Abstract
Background
Although temperament has been recognized as an important contributor to childhood psychopathology, its role in emergent autism spectrum phenotypes is not well-understood. This study examined whether toddlers with Autism Spectrum Disorder (ASD) display temperamental vulnerabilities compared to toddlers with other developmental challenges, whether these characteristics are distinct from core autism symptoms, if they are stable over time, and if they contribute to social outcomes in preschool.
Methods
Parents of 165 toddlers with ASD, 58 nonverbal ability- and chronological age- (CA) matched developmentally delayed (DD) toddlers and 92 CA-matched typically developing (TD) toddlers completed the Toddler Behavior Assessment Questionnaire-Supplemental (TBAQ-S) at 26 months (SD=6; Time 1). TBAQ-S data were also available for a subset of toddlers with ASD (n=126) at 43 months (SD=9; Time 2).
Results
Compared to the DD and TD groups, toddlers with ASD exhibited vulnerabilities within the Effortful Control domain as well as the Surgency domain. They also displayed greater Negative Emotionality compared to TD peers. In the ASD group, temperamental characteristics were not concurrently related to autism severity or developmental level and individual differences were highly stable over time. Changes in Perceptual Sensitivity, Inhibitory Control, and Low-Intensity Pleasure from age 2 to 3 ½ uniquely predicted autism symptom severity and adaptive social skill level at Time 2.
Conclusions
Temperamental vulnerabilities in toddlers with ASD are stable over time and involve attentional and behavioral control as well as affective reactivity. They contribute uniquely to social outcomes in preschool and are likely to signal risk for developing later maladaptive attentional, affective, and behavioral symptoms. Considering biologically-based dimensions may shed light on non-core facets of the early ASD phenotype that are potentially relevant to the emergence of comorbid conditions later in childhood.
Keywords: Autism spectrum disorders, temperament
Introduction
There is a long tradition of interest in early temperament as central to both later behavioral disorders and healthy outcomes (Caspi, Henry, McGee, Moffitt, & Silva, 1995; Chess, Thomas, Rutter, & Birch, 1963; Rutter, 1987). Temperament, thought to serve as the foundation for subsequent personality development (Goldsmith et al., 1987), consists of neurobiologically-based individual differences in reactivity and regulation in the domains of emotion, motor activity, and attention (Rothbart & Derryberry, 1981). Temperamental patterns are already apparent in infancy, and, while influenced to some degree by maturation and experience, are largely stable over time (Plomin, 1983; Rothbart, 1986). There is considerable evidence that specific constellations of temperamental characteristics in toddlerhood are linked with later social competence, peer relationships, and school adjustment (Sanson, Hemphill, & Smart, 2004), as well as anxiety, externalizing behaviors, and social withdrawal (Booth-LaForce & Oxford, 2008; Pérez-Edgar et al., 2011; Rubin, Burgess, Dwyer, & Hastings, 2003).
Theories of temperament include those focusing on stylistic aspects of behavior (Chess, Thomas, & Birch, 1959), regulatory functions (Rothbart & Derryberry, 1981) and emotional aspects of behavior (Goldsmith & Campos, 1982). Goldsmith's Toddler Behavior Assessment Questionnaire (TBAQ; Goldsmith, 1996) was grounded in the emotion-based approach; a subsequent version employed in the current study, the TBAQ-Supplemental (TBAQ-S; Goldsmith, & Rothbart, 1999), incorporated scales developed by Rothbart and colleagues. Its three broad temperament dimensions are: Surgency, including high-energy activation and positive approach behaviors; Negative Emotionality, comprising a range of distress reactions; and Attention, which involves the self-regulation of attention and behavior (Backen-Jones, Gartstein, Rothbart, & Chasman, 1999). The TBAQ-S's factor structure parallels that of a more recent version of the parent-report instrument, the Early Childhood Behavior Questionnaire (ECBQ; Putnam, Gartstein, & Rothbart, 2006), in which the Attention factor is called Effortful Control. Surgency and Negative Emotionality consist of reactive traits, whereas Effortful Control captures self-regulatory features (Rothbart, 1989). These dimensions align with some of the “Big Five” factors in adult personality research, namely, extraversion, neuroticism, and conscientiousness (Rothbart & Ahadi, 1994).
Reactivity and regulation of attention and emotion are subject to maturational changes over the first few years of life. As the neural mechanisms underlying effortful control develop, normative improvements occur in abilities such as endogenous attention during play (Kannass & Oakes, 2008) and inhibitory control (Jones, Rothbart, & Posner, 2003). During these early epochs, mean-level increases over time have been reported for both negative affectivity (Putnam et al., 2006) and surgency (Goldsmith, 1996). Similar to personality traits, however (e.g., Roberts & DelVecchio, 2000), rank-order stability of individual differences in temperamental traits is moderately high, particularly from late infancy onward (Goldsmith, 1996; Pedlow et al., 1993; Putnam et al., 2006; Rothbart, 1981).
Autism Spectrum Disorder (ASD) is an early-onset neurodevelopmental disorder characterized by deficits in social communication and the presence of repetitive behaviors and interests. Several studies have identified temperamental atypicalities in preschoolers and school-age children with ASD. Compared to a typically-developing (TD) reference group, the children were rated by parents as less reactive to small changes in their environment, less adaptable, and less persistent (Hepburn & Stone, 2006). Another ASD cohort exhibited less distractibility, intensity, approach behavior, and physiological rhythmicity than TD peers (Bailey, Hatton, Mesibov, Ament, & Skinner, 2000). A third study reported lower attentional focus, attentional shifting, inhibitory control, and soothability in children with ASD compared to age-matched controls (Konstantareas & Stewart, 2006).
Several studies also examined temperamental characteristics during prodromal and early syndromal stages of ASD in younger siblings of children with ASD, who, due to familial factors, are at increased risk of developing the disorder themselves (Ozonoff et al., 2011). Six high-risk infants later diagnosed with ASD were reported to exhibit passivity and diminished activity level at six months of age, followed by elevated distress reactions and longer durations of attentional focusing at 12 months (n=10) relative to other high-risk siblings and low-risk controls (Zwaigenbaum et al., 2005). In another study, high-risk siblings with ASD at 14 months were described as smiling less than low-risk peers, being more perceptually sensitive than high-risk typical peers, and being less cuddly than high-risk and low-risk peers (Clifford, Hudry, Elsabbagh, Charman, & Johnson, 2013). By 24 months, toddlers with ASD exhibited more sadness and less cuddliness than low-risk peers, less enjoyment of quiet activities than both groups of TD controls, more shyness and lower soothability than all controls (Clifford et al., 2013), reduced ability to shift attention in response to social cues, lower inhibitory control, and less positive anticipation than high-risk and low-risk peers (Garon et al., 2009; Zwaigenbaum et al., 2005). Another study noted significant declines in adaptability and approach behaviors and increases in activity level over the first three years in siblings with ASD compared to other high-risk siblings (Rosario, Gillespie-Lynch, Johnson, Sigman, & Hutman, 2013).
