Abstract
Background and Purpose:
Patulin is a mycotoxin produced by some molds, especially Aspergillus and Penicilium, and is responsible for mycotoxicosis in animals and humans.
There is still not very detailed data about the anti-cancer potency of patulin, but some reports demonstrated that it induces cellular apoptosis and toxicity.
Materials and Methods:
To determine the efficacy of patulin as a therapeutic strategy for cervical and colorectal cancers, we investigated its effects on HeLa,SW-48, and MRC-5 cell lines. Cell lines were exposed to various concentrations of patulin (i.e., 0.5, 1, 2, and 4 µM), then using methyl thiazolyl tetrazolium (MTT) and bromo-2′-deoxyuridine (BrdU) assays, the rates of apoptosis and cell viability were determined.
Results:
The obtained results showed a significant reduction in cell viability and apoptosis induction in a dose-dependent manner. Among all the cell lines, the highest growth inhibition rate was obtained at the 4 μM concentration of patulin.
Conclusion:
Our results suggested that patulin could significantly decrease tumor growth in human cervical and colorectal cancer models.
Key Words: Cancer, HeLa cells, Mycotoxin, Patulin
Introduction
Patulin is a toxic secondary metabolite produced by molds, especially Penicillium and Aspergillus, and is usually found in apples and apple-derived products including juice and compote. Several reports have demonstrated that exposure to different levels of this polyketide metabolite is associated with immunotoxicity, genotoxicity, embryotoxicity, and teratogenicity in animals and some microorganisms [1-6].
Regarding many disadvantages of the routine anticancer drugs, researchers are seeking new compounds and formulations with better activities and lower side effects [7, 8]. In this regard, the use of toxins and secreted compounds of fungi and bacteria can be beneficial.
Patulin induces toxicity through two mechanisms, first by creating oxidative stress response in cells and additionally binding to reactive sulfhydryl groups in cellular proteins through covalent bonds [8, 9]. Patulin is known to affect the testis tissue of rats and cause a significant increase in serum testosterone level, but there are no reports on the toxic or apoptotic effects of this toxin on the female reproductive system [10].
Colorectal and cervical cancers are respectively the second and third most frequently diagnosed cancers in women worldwide [11, 12]. In spite of comprehensive understanding regarding the management of various stages of these cancers, definitive treatment remains clinically challenging; thus, finding novel inhibitors and compounds seems to be essential.
Patulin has been reported to activate apoptosis in several mammalian cell lines, such as human leukemia cells (HL-60), through the generation of reactive oxygen species (ROS) and human embryonic kidney cells 293 (HEK293) by phosphorylation of two major mitogen-activated protein kinases (MAPKs), p38 kinase and c-Jun N-terminal kinase (JNK) [9]. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) assay, a reliable and common colorimetric test for in vitro evaluation of cell viability, has been utilized to analyze cell cultures subjected to different doses of patulin [13].
In addition, bromodeoxyuridine (5-bromo-2'-deoxyuridine, BrdU), the analog of the DNA base thymidine, is usually used for the detection of cell apoptosis and proliferation [14]. Therefore, in the present study, HeLa, the human cervical cancer cell line, was treated with different levels of patulin to examine its effect on cell proliferation and toxicity. In addition, we assessed the effect of patulin on two other cell lines, including Medical Research Council cell strain 5 (MRC-5; normal human lung cells) and SW-48 (colonic adenocarcinoma cells), using MTT and BrdU assays.
Materials and Methods
Chemicals and cell culture
The reference powder of patulin, (4-hydroxy-4H-furo[3, 2-c]pyran-2 (6H)-one), was obtained from Sigma Chemical Company (St. Louis, Missouri, USA) and dissolved in 15% ethanol at a concentration of 10 mM and stored at −20°C until use. All other solvents and reagents were used as chemical grade.
Three cell lines, SW-48, HeLa, and MRC-5, were purchased from the National Cell Bank of Iran deposited in Pasteur Institute (Tehran, Iran). All the cell lines were cultured in RPMI 1640 medium (Gibco, Germany) supplemented with 10% fetal bovine serum, 0.1 mg/ml streptomycin, and 100 U/ml penicillin, and then incubated at 37°C in a 5% CO2 incubator.
