tonascia J, Hamilton FA, Pasricha PJ Consortium NGCR. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology. 2011;140:1575–1585. e1578. doi: 10.1053/j.gastro.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GD, Edwards FR. Generation of slow waves in the antral region of guinea-pig stomach--a stochastic process. J Physiol. 2001;535:165–180. doi: 10.1111/j.1469-7793.2001.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Blair PJ, Britton FC, O’Driscoll KE, Hennig G, Bayguinov YR, Rock JR, Harfe BD, Sanders KM, Ward SM. Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol. 2009;587:4887–4904. doi: 10.1113/jphysiol.2009.176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kito Y, Fukuta H, Suzuki H. Components of pacemaker potentials recorded from the guinea pig stomach antrum. Pflugers Arch. 2002;445:202–217. doi: 10.1007/s00424-002-0884-z. [DOI] [PubMed] [Google Scholar]
- Kito Y, Kurahashi M, Mitsui R, Ward SM, Sanders KM. Spontaneous transient hyperpolarizations in the rabbit small intestine. J Physiol. 2014;592:4733–4745. doi: 10.1113/jphysiol.2014.276337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kito Y, Suzuki H. Properties of pacemaker potentials recorded from myenteric interstitial cells of Cajal distributed in the mouse small intestine. J Physiol. 2003;553:803–818. doi: 10.1113/jphysiol.2003.051334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koncz C, Daugirdas JT. Use of MQAE for measurement of intracellular [Cl-] in cultured aortic smooth muscle cells. Am J Physiol. 1994;267:H2114–2123. doi: 10.1152/ajpheart.1994.267.6.H2114. [DOI] [PubMed] [Google Scholar]
- Lee KS, Tsien RW. Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature. 1983;302:790–794. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- Lee MY, Ha SE, Park C, Park PJ, Fuchs R, Wei L, Jorgensen BG, Redelman D, Ward SM, Sanders KM, Ro S. Transcriptome of interstitial cells of Cajal reveals unique and selective gene signatures. PLoS One. 2017;12:e0176031. doi: 10.1371/journal.pone.0176031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MY, Park C, Berent RM, Park PJ, Fuchs R, Syn H, Chin A, Townsend J, Benson CC, Redelman D, Shen TW, Park JK, Miano JM, Sanders KM, Ro S. Smooth Muscle Cell Genome Browser: Enabling the Identification of Novel Serum Response Factor Target Genes. PLoS One. 2015;10:e0133751. doi: 10.1371/journal.pone.0133751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LW, Huizinga JD. Electrical coupling of circular muscle to longitudinal muscle and interstitial cells of Cajal in canine colon. J Physiol. 1993;470:445–461. doi: 10.1113/jphysiol.1993.sp019868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyford GL, He CL, Soffer E, Hull TL, Strong SA, Senagore AJ, Burgart LJ, Young-Fadok T, Szurszewski JH, Farrugia G. Pan-colonic decrease in interstitial cells of Cajal in patients with slow transit constipation. Gut. 2002;51:496–501. doi: 10.1136/gut.51.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H, Yamagata A, Nishikawa S, Yoshinaga K, Kobayashi S, Nishi K. Requirement of c-kit for development of intestinal pacemaker system. Development. 1992;116:369–375. doi: 10.1242/dev.116.2.369. [DOI] [PubMed] [Google Scholar]
- Malysz J, Gibbons SJ, Saravanaperumal SA, Du P, Eisenman ST, Cao C, Oh U, Saur D, Klein S, Ordog T, Farrugia G. Conditional genetic deletion of Ano1 in interstitial cells of Cajal impairs Ca2+ transients and slow waves in adult mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2017;312:G228–G245. doi: 10.1152/ajpgi.00363.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone A, Bernard CE, Strege PR, Beyder A, Galietta LJ, Pasricha PJ, Rae JL, Parkman HP, Linden DR, Szurszewski JH, Ordog T, Gibbons SJ, Farrugia G. Altered expression of Ano1 variants in human diabetic gastroparesis. J Biol Chem. 2011;286:13393–13403. doi: 10.1074/jbc.M110.196089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld DS, Wright SH. Basolateral transport of taurine in epithelial cells of isolated, perfused Mytilus californianus gills. J Exp Biol. 1995;198:465–473. doi: 10.1242/jeb.198.2.465. [DOI] [PubMed] [Google Scholar]
- O’Grady G, Angeli TR, Du P, Lahr C, Lammers W, Windsor JA, Abell TL, Farrugia G, Pullan AJ, Cheng LK. Abnormal initiation and conduction of slow-wave activity in gastroparesis, defined by high-resolution electrical mapping. Gastroenterology. 2012;143:589–598. e583. doi: 10.1053/j.gastro.2012.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen NE, Prastein ML. Na/K/Cl cotransport in cultured human fibroblasts. J Biol Chem. 1985;260:1445–1451. [PubMed] [Google Scholar]
- Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592–1622. doi: 10.1053/j.gastro.2004.09.055. [DOI] [PubMed] [Google Scholar]
- Pezier A, Grauso M, Acquistapace A, Monsempes C, Rospars JP, Lucas P. Calcium activates a chloride conductance likely involved in olfactory receptor neuron repolarization in the moth Spodoptera littoralis. J Neurosci. 2010;30:6323–6333. doi: 10.1523/JNEUROSCI.0261-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes JP, Lopez-Rodriguez A, Espino-Saldana AE, Huanosta-Gutierrez A, Miledi R, Martinez-Torres A. Anion permeation in calcium-activated chloride channels formed by TMEM16A from Xenopus tropicalis. Pflugers Arch. 2014;466:1769–1777. doi: 10.1007/s00424-013-1415-9. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Ward SM, Koh SD. Interstitial cells: regulators of smooth muscle function. 2014;94:859–907. doi: 10.1152/physrev.00037.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha L, Farrugia G, Harmsen WS, Szurszewski JH. Membrane potential gradient is carbon monoxide-dependent in mouse and human small intestine. Am J Physiol Gastrointest Liver Physiol. 2007;293:G438–445. doi: 10.1152/ajpgi.00037.2007. [DOI] [PubMed] [Google Scholar]
- Singh RD, Gibbons SJ, Saravanaperumal SA, Du P, Hennig GW, Eisenman ST, Mazzone A, Hayashi Y, Cao C, Stoltz GJ, Ordog T, Rock JR, Harfe BD, Szurszewski JH, Farrugia G. Ano1, a Ca2+-activated Cl− channel, coordinates contractility in mouse intestine by Ca2+ transient coordination between interstitial cells of Cajal. J Physiol. 2014;592:4051–4068. doi: 10.1113/jphysiol.2014.277152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibsted LH, Kilde G. Dissociation constant of calcium gluconate. Calculations from hydrogen ion and calcium ion activities. Dan Tidsskr Farm. 1972;46:41–46. [PubMed] [Google Scholar]
- Strege PR, Ou Y, Sha L, Rich A, Gibbons SJ, Szurszewski JH, Sarr MG, Farrugia G. Sodium current in human intestinal interstitial cells of Cajal. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1111–1121. doi: 10.1152/ajpgi.00152.2003. [DOI] [PubMed] [Google Scholar]
- Szurszewski JH. Electrophysiological basis of gastrointestinal motility. In: Johnson LR, Christensen J, Jackson MJ, Jacobson ED, Walsh JH, editors. Physiology of the Gastrointestinal Tract. Raven; New York: 1987. pp. 383–422. [Google Scholar]
- Tokutomi N, Maeda H, Tokutomi Y, Sato D, Sugita M, Nishikawa S, Nakao J, Imamura T, Nishi K. Rhythmic Cl− current and physiological roles of the intestinal c-kit-positive cells. Pflugers Arch. 1995;431:169–177. doi: 10.1007/BF00410188. [DOI] [PubMed] [Google Scholar]
- Tomita T, Hata T. Effects of removal of Na(+) and Cl(-) on spontaneous electrical activity, slow wave, in the circular muscle of the guinea-pig gastric antrum. Jpn J Physiol. 2000;50:469–477. doi: 10.2170/jjphysiol.50.469. [DOI] [PubMed] [Google Scholar]
- Tomita T, Hata T, Tokuno H. Effects of removal and reapplication of K(+) and Cl(-) on spontaneous electrical activity, slow wave, in the circular muscle of the guinea-pig gastric antrum. Jpn J Physiol. 2000;50:191–198. doi: 10.2170/jjphysiol.50.191. [DOI] [PubMed] [Google Scholar]
- Ward SM, Beckett EA, Wang X, Baker F, Khoyi M, Sanders KM. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci. 2000;20:1393–1403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480(Pt 1):91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Dixon RE, de Faoite A, Sanders KM. Voltage-dependent calcium entry underlies propagation of slow waves in canine gastric antrum. J Physiol. 2004;561:793–810. doi: 10.1113/jphysiol.2004.076067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, McLaren GJ, Sanders KM. Interstitial cells of Cajal in the deep muscular plexus mediate enteric motor neurotransmission in the mouse small intestine. J Physiol. 2006;573:147–159. doi: 10.1113/jphysiol.2006.105189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won KJ, Sanders KM, Ward SM. Interstitial cells of Cajal mediate mechanosensitive responses in the stomach. Proc Natl Acad Sci U S A. 2005;102:14913–14918. doi: 10.1073/pnas.0503628102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouters M, De Laet A, Donck LV, Delpire E, van Bogaert PP, Timmermans JP, de Kerchove d’Exaerde A, Smans K, Vanderwinden JM. Subtractive hybridization unravels a role for the ion cotransporter NKCC1 in the murine intestinal pacemaker. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1219–1227. doi: 10.1152/ajpgi.00032.2005. [DOI] [PubMed] [Google Scholar]