Taken together, these studies suggest that, compared to typically-developing children, young children with ASD exhibit a range of temperamental challenges in areas involved in attention regulation and affective functioning. However, many of the described studies are relatively small and, given the lack of non-high-risk developmentally-delayed control groups, the specificity of temperamental vulnerabilities to toddlers with ASD is unclear. Moreover, it is unknown whether the challenges are stable and likely to affect development into preschool and early school-age. The extent to which the observed temperamental vulnerabilities are related to or distinct from autism symptoms or developmental level is also unclear. And finally, little is known about how early temperamental features may shape emergent ASD phenotypes over and above initial developmental and social characteristics (Garon et al., 2015). The quintessential feature of the autism phenotype is social impairment, indexed by performance on a direct assessment measure such as the ADOS. Equally central is social adaptation, or the capacity to apply one's cognitive and verbal skills in service of social adaptation in real-world, everyday situations, as reported by parents during interviews like the Vineland Adaptive Behavior Scales. Though it might be assumed that social ability and social disability represent opposite ends of a continuum, these have been demonstrated to be only very weakly correlated with one another in children with ASD (Kanne et al., 2011; Klin et al., 2007; Saulnier & Klin, 2007). Thus, measuring both social adaptive ability as well as social impairment serves to capture distinct aspects of social functioning.
This study examines temperament in a large clinic-referred sample of toddlers with ASD compared to peers with developmental delays (DD) or typical development (TD). The study's aims were to identify ASD-specific temperamental patterns in toddlers with ASD at the time of their first diagnosis, evaluate the independence of temperament from core symptoms of ASD and developmental functioning, assess stability of temperament in ASD, and determine the unique contributions of changes in temperament during toddler years to social skills and autism severity at preschool-age. Based on previous research, we formulated the following hypotheses: (1) parents of toddlers with ASD would report poorer Effortful Control and higher Negative Emotionality compared to parents of controls; (2) temperament would be independent from the core symptoms of ASD; (3) similar to TD children, individual differences in temperament in toddlers with ASD would be stable over time. The fourth aim of the study was exploratory; we examined whether changes in temperamental characteristics between 2 and 3 ½ years contributed to children's social functioning at outcome.
Method
Participants
The study was approved by the Human Investigations Committee and parents granted permission for their children to participate in research studies on early social development at a university-based clinic. Toddlers with suspected delays were referred to the studies by parents or professionals and typically-developing toddlers were recruited from the community. Participants included 315 toddlers between 16 and 36 months of age (Time 1), of whom 165 were diagnosed with autism spectrum disorder (ASD; 135 (81.8%) males) and 58 with developmental delays (DD; 45 (77.5%) males; 25 with global developmental delays and 33 with specific developmental delays); 92 were typically-developing (TD; 70 (76.1%) males) (Table 1). The TD toddlers were chosen from a larger sample to more closely match the gender composition of the other groups. Of the 165 toddlers with ASD, 126 (76%) returned for a follow-up visit (Time 2) at 43.4 months of age (SD=9.1), with an average gap of 17.2 months (SD=6.4) between visits. Consistent with the design of the original studies, DD and TD participants were seen only at Time 1.
Table 1. Sample characterization at Time 1 and Time 2.
| ASD | DD | TD | ||
|---|---|---|---|---|
| Time 1 | N | 165 | 58 | 92 |
| Age in months | 26.46(5.77)a | 26.40(6.17)a | 24.88(5.57)a | |
| Gender (% male) | 81.8%a | 77.5%a | 76.1%a | |
| Mullen Verbal DQ | 51.73(27.90)a | 61.82(25.03)b | 98.57(19.41)c | |
| Mullen Nonverbal DQ | 78.42(18.74)a | 83.80(20.30)a | 99.84(19.74)b | |
| ADOS-G* Social Affect | 15.19(4.10)a | 7.15(5.67)b | - | |
| ADOS-G* Restricted Repetitive Behavior | 4.17(2.06)a | 1.73(1.69)b | - | |
| Vineland Communication | 72.76(12.01)a | 77.55(11.53)b | 100.51(11.62)c | |
| Vineland Socialization | 69.58(10.01)a | 77.13(10.44)b | 92.24(7.91)c | |
| Vineland Daily Living | 71.94(9.01)a | 79.36(13.04)b | 93.96(9.26)c | |
| Vineland Motor | 82.14(12.08)a | 83.98(15.62)a | 96.49(11.25)b | |
|
| ||||
| Time 2 | N | 126 | ||
| Age in months | 43.38(9.13) | |||
| Gender (% male) | 81.7% | |||
| Mullen Verbal DQ | 71.33(31.70) | |||
| Mullen Nonverbal DQ | 77.80(20.85) | |||
| ADOS-G Calibrated Severity Score | 7.36(2.08) | |||
| Vineland Communication | 81.30(19.08) | |||
| Vineland Socialization | 69.80(11.50) | |||
| Vineland Daily Living | 69.25(12.27) | |||
| Vineland Motor | 73.88(14.94) | |||
Note. Means with different superscripts within each row differ significantly at least at the p<.05 level.
Module 1 Algorithm Scores, administered to all but two children in the ASD group.
In 73% of the sample, parents reported a Caucasian background, 2% Asian, 3% African American, and 6% mixed racial heritage; for 16%, racial information was not provided. Mothers were, on average, 33 years of age (SD=4.8) and fathers 35 (SD=5.5), with no differences across groups. Approximately 76% of mothers and 74% of fathers reported some college or more, similar across groups.
