Abstract
Background
Pathologic alterations in resting-state brain activity patterns exist among individuals with Parkinson’s disease (PD). Since physical exercise alters resting-state brain activity in non-PD populations and improves PD symptoms, we assessed the acute effect of exercise on resting-state brain activity in exercise-trained individuals with PD.
Material/Methods
Resting-state functional magnetic resonance imaging (fMRI) was collected twice for 17 PD participants at the conclusion of an exercise intervention. The acute effect of exercise was examined for PD participants using the amplitude of low frequency fluctuation (ALFF) before and after a single bout of exercise. Correlations of clinical variables (i.e., PDQ-39 quality of life and MDS-UPDRS) with ALFF values were examined for the exercise-trained PD participants.
Results
An effect of acute exercise was observed as an increased ALFF signal within the right ventromedial prefrontal cortex (PFC), left ventrolateral PFC, and bilaterally within the substantia nigra (SN). Quality of life was positively correlated with ALFF values within the vmPFC and vlPFC.
Conclusions
Given the role of the SN and PFC in motor and non-motor symptoms in PD, the acute increases in brain activity within these regions, if repeated frequently over time (i.e., exercise training), may serve as a potential mechanism underlying exercise-induced PD-specific clinical benefits.
MeSH Keywords: Functional Neuroimaging, Parkinson Disease, Resistance Training
Background
Parkinson’s disease (PD) is a multisystem disorder that results in progressive deterioration of multiple structures in the brain/nervous system [1,2]. As a result, PD presents clinically with both motor and non-motor deficits. Although symptomatic progression is largely monitored using the Movement Disorders Society – Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), currently there are no reliable tests to monitor biological disease progression. However, recent advances in neuroimaging, specifically resting-state functional magnetic resonance imaging (rs-fMRI), offer promise by revealing PD-induced changes in regionally specific brain activity [3,4].
Utilization of rs-fMRI to measure the amplitude of low-frequency fluctuation (ALFF), which serves as an index of spontaneous brain activity [5–7], has provided insight into how altered resting-state activity patterns in certain brain regions may be associated with distinct motor and non-motor symptoms [6] or with disease progression [8] in PD. A handful of rs-fMRI studies have also investigated changes in brain activity associated with common PD medications (i.e., levodopa) [3,4,9,10]. However, despite the substantial literature base supporting voluntary physical exercise as an effective adjuvant therapy for PD [11–19], the impact of exercise on resting-state activity patterns has yet to be evaluated in this patient population.
Studies investigating the short- and long-term impacts of exercise on resting-state brain activity in non-PD populations are sparse. Only two studies have evaluated the effect of exercise alone on adults [20,21] with just one assessing individuals with a recognized dysregulation of resting-state brain activity [20]. Results show that a single session of aerobic exercise alters resting-state brain activity in young healthy adults [21] while six months of aerobic exercise training exerts a normalizing effect on resting-state brain activity in overweight/obese individuals [20]. Taken together, these findings suggest that exercise induces immediate changes in intrinsic brain activity and may also exhibit a cumulative restorative effect on dysregulated brain regions in a pathological context.
In this study, we hypothesized that an acute bout of exercise will alter whole-brain activity in exercise-trained individuals with PD. Given the role of the substantia nigra (SN) in PD, and small volume of this brain region, we anatomically defined this area for analysis. Resting-state brain activity was correlated with clinical measures that evaluated symptom severity in exercise-trained individuals with PD.
Material and Methods
Participants
Eighteen patients with idiopathic PD were recruited from the Movement Disorders Clinic at the University of Alabama at Birmingham (UAB) Department of Neurology (detailed eligibility criteria are published elsewhere [13]). One participant was excluded due to motion artifact, therefore participant demographic and clinical characteristics for n=17 are presented in Table 1. This study was approved by the UAB Institutional Review Board and each participant provided written, informed consent before participation.
Table 1.
