Abstract
This case report of an infant with severe early-onset obesity illustrates the societal condemnation of persons with obesity. In addition, it underlines the importance of diagnosing rare forms of monogenic obesity, even if no drug treatment is available. Here, we describe a 2-year-old girl with severe progressive obesity from birth onwards due to insatiable hunger. Genetic studies eventually reveal that the girl has a monogenic form of obesity caused by two mutations in the LEPR gene. No drug treatment is available (as yet) for this disease. Parents describe the stigmatic remarks they have to deal with every day. Diagnosing this rare genetic disorder was very important for understanding that satiety regulation is a complex system, of which willpower is only a small portion. In these patients, reduction of obesity can be achieved, but a different approach to lifestyle intervention is needed.
Keywords: endocrinology, genetics, obesity (nutrition), obesity (public Health), childhood nutrition (paediatrics)
Background
Obesity is a common problem that almost every physician or healthcare professional encounters. Genetic obesity however, is less known among clinicians. In these rare cases, genetic factors play a larger role than the behavioural and environmental factors that we usually associate with the causes of obesity. Many people still assume that obesity is just a matter of lacking the willpower to comply to a diet. However, satiety regulation is a much more complex system, and most non-syndromic monogenic obesity genes are involved in the brain’s neuroendocrine satiety system. The patient described in this article is a striking example of the problems that occur when this satiety system is not functioning properly. Currently, we are not able to treat these diseases, except for leptin deficiency, but the first clinical trial with MC4R-agonists for patients with a specific type of monogenetic obesity (proopiomelanocortin deficiency) was recently performed with positive results.1 By diagnosing the exact cause in genetic obesity, personalised treatment might be realised for all these different gene defects.
Case presentation
The girl was born premature at 34 weeks of gestation with birth weight of 2605 g (+1.9 SD for age) to non-consanguineous parents. Because of her preterm birth, she was admitted to the hospital and needed respiratory support for a couple of days. After 10 days, she was discharged.
A couple of weeks later, the girl had changed from a quiet neonate into an inconsolable baby. She cried day and night and could only be consoled with extra bottle feeding. At 11 weeks, she weighed more than 6 kg, and rolls of fat were appearing around her arms and legs. Her parents sought advice from the healthcare professionals of the community centre and were referred to a paediatrician.
Meanwhile, the parents tried harder and harder to follow the nutrition guidelines, with continuous crying and screaming of the girl as a result. Unfortunately, she did not lose weight at all, she gained weight at a more alarming rate than ever before. At the age of 6 months, the infant weighed almost 15 kg and she could not fit in her baby carriage.
The couple returned to the paediatrician with their daughter when she was 9 months old. The doctor was disturbed by the girl’s weight and immediately referred her to an academic hospital.
Investigations
Hormonal disorders like hypothyroidism, hypocortisolism and hypercortisolism were excluded. Other laboratory tests showed that the girl’s health status was already suffering from her obesity: hypercholesterolaemia and insulin resistance were detected. Her basal energy expenditure, measured by indirect calorimetry, was 24% lower than normal for her weight (972 kcal, measured with indirect calorimetry using the Schofield equation).
Because of the combination of severe early-onset obesity and hyperphagia, we suspected the girl of a monogenic form of obesity. We tested her for the most common early-onset genetic obesity type, melanocortin-4 receptor deficiency,2 but no mutations were found in the MC4R gene. Her leptin levels, measured in blood using a radioimmunoassay, were appropriately elevated due high fat mass, excluding leptin deficiency. The blood was not analysed for bioinactive leptin. There were no signs indicating a syndromic form of genetic obesity, for example, developmental delay, short stature, macrocephaly, dysmorphic signs or visual or hearing impairment.
The girl was admitted to the hospital for various diagnostic tests and the fine tuning of a low caloric diet. At that time, aged 1 year and 9 months, she was 88 cm tall (+1 SD), weighed 30 kg (+7.9 SD) and had a body mass index (BMI) of 38.7 kg/m2 (+8.2 SD). Serious motor development limitation and genua vara were identified, caused by the extreme amount of fat tissue.
In the meantime, a diagnostic next-generation sequencing panel for genetic obesity became available. This test is aimed at the sequencing 52 obesity-related genes. With the use of the obesity gene panel, we could diagnose the girl with a monogenic form of obesity: leptin receptor deficiency. Two different (compound heterozygous) mutations in the LEPR gene were identified: c.1985T>C p.(Leu662Ser) and c.2168C>T p.(Ser723Phe), confirmed by Sanger sequencing. Sanger sequencing also showed that the parents are both carrier for one of the mutations. The variants are not previously found in other obese patients nor in the ExAC database (http://exac.broadinstitute.org/) of healthy controls. The LEPR mutations occur in highly conserved regions of the gene, suggesting an important role in the functioning of the receptor.