Procedures
The participants with ASD and DD were referred to a university-based clinic due to developmental concerns. The evaluation included standardized assessments of verbal and nonverbal development, adaptive functioning, and autism symptoms (see Measures below). Clinical best estimate (CBE) diagnosis was determined based on this evaluation in conjunction with all available evidence (e.g., developmental and medical history), DSM-IV criteria, and clinical judgment (Chawarska, Klin, Paul, Macari, & Volkmar, 2009). CBE diagnosis of DD children was based on the evaluation at Time 1 (T1). Final diagnostic classification of the ASD group was based on the Time 2 (T2) CBE diagnosis unless a T2 visit was unavailable, in which case the T1 diagnosis was used (23.6%). Previous work suggests high stability (∼90%) of an early ASD diagnosis (Chawarska et al., 2009; Guthrie, Swineford, Nottke, & Wetherby, 2013; Kim, Macari, Koller, & Chawarska, 2016). Thus, among those with ASD who were not evaluated at T2 (n=39), approximately four children would be expected not to retain the ASD diagnosis. Given the sample size, this is unlikely to threaten the integrity of the overall results. TD controls were recruited through advertisements in the local community. Their developmental status was confirmed through parent interview and assessment of verbal and nonverbal skills (MSEL). All parents completed the Toddler Behavior Assessment Questionnaire-Supplemental (TBAQ-S; Goldsmith & Rothbart, 1999) at T1. In the ASD group, the mothers were informants for 74.5% of the group at T1 and for 71.4% at T2, with the remaining informants consisting of fathers and other caregivers. Informant distribution was similar at T1 in the control groups (p =.31). Parents/caregivers of the children with ASD returning at T2 completed the TBAQ-S a second time, and respondents were consistent at both time points for 73%. There were no significant differences on TBAQ scales at T1 between children who did and did not have a T2 visit (all p-values =.13 and higher).
Measures
Temperament
The TBAQ-S is a 144-item parent-report questionnaire consisting of 13 scales comprising three domains. The Attention/Effortful Control domain captures the ability to regulate behavior and attention by overriding reflexive response tendencies and substituting them with adaptive responses and includes: Attentional Focusing, Attentional Shifting, Inhibitory Control, Low-Intensity Pleasure, and Perceptual Sensitivity. The Negative Emotionality domain covers a range of negative emotional reactions, including: Anger, Discomfort, Sadness, Social Fear, and Soothability. Finally, the Surgency domain represents high-energy activation and approach behaviors and includes: Activity Level, High-Intensity Pleasure, and Positive Anticipation (see Supplemental Table 1). Each item is rated on a 7-point Likert scale ranging from “never” to “always.” Scale scores were computed by summing the item scores and dividing by the number of items completed; domain scores were sums of the respective scale scores.
Clinical characterization
The Mullen Scales of Early Learning (Mullen, 1995) was used to assess verbal and non-verbal development, with developmental quotient (DQ) scores derived from age equivalent (AE) scores in Receptive and Expressive Language (verbal) and in Fine Motor and Visual Reception (nonverbal) (DQ=[mean of AE scores/chronological age]*100). Adaptive functioning was evaluated using the Vineland Adaptive Behavior Scales (Sparrow, Balla, & Cicchetti, 1984), a structured interview providing scores in Socialization, Communication, Daily Living Skills, and Motor Skills (M=100, SD=15). The Autism Diagnostic Observation Schedule-Generic (ADOS-G; Lord et al., 2000) quantifies autism-related symptoms, with Social Affect and Restricted Repetitive Behavior algorithm scores, as well as standard scores (Calibrated Severity Scores (CSS); Gotham, Pickles, & Lord, 2009) representing overall symptom severity.
Statistical Analysis
To examine which components of the TBAQ-S were specifically affected in toddlers with ASD, we utilized a generalized linear mixed model (SPSS GLMM) with the three domains (Effortful Control, Negative Emotionality, and Surgency) as the within-subject factor and diagnosis as the between-subject factor, with nonverbal DQ as a covariate. Planned contrasts were performed when a significant interaction was found, followed by univariate ANOVAs on the individual scales, with nonverbal DQ as a covariate. The univariate ANOVA results were adjusted for these 13 comparisons using a Holm-Bonferroni correction, and when group differences were significant, post-hoc comparisons were performed and Bonferroni-corrected. Cohen's d effect sizes were calculated where appropriate. Pearson's r correlation coefficient analysis was used to examine concurrent relationships between the temperament domains and autism severity, developmental functioning, and adaptive behavior, and to evaluate stability of temperamental characteristics over time in the ASD sample. To examine if changes in temperamental characteristics from Time 1 to Time 2 contributed to Time 2 autism severity (ADOS-G CSS) and social functioning (Vineland Socialization Scale), we used multivariate linear regression. Hierarchical regression was performed, first including a block of Time 1 characterization variables (Mullen, ADOS-G algorithm scores, and Vineland Socialization and Communication scores) followed by a block of TBAQ-S difference scores (Scale score T2-Scale score T1). Each block of IVs was arranged to let the variables compete stepwise for order of entry. Thus the regression was hierarchical over blocks, but stepwise within the blocks.
Statistical analyses were performed in SPSS version 21 (IBM, 2012).
Results
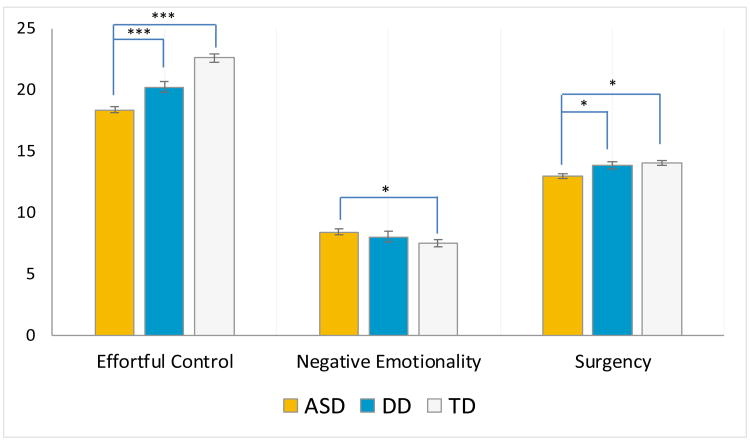
The GLMM analysis indicated a main effect of group (F(2,932)=12.37, p<.001), a main effect of domain (F(2,932)=937.86, p<.001), no main effect of NVDQ (F(1,932)=2.54, p=.11), and a significant group by temperament domain interaction (F(4,932)=17.57, p<.001). Planned contrasts revealed that, for Effortful Control, toddlers with ASD had lower scores than toddlers with DD (p<.001, d=.50) and TD (p<.001, d=1.14); for Negative Emotionality, toddlers with ASD received higher scores than toddlers with TD (p=.029, d=.34); and for Surgency, scores of toddlers with ASD were lower than those of DD (p=.020, d=.40) and TD toddlers (p=.020, d=.37) (Supplemental Table 2).
