Abstract
Steroid-refractory acute graft-versus-host disease (aGVHD) remains a frequent and often fatal complication of allogeneic hematopoietic cell transplantation (HCT). Recent evidence suggests that angiogenic factors – growth factors that contribute to blood vessel development – may be involved in tissue healing and restitution after inflammatory insults such as aGVHD. However, some angiogenic factors may also be involved in inflammation and worsen clinical outcomes. In this review, we summarize the data relevant to angiogenic factors that may contribute to healing after aGVHD (epidermal growth factor and vascular endothelial growth factor-A) and angiogenic factors that may promote inflammation after aGVHD (placental growth factor and follistatin). It is currently unknown whether changes in these factors are a cause or a consequence of aGVHD. Mechanistic studies in the coming years will clarify their roles and identify new pathways for improving outcomes in steroid-refractory aGVHD.
Keywords: Allogeneic hematopoietic cell transplantation, acute GVHD, angiogenic factor, epidermal growth factor, vascular endothelial growth factor, placental growth factor, follistatin
Background
Steroid-refractory acute graft-versus-host disease (aGVHD) is a life-threatening complication of allogeneic hematopoietic cell transplantation (HCT) affecting 11% of transplant recipients [1]. In this condition, an immunocompromised host’s organs are attacked by immunocompetent lymphocytes from the donor graft without clinical improvement after the accepted first-line therapy, corticosteroids. As a result of the immunologic attack, target organs and tissues can be badly damaged, leading to inflammatory cytokine release (e.g., tumor necrosis factor-alpha) into the circulation, which can fuel ongoing immune activation in a vicious cycle [2].
Damage to the gastrointestinal (GI) tract is the major cause of morbidity and mortality in most patients with steroid-refractory aGVHD (although severe skin aGVHD presenting with erythroderma, bulla formation, and desquamation and severe liver aGVHD presenting with marked cholestasis can also be observed). Patients with steroid-refractory aGVHD of the GI tract typically have severe diarrhea (often >2 liters daily), with abdominal pain and cramping, intermittent ileus, and at times, overt GI bleeding. They endure prolonged hospital stays measured in weeks to months, are often unable to eat, receive intravenous nutrition support, develop anasarca related to hypoalbuminemia, are at risk for bacteremia due to compromised gut barrier function, and are often debilitated by steroid myopathy and malnutrition. Endoscopically, the intestinal tract of patients with severe GI aGVHD often demonstrates mucosal erythema, loss of vascular markings, and ulceration [3]. Histologically, crypt loss, epithelial and endothelial cell apoptosis, and precapillary hemorrhage are observed in these patients [4, 5]. Intensification of immunosuppression, the standard approach to steroid-refractory aGVHD at present, results in neither complete correction of malabsorption nor long-term survival in the majority of patients [6, 7]. In addition, intensification of immunosuppression can have a profoundly negative effect on infectious immunity, significantly increasing risk of life-threatening infections. New approaches that can promote restoration of epithelial and endothelial integrity and promote normal mucosal immune homeostasis without impairing the immune response to infections are urgently needed. Aside from mucoadherent platelet lysates [8] and intralesional injection of mesenchymal stromal cells into oral surfaces damaged by steroid-refractory chronic GVHD (NCT02055625), the concept of mucosal healing as an endpoint has not been extensively studied in allogeneic HCT recipients.
Both endothelial cell damage and neovascularization play a role in the pathophysiology of aGVHD. In the 1970s, the concept of “lymphocyte-induced angiogenesis” was introduced, where adoptive transfer of thymus-derived lymphocytes was observed to cause a quantifiable increase in vascular reticulation in immunocompromised recipients [9]. Although it was clear from historical studies that mature lymphocytes were the main effectors in the lymphocyte-induced angiogenesis reaction, the soluble mediators involved in host vascular proliferation were unknown. In the years that followed, several factors critical for angiogenic responses were discovered, with the first prototypic angiogenic factor, vascular endothelial growth factor-A (VEGF-A, initially known as vascular permeability factor), discovered in the 1980s [10, 11]. In general, angiogenic factors are characterized by their participation in blood vessel development, wound healing, and tissue regeneration after injury. More recently, vascular endothelial trophic factors have also been described for modulating immune responses [12, 13], which could have significant implications for the pathophysiology and treatment of steroid-refractory aGVHD.