Cell viability assay
The MTT colorimetric assay was used to determine the in vitro growth inhibitory effect of patulin on cell lines. The cell lines at a concentration of 5×103 cells/well were seeded in 96-well flat-bottom plates (SPL Life Sciences, South Korea) containing 100 µl of RPMI medium. After 24 h, the cell lines were treated with a serial doubling dilution of patulin. Afterwards, patulin was discarded and the cells received 10 µl of MTT (5 mg/ml stock solution; Roche Applied Science, Germany). All the plates were incubated at 37°C for 4 h, and formazan blue was dissolved in 200 µl of dimethyl sulfoxide (DMSO). Finally, absorbance was measured at a wavelength of 570 nm using a spectrophotometric microplate reader (BioTek Elx 808). All the data are represented as two independent experiments.
Cell apoptosis assay
The proliferation of the three cell lines, SW-48, HeLa, and MRC-5, was determined using a colorimetric test based on the evaluation of BrdU (Roche Applied Science, Germany) incorporation during cell division. Briefly, the cells were seeded in plates at a concentration of 5000 cells per insert well, cultured for 24 h to allow the growth of a confluent monolayer, treated with a serial dilution of patulin, and finally, incubated with 10 μM of BrdU for 5 h at 37°C under 5% CO2 before analysis. Absorbance values were measured using a spectrophotometric microplate reader (BioTek Elx 808) at 490 nm. All the tests were performed in duplicate.
Growth inhibition rate and cell proliferation were calculated by using the following formula:
Growth inhibition rate % = 1− (OD drug exposure / OD control ) ˟ 100
Cell proliferation % = 1− (OD drug exposure / OD control ) ˟ 100(7)
Statistical analysis
IC50 of formulations were calculated using CalcuSyn version 2 software (BIOSOFT, UK). Differences in inhibition and proliferation were analyzed using Student’s t-test. P-values less than 0.05 were considered statistically significant.
Results
To determine the growth inhibitory effect of patulin, all the cell lines, SW-48, HeLa, and MRC-5, were treated with various concentrations of patulin (i.e., 1 μM, 2 μM, 4 μM, and 8 μM) for 24 h and cell viability and proliferation were determined by MTT and BrdU assays and finally presented as IC50 and proliferation rate.
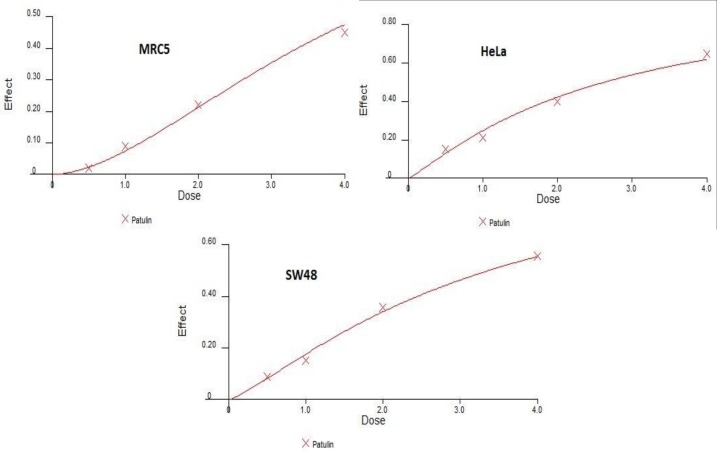
The results of MTT and BrdU assays indicated that patulin had cytotoxic effects on all the cell lines in a dose-dependent manner. As illustrated in Figure 1, assessment of in vitro growth inhibition by MTT assay revealed significant changes in the treated SW-48, HeLa, and MRC-5 cell lines versusthe untreated cells (P<0.05). Patulin caused dose-dependent induction of cytotoxicity at the concentrations of 0.5, 1, 2, and 4 μM after 24 h of treatment.
Figure 1.
Assessment of in vitro growth inhibition by MTT test. Results revealed significant changes in the treated SW-48, HeLa, and MRC-5 cell lines versus the untreated ones (P<0.05).
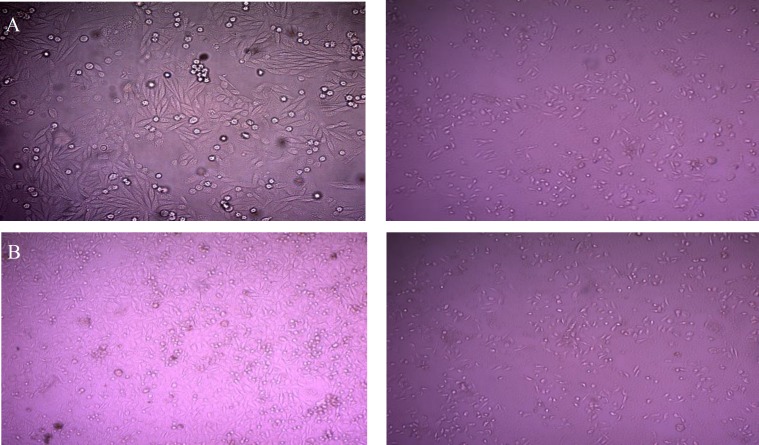
Figure 2 demonstrated that the treatment of HeLa and SW-48 cell lines with patulin resulted in reduction in cell numbers.