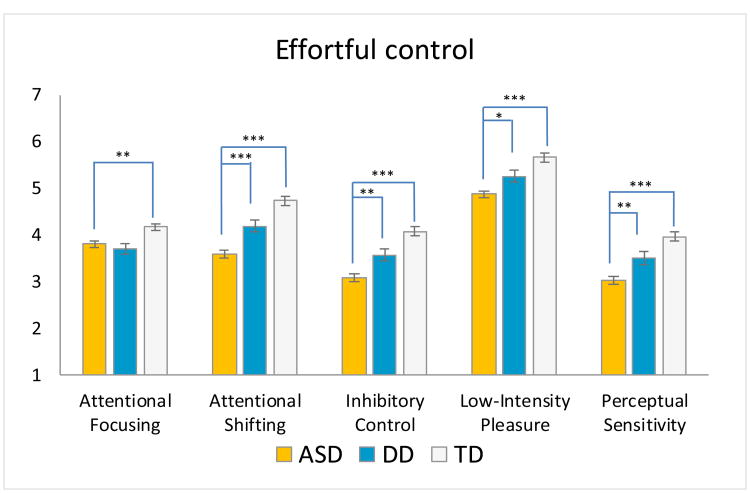
To better understand what was driving the domain-level results, we conducted direct comparisons of the 13 TBAQ-S scales (Figure 1b/Supplemental Table 3). Within the Effortful Control domain, there were significant group differences on all five scales. Post-hoc comparisons revealed that toddlers with ASD had greater difficulty shifting attention across activities when directed (Attentional Shifting) compared to toddlers with DD and TD (d=.57, d=.94, respectively); refrained from actions less often when instructed to do so (Inhibitory Control), (d=.48, d=.86); enjoyed low-intensity activities less (Low-Intensity Pleasure), (d=.38, d=.75); and were less aware of minor perceptual stimuli in the environment (Perceptual Sensitivity), (d=.45, d=.76). The ASD group exhibited a diminished ability to focus attention (Attentional Focusing) compared to TD controls, d=.42.
Figure 1.
a. TBAQ-S domain scores (marginal means and +/-1 standard error) for toddlers with ASD, DD, and TD.
b. TBAQ-S scale scores (marginal means and +/-1 standard error) for toddlers with ASD, DD, and TD, by domain.
Legend: (***) p<0.001;(**) p<0.01;(*) p<0.05. Significance of post-hoc tests is reported with Bonferroni correction. Nonverbal DQ was included as a covariate.
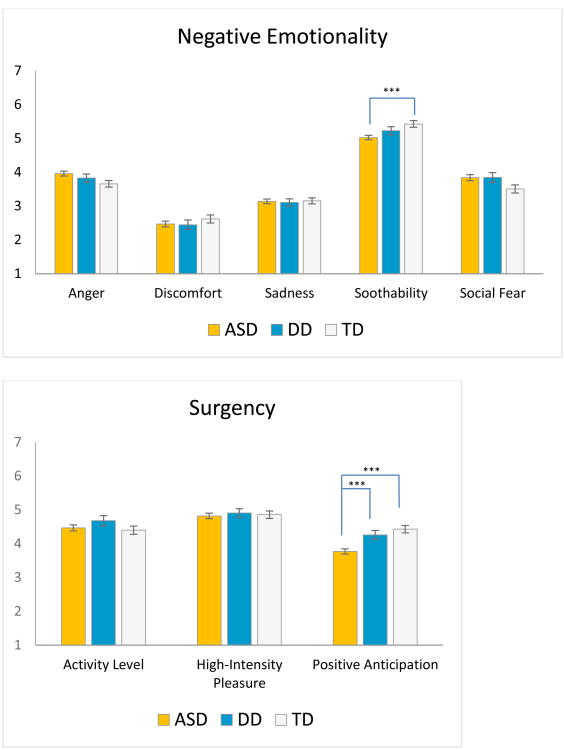
In the Negative Emotionality domain, group differences emerged on Soothability. Post-hoc tests showed that toddlers with ASD differed from the TD group in terms of experiencing slower recovery from excitement and distress, d=.44. Positive Anticipation carried the group differences in the Surgency domain, with post-hoc tests revealing that the ASD group showed lower levels of excitement about current and future pleasurable activities than the DD and TD groups, (d=.48, d=.61).
Relationship between Temperament, ASD Severity, and Verbal/Nonverbal Skills
Correlation analysis within the ASD group (n=165) revealed negligible associations between ADOS-G Module 1 SA algorithm scores and TBAQ domain scores: Effortful Control, r=-.11, p=.15; Negative Emotionality, r=-.14, p=.07; and Surgency, r=-.12, p=.13. Correlations of similar magnitude were noted between ADOS RRB algorithm scores and TBAQ domain scores: Effortful Control, r=-.02, p=.79; Negative Emotionality, r=.03, p=.73; and Surgency, r=-.14, p=.08. Similarly, near-zero correlations were observed across temperament domains and the Mullen Verbal DQ: Effortful Control, r=.08, p=.32; Negative Emotionality, r=-.01, p=.90; Surgency, r=.05, p=.49, and Mullen Nonverbal DQ: Effortful Control, r=-.02, p=.76; and Negative Emotionality, r=-.06, p=.46. One exception was the weak but significant correlation between NVDQ and Surgency, r=.16, p=.04, driven by the subscale Positive Anticipation, r=.18, p=.02.
Stability of Temperamental Characteristics in ASD
Correlation analysis1 on the subsample of children with ASD who returned for T2 (n=126) indicated high differential stability of TBAQ-S scores: Effortful Control, r=.56, p<.001; Negative Emotionality, r=.61, p<.001; Surgency, r=.54, p<.001. There was evidence of moderate to high stability over time for individual scales as well (r=.30 to r=.60, ps<.001; Supplemental Table 5). Mean scores on the TBAQ domains and scales at T1 and T2 are listed in Supplemental Table 4.
Predicting Time 2 Autism Symptom Severity and Adaptive Social Skills
The prediction of Time 2 ADOS-G CSS from T1 clinical characterization variables and TBAQ-S difference scores yielded an R2 value of 0.32 (Table 2). In addition to T1 ADOS-G SA and RRB, one TBAQ-S difference score, Perceptual Sensitivity, contributed significantly to the model. Higher scores on ADOS-G SA and RRB and lower change scores (less improvement or decline) for TBAQ-S Perceptual Sensitivity predicted higher autism severity at T2. The model predicting T2 Vineland adaptive socialization scores yielded an R2 value of 0.50. Along with T1 Vineland Socialization and ADOS-G SA, two TBAQ-S difference scores were retained after stepwise selection: Inhibitory Control and Low-Intensity Pleasure. Higher scores on T1 Vineland Socialization and Mullen Verbal DQ as well as positive change in Inhibitory Control and Low-Intensity Pleasure predicted higher Vineland Socialization scores at T2.
Table 2.