Demographics and clinical characteristics.
| PD | |
|---|---|
| Age, y, mean (SD) | 66.6 (5.8) |
| Sex | 14 M, 3 F |
| Years since diagnosis, mean (SD) | 4.5 (4.4) |
| Hoehn and Yahr stage, n (2, 3) | 11, 6 |
| LED, mg/d, mean (SD) | 494.4 (370.3) |
| MDS-UPDRS, mean (SD) | |
| Part I | 9.0 (5.0) |
| Part II | 10.8 (5.2) |
| Part III | 32.9 (9.7) |
| Part IV | 1.4 (2.2) |
| Total | 54.2 (11.9) |
| PDQ-39, mean (SD) | |
| Mobility | 15.3 (13.3) |
| Activities of daily living | 15.9 (9.6) |
| Emotional wellbeing | 20.8 (19.2) |
| Stigma | 18.8 (15.8) |
| Social support | 14.7 (11.6) |
| Cognitive impairment | 25.7 (15.7) |
| Communication | 24.5 (16.5) |
| Bodily discomfort | 28.4 (17.4) |
| Index score | 20.5 (11.4) |
PD – Parkinson’s disease; LED – Levodopa equivalent dose; MDS-UPDRS – Movement Disorder Society – Unified Parkinson’s Disease Rating Scale; PDQ-39 – Parkinson’s Disease Questionnaire – 39. Reported clinical assessments were conducted after 16 weeks of exercise training and before fMRI acquisition.
Exercise intervention
Prior to the current imaging study, the PD participants completed 16 weeks of supervised high-intensity exercise training three days per week. Detailed methods describing the exercise intervention and functional and clinical outcomes due to the intervention were published previously [13]. Briefly, the acute bout of exercise consisted of a five minute warm up followed by five resistance training (RT) exercises (leg press, knee extension, chest press, overhead press, lat pull down) performed for three sets of 8–12 repetitions each. Instead of traditional rest periods between sets, participants completed bodyweight exercises or treadmill walking/stationary cycling for 60 seconds after each set of two back-to-back RT exercises to maintain an aerobic stimulus, averaging >60% heart rate reserve (HRR), throughout the entire 35–45 minute workout.
Clinical assessments
PD participants completed a battery of clinical questionnaires before and after the 16 week exercise intervention, as reported previously [13]. All PD participants were evaluated by a trained clinician during completion of the MDS-UPDRS and Parkinson’s Disease Questionnaire – 39 (PDQ-39) [14]. The MDS-UPDRS evaluates symptom severity during the past week using a scale 0–4 where higher scores indicate greater symptom severity. The MDS-UPDRS includes patient self-report and a motor examination administered by a trained clinician. This clinical tool is comprised of four sections: Part I is mentation, mood, behavior; Part II is non-motor symptoms; Part III is motor examination; Part IV is motor complications, and total score. The PDQ-39 is a quality of life measure that consists of 39 items in which patients use a scale 1–5 (never to always) to rate how often they have experienced PD-related symptoms and difficulties over the last month where higher scores indicate greater symptom severity. The PDQ-39 evaluates domains of mobility, activities of daily living (ADL), emotional disturbances, stigma, lack of social support, cognitive impairment, bodily discomfort, and an index score is calculated. Data was collected for these two measures and used to examine the relationship between neuronal brain activity and symptom severity in exercise-trained PD participants.