Approximately five decades after the first description of a host vascular proliferative response accompanying aGVHD [14], Luft et al. provided critical evidence that endothelial damage, not recalcitrant T-cell activity, characterizes refractory aGVHD [15], where patients with refractory aGVHD had increasing levels of serum thrombomodulin and elevated angiopoietin-2/VEGF ratios, indicating endothelial vulnerability in refractory patients. In a multivariate analysis of non-relapse mortality, elevated angiopoietin-2/VEGF ratio >10 was associated with a 17.5-fold increased risk of death [15]. The phenomenon of endothelial cell damage and subsequent vascular response possibly arises in a manner similar to the classic description of the pathogenesis of aGVHD itself, with endothelial damage as a result of the conditioning regimen, followed by T-cell activation against host endothelial cells, followed by apparent neovascularization in an effort to repair damaged tissues. Interestingly, epithelial injury – the clinical hallmark of aGVHD – might be considered a secondary event after initial endothelial cell damage caused by alloreactive T cells [16, 17]. The dichotomy of endothelial cell damage and neovascularization in aGVHD remains an area of active investigation.
Recently, studies involving patient samples from multicenter aGVHD treatment trials have expanded the number of angiogenic factors of interest in the pathophysiology of steroid-refractory aGVHD. Alterations in VEGF-A and 3 other circulating angiogenic factors — epidermal growth factor (EGF), placental growth factor (PlGF), and follistatin (FS) — were associated with important clinical outcomes, including response to therapy and survival in a pilot study and two validation cohorts [18]. In samples collected from patients with aGVHD compared to controls, (a) plasma levels of EGF were markedly lower in patients with aGVHD, in particular those without a complete response (CR) to first-line therapy with corticosteroids, and EGF levels decreased after 28 days in patients with no response (NR) to corticosteroids; (b) plasma VEGF-A was low at the onset of aGVHD, but increased after 28 days in patients with CR to first-line corticosteroids; (c) plasma and serum PlGF and FS were elevated at the onset of aGVHD compared to controls; and (d) elevated FS predicted poor 6-month survival after aGVHD. These findings, as summarized in Table 1, suggest that some angiogenic factors may attenuate, while others may exacerbate, inflammation in aGVHD.
Table 1.
Summary of recently described clinical associations and tissue expression of angiogenic factors in aGVHD.
| Angiogenic Factor |
Circulating Level in aGVHD |
Clinical Associations | Cellular/Tissue Expression in aGVHD |
|---|---|---|---|
| EGF | Low | Decreases in patients with no response to steroids at day 28 | Unknown |
| VEGF-A | Low | Increases in patients with complete response to steroids at day 28 | Megakaryocytes, likely others |
| PlGF | High | Highest in patients with aGVHD after HCT from unrelated donors compared to sibling donors | Increased in aGVHD skin, decreased in aGVHD colon compared to normal controls |
| FS | High | Elevated levels associated with mortality at 6 months | Unknown |
With neovascularization and endothelial damage both involved in the pathophysiology of aGVHD [15, 19], interest in studying angiogenic factors for their potential healing and inflammatory roles in aGVHD has grown. Angiogenic factors in general are classified as such by their ability to contribute to the growth of new blood vessels, although the balance of some angiogenic factors may also determine clinical outcomes — repair and regeneration versus ongoing damage and inflammation — in aGVHD. In this review, we will discuss recent findings in the context of what is currently known regarding the role of EGF, VEGF-A, PlGF, and FS in tissue repair and inflammation in models that are relevant to aGVHD. It is possible that EGF and VEGF-A predominantly support tissue repair, while PlGF and FS predominantly reflect tissue damage and unresolved inflammation in aGVHD.