Results of linear regression predicting ADOS-G Calibrated Severity Score (CSS) and Vineland Socialization Score at Time 2 in children with ASD using Time 1 characterization variables and change (Δ) in TBAQ-S scale scores (N=126).
| Time 2 predicted variable | Time 1 predictor variable | B | SE(B) | β | p-value |
|---|---|---|---|---|---|
| ADOS-G CSS R2: 0.32 |
Intercept | 3.21 | 0.63 | <0.001 | |
| ADOS-G Social Affect | 0.23 | 0.04 | 0.44 | <0.001 | |
| ADOS-G Restricted Repetitive Behavior | 0.20 | 0.08 | 0.21 | 0.012 | |
| TBAQ-S Perceptual Sensitivity Δ | -0.31 | 0.16 | -0.15 | 0.050 | |
| Vineland Socialization SS R2: 0.50 |
Intercept | 18.73 | 5.57 | 0.001 | |
| Vineland Socialization | 0.63 | 0.09 | 0.52 | <0.001 | |
| Mullen Verbal DQ | 0.11 | 0.03 | 0.24 | 0.001 | |
| TBAQ-S Inhibitory Control Δ | 2.42 | 0.90 | 0.19 | 0.008 | |
| TBAQ-S Low-Intensity Pleasure Δ | 2.09 | 0.82 | 0.18 | 0.012 |
Note: Variables listed are those remaining in the model after hierarchical/stepwise selection.
Discussion
Temperament functions as an “organizer of development” (Marshall, Fox, & Henderson, 2000) and, as such, plays an important role in typical and atypical developmental trajectories. Results of our study revealed that there are ASD-specific vulnerabilities in the domain of Effortful Control (EC) compared to peers with and without developmental delays, when controlling for nonverbal ability. Per their parents' report, toddlers with ASD have difficulties transitioning between activities when prompted (e.g., stopping play when told it is bedtime) and inhibiting actions when directed (e.g., refraining from touching an attractive object when told not to). They are also less likely to enjoy and maintain attention to low-intensity activities (e.g., listening to a story), and demonstrate less awareness of subtle environmental stimuli (e.g., an insect crawling in the garden). Some temperamental vulnerabilities in ASD are shared with toddlers who have other developmental delays: lower soothability (recovering from extreme emotions) and diminished attention focusing. Similar to non-clinical populations (Putnam et al., 2006), individual differences in many temperamental traits are highly stable in children with ASD from the toddler years to preschool-age. Temperamental traits appear to be independent of concurrent severity of social impairments and levels of cognitive functioning at 2 years of age, and changes in EC traits over time contribute uniquely to later severity of autism symptoms and social adaptive functioning. These results replicate and extend previous reports (Clifford et al., 2013; Garon et al., 2009; Hepburn & Stone, 2006; Konstantareas & Stewart, 2006; Zwaigenbaum et al., 2005; but see Bailey et al., 2000), suggesting that pervasive temperamental challenges in the EC domain are already present in toddlerhood for those with ASD, cannot be fully attributed to cognitive delays, and have consequences for future social development.
The importance of these findings is underscored by a large body of work that links early EC vulnerabilities with a wide range of non-optimal developmental outcomes in children without ASD. Early childhood difficulties in the self-regulatory capacity to engage executive control processes have been linked with later aggressive, antisocial, and externalizing behaviors (Kochanska & Knaack, 2003; Rothbart & Ahadi, 1994), as well as ADHD symptoms (Carlson, Jacobvitz, & Sroufe, 1995). Aspects of EC relate to different features of ADHD in school-age children, with impaired attentional control predicting attention deficits and low inhibitory control correlating with hyperactivity/impulsivity (Martel, Nigg, & Von Eye, 2009). This is highly relevant to ASD, as attentional and behavioral problems affect as many as 48% of children on the autism spectrum (Simonoff et al., 2008). Closely associated with EC, both in terms of underlying brain networks and genetic underpinnings (Rothbart, Sheese, & Posner, 2007), is executive function (EF), a construct that encompasses skills relating to attentional control. EF skills are known to be impaired in older children with ASD, impacting their ability to navigate fast-paced and complex real-life settings (Hill, 2004; Rosenthal et al., 2013).
Negative emotionality (NE), hedonically-negative reactive responses to novelty and other challenges, was impacted in both toddlers with ASD and DD compared to TD peers: both groups were vulnerable in one facet of this domain, slower recovery from extreme emotions (Soothability). This result is consistent with previous reports of high-risk siblings and school-age children with ASD (Clifford et al., 2013; Konstantareas & Stewart, 2006). Importantly, studies of typically-developing preschoolers suggest that greater NE in early childhood predicts lower social competency at older ages (Nelson, Martin, Hodge, Havill, & Kamphaus, 1999). Current models of the emergence of anxiety symptoms in childhood and adolescence stress the role of early temperamental factors, among them NE (Kagan, Reznick, & Snidman, 1987; Lonigan, Vasey, Phillips, & Hazen, 2004; Pérez-Edgar et al., 2011). Moreover, as temperamental risk for anxiety appears to be a function of the interaction between reactive (NE) and effortful (EC) traits (Lonigan et al., 2004), the combination of high NE and low EC reported here in toddlers with ASD may be consequential for the development of anxiety disorders, which affect up to 50% of children with ASD (Kerns & Kendall, 2012; Simonoff et al., 2008).
Diminished excitement about upcoming pleasant activities (Positive Anticipation) drove the lower surgency scores in the ASD group compared to controls, as reported previously (Bailey et al., 2000; Garon et al., 2009; Rosario et al., 2013; Zwaigenbaum et al., 2005). Notably, Positive Anticipation (PA) reflects more than exuberance, it also taps into excitement over anticipation of an imminent event (e.g., “How often did your child get very excited when told that loved adults would visit?”), an emotion that involves mental representation. It is thus not surprising that PA was the only TBAQ-S scale to be significantly correlated with nonverbal DQ, albeit weakly. Like anxiety, depression in middle childhood is associated with early temperamental precursors including lower positive emotionality, a relationship moderated by the presence of NE (Dougherty, Klein, Durbin, Hayden, & Olino, 2010). Given these specific longitudinal associations between the major domains of temperament and later psychopathology, early measurement of temperamental characteristics may help identify children with ASD who are at highest risk for developing a range of attentional, affective, and behavioral problems later on.
While several facets of temperament evidenced ASD-specific differences, two areas did not distinguish toddlers with ASD from their DD peers: Attention Focusing and Soothability. The ability to sustain attention to activities for longer periods of time increases from the second to third year in typically-developing children (Putnam et al., 2006), thus the lower scores on Attention Focusing reported here in the two delayed groups is likely to be associated with their lower cognitive maturity. Such a link between Soothability and lower developmental level in the delayed groups is less evident, as this trait reportedly declines over this period in non-clinical samples (Putnam et al., 2006) but was already lower in the ASD and DD groups at age 2 compared to their TD peers.
