Abstract
We present a case of a 59-year-old man with left upper alveolar numbness of 2 years’ duration in the absence of sinonasal symptoms. On physical examination, he demonstrated mild left facial asymmetry and diminished sensation of his left upper alveolus from the left second upper incisor to first canine. CT imaging revealed chronic sinusitis changes of the left maxillary sinus, with reduced volume and depressed anterior wall. The patient underwent functional endoscopic sinus surgery to re-establish maxillary sinus ventilation. He was noted to have some improvement of his upper alveolar paraesthesia postoperatively. Silent sinus syndrome is part of the spectrum of chronic maxillary atelectasis. In the presented case, chronic osteitic bony sclerosis, as opposed to osteopenic change of the maxillary sinus, was seen. We postulate that bony encasement of the anterior superior alveolar nerve resulted in chronic nerve compression and the patient’s unusual symptom of upper alveolar paraesthesia.
Keywords: Ear, nose and throat/otolaryngology; Otolaryngology/ENT; Surgery
Background
Silent sinus syndrome (SSS) is a rare and underdiagnosed condition within the spectrum of chronic maxillary atelectasis (CMA). The purpose of this case report is to highlight the unusual symptom of isolated alveolar numbness likely due to bony encasement and compression of the anterior superior alveolar nerve (ASAN) from chronic maxillary sinus wall bony sclerosis. It serves to better clinicians’ understanding of the spectrum of clinical and radiological characteristics within SSS. We also discuss the various stages of CMA and the inclusion of SSS within the staging system.
Case presentation
A 59-year-old Chinese man presented to the Otorhinolaryngology (ear, nose and throat) department of our hospital with left upper alveolar numbness for 2 years. He had been referred by neurosurgery for incidental findings of left maxillary sinusitis on MR brain imaging performed for surveillance of his right parafalcine meningioma.
On physical examination, he demonstrated left facial asymmetry with a hypoplastic left midface noted. Sensation of his left upper alveolus between the second left upper incisor and first upper premolar was diminished. He was edentulous on his left maxilla. Flexible nasoendoscopy revealed a possible concha bullosa of the left middle turbinate with no mucopus seen.
Investigations
CT of the paranasal sinuses revealed reduced volume of the left maxillary sinus and a depressed anterior wall (figure 1). Bony sclerosis of the maxillary sinus was noted with inwards traction of the lateral, anterior and posterior walls. Mucosal thickening was seen occupying almost the entire left maxillary sinus with a small air-fluid level seen. The left uncinate process was retracted laterally. Inferior bowing of the left orbital floor was also noted. Thick bony encasement and significant stenosis of the left ASAN within the anterior wall of the left maxillary sinus was demonstrated (figure 2).
Figure 1.
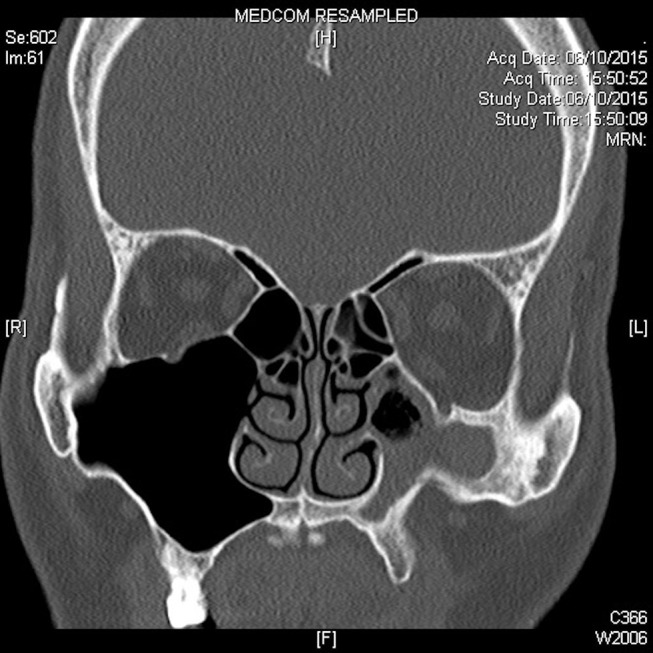
Coronal CT images showing features of left silent sinus syndrome smooth in-bowing of anterior and lateral maxillary walls, depressed orbital floor. Left maxillary sinusitis with bony sinus wall osteitic changes is demonstrated.
Figure 2.