Repair and Regeneration Factors in aGVHD
EGF
Epidermal growth factor (EGF) levels are markedly lower in allogeneic HCT recipients with aGVHD than in those without aGVHD, and higher EGF levels are associated with a complete response to first-line therapy with corticosteroids and improved 2-year survival [20, 21]. EGF is a well-described trophic factor for gastrointestinal and other tissues, but it has not been extensively studied for its pathophysiologic role in aGVHD. However, low circulating EGF levels have been identified in inflammatory bowel disease [22], an autoimmune disorder that shares many clinical manifestations with aGVHD. The major luminal (exocrine) sources of EGF in the gastrointestinal tract are salivary glands and Brunner’s glands in the duodenum. Paneth cells in the small intestine, a recently described target of aGVHD, are also producers of EGF [23, 24], raising the possibility of an alloimmune attack on EGF-producing cells as one mechanism underlying the observation that EGF levels are markedly lower in patients with aGVHD. Damaged intestinal stem cells also produce EGF which, like EGF from Paneth cells, may work in a paracrine or autocrine manner to heal ulceration [25].
EGF has been shown to enhance gut epithelial restitution after radiation injury [26] and protect against the development of colitis in a rat model [27]. Furthermore, EGF treatment can improve ion transport capabilities, especially sodium reabsorption, in inflamed colonic mucosa [28]. In addition to providing mitogenic signals for intestinal epithelial cells, EGF also has been shown to regulate inflammation, intestinal epithelial apoptosis, and autophagy in the setting of necrotizing enterocolitis [29]. The EGF receptor (EGFR) is activated in intestinal macrophages in both experimental and in human ulcerative colitis, suggesting that EGFR signaling may play a critical role in mucosal immune homeostasis [30].
Based upon the evidence suggesting a deficiency of EGFR signaling in various forms of intestinal inflammation, supplementation of EGF has been tested in pre-clinical models and small clinical trials. Chronic administration of intraluminal EGF enhanced colonic mucosal growth in both a rodent model [31] and in humans [32]. However, rectal administration is unlikely to be adequate to treat aGVHD due to widespread gastrointestinal damage. Feasibility of systemic treatment with EGF has recently been demonstrated. In a randomized, placebo-controlled trial of intravenous recombinant EGF in premature infants with necrotizing enterocolitis, 6-day continuous IV administration of EGF improved gut mucosal thickness by 54% over baseline as early as day 4 of therapy [33]. No significant infusional or other systemic side effects from EGF administration were noted in the trial. It is not known whether similar responses could be elicited in aGVHD, with both preclinical and clinical studies currently lacking. However, such an approach is attractive, as current immunosuppression strategies do not directly address the issue of intestinal damage. Rather, clinical improvement is expected to spontaneously occur with recovery of endogenous repair mechanisms. Lack of responses to immunosuppressive therapy and poor survival after steroid-refractory aGVHD suggest that EGF-mediated repair pathways do not spontaneously activate after reduction of inflammation in most patients, although this requires further study.
Interestingly, a potential role of intestinal microbiota may exist in the availability of EGF receptor ligands in the gut. A probiotic, Lactobacillus rhamnosus GG, has been shown to increase EGF receptor signaling by enhancing the activity of an EGF receptor ligand sheddase, ADAM17 [34]. EGF may also play a role in host recovery from infections. EGF can attenuate the severity of several experimental gastrointestinal infections, including rotavirus [35], Clostridium difficile colitis [36], and enteropathogenic E. coli [37]. While the ability to resist an infection is important for survival, robust mechanisms that promote the capacity of the host to survive an infection (e.g., mucosal repair) are equally important and less methodically studied [38].