A major question of interest in the field focuses on early predictors of the severity of social impairments as well as social competence in ASD. Changes in several temperament scales within the EC domain (Garon et al., 2015) contributed uniquely to social outcomes in the children with ASD, over and above the effects of initial verbal and nonverbal ability, severity of autism symptoms, and adaptive social and communication behavior. Greater severity of autism symptoms at outcome was predicted by minimal improvement in the awareness of and reactivity to low-intensity stimuli (e.g., the sound of a plane overhead or changes in another's appearance; Perceptual Sensitivity) from age 2 to age 3 ½. Conversely, improvements in the ability to inhibit behavior (Inhibitory Control) when instructed and increases in the enjoyment of low-key activities (Low-Intensity Pleasure) were both related to better social adaptive skills one to two years later. These differential associations support the notion that functional social ability and social disability represent distinct dimensions, not two ends of a continuum (Klin et al., 2007).
How can these prospective relationships be understood? Some temperamental features may reflect behavioral styles or inherent capacities that promote learning. For example, given that attention gates learning (Posner & Rothbart, 2005), attention regulation is advantageous for the acquisition of skills such as expressive language (Salley, Panneton, & Colombo, 2013) and for monitoring the activities of others, the key to observational learning (Shic, Bradshaw, Klin, Scassellati, & Chawarska, 2011). Attention in a dyadic context in late infancy affects the development of verbal skills and severity of autism symptoms (Campbell, Shic, Macari, & Chawarska, 2013). Similarly, the current data indicates that improvement in attentional and behavioral regulation heralds more positive social outcomes. Higher ratings of awareness of minor aspects of the environment at age 3½ relative to age 2, signaling greater attention to details about people and objects (both animate and inanimate), were associated with lower autism severity at preschool age. Increased pleasure derived from quiet activities requiring well-regulated attention, such as being read to or spoken to, was associated with greater social adaptive skills at later ages. Finally, improved inhibitory control predicted greater social competence; perhaps through its role in the emerging understanding of mental states (Carlson & Moses, 2001), inhibitory control becomes pivotal to navigating everyday social interactions.
Conclusions
Taken together, these findings suggest that, as very young children with ASD gain greater control over attention and behavior over time, the more likely they are to experience lessening of autism symptoms and the more apt they are to make strides in adaptive social functioning. Both point to the consequential nature of attention and behavioral regulation in early childhood for later outcomes and suggest important treatment targets for early and potentially preventative interventions in ASD. Although further longitudinal studies are needed, given the established links between early temperamental characteristics and later behavioral and affective outcomes in other populations, vulnerabilities in the Effortful Control and Negative Emotionality domains in toddlers with ASD may signal risk for developing attentional, affective, and behavioral symptoms that are likely to hinder their learning and adaptation over and above their social and cognitive disability. Given the ubiquity of comorbid conditions in children and adolescents with ASD, particularly ADHD and anxiety (Simonoff et al., 2008), this study helps illuminate the early childhood predictors of such problems. Therefore, repeated assessment of temperament in early childhood may help identify those at the greatest risk for more severe variants of social disability as well as for comorbid behavioral and affective problems, ADHD, and EF dysfunction. It may also inform the design of individualized preventative intervention approaches for symptoms that may not constitute core impairments, but nonetheless, can be very consequential for the child's ability to learn and adapt. Our findings add to the emerging body of literature implicating characteristics outside of the realm of core autism symptoms or language domain as predictors of outcome (Campbell et al., 2013; Garon et al., 2015; Klintwall, Macari, Eikeseth, & Chawarska, 2015; Schreibman, Stahmer, Barlett, & Dufek, 2009; Vivanti, Dissanayake, Zierhut, & Rogers, 2013). Moreover, our study highlights the importance of investigations into biologically-based dimensions that may shed light on non-core facets of the early ASD phenotype that are potentially relevant to the emergence of comorbid conditions later in childhood.
Supplementary Material
What is Known
Temperament has rarely been studied in clinic-referred toddlers with ASD.
What is New
Two-year-olds with ASD exhibited temperamental differences across domains compared with TD and DD peers, with the most striking vulnerabilities within the effortful control (EC) domain.
Temperament was not associated with concurrent autism symptom severity or developmental level in toddlers with ASD and was highly stable from age 2 to 3½.
Developmental changes in traits within the EC domain uniquely predicted social adaptive skills and autism severity at preschool-age.
What is Clinically Relevant
Assessment of temperament in early childhood may help identify those at the greatest risk for more severe variants of social disability as well as for later behavioral problems and comorbid disorders.
Acknowledgments
This study was supported by the National Institute of Mental Health P50 MH081756, Project 2 (KC); NIMH R01MH087554 (KC); NIMH R03MH086732 (SM); DOD W81XWH-12-ARP-IDA (KC), NIMH U54 MH66494 (KC); Autism Speaks, NAAR, the V & L Marx Foundation, and the Associates of the Child Study Center. Portions of this study were presented at the International Meeting for Autism Research, 2012. We wish to express our appreciation to the families and their children for their participation. We thank Kelly Powell and Sophy Kim for their comments, and Lauren DiNicola and Rebecca Shalev for their edits.
Abbreviations
- ASD
Autism Spectrum Disorder
- DD
Developmental Delays
- TD
Typical Development
- TBAQ-S
Toddler Behavior Assessment Questionnaire–Supplemental
- ADOS-G
Autism Diagnostic Observation Schedule-Generic
Footnotes
Note that Pearson's correlations index the stability of individual differences within the sample over time, not absolute stability of the trait at a group level.