Figure 2.
Effects of patulin on morphology of SW48 (A) and HeLa (B) cells by BrdU assay; right: untreated cell, left: cells after treatment by patulin
The highest IC50 values for the SW-48, HeLa, and MRC-5 cell lines, as indicated by the MTT assay, were 55%, 65%, and 45%, respectively, at a concentration of 4 μM (Table 1).
Table 1.
Effects of patulin on growth of SW-48, HeLa, MRC5 cell lines
|
MTT assay
| |||
|---|---|---|---|
|
Inhibition rate (IR)%
| |||
| SW-48 | HeLa | MRC5 | Concentration of patulin (μM) |
| 8 | 15 | 2 | 0.5 |
| 15 | 21 | 9 | 1 |
| 30 | 40 | 22 | 2 |
| 55 | 65 | 45 | 4 |
As presented in Figure 1, the cell proliferation rates of SW-48 and HeLa cells decreased in a dose-dependent manner. The lowest cell proliferation rates of SW-48, HeLa, and MRC-5, as revealed by the
BrdU assay, were 29%, 17%, and 30%, respectively, at a concentration of 4 μM (Table 2).
Table 2.
Effects of patulin on growth of SW-48, HeLa, MRC5 cell lines
|
BrdU assay
| |||
|---|---|---|---|
|
Cell proliferation%
| |||
| SW-48 | HeLa | MRC5 | Concentration of patulin (μM) |
| 70 | 97 | 87 | 0.5 |
| 49 | 52 | 45 | 1 |
| 41 | 32 | 37 | 2 |
| 29 | 17 | 30 | 4 |
Discussion
Currently, the incidence of colon and cervical cancers is increasing worldwide and are the third and fourth leading causes of female mortality, respectively [15, 16]. In spite of advances in the treatment of these cancers in recent years, the therapeutic strategies pose various challenges to specialists, such as unwanted side effects, incompliancy of patients, as well as development of metastasis and resistance to different drugs [17-19]. Therefore, it is essential to look for potential efficient compounds to design novel drugs targeting colon and cervical cancers.
Patulin is a toxic substance produced by some fungi that has potent anticancer activity; however, its exact biological target remains inconspicuous [1, 6]. Rare reports have been published on the effects of patulin on human cell lines, particularly tumor cells.
In this study, the anti-tumor effects of patulin on a colonic adenocarcinoma cell line (SW-48) and a cervical cancer cell line (HeLa) in an in vitro model (MTT and BrdU assay) were evaluated. Our results demonstrated that treatment with patulin significantly prevented the growth of tumors, as revealed in figures 1 and 2, as well as tables 1 and 2.
Previous studies revealed that patulin induced toxicity in human cells including human embryonic kidney 293 (HEK293) [9] and human immortalized keratinocyte (HaCaT) [20], as well as animal cells such as Chinese hamster ovary (CHO-K1) [13], V79 Chinese hamster cells [21], and spontaneously immortalized rat granulosa cells (SIGC) [22]. In a study by Wu et al., the lowest cell viability and greatest morphological changes of HL-60 cells were observed at the concentration of 2.5 μM [8].
In the current study, MTT assay demonstrated that the inhibition rates of HeLa and SW-48 cell lines, which were incubated with 2 μM of patulin, were 40% and 30%, respectively (Table 1). Patulin at a concentration of 4 μM also reduced the viability of tumor cells to 55% for SW-48 cell lines and 65% for HeLa cell lines, respectively.
However, a study by Luft et al. (23), it was reported that the treatment of peripheral blood mononuclear cells (PBMCs) with a lower concentration of patulin (0.6 μM) resulted in the reduction of cell viability. The results of this study (Table1) indicated that cell viability reduced in MRC-5, normal cell lines of the lungs, which is in agreement with the findings of Liu et al. [9] who found that patulin reduced cell viability in HEK cells.
It is worth mentioning that MTT assay indirectly quantifies cell number and cannot obviously confirm the induction of apoptosis and reduced cell number; thus, the apoptotic effect of patulin on tumor cell growth was evaluated using an ELISA-based BrdU incorporation assay.