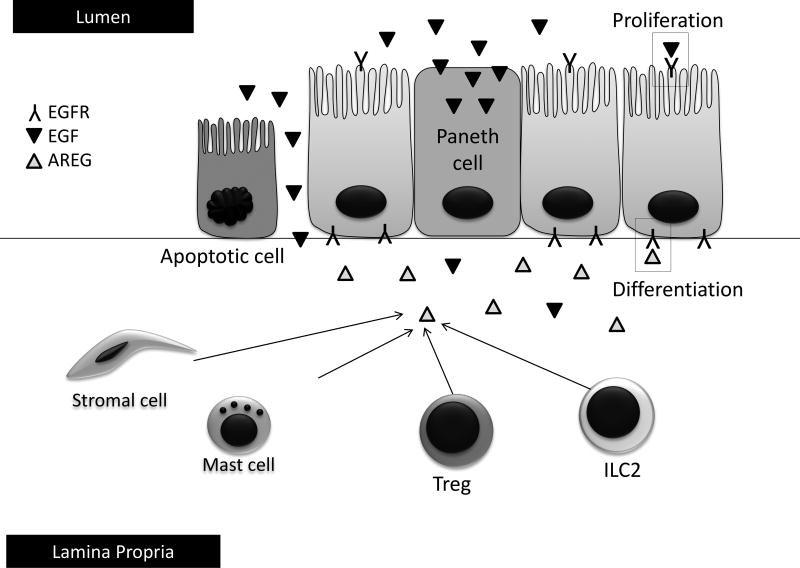
Other EGF receptor ligands produced in the gastrointestinal tract work in concert to maintain mucosal immune homeostasis. One of these ligands, amphiregulin (AREG), is produced by an immune cellular subset, innate lymphoid cells (ILCs, Figure 2), and other immune cellular subsets in the intestine [39]. AREG differs from EGF in that it is a low affinity ligand for EGFR, potentially creating a tonic signal that can produce not only proliferation but also differentiation of target cells [39]. Studies are ongoing to determine the role of AREG and other EGFR ligands as produced by ILCs and other cellular subsets in aGVHD. ILCs are also of significant interest in the transplant community as producers of IL-22, a tissue-protective cytokine of the IL-10 superfamily which may work to protect intestinal stem cells from damage and improve outcomes in aGVHD [40]. Much work remains in further defining the mechanisms by which EGF and related EGF receptor ligands may aid in the prevention or treatment of aGVHD.
Figure 2.
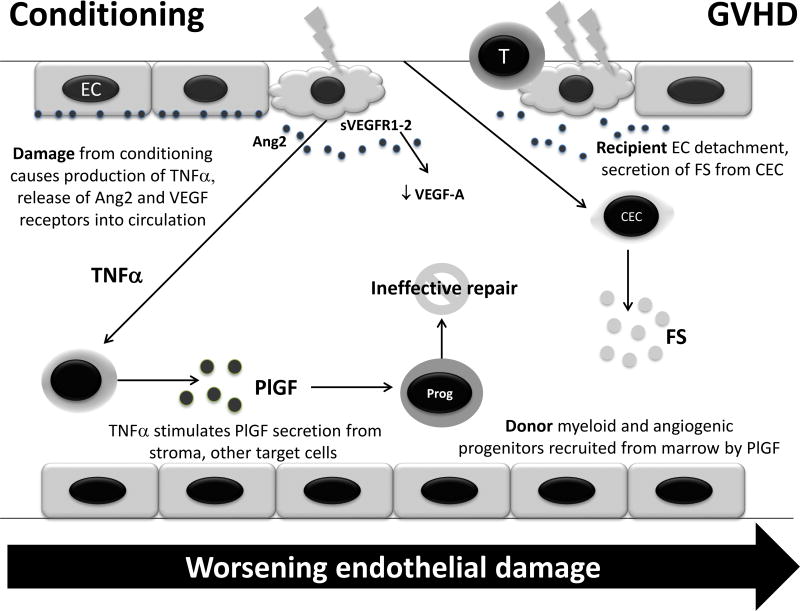
Potential mechanism of alterations in levels of angiogenic factors after conditioning prior to HCT and during GVHD. Damage to endothelial cells (EC) can cause release of angiopoietin-2 (Ang2) and vascular endothelial growth factor receptors (VEGFR) 1–2, the latter of which possibly leads to sequestration of VEGF-A in circulation. Damage also increases tumor necrosis factor-alpha (TNF-α) production, leading to increased production of placental growth factor (PlGF) in target tissues. PlGF causes chemotaxis of donor myeloid and angiogenic progenitors (Prog) to repair the damage, which is not completely effective due to ongoing inflammation. This cascade is amplified in GVHD, where endothelial damage is sufficiently severe to cause circulation of endothelial cells (CEC), which release follistatin (FS) as an autocrine enhancer of proliferation. Thus, although these alterations are observed after transplant, the cascade is exaggerated in GVHD, which reflects a greater degree of endothelial damage.