Image acquisition and processing
After the 16 week exercise intervention, the PD participants underwent a scanning session immediately before (Trained ALFF) and after (Acute ALFF) (within one hour) a single bout of exercise. Structural and functional imaging was completed on a 3.0 Tesla Siemens Magnetom Allegra scanner. High-resolution structural images were obtained in the sagittal plane using a T1-weighted series to serve as an anatomical reference (TR=2,200 ms, TE=2.6 ms, flip angle=12°, FOV=25.6 cm, matrix=256×256, slice thickness=1 mm, 0.5 mm gap). Blood oxygen level-dependent resting-state fMRI of the entire brain was conducted using a gradient-echo echoplanar pulse sequence in an oblique-axial orientation (TR=2,000 ms, TE=30 ms, flip angle=70°, FOV=19.2 cm, matrix=192×192, slice thickness=4 mm, no gap). Initial fMRI included 208 time points (including initial signal). The first five time points were excluded from analysis, leaving 203 time points (approximately 6.8 minutes) for analysis. Analysis of Functional NeuroImages (AFNI) software package [22] was used to correct for slice timing offset, motion correction, manual alignment in native space with the structural image, and spatial normalization to a standard Montreal Neurological Institute (MNI) axis.
Resting-state fMRI data were analyzed at the individual subject level to adjust for movement and artifacts, following suggestions made by Power et al. [23]. Specifically, time points concerning for artifact or motion were censored from the analysis as described previously [24]. While BOLD signal values at censored time points were censored, the time-stamp was retained to retain frequency information. An average of 0.6% of the time points were censored in the sample. Head motion parameters were also included as regressors and the remaining residual was used to evaluate the ALFF signal, using a gamma variate function to model the effect of motion. This study uses an individual subject repeated measure design.
Determining ALFF
Based on prior work showing significant differences in a clinical population, we empirically restricted our analyses of the ALFF signal to the frequency band of 0.01–0.08 Hz [25]. The ALFF signal was obtained using a bandpass filter to remove the effects of very-low frequency drift and high frequency noise [5,26], and ALFF was computed in accordance with methodology published by Biswal et al. [5].
Statistical analyses
Based on a priori hypotheses, group level analyses were restricted to cortical grey matter and the SN and paired t-tests were performed to determine the acute effect of exercise. A voxel-wise threshold of p<0.05 (corrected) was employed by using an uncorrected threshold of p<0.01 and a cluster volume larger than 472 mm3 (13 voxels of 3.0×3.0×4.0 mm dimension). Cluster size within the cortical grey matter was determined by Monte Carlo simulations to correct for multiple comparisons [27,28]. Finally, Pearson correlations were conducted to assess the relationship between resting-state brain activity (ALFF signal) and symptom severity (MDS-UPDRS and PDQ-39) upon completion of a 16 week exercise training intervention. Recent work has generated debate regarding the effectiveness of cluster-wise inference in fMRI research [29], resulting in updates to statistical algorithms [30]. In this work, we used the most recent statistical algorithms from AFNI. Our group has formally evaluated methods to clarify reliability of MRI findings in brain imaging data [6,31], and reliability of regions defined in the full brain analyses was analyzed using leave-one-out cross-validation. As our nigral analysis was hypothesis focused and not subject to cluster-thresholding, a similar cluster-reliability analysis was not required. Instead, paired t-tests were performed on the mean ALFF signal extracted from this region using a WFU Pickatlas [32,33] anatomical mask.