Differential diagnosis
Young children with early-onset obesity and hyperphagia may have an underlying genetic defect causing these problems. It is important to assess whether it is more likely a lifestyle related, a syndromic or non-syndromic type of obesity. The extreme early onset of the obesity is suggestive for a non-lifestyle caused obesity. Syndromal genetic obesity was not likely, because the infant showed no dysmorphic facial features or congenital anomalies, had a normal head circumference and there was no developmental delay. Therefore, monogenic obesity was suspected. Important types of monogenic obesity to diagnose are leptin and proopiomelanocortin (POMC) deficiency, since these can be effectively treated with leptin or setmelanotide injections, respectively. Leptin deficiency was excluded because of adequate elevated levels of leptin. POMC deficiency was excluded because of the absence of hypocortisolism and red hair. At the moment it is not possible to differentiate very easily between the other types of monogenic obesity based on phenotype or clinical chemistry tests, so we used a multigene sequencing panel to establish the diagnosis of leptin receptor deficiency.
Treatment
No drug treatment is available as yet for patients with leptin receptor deficiency. However, it was a relief for the girl’s parents to finally understand her problem and explain it to their family and friends. We tried to find supportive treatment for their daughter in various ways, referring for parental support for coping with the hyperphagic behaviour and the hurtful stigmatising comments made by strangers. We also referred the girl to the rehabilitation physician who designed adapted shoes and a custom-built stroller, as three regular strollers had already collapsed under her weight.
Outcome and follow-up
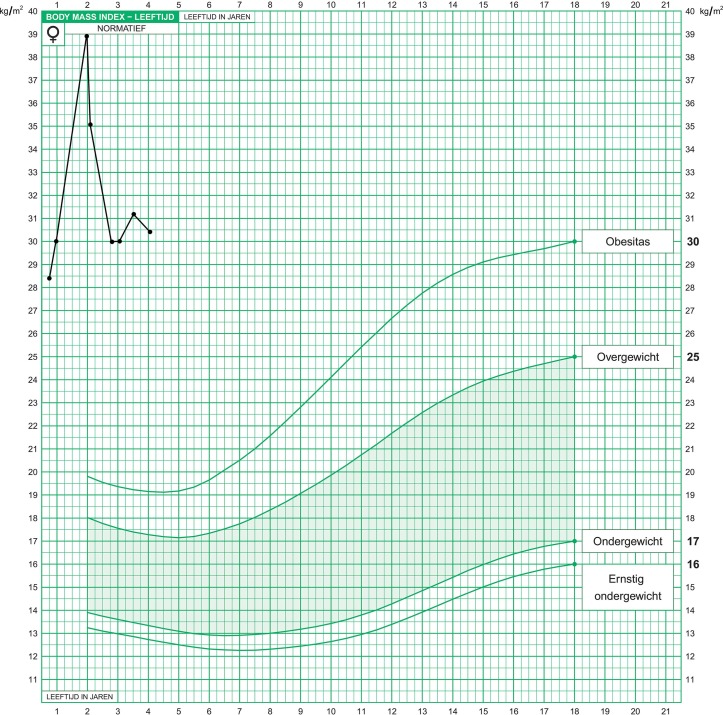
After the diagnosis was made, the girl’s extreme weight slowly stabilised. At the age of 2 years (figure 2), her BMI was 38.7 kg/m2 (+8.7 SD for her age group). In the first 4 months after the diagnosis her BMI lowered to 30 kg/m2 (+6 SD), as seen in figure 1.
Figure 2.
The patient at the age of two.
Figure 1.
The patient’s growth chart: body Mass index for age (0–4 years).
The identification of the LEPR mutations and thus the end of the diagnostic odyssey helped in the control of her weight, even without the availability a specific treatment for leptin receptor deficiency. To our knowledge there are no other examples of this observation published in literature, but we see this quite regularly in our genetic obesity clinics. Both the supportive treatment and the better understanding of the hyperphagic behaviour may enhance this effect.
The girl is now 4 years old and the fight against hunger remains, but her parents are doing the best they can to support their daughter. Diagnosing this rare genetic disorder has been of great importance even though drug treatment is not yet available for this genetic disorder.
Discussion
Our patient was diagnosed with leptin receptor deficiency. This is a rare condition that causes early-onset obesity, mostly because of increased hunger and the accompanying overeating.3 Leptin receptor deficiency is caused by homozygous or compound heterozygous mutations in the LEPR gene. Normally, the leptin receptor is activated by the hormone leptin, which is secreted by adipocytes. The amount of leptin in blood rises when adipocytes increase in size. Leptin binds to the leptin receptor causing various reactions in the hypothalamus that affect the energy balance by inhibiting appetite. Because the leptin receptor does not respond properly to leptin, it is not possible to treat these patients with leptin injections, as can be done for patients with a leptin deficiency.3 Future studies are awaited to see if setmelanotide is effective in these patients. It is questionable if patients with homozygous leptin receptor mutations might be candidates for bariatric surgery taking into account the underlying defect. One case report from 2013 described a patient with successful weight loss and maintenance of the weight loss at 12 months after bariatric surgery.4 Another case report described a patient with a homozygous LEPR mutation that showed limited weight loss and weight regain a year after bariatric surgery.4 5
Even though leptin receptor deficiency is rare, the 25 children reported in literature illustrate the complexity of the disturbed food intake and satiety regulation causing obesity.6 The insatiability of LEPR-deficient children demonstrates the power of the brain’s neuroendocrine satiety system. It is important that clinicians are aware of genetic causes of obesity and the new technologies available to test for the diseases. Since specific treatment options for monogenetic obesity are now being used in medical trials, the diagnosis can be of great importance for the patient. Moreover, a diagnosis helps in treatment strategy by using tailored lifestyle interventions and it can support the patients or their parents in coping with the social stigma of obesity. The hyperphagic behaviour associated with this disease is not a ‘character flaw’ after all but part of the disease.