Several studies have confirmed that patulin can induce apoptosis in various cell lines [9]. Wu et al. reported that apoptosis was initiated by patulin in intrinsic pathway by the Bcl-2 family, in which cytochrome c is released into cytosol and the level of cleaved caspase-9 is elevated [24]. Additionally, the activation of p38 kinase and c-Jun N-terminal kinase signaling was introduced as apoptotic pathways in HEK cells induced by patulin [9]. Exposure of HeLa and SW-48 cell lines to patulin led to the induction of apoptosis as presented in BrdU assay (Table 2 and Figure 2). As presented by BrdU assay, the best apoptotic effect on all the cell lines was obtained at the concentration of 4 μM of patulin, and among all the cells lines, the rate of apoptosis was the highest in HeLa.
Previously, BrdU has been used as a rapid test to determine DNA synthesis in proliferating cells exposed to different mycotoxins. In the study of Nagashima et al. using this assay, the IC50 value of nivalenol against the promyelocyte-derived cell line, HL60, was 0.51 µM [25]. Also, fusarochromanone, a mycotoxin produced by Fusarium equiseti, exhibited IC50 values ranging from 10 nM to 2.5 μM against HaCat, P9-WT, MCF-7, MDA-231, SV-HUC, UM-UC14, and PC3 cell lines [26].
The current results regarding patulin are in line with those obtained by Wu and Liu, who concluded that patulin induced apoptosis in HL-60 and HEK293 cells as well as similar to other mycotoxins [25, 26].
Conclusion
We believe that because of verified induction of toxicity and apoptosis in our report and other studies, patulin can be considered an extreme candidate for anti-cancer agents. Fungus usually produces many important metabolites that may have anti-cancer benefits. For the first time we have demonstrated that patulin was able to decrease tumor growth in human colorectal and cervical cancer models.
Acknowledgments
This study was financially supported by grant number 1519, with ethical code IR.MAZUMS.REC.93-1519 from Invasive Fungi Research Center (IFRC), Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
Author’s contribution
J. A. and M. A. designed and supervised the study, S. Gh., A. A., H. Gh., and H. M. performed the tests. Furthermore, M. A. wrote the first draft, and M. H., T. S., and J. A. edited the final manuscript.
Conflicts of interest
The authors declare no conflicts of interest. The authors are responsible for the content and writing of the paper
Financial disclosure
The authors declare no financial interests related to the materials of the study.
References
- 1.Harrison MA. Presence and stability of patulin in apple products: a review. J Food Saf. 1988;9(3):147–53. [Google Scholar]
- 2.Würgler FE, Friederich U, Schlatter J. Lack of mutagenicity of ochratoxin A and B, citrinin, patulin and cnestine in Salmonella typhimurium TA102. Mutat Res. 1991;261(3):209–16. doi: 10.1016/0165-1218(91)90069-x. [DOI] [PubMed] [Google Scholar]
- 3.International Agency for Research on Cancer. Some naturally occurring and synthetic food components, furocoumarins and ultraviolet radiation. Geneva: World Health Organization; 1998. [Google Scholar]
- 4.Dailey R, Brouwer E, Blaschka A, Reynaldo E, Green S, Monlux W, et al. Intermediate‐duration toxicity study of patulin in rats. J Toxicol Environ Health. 1977;2(3):713–25. doi: 10.1080/15287397709529471. [DOI] [PubMed] [Google Scholar]
- 5.Reddy CS, Chan PK, Hayes AW. Teratogenic and dominant lethal studies of patulin in mice. Toxicology. 1978;11(3):219–23. doi: 10.1016/s0300-483x(78)91339-2. [DOI] [PubMed] [Google Scholar]
- 6.Puel O, Galtier P, Oswald IP. Biosynthesis and toxicological effects of patulin. Toxins. 2010;2(4):613–31. doi: 10.3390/toxins2040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akhtari J, Rezayat SM, Teymouri M, Alavizadeh SH, Gheybi F, Badiee A, et al. Targeting, bio distributive and tumor growth inhibiting characterization of anti-HER2 affibody coupling to liposomal doxorubicin using BALB/c mice bearing TUBO tumors. Int J Pharm. 2016;505(1-2):89–95. doi: 10.1016/j.ijpharm.2016.03.060. [DOI] [PubMed] [Google Scholar]