VEGF-A
VEGF-A is the prototypic angiogenic factor, and like EGF, plays an important role in healing of intestinal mucosa after damage [41]. VEGF-A inhibitors have been available for clinical use since 2004 and have revolutionized treatment of many solid tumors by both interfering with the blood supply to tumors as well as correcting the VEGF-induced immune suppression that accompanies chronic inflammation in malignancies [42]. The preponderance of studies available regarding the role of VEGF in immune responses suggests that it is more regulatory and immune suppressive as opposed to immune activating. VEGF-A at physiologic concentrations can impair thymic output [43], and continuous infusion of VEGF-A in a steady-state serum level of 120 to 160 pg/mL leads to inhibition of dendritic cell maturation with a concomitant expansion of B cells and immature myeloid cells [44]. The functional impairment of dendritic cells matured in the presence of VEGF-A can be restored by VEGF-A inhibitors, such as bevacizumab and sorafenib [45]. Recently, Voron et al. identified enhanced inhibitory checkpoint receptors on cytotoxic CD8+ T-lymphocytes – a mechanism by which VEGF-A exerts its immune modulatory effects on T cells [46].
The above observations suggest that enhancing VEGF-A signaling would be helpful in attenuating an alloimmume attack in aGVHD. Indeed, in addition to our own studies, others have shown that higher VEGF levels appear to be protective against aGVHD [47, 48]. It is possible that a patient’s capacity to produce VEGF-A in response to damage or injury to peripheral tissues underlies that observation. Accordingly, patients with VEGF single nucleotide polymorphisms associated with low VEGF production have increased risk of aGVHD [49]. Another line of evidence suggesting a protective role of VEGF-A in aGVHD is the observation that VEGF-A blockade worsens aGVHD in experimental transplantation [50]. However, not all studies evaluating VEGF-A in aGVHD are completely consistent with a protective effect [51]. One recent study of pediatric recipients found that VEGF-A at day 21 post-HCT was significantly higher in patients destined to develop skin or intestine aGVHD compared to those who did not develop aGVHD, and when coupled with a higher angiopoietin-2 level also associated with a significantly higher risk of relapse or death after allogeneic HCT [52]. The range of tissues with increased expression of VEGF-A after aGVHD are not completely known, although bone marrow megakaryocytes have been shown to increase VEGF-A expression after aGVHD [53]. Further mechanistic studies will be required to clarify the role of VEGF-A in recovery from aGVHD.
Damage and Inflammation Factors in GVHD
PlGF
PlGF is a member of the VEGF family of angiogenic factors. Although PlGF is redundant as a growth factor in homeostasis, it is required for angiogenesis in settings such as pregnancy and wound healing [54]. Like VEGF-A, PlGF signals through VEGF receptor 1 (VEGFR1). Due to its production by placental tissues [55], it would seem as though PlGF would play a role in immunologic tolerance. However, PlGF appears to exert a more inflammatory role in pathologic conditions than VEGF-A. PlGF is involved in regulation of cutaneous inflammation and edema [56], and it causes an increase in production of TNF-alpha, IL-1, IL-8, MIP-1 beta, and MCP-1 from monocytes [57]. In the circulation, PlGF levels have been shown to increase with VEGF-A blockade for treatment of solid tumors [58] as well as in several inflammatory conditions [59].
Recently, we have shown that circulating PlGF levels in allogeneic HCT patients are elevated by over 10-fold compared to healthy donors, with PlGF levels further elevated in the setting of aGVHD especially after unrelated donor HCT, regardless of organ involvement or severity [20, 60]. The source of PlGF in allogeneic HCT is not completely known. Our preliminary studies indicate that during severe aGVHD PlGF expression is increased in the skin, but decreased in the colon [61]. A unifying mechanism that results in lower circulating VEGF-A and higher PlGF in aGVHD has not yet been confirmed. Soluble VEGFR1, released from cell types such as endothelial cells (Figure 2) and mononuclear phagocytes, can bind VEGF-A with higher affinity than PlGF [62] and thus act as a sink for VEGF-A [63]. VEGFR2 from endothelial and epithelial cells can also circulate, providing an additional mechanism for VEGF-A sequestration [64]. Studies are ongoing to determine whether a VEGF-A sequestration mechanism or other possibilities explain the observations of elevated PlGF in aGVHD.