References
- Bailey DB, Hatton DD, Mesibov G, Ament N, Skinner M. Early development, temperament, and functional impairment in autism and fragile x syndrome. Journal of Autism & Developmental Disorders. 2000;30(1):49–49. doi: 10.1023/a:1005412111706. [DOI] [PubMed] [Google Scholar]
- Backen-Jones L, Gartstein M, Rothbart MK, Chasman J. Presented at the Society for Research in Child Development. Albuquerque, NM: 1999. Apr, Developing Fine-Grained Assessments of Caregiver Report of Temperament in Infants and Toddlers. [Google Scholar]
- Booth-LaForce C, Oxford ML. Trajectories of Social Withdrawal From Grades 1 to 6: Prediction From Early Parenting, Attachment, and Temperament. Developmental Psychology. 2008;44(5):1298–1313. doi: 10.1037/a0012954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DJ, Shic F, Macari S, Chawarska K. Gaze Response to Dyadic Bids at 2 Years Related to Outcomes at 3 Years in Autism Spectrum Disorders: A Subtyping Analysis. Journal of Autism and Developmental Disorders. 2013;44(2):431–442. doi: 10.1007/s10803-013-1885-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson EA, Jacobvitz D, Sroufe L Alan. A Developmental Investigation of Inattentiveness and Hyperactivity. Child Development. 1995;66(1):37–54. doi: 10.1111/j.1467-8624.1995.tb00854.x. [DOI] [PubMed] [Google Scholar]
- Caspi A, Henry B, McGee RO, Moffitt TE, Silva PA. Temperamental Origins of Child and Adolescent Behavior Problems: From Age Three to Age Fifteen. Child Development. 1995;66(1):55–68. doi: 10.1111/j.1467-8624.1995.tb00855.x. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Klin A, Paul R, Macari S, Volkmar F. A prospective study of toddlers with ASD: short-term diagnostic and cognitive outcomes. Journal of Child Psychology and Psychiatry. 2009;50(10):1235–1245. doi: 10.1111/j.1469-7610.2009.02101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess S, Thomas A, Birch H. Characteristics of the Individual Child's Behavioral Responses to the Environment*. American Journal of Orthopsychiatry. 1959;29(4):791–802. doi: 10.1111/j.1939-0025.1959.tb00249.x. [DOI] [PubMed] [Google Scholar]
- Chess S, Thomas A, Rutter M, Birch HG. Interaction of temperament and environment in the production of behavioral disturbances in children. American Journal of Psychiatry. 1963;120(2):142–148. doi: 10.1176/ajp.120.2.142. [DOI] [PubMed] [Google Scholar]
- Clifford SM, Hudry K, Elsabbagh M, Charman T, Johnson MH. Temperament in the First 2 Years of Life in Infants at High-Risk for Autism Spectrum Disorders. Journal of Autism and Developmental Disorders. 2013;43(3):673–686. doi: 10.1007/s10803-012-1612-y. [DOI] [PubMed] [Google Scholar]
- Dougherty LR, Klein DN, Durbin CE, Hayden EP, Olino TM. Temperamental Positive and Negative Emotionality and Children's Depressive Symptoms: A Longitudinal Prospective Study from Age Three to Age Ten. Journal of Social & Clinical Psychology. 2010;29(4):462–488. [Google Scholar]
- Garon N, Bryson SE, Zwaigenbaum L, Smith IM, Brian J, Roberts W, Szatmari P. Temperament and its Relationship to Autistic Symptoms in a High-Risk Infant Sib Cohort. Journal of Abnormal Child Psychology. 2008;37(1):59–78. doi: 10.1007/s10802-008-9258-0. [DOI] [PubMed] [Google Scholar]
- Garon N, Zwaigenbaum L, Bryson S, Smith IM, Brian J, Roncadin C, et al. Roberts W. Temperament and its Association with Autism Symptoms in a High-risk Population. Journal of Abnormal Child Psychology. 2015:1–13. doi: 10.1007/s10802-015-0064-1. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH. Studying Temperament via Construction of the Toddler Behavior Assessment Questionnaire. Child Development. 1996;67(1):218–235. [PubMed] [Google Scholar]
- Goldsmith HH, Buss AH, Plomin R, Rothbart MK, Thomas A, Chess S, et al. McCall RB. Roundtable: What Is Temperament? Four Approaches. Child Development. 1987;58(2):505–529. [PubMed] [Google Scholar]
- Goldsmith HH, Campos JJ. Toward a Theory of Infant Temperament. In: Emde RN, Harmon RJ, editors. The Development of Attachment and Affiliative Systems. Springer; 1982. pp. 161–193. [Google Scholar]
- Goldsmith HH, Rothbart MK. Toddler Behavior Assessment Questionnaire-Supplemental 1999 [Google Scholar]
- Gotham K, Pickles A, Lord C. Standardizing ADOS Scores for a Measure of Severity in Autism Spectrum Disorders. Journal of Autism and Developmental Disorders. 2008;39(5):693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie W, Swineford LB, Nottke C, Wetherby AM. Early diagnosis of autism spectrum disorder: stability and change in clinical diagnosis and symptom presentation. Journal of Child Psychology and Psychiatry. 2013;54(5):582–590. doi: 10.1111/jcpp.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepburn SL, Stone WL. Using Carey Temperament Scales to Assess Behavioral Style in Children with Autism Spectrum Disorders. Journal of Autism & Developmental Disorders. 2006;36(5):637–642. doi: 10.1007/s10803-006-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill EL. Executive dysfunction in autism. Trends in Cognitive Sciences. 2004;8(1):26–32. doi: 10.1016/j.tics.2003.11.003. [DOI] [PubMed] [Google Scholar]
- IBM. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM; 2012. [Google Scholar]
- Jones LB, Rothbart MK, Posner MI. Development of executive attention in preschool children. Developmental Science. 2003;6(5):498–504. [Google Scholar]
- Kagan J, Reznick JS, Snidman N. The Physiology and Psychology of Behavioral Inhibition in Children. Child Development. 1987;58(6):1459–1473. [PubMed] [Google Scholar]
- Kannass KN, Oakes LM. The development of attention and its relations to language in infancy and toddlerhood. Journal of Cognition and Development. 2008;9(2):222–246. [Google Scholar]
- Kanne SM, Gerber AJ, Quirmbach LM, Sparrow SS, Cicchetti DV, Saulnier CA. The role of adaptive behavior in autism spectrum disorders: Implications for functional outcome. Journal of autism and developmental disorders. 2011;41(8):1007–1018. doi: 10.1007/s10803-010-1126-4. [DOI] [PubMed] [Google Scholar]
- Kerns CM, Kendall PC. The Presentation and Classification of Anxiety in Autism Spectrum Disorder. Clinical Psychology: Science and Practice. 2012;19(4):323–347. [Google Scholar]
- Kim SH, Macari S, Koller J, Chawarska K. Examining the phenotypic heterogeneity of early autism spectrum disorder: subtypes and short-term outcomes. Journal of Child Psychology and Psychiatry. 2016;57(1):93–102. doi: 10.1111/jcpp.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A, Saulnier CA, Sparrow SS, Cicchetti DV, Volkmar FR, Lord C. Social and Communication Abilities and Disabilities in Higher Functioning Individuals with Autism Spectrum Disorders: The Vineland and the ADOS. Journal of Autism & Developmental Disorders. 2007;37(4):748–759. doi: 10.1007/s10803-006-0229-4. [DOI] [PubMed] [Google Scholar]