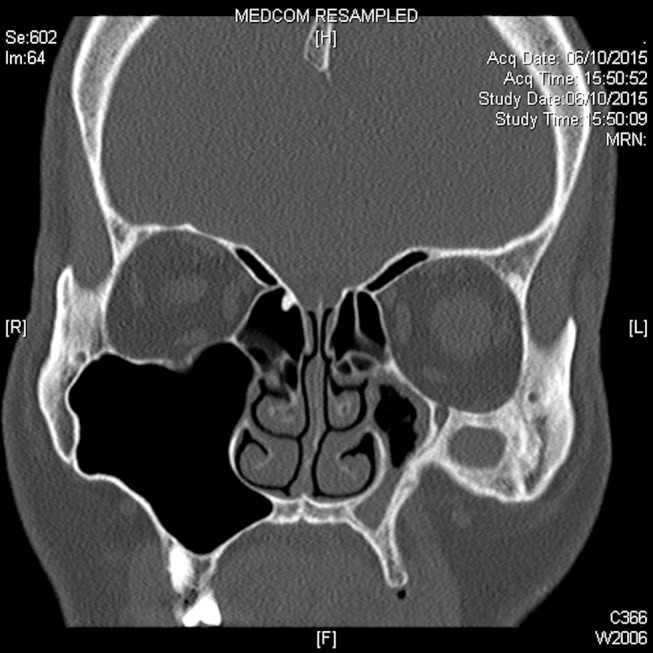
Coronal CT images showing the left anterior superior alveolar nerve (ASAN) branching inferiorly from the infraorbital nerve. The ASAN pathway is disrupted distally within the anterior maxillary wall due to bony sclerosis.
Bilateral concha bullosa was observed. No mucosal thickening or fluid levels were reported of the other paranasal sinuses.
Differential diagnosis
Our case presented with left upper alveolar numbness and facial asymmetry on the background of an incidental finding of left maxillary sinusitis. The differential diagnoses made based on these clinical findings include pathologies of isolated maxillary sinusitis. Inflammatory and infective aetiologies such as chronic sinusitis, odontogenic maxillary sinusitis, osteomyelitis and CMA/SSS were considered. Idiopathic, iatrogenic and traumatic causes of SSS were also included while neoplastic maxillary lesions (both primary and secondary) were to be excluded.
Our consideration for differential diagnoses also encompassed non-sinusitis-related causes of both congenital and acquired aetiologies. These include maxillary hypoplasia, metabolic bone disease, previous surgery/radiation and trauma.
Treatment
The patient underwent left functional endoscopic sinus surgery (FESS) to restore left maxillary sinus ventilation and drainage. Surgery performed consisted of left concha bullectomy, uncinectomy, middle meatal antrostomy, aspiration of retained secretions and maxillary sinus mucus retention cyst decompression and irrigation.
Outcome and follow-up
The patient was last reviewed 4 months after surgery and noted some improvement in his upper alveolar numbness. No significant improvement was seen in his facial asymmetry. Endoscopic examination demonstrated a widely patent middle maxillary antrostomy and a small mucus retention cyst within the left maxillary sinus.
Discussion
SSS is a rare and underdiagnosed entity typically characterised by unilateral painless enophthalmos, hypoglobus and facial deformity with the peak incidence in the fourth decade. It was first described by Montgomery in 1964 and subsequently termed as SSS by Soparkar et al in 1994.1 2 Besides hypoglobus and enophthalmos, the spectrum of ophthalmic presentation of SSS also includes transient vertical diplopia, upper lid retraction, lagophthalmos and blurred vision.3 Confusion of the description of SSS and CMA as independent and isolated entities exists.4
CMA, like SSS, is an uncommon acquired condition with progressive inflexion of the maxillary antral walls resulting in volume loss and resultant clinical signs/symptoms. CMA is also accompanied by sinonasal symptoms such as nasal congestion, facial pain, discomfort or pressure, headaches and rhinorrhoea. Patients with CMA present in their late 30s to early 40s with no gender predilection or laterality bias.4 5 Clinical presentation of CMA is a range based on the evolution and severity of disease. Kass et al 5 proposed that CMA may be divided into three stages representing the spectrum of the disease: stage 1, membranous deformity with lateralised maxillary fontanelle; stage 2, bony deformity with inward bowing of one or more osseous wall; stage 3, clinical deformity with enophthalmos, hypoglobus and/or midfacial deformity.5 SSS has been proposed to be a subtype of stage 3 CMA, whereby there is absence of sinonasal symptoms.4
The exact aetiology and pathogenesis of CMA/SSS are not fully elucidated. Two main theories have been proposed to explain the pathophysiological process. The main pathogenesis theory is that of persistent ostial obstruction resulting in maxillary sinus hypoventilation and negative sinus pressure build-up.5 6 Gas resorption by the sinus mucosa leads to subatmospheric pressure build-up, while chronic subclinical mucosal inflammation results in remodelling and resorption of the bony sinus walls.7 The negative pressure gradient within the maxillary sinus acts on osteopenic sinus walls to cause inwards traction and loss of volume. Histological analyses of affected maxillary sinus mucosa have revealed chronic inflammatory infiltrates. Based on detailed histological examination of affected mucosa, Dumitrescu et al found association of chronic inflammatory elements and areas of neovascularisation, suggesting that chronic inflammation and angiogenesis occurred codependently in SSS.8 The alternative theory, known as the ‘mechanical theory’, attributes aspiration of a closed maxillary sinus by repeated contraction and relaxation of masticatory muscles. This results in generation of negative pressure and consequential inflexion of the sinus walls seen in SSS.9