- 8.Alavizadeh SH, Akhtari J, Badiee A, Golmohammadzadeh S, Jaafari MR. Improved therapeutic activity of HER2 Affibody-targeted cisplatin liposomes in HER2-expressing breast tumor models. Expert Opin Drug Deliv. 2016;13(3):325–36. doi: 10.1517/17425247.2016.1121987. [DOI] [PubMed] [Google Scholar]
- 9.Liu BH, Wu TS, Yu FY, Wang CH. Mycotoxin patulin activates the p38 kinase and JNK signaling pathways in human embryonic kidney cells. Toxicol Sci. 2005;89(2):423–30. doi: 10.1093/toxsci/kfj049. [DOI] [PubMed] [Google Scholar]
- 10.Selmanoglu G, Koçkaya EA. Investigation of the effects of patulin on thyroid and testis, and hormone levels in growing male rats. Food Chem Toxicol. 2004;42(5):721–7. doi: 10.1016/j.fct.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Kohler BA, Sherman RL, Howlader N, Jemal A, Ryerson AB, Henry KA, et al. Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107(6):djv048. doi: 10.1093/jnci/djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 13.Ferrer E, Juan-García A, Font G, Ruiz M. Reactive oxygen species induced by beauvericin, patulin and zearalenone in CHO-K1 cells. Toxicol Vitro. 2009;23(8):1504–9. doi: 10.1016/j.tiv.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Lehner B, Sandner B, Marschallinger J, Lehner C, Furtner T, Couillard-Despres S, et al. The dark side of BrdU in neural stem cell biology: detrimental effects on cell cycle, differentiation and survival. Cell Tissue Res. 2011;345(3):313–28. doi: 10.1007/s00441-011-1213-7. [DOI] [PubMed] [Google Scholar]
- 15.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64(1):52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 16.Bosch FX. The path to eliminate cervical cancer in the world and the challenges of professional education. Vaccine. 2013;31:7. doi: 10.1016/j.vaccine.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Nicolay NH, Berry DP, Sharma RA. Liver metastases from colorectal cancer: radioembolization with systemic therapy. Nat Rev Clin Oncol. 2009;6(12):687–97. doi: 10.1038/nrclinonc.2009.165. [DOI] [PubMed] [Google Scholar]
- 18.Wiela-Hojeńska A, Kowalska T, Filipczyk-Cisarż E, Łapiński Ł, Nartowski K. Evaluation of the toxicity of anticancer chemotherapy in patients with colon cancer. Adv Clin Exp Med. 2015;24(1):103–11. doi: 10.17219/acem/38154. [DOI] [PubMed] [Google Scholar]
- 19.Remesh A. Toxicities of anticancer drugs and its management. Int J Basic Clin Pharm. 2012;1(1):2–12. [Google Scholar]
- 20.Guo X, Dong Y, Yin S, Zhao C, Huo Y, Fan L, et al. Patulin induces pro-survival functions via autophagy inhibition and p62 accumulation. Cell Death Dis. 2013;4(10):e822. doi: 10.1038/cddis.2013.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alves I, Oliveira N, Laires A, Rodrigues A, Rueff J. Induction of micronuclei and chromosomal aberrations by the mycotoxin patulin in mammalian cells: role of ascorbic acid as a modulator of patulin clastogenicity. Mutagenesis. 2000;15(3):229–34. doi: 10.1093/mutage/15.3.229. [DOI] [PubMed] [Google Scholar]
- 22.Burghardt RC, Barhoumi R, Lewis EH, Bailey RH, Pyle KA, Clement BA, et al. Patulin-induced cellular toxicity: a vital fluorescence study. Toxicol Appl Pharmacol. 1992;112(2):235–44. doi: 10.1016/0041-008x(92)90193-v. [DOI] [PubMed] [Google Scholar]
- 23.Luft P, Oostingh GJ, Gruijthuijsen Y, Horejs‐Hoeck J, Lehmann I, Duschl A. Patulin influences the expression of Th1/Th2 cytokines by activated peripheral blood mononuclear cells and T cells through depletion of intracellular glutathione. Environ Toxicol. 2008;23(1):84–95. doi: 10.1002/tox.20309. [DOI] [PubMed] [Google Scholar]
- 24.Wu TS, Liao YC, Yu FY, Chang CH, Liu BH. Mechanism of patulin-induced apoptosis in human leukemia cells (HL-60) Toxicol Lett. 2008;183(1):105–11. doi: 10.1016/j.toxlet.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 25.Nagashima H, Nakagawa H, Kushiro M, Iwashita K. The in vitro approach to the cytotoxicity of a trichothecene mycotoxin nivalenol. Japan Agricul Res Quart. 2009;43(1):7–11. [Google Scholar]
- 26.Mahdavian E, Palyok P, Adelmund S, Williams-Hart T, Furmanski BD, Kim YJ, et al. Biological activities of fusarochromanone: a potent anti-cancer agent. BMC Res Notes. 2014;7(1):601. doi: 10.1186/1756-0500-7-601. [DOI] [PMC free article] [PubMed] [Google Scholar]