Results
Acute effect of exercise on trained PD resting-state activity
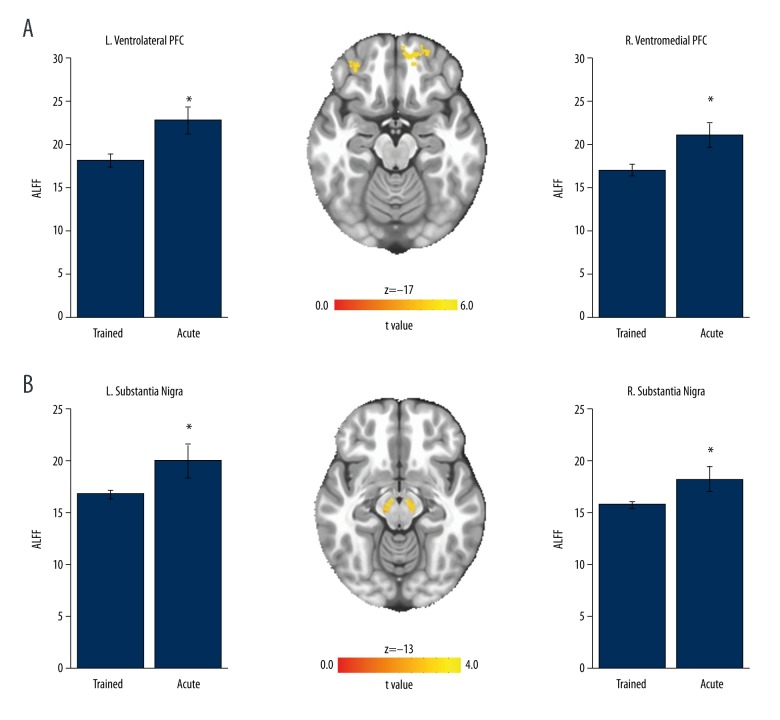
The voxel-wise paired t-test revealed significant differences in ALFF signal following a single bout of exercise within two regions of the prefrontal cortex (PFC) (Figure 1A). Increased resting-state brain activity was observed within the right ventromedial (vmPFC) and left ventrolateral PFC (vlPFC) (t[16]>2.92; p<0.05 corrected). Location, volumes, and coordinates are from the MNI axis for the peak voxel of activation. Paired t-tests on the mean ALFF signal within the SN also revealed a significant increase in resting-state brain activity following a single bout of exercise within the left (t[16]=−2.15, p<0.05) and right (t[16]=−2.17, p<0.05) SN (Figure 1B).
Figure 1.
Neural response to a single bout of exercise within trained PD patients. (A) Resting-state brain activity (ALFF signal) was increased within the left ventrolateral PFC (volume mm3=32,220, x=38.0, y=−54, z=1) and right ventromedial PFC (volume mm3=20,556, x=−7, y=−53, z=−12). (B) ALFF signal was increased within the left and right substantia nigra; * p<0.05, values are mean ± SEM ALFF signal of all voxels within the volumes of activation.
Relating clinical measures to resting-state activity in exercise-trained PD participants
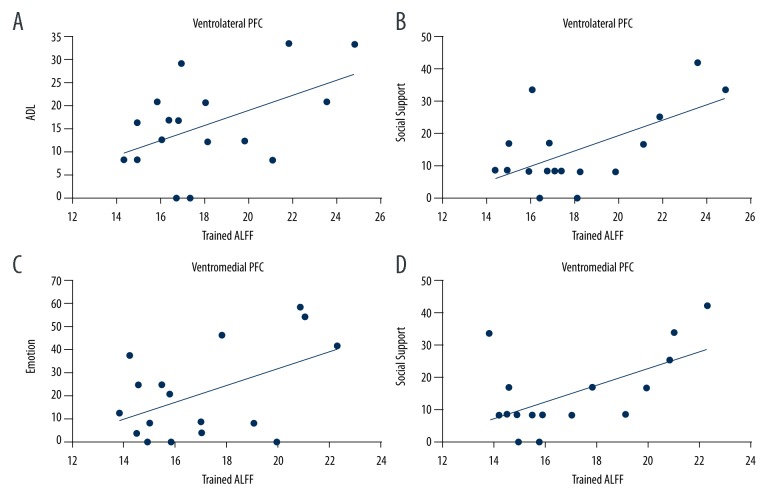
Pearson correlations showed a positive relationship between the trained PDQ-39 ratings (i.e., after 16 weeks of exercise) and the Trained ALFF signal within the vlPFC and vmPFC. Specifically, the Trained ALFF signal within the vlPFC was correlated with ADL (Figure 2A; r=0.50, p<0.042) and social support (Figure 2B; r=0.61, p=0.009). Positive relationships were also observed between the Trained ALFF signal within the vmPFC and emotional disturbance (Figure 2C; r=0.49, p<0.047) and social support (Figure 2D; r=0.58, p=0.015). No relationships were revealed between Trained ALFF signal and the MDS-UPDRS.
Figure 2.