The diagnosis of SSS can be made clinically, however, radiological imaging is useful in both confirming the diagnosis as well as for surgical planning. Pathognomonic findings include the smooth inbowing of the maxillary antral walls and depressed orbital floor, resulting in volume reduction and secondary enophthalmos/hypoglobus. Soft tissue changes are consistently seen in the affected sinus. The antral walls are osteopenic in the majority of cases.10 Obstruction of the maxillary infundibulum with lateralisation of the uncinate process against the inferiomedial orbital wall is also noted.11
The treatment for SSS aims to achieve two goals: first to restore maxillary sinus drainage and second to restore normal orbit anatomy and function. Sinus procedures performed aim to re-establish physiological maxillary sinus drainage and ventilation, enabling aspiration of retained secretions and sinus irrigation.12 Sinus procedures described include FESS, Caldwell-Luc procedure and balloon sinuplasty. Spontaneous resolution of ocular symptoms after restoration of sinus ventilation via FESS alone is possible.13 Orbital floor reconstruction, if indicated, may be performed in the same setting as sinus surgery or as a two-stage operation a few months after FESS.
In our case presented, two unusual observations were of particular interest. First, thickened, sclerotic bony sinus walls were demonstrated instead of osteopenic bone often noted on radiological examination in SSS (figure 1). This suggested to us the possibility of progressive chronic inflammation, such as chronic sinusitis with osteitic bony remodelling, with ostiomeatal complex obstruction as the inciting factor for sinus inflexion. This postulation would be consistent with the theory of SSS being a progressive yet asymptomatic late-stage of CMA.
Second, the presenting symptom of unilateral upper alveolar numbness in SSS is, to our knowledge, the first ever described. We attribute this unusual symptom to the loss of sensory innervation of the ASAN. The ASAN is a somatosensory nerve that originates from the infraorbital nerve. It innervates the upper canine and incisors as well as surrounding soft tissue. The ASAN originates from the infraorbital nerve posterior to the infraorbital foramen and runs in a thin bony canal called the canalis sinuosus. It first courses anteriolaterally to the anterior wall of maxilla before turning to run medially and inferiorly within the anterior wall.14 The ASAN descends towards the pyriform aperture before branching to form the superior dental plexus within the alveolar process of the maxilla.15
From the coronal-cut CT images of the left anterior maxilla displayed in this report, the canalis sinosus is stenotic with adjacent thickened bony encasement. The ASAN distal pathway is subsequently absent due to significant bony sclerosis as it descends inferiomedially within the contracted anterior maxillary wall of the patient (figure 2). This leads us to postulate that the patient’s unusual symptom of left upper incisor and canine numbness was attributed to sensory denervation of the ASAN due to chronic bony nerve encasement secondary to the anterior maxilla bony sclerosis. To our knowledge, this is the first-ever reported case of isolated unilateral upper alveolar numbness as a presenting symptom of SSS.
In summary, this case highlights an unusual presenting symptom of SSS with associated facial asymmetry. Given the known course of the ASAN within the anterior wall of the maxilla, chronic sinusitis with bony sclerosis can possibly result in ASAN injury. CT imaging was useful in aiding diagnosis and also postulation of the pathogenesis of the patient’s symptom.
Learning points.
Silent sinus syndrome (SSS) is a rare but underdiagnosed condition.
Unilateral isolated upper alveolar numbness with facial asymmetry may be a presenting symptom of SSS.
SSS forms part of the spectrum of chronic maxillary atelectasis (asymptomatic stage 3 with clinical deformity).