Follistatin (FS)
FS is an angiogenic factor with clinical relevance to aGVHD. We recently found that FS is elevated post-HCT, especially in the setting of aGVHD, where it is independently prognostic for 6-month survival [20, 60]. FS was first described as an inhibitor of follicle stimulating hormone in ovarian follicular fluid [65]. Aside from its hormone regulatory and pro-angiogenic role, FS is also a specific binding protein of activin-A, a protein with inflammatory and pro-fibrotic properties [66, 67]. Studies are ongoing to determine whether FS increases are related to or independent of activin-A. It is possible that elevations in FS are reflective of endothelial damage [68, 69], a known manifestation of steroid refractory aGVHD [15]. It is not known why FS levels were associated with poor survival after treatment of aGVHD. In the Blood and Marrow Transplant Clinical Trials Network 0302 and 0802 trials, elevated FS was an independent predictor of poor survival and was not related to day 28 response after initial aGVHD therapy [18]. We recently identified that circulating FS is significantly higher in healthy pregnant women carrying a male fetus as opposed to a female fetus [70]. This sex-based disparity raises the possibility of chronic GVHD contributing to poor outcomes. This possibility will be tested in future studies.
Proposed Model of Circulating Angiogenic Factor Alterations in aGVHD
We propose a model to explain our findings of altered levels of angiogenic factors after allogeneic HCT, exacerbated by aGVHD (Figure 2). In our hypothesized model, the conditioning regimen and immunosuppressive medications for GVHD prophylaxis can damage the endothelium [71–75]. This damage leads to the production and release of tumor necrosis factor-alpha (TNFα) as previously described [2]. PlGF production is triggered in target tissues in response to inflammation and leads to recruitment of donor myeloid and angiogenic progenitors for endothelial repair [76, 77], although repair from these marrow-derived progenitors may not be completely effective in the setting of GVHD, possibly due to a relative deficiency of trophic angiogenic growth factors (i.e., EGF and VEGF-A) for repair. Failure of donor progenitors to heal endothelium was demonstrated by Mueller et al., who recently showed using short tandem repeat analysis of laser-captured endothelial cells that donor bone marrow-derived cells do not systematically integrate into the recipient’s endothelium despite complete donor chimerism in the bone marrow [78]. Soluble VEGFR1, released from cell types such as endothelial cells and mononuclear phagocytes, can bind VEGF-A with higher affinity than PlGF [62] and thus act as a sink, contributing to an anti-angiogenic phenotype [63]. VEGF-A also binds to VEGFR2, whereas PlGF does not [79]. VEGFR2 from endothelial and epithelial cells can also circulate, providing an additional mechanism for VEGF sequestration [64]. When this endothelial damage cascade is amplified in GVHD, endothelial cell detachment and release into the circulation may occur [68, 69, 80]. As a result, circulating endothelial cells release follistatin for autocrine enhancement of endothelial cell proliferation in ongoing, but unsuccessful, attempts at repair. Thus, evidence of severe endothelial damage reflects poor outcomes in aGVHD, and a shift of the anigiogenic factor milieu back to one that is able to facilitate repair and regeneration may be needed to overcome the vicious cycle.
Conclusions
In the next few years, our understanding of the role of angiogenic factors in recovery from allogeneic HCT – both with and without aGVHD – will be significantly enhanced. Preliminary evidence suggests that circulating angiogenic factors involved in healing, EGF and VEGF-A, are deficient in steroid-refractory aGVHD. Furthermore, elevated PlGF and follistatin appear to contribute to, or reflect, inflammation, which could contribute to poor clinical outcomes. Mechanistic studies of these factors are justified to identify new, non-immunosuppressive therapies to improve outcomes in steroid-refractory aGVHD.
Figure 1.
Intestinal cells require epidermal growth factor (EGF) receptor signaling for proliferation and differentiation to support healing in response to mucosal damage. Exocrine EGF in the intestinal lumen comes from predominantly submandibular and Brunner’s glands, although some luminal EGF may also be supplied by Paneth cells and damaged epithelial cells. EGF plays a key