Regions that showed a relationship with chronic exercise and the PDQ-39. Resting-state brain activity (ALFF signal) within the left ventrolateral PFC was positively correlated with (A) activities of daily living (ADL) and (B) lack of social support. Similar relationships were observed for right ventromedial PFC resting-state activity and (C) emotional disturbances and (D) a lack of social support.
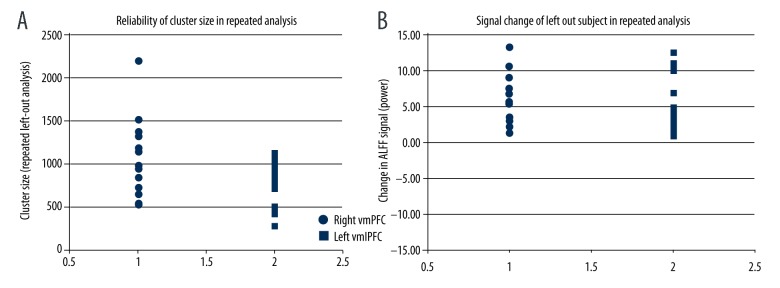
Robustness of whole brain analysis results under cross-validation
In a serial leave-one-out analysis, using a voxel-wise threshold of p<0.01 the right vmPFC in all cases met cluster thresholding criteria of 472 voxels (regional size=1,002±420 voxels, range 511–2,195 voxels). The left vlPFC met cluster thresholding criteria in 14 of 17 analyses (regional size=702±252 voxels, range 283–1,121) (Figure 3A). In a separate analysis using a voxel-wise threshold of p<0.05, both clusters met criteria in all repeated analyses. In all cases, mean signal in the left-out subject was increased in a region derived the 16 subjects in the analysis (right vmPFC, increase in ALFF signal power=5.23±3.45, range 1.63–13.43; left vlPFC increase in ALFF power=4.40±3.68, range 1.12–12.65) (Figure 3B). The cross-validation results indicate that ALFF is reliably increased in the detected regions within the sample, and that the finding is not due to an outlier effect.
Figure 3.
Reliability finding in repeated analysis. (A) The right vmPFC met cluster thresholding criteria in all cases, whereas the left vlPFC met cluster thresholding criteria in 14 of 17 analyses. (B) In all cases, signal change in the left out subject was increased in a region derived from 16 subjects in the analysis.
Discussion
This pilot trial is the first to report acute effects of exercise on neuronal resting-state brain activity in exercise-trained individuals with PD. We demonstrate exercise-induced increases in resting-state activity within the SN, right vmPFC, and left vlPFC. Our results are robust under cross-validation. Among PD patients in the chronically trained state quality of life (i.e., PDQ-39) was positively correlated with right vmPFC and left vlPFC resting-state brain activity. It is important to take into account the small sample size when considering the implications of our study; however, these pilot findings are relevant considering the well characterized neurodegeneration of the SN, deterioration of vlPFC-related executive functions [34] and disturbances within the vlPFC [35] and vmPFC [36] in PD. Finally, these findings may suggest a potential mechanism underlying exercise-induced non-motor improvements in individuals with PD.
Dopaminergic neurons within the SN play an integral role in signaling movement initiation and motor learning. Current evidence indicates that SN dopaminergic neurons are more susceptible to cytotoxic stimuli in PD [37] and depend on depolarizing stimuli (such as generated by physical activity) for survival; especially under cytotoxic conditions [38]. In fact, several studies have shown that exercise training preserves SN dopaminergic neuron viability and reduces motor disruption in animal models of cytotoxin-induced PD [19,39]. This study provides preliminary evidence that exercise increases SN dopaminergic neuronal activity, suggesting a mechanism for the effect of exercise on PD motor function. In this context, our current finding of acutely increased resting-state SN activity provides a hypothetical mechanism for our previously reported findings [13] of chronic motor improvement (i.e., MDS-UPDRS part III) upon completion of 16 weeks of high-intensity exercise training. Based on our findings, evaluating the impact of repeated bouts of transient increases in SN activity over time (i.e., through chronic exercise training) on disease progression in PD is a viable and potentially fruitful future research direction.
In addition to the commonly described motor symptoms associated with PD, there are a host of non-motor symptoms that manifest simultaneously. For example, executive dysfunction and emotional disturbances (e.g., depression) are non-motor symptoms that have been associated with perturbations in vlPFC and vmPFC function, respectively [6,38,40]. Similar to the SN, decreased event-related fMRI response within the left and right vlPFC is associated with impaired performance on an executive function task in unmedicated PD patients [34]. Yet, increased ALFF within the right vlPFC is positively associated with disease duration as well as levodopa dose [41] suggesting greater neuronal activity at rest within this brain region is associated with symptom severity. In contrast, activity within the vmPFC is reduced due to dopaminergic medication usage and this reduction has been associated with depression among individuals with PD [38]. Therefore, brief exercise-induced increases in vlPFC and vmPFC activity may promote executive function, as well as counteract medication-induced emotional disturbances in PD patients. The current study is the first to demonstrate that a trained exercise-induced decrease in neuronal activity within the prefrontal cortex is associated with improved non-motor symptoms (i.e., emotional disturbance) in PD. Our small sample size should be considered in the interpretation of these findings and that a larger randomized controlled trial is warranted to confirm the observed effects. However, taken together these data provide a possible link between frequent, repeated increases in vlPFC/vmPFC activity and cognitive/emotional wellbeing among PD patients.
Conclusions
The present study demonstrates that an acute bout of high-intensity exercise increases resting-state brain activity within the bilateral SN, right vmPFC, and left vlPFC in exercise trained individuals with PD. Importantly, prior work has shown that decreased neural activity within these brain areas may be associated with increased severity of some motor and non-motor symptoms in PD [6,36–38,40]. Upon completion of a 16 week exercise intervention, positive relationships were observed between our cortical brain areas and some measures of quality of life. One limitation of the current study is the lack of comparison between pre- and post-intervention resting-state brain activity. Further, the study would have been strengthened by comparison to a group of PD patients who did not exercise and by assessment of clinical measures before and immediately following the acute exercise bout. Finally, without a healthy control group, we cannot comment on whether our observed effects are limited to PD or are a general effect of exercise, although in either case an intervention that both improves motor function measurably in PD, and increases regional blood flow in regions of known importance, would appear to be generally of value. This pilot study is therefore only a first step toward establishing a better understanding of the underlying neural mechanisms that support exercise-induced changes in PD. Future studies are needed to assess chronic changes in regional brain activity due to long-term exercise training within the same participants and to link observed changes in brain activity to clinical and behavioral outcomes in PD to examine differences pre- and post-intervention.
Acknowledgements
We are indebted to the participants for their tireless effort and dedication.
Footnotes
Source of support: This work was supported by the UAB School of Medicine, UAB Center for Exercise Medicine, National Institutes of Health [grant numbers 1T32 HD071866 (NAK, KHW), 5K23NS083620 (FMS) K23NS080912 (AWA), and the UAB Center for Clinical and Translational Science [grant number UL1 TR000165]
Conflict of interest
None.