- Klintwall L, Macari S, Eikeseth S, Chawarska K. Interest level in 2-year-olds with autism spectrum disorder predicts rate of verbal, nonverbal, and adaptive skill acquisition. Autism. 2015;19(8):925–933. doi: 10.1177/1362361314555376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanska G, Knaack A. Effortful Control as a Personality Characteristic of Young Children: Antecedents, Correlates, and Consequences. Journal of Personality. 2003;71(6):1087. doi: 10.1111/1467-6494.7106008. [DOI] [PubMed] [Google Scholar]
- Konstantareas MM, Stewart K. Affect Regulation and Temperament in Children with Autism Spectrum Disorder. Journal of Autism & Developmental Disorders. 2006;36(2):143–154. doi: 10.1007/s10803-005-0051-4. [DOI] [PubMed] [Google Scholar]
- Lonigan CJ, Vasey MW, Phillips BM, Hazen RA. Temperament, Anxiety, and the Processing of Threat-Relevant Stimuli. Journal of Clinical Child & Adolescent Psychology. 2004;33(1):8–20. doi: 10.1207/S15374424JCCP3301_2. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. Rutter M. The Autism Diagnostic Observation Schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Marshall PJ, Fox NA, Henderson HA. Temperament as an Organizer of Development. Infancy. 2000;1(2):239–244. doi: 10.1207/S15327078IN0102_5. [DOI] [PubMed] [Google Scholar]
- Martel MM, Nigg JT, Von Eye A. How Do Trait Dimensions Map onto ADHD Symptom Domains? Journal of Abnormal Child Psychology. 2009;37(3):337–348. doi: 10.1007/s10802-008-9255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen EP. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- Nelson B, Martin RP, Hodge S, Havill V, Kamphaus R. Modeling the prediction of elementary school adjustment from preschool temperament. Personality and Individual Differences. 1999;26(4):687–700. [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, et al. Stone WL. Recurrence risk for autism spectrum disorders: a baby siblings research consortium study. Pediatrics. 2011 doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedlow R, Sanson A, Prior M, Oberklaid F. Stability of maternally reported temperament from infancy to 8 years. Developmental Psychology. 1993;29(6):998. [Google Scholar]
- Pérez-Edgar K, Reeb-Sutherland BC, McDermott JM, White LK, Henderson HA, Degnan KA, et al. Fox NA. Attention Biases to Threat Link Behavioral Inhibition to Social Withdrawal over Time in Very Young Children. Journal of Abnormal Child Psychology. 2011;39(6):885–895. doi: 10.1007/s10802-011-9495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R. Childhood Temperament. In: Lahey BB, Kazdin AE, editors. Advances in Clinical Child Psychology. Springer; 1983. pp. 45–92. [Google Scholar]
- Posner MI, Rothbart MK. Developing mechanisms of self-regulation. Development and Psychopathology. 2000;12:427–441. doi: 10.1017/s0954579400003096. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Influencing brain networks: implications for education. Trends in Cognitive Sciences. 2005;9(3):99–103. doi: 10.1016/j.tics.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Putnam SP, Gartstein MA, Rothbart MK. Measurement of fine-grained aspects of toddler temperament: The Early Childhood Behavior Questionnaire. Infant Behavior and Development. 2006;29(3):386–401. doi: 10.1016/j.infbeh.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts BW, DelVecchio WF. The rank-order consistency of personality traits from childhood to old age: a quantitative review of longitudinal studies. Psychological Bulletin. 2000;126(1):3. doi: 10.1037/0033-2909.126.1.3. [DOI] [PubMed] [Google Scholar]
- Rosario M del, Gillespie-Lynch K, Johnson S, Sigman M, Hutman T. Parent-Reported Temperament Trajectories Among Infant Siblings of Children with Autism. Journal of Autism and Developmental Disorders. :1–13. doi: 10.1007/s10803-013-1876-x. n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal M, Wallace GL, Lawson R, Wills MC, Dixon E, Yerys BE, Kenworthy L. Impairments in real-world executive function increase from childhood to adolescence in autism spectrum disorders. Neuropsychology. 2013;27(1):13–18. doi: 10.1037/a0031299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart MK. Measurement of temperament in infancy. Child Development. 1981:569–578. [Google Scholar]
- Rothbart MK. Longitudinal observation of infant temperament. Developmental Psychology. 1986;22(3):356–365. [Google Scholar]
- Rothbart MK, Ahadi SA. Temperament and the Development of Personality. Journal of Abnormal Psychology. 1994;103(1):55–66. doi: 10.1037//0021-843x.103.1.55. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Derryberry D. Theoretical Issues in Temperament. In: Lewis M, editor. Developmental Disabilities. Springer; 1981. pp. 383–400. [Google Scholar]
- Rothbart MK, Sheese BE, Posner MI. Executive Attention and Effortful Control: Linking Temperament, Brain Networks, and Genes. Child Development Perspectives. 2007;1(1):2–7. [Google Scholar]
- Rubin KH, Burgess KB, Dwyer KM, Hastings PD. Predicting preschoolers' externalizing behaviors from toddler temperament, conflict, and maternal negativity. Developmental Psychology. 2003;39(1):164–176. [PubMed] [Google Scholar]
- Rutter M. Temperament, personality, and personality disorder. The British Journal of Psychiatry. 1987;150:443–458. doi: 10.1192/bjp.150.4.443. [DOI] [PubMed] [Google Scholar]
- Salley B, Panneton RK, Colombo J. Separable Attentional Predictors of Language Outcome. Infancy. 2013;18(4):462–489. doi: 10.1111/j.1532-7078.2012.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanson A, Hemphill SA, Smart D. Connections between Temperament and Social Development: A Review. Social Development. 2004;13(1):142–170. [Google Scholar]
- Saulnier CA, Klin A. Brief report: Social and communication abilities and disabilities in higher functioning individuals with autism and Asperger syndrome. Journal of autism and developmental disorders. 2007;37(4):788–793. doi: 10.1007/s10803-006-0288-6. [DOI] [PubMed] [Google Scholar]
- Schreibman L, Stahmer AC, Barlett VC, Dufek S. Brief report: Toward refinement of a predictive behavioral profile for treatment outcome in children with autism. Research in Autism Spectrum Disorders. 2009;3(1):163–172. doi: 10.1016/j.rasd.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shic F, Bradshaw J, Klin A, Scassellati B, Chawarska K. Limited activity monitoring in toddlers with autism spectrum disorder. Brain Research. 2011;1380:246–254. doi: 10.1016/j.brainres.2010.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric Disorders in Children With Autism Spectrum Disorders: Prevalence, Comorbidity, and Associated Factors in a Population-Derived Sample. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47(8):921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV. Vineland Adaptive Behavior Scales: Interview Edition. American Guidance Service; 1984. [Google Scholar]
- Vivanti G, Dissanayake C, Zierhut C, Rogers S. Brief Report: Predictors of Outcomes in the Early Start Denver Model Delivered in a Group Setting. Journal of Autism & Developmental Disorders. 2013;43(7):1717–1724. doi: 10.1007/s10803-012-1705-7. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23(2–3):143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.