Radiological imaging can aid in diagnosing SSS. Features of sclerotic bony sinus walls may also be observed rather than the typical osteopenic changes.
Footnotes
Contributors: According to the definition given by the International Committee of Medical Journal Editors (ICMJE), the authors have made one or more of the substantial contributions to the intellectual content as follows:
conception or design of the work: HTL, KHL.
Data collection: HTL, KHL.
Data analysis and interpretation: HTL.
Drafting the article: HTL.
Critical revision of the article: KHL.
Final approval of the version to be published: HTL, KHL.
Competing interests: None declared.
Patient consent: Not obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Montgomery WW. Mucocele of the maxillary sinus causing enophthalmos. Eye Ear Nose Throat Mon 1964;43:41–4. [PubMed] [Google Scholar]
- 2. Soparkar CN, Patrinely JR, Cuaycong MJ, et al. The silent sinus syndrome. A cause of spontaneous enophthalmos. Ophthalmology 1994;101:772–8. [DOI] [PubMed] [Google Scholar]
- 3. Wan MK, Francis IC, Carter PR, et al. The spectrum of presentation of silent sinus syndrome. J Neuroophthalmol 2000;20:207–12. 10.1097/00041327-200020030-00010 [DOI] [PubMed] [Google Scholar]
- 4. Brandt MG, Wright ED. The silent sinus syndrome is a form of chronic maxillary atelectasis: a systematic review of all reported cases. Am J Rhinol 2008;22:68–73. 10.2500/ajr.2008.22.3118 [DOI] [PubMed] [Google Scholar]
- 5. Kass ES, Salman S, Rubin PA, et al. Chronic maxillary atelectasis. Ann Otol Rhinol Laryngol 1997;106:109–16. [DOI] [PubMed] [Google Scholar]
- 6. Davidson JK, Soparkar CN, Williams JB, et al. Negative sinus pressure and normal predisease imaging in silent sinus syndrome. Arch Ophthalmol 1999;117:1653–4. [PubMed] [Google Scholar]
- 7. Boyd JH, Yaffee K, Holds J. Maxillary sinus atelectasis with enophthalmos. Ann Otol Rhinol Laryngol 1998;107:34–9. 10.1177/000348949810700107 [DOI] [PubMed] [Google Scholar]
- 8. Dumitrescu D, FănuŢă B, Stepan AE, et al. Silent sinus syndrome–report of a case. Rom J Morphol Embryol 2015;56:229–37. [PubMed] [Google Scholar]
- 9. Baujat B, Derbez R, Rossarie R, et al. Silent sinus syndrome: a mechanical theory. Orbit 2006;25:145–8. 10.1080/01676830600669502 [DOI] [PubMed] [Google Scholar]
- 10. Rose GE, Sandy C, Hallberg L, et al. Clinical and radiologic characteristics of the imploding antrum, or ‘silent sinus,’ syndrome. Ophthalmology 2003;110:811–8. 10.1016/S0161-6420(02)01993-0 [DOI] [PubMed] [Google Scholar]
- 11. Illner A, Davidson HC, Harnsberger HR, et al. The silent sinus syndrome: clinical and radiographic findings. AJR Am J Roentgenol 2002;178:503–6. 10.2214/ajr.178.2.1780503 [DOI] [PubMed] [Google Scholar]
- 12. Bossolesi P, Autelitano L, Brusati R, et al. The silent sinus syndrome: diagnosis and surgical treatment. Rhinology 2008;46:308–16. [PubMed] [Google Scholar]
- 13. Sivasubramaniam R, Sacks R, Thornton M. Silent sinus syndrome: dynamic changes in the position of the orbital floor after restoration of normal sinus pressure. J Laryngol Otol 2011;125:1239–43. 10.1017/S0022215111001952 [DOI] [PubMed] [Google Scholar]
- 14. Song WC, Kim JN, Yoo JY, et al. Microanatomy of the infraorbital canal and its connecting canals in the Maxilla using 3-D reconstruction of microcomputed tomographic images. J Craniofac Surg 2012;23:1184–7. 10.1097/SCS.0b013e3182587a4f [DOI] [PubMed] [Google Scholar]
- 15. Robinson S, Wormald PJ. Patterns of innervation of the anterior maxilla: a cadaver study with relevance to canine fossa puncture of the maxillary sinus. Laryngoscope 2005;115:1785–8. 10.1097/01.mlg.0000176544.72657.a6 [DOI] [PubMed] [Google Scholar]