References
- 1.Braak H, Del Tredici K, Rüb U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 2.Braak H, Ghebremedhin E, Rüb U, et al. Stages in the development of Parkinson’s disease – related pathology. Cell Tissue Res. 2004;318(1):121–34. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 3.Niethammer M, Feigin A, Eidelberg D. Functional neuroimaging in Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2(5):a009274. doi: 10.1101/cshperspect.a009274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prodoehl J, Burciu RG, Vaillancourt DE. Resting state functional magnetic resonance imaging in Parkinson’s disease. Curr Neurol Neurosci Rep. 2014;14(6):448. doi: 10.1007/s11910-014-0448-6. [DOI] [PubMed] [Google Scholar]
- 5.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 6.Skidmore FM, Yang M, Baxter L, et al. Apathy, depression, and motor symptoms have distinct and separable resting activity patterns in idiopathic Parkinson disease. Neuroimage. 2013;81:484–95. doi: 10.1016/j.neuroimage.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Zou QH, Zhu CZ, Yang Y, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: Fractional ALFF. J Neurosci Methods. 2008;172(1):137–41. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo C, Guo X, Song W, et al. The trajectory of disturbed resting-state cerebral function in Parkinson’s disease at different Hoehn and Yahr stages. Hum Brain Mapp. 2015;36(8):3104–16. doi: 10.1002/hbm.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esposito F, Tessitore A, Giordano A, et al. Rhythm-specific modulation of the sensorimotor network in drug-naive patients with Parkinson’s disease by levodopa. Brain. 2013;136(Pt 3):710–25. doi: 10.1093/brain/awt007. [DOI] [PubMed] [Google Scholar]
- 10.Krajcovicova L, Mikl M, Marecek R, Rektorova I. The default mode network integrity in patients with Parkinson’s disease is levodopa equivalent dose-dependent. J Neural Transm. 2012;119(4):443–54. doi: 10.1007/s00702-011-0723-5. [DOI] [PubMed] [Google Scholar]
- 11.Allen NE, Sherrington C, Paul SS, Canning CG. Balance and falls in Parkinson’s disease: A meta-analysis of the effect of exercise and motor training. Mov Disord. 2011;26(9):1605–15. doi: 10.1002/mds.23790. [DOI] [PubMed] [Google Scholar]
- 12.Earhart GM, Falvo MJ. Parkinson disease and exercise. Compr Physiol. 2013;3(2):833–48. doi: 10.1002/cphy.c100047. [DOI] [PubMed] [Google Scholar]
- 13.Kelly NA, Ford MP, Standaert DG, et al. Novel, high-intensity exercise prescription improves muscle mass, mitochondrial function, and physical capacity in individuals with Parkinson’s disease. J Appl Physiol (1985) 2014;116(5):582–92. doi: 10.1152/japplphysiol.01277.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peto V, Jenkinson C, Fitzpatrick R, Greenhall R. The development and validation of a short measure of functioning and well being for individuals with Parkinson’s disease. Qual Life Res. 1995;4(3):241–48. doi: 10.1007/BF02260863. [DOI] [PubMed] [Google Scholar]
- 15.Uc EY, Doerschug KC, Magnotta V. Phase I/II randomized trial of aerobic exercise in Parkinson disease in a community setting. Neurology. 2014;83:413–25. doi: 10.1212/WNL.0000000000000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uhrbrand A, Stenager E, Pedersen MS, Dalgas U. Parkinson’s disease and intensive exercise therapy – a systematic review and meta-analysis of randomized controlled trials. J Neurol Sci. 2015;353(1–2):9–19. doi: 10.1016/j.jns.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 17.van der Kolk NM, King LA. Effects of exercise on mobility in people with Parkinson’s disease. Mov Disord. 2013;28(11):1587–96. doi: 10.1002/mds.25658. [DOI] [PubMed] [Google Scholar]
- 18.Skidmore FM, Patterson SL, Shulman LM, et al. Pilot safety and feasibility study of treadmill aerobic exercise in Parkinson disease with gait impairment. J Rehabil Res Dev. 2008;45(1):117–24. doi: 10.1682/jrrd.2006.10.0130. [DOI] [PubMed] [Google Scholar]
- 19.Zigmond MJ, Cameron JL, Hoffer BJ, Smeyne RJ. Neurorestoration by physical exercise: Moving forward. Parkinsonism Relat Disord. 2012;18(Suppl 1):S147–50. doi: 10.1016/S1353-8020(11)70046-3. [DOI] [PubMed] [Google Scholar]
- 20.McFadden KL, Cornier MA, Melanson EL, et al. Effects of exercise on resting-state default mode and salience network activity in overweight/obese adults. Neuroreport. 2013;24(15):866–71. doi: 10.1097/WNR.0000000000000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajab AS, Crane DE, Middleton LE, et al. A single session of exercise increases connectivity in sensorimotor-related brain networks: A resting-state fMRI study in young healthy adults. Front Hum Neurosci. 2014;8:625. doi: 10.3389/fnhum.2014.00625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 23.Power JD, Mitra A, Laumann TO, et al. NeuroImage methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage. 2014;84:320–41. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood KH, Wheelock MD, Shumen JR, et al. Controllability modulates the neural response to predictable but not unpredictable threat in humans. Neuroimage. 2015;119:371–81. doi: 10.1016/j.neuroimage.2015.06.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zang YF, He Y, Zhu CZ, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29(2):83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7(2):119–32. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- 27.Forman SD, Cohen JD, Fitzgerald M, et al. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magn Res Med. 1995;33(5):636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 28.Saad ZS, Chen G, Reynolds RC, et al. Functional imaging analysis contest (FIAC) analysis according to AFNI and SUMA. Hum Brain Mapp. 2006;27(5):417–24. doi: 10.1002/hbm.20247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci. 2016;113(28):7900–5. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cox RW, Reynolds RC, Taylor PA. AFNI and clustering: False positive rates redux. bioRxiv. 2016:1–15. [Google Scholar]
- 31.Skidmore FM, Yang M, Baxter L, et al. Reliability analysis of the resting state can sensitively and specifically identify the presence of Parkinson disease. Neuroimage. 2013;75:249–61. doi: 10.1016/j.neuroimage.2011.06.056. [DOI] [PubMed] [Google Scholar]
- 32.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19(3):1233–39. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 33.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 34.Gerrits NJHM, van der Werf YD, Verhoef KMW, et al. Compensatory fronto-parietal hyperactivation during set-shifting in unmedicated patients with Parkinson’s disease. Neuropsychologia. 2015;68:107–16. doi: 10.1016/j.neuropsychologia.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 35.Hughes LE, Altena E, Barker RA, Rowe JB. Perseveration and choice in parkinson’s disease: The impact of progressive frontostriatal dysfunction on action decisions. Cereb Cortex. 2013;23(7):1572–81. doi: 10.1093/cercor/bhs144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doruk D, Gray Z, Bravo GL, et al. Effects of tDCS on executive function in Parkinson’s disease. Neurosci Lett. 2014;582:27–31. doi: 10.1016/j.neulet.2014.08.043. [DOI] [PubMed] [Google Scholar]
- 37.Manenti R, Brambilla M, Benussi A, et al. Mild cognitive impairment in Parkinson’s disease is improved by transcranial direct current stimulation combined with physical therapy. Mov Disord. 2016;31(5):715–24. doi: 10.1002/mds.26561. [DOI] [PubMed] [Google Scholar]
- 38.Andersen AH, Smith CD, Slevin JT, et al. Dopaminergic modulation of medial prefrontal cortex deactivation in Parkinson depression. Parkinsons Dis. 2015;2015:513452. doi: 10.1155/2015/513452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Archer T, Fredriksson A, Johansson B. Exercise alleviates Parkinsonism: Clinical and laboratory evidence. Acta Neurol Scand. 2011;123(2):73–84. doi: 10.1111/j.1600-0404.2010.01360.x. [DOI] [PubMed] [Google Scholar]
- 40.Putcha D, Ross RS, Cronin-Golomb A, et al. Altered intrinsic functional coupling between core neurocognitive networks in Parkinson’s disease. NeuroImage Clin. 2015;7:449–55. doi: 10.1016/j.nicl.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hou Y, Wu X, Hallett M, Chan P, Wu T. Frequency-dependent neural activity in Parkinson’s disease. Hum Brain Mapp. 2014;35(12):5815–33. doi: 10.1002/hbm.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]