Genetic testing is currently advised in the guideline of the Endocrine Society for obese patients with an onset before the age of 5, combined with clinical signs of a genetic obesity syndrome like hyperphagia or a family history that is suggestive of a genetic cause.7 Because there is no distinct phenotype for every genetic defect in the leptin-melanocortin pathway, it may be the most effective to use a multigene panel or whole exome sequencing analysis for genetic testing in these patients. Even though the non-syndromic monogenic obesity disorders are rare, the yield of these tests may be higher in selected populations.
Patient’s Perspective.
The story of the patient’s mother:
“I felt so insecure and I often worried that I was to blame for my daughter’s obesity. Was I overfeeding her? Did I give her the wrong food? Was this all my fault? Every time the doorbell rang when I was not expecting visitors, I feared that child protection workers would take away my daughter under suspicion of abuse or neglect. After all, many people told me that I was a bad parent by letting her become so obese. I was also extremely worried about my daughter’s health problems, I was so scared that her heart would stop beating, breaking down under the burden of her obese body. Every morning when my little girl slept longer than expected, I just could not enter her bedroom. In fear that I would find her not breathing. I started to avoid to take her outside to prevent the emotional burden of the remarks people made. Strangers asked if we were feeding my daughter “frying fat shakes’. It is a daily fight against both our daughter’s hunger and the comments we get from strangers. After the diagnosis was finally made, we felt more capable to take on this battle.’
Learning points.
Satiety regulation is not simply a matter of willpower. In patients with monogenetic obesity, the hypothalamic neuroendocrine satiety system is affected, leading to hyperphagia and early-onset obesity.
Diagnosing a genetic obesity disorder can be of great importance even though drug treatment is not (yet) available in most cases. A diagnosis helps in treatment strategy by using tailored lifestyle intervention and it helps the patient and parents in their fight against stigmatisation.
Continuous research will hopefully further elucidate the underlying genetic pathways with ultimately personalised treatment (lifestyle interventions or medication) based on the genetic cause of this and other genetic obesity disorders.
Footnotes
Contributors: All authors approve the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Detailed contributions:. 1. LK: main author, drafted the case report, collected the data of the patient, worked on the interpretation of the test results, communicated with the parents about the case report and searched for and selected the relevant literature and guidelines. 2. MH: clinical genetics counselling of the patient and parents, designed and developed the genetic test, analysis of the genetic test result, language editing and revising of the work for important intellectual content. ELTvdA: cared for the patient, collected data about the patient, interpretations of the endocrine lab results, assistance selecting literature and guidelines, writing assistance and revising of the work for important intellectual content.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Kühnen P, Clément K, Wiegand S, et al. . Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. N Engl J Med 2016;375:240–6. 10.1056/NEJMoa1512693 [DOI] [PubMed] [Google Scholar]
- 2.Farooqi IS, Keogh JM, Yeo GS, et al. . Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med 2003;348:1085–95. 10.1056/NEJMoa022050 [DOI] [PubMed] [Google Scholar]
- 3.Farooqi IS, Wangensteen T, Collins S, et al. . Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med 2007;356:237–47. 10.1056/NEJMoa063988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Beyec J, Cugnet-Anceau C, Pépin D, et al. . Homozygous leptin receptor mutation due to uniparental disomy of chromosome 1: response to bariatric surgery. J Clin Endocrinol Metab 2013;98:E397–E402. 10.1210/jc.2012-2779 [DOI] [PubMed] [Google Scholar]
- 5.Clément K, Vaisse C, Lahlou N, et al. . A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 1998;392:398–401. 10.1038/32911 [DOI] [PubMed] [Google Scholar]
- 6.Hannema SE, Wit JM, Houdijk ME, et al. . Novel leptin receptor mutations identified in two girls with severe obesity are associated with increased bone mineral density. Horm Res Paediatr 2016;85:412–20. 10.1159/000444055 [DOI] [PubMed] [Google Scholar]
- 7.Styne DM, Arslanian SA, Connor EL, et al. . Treatment, and prevention: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2017;102:709–57. [DOI] [PMC free article] [PubMed] [Google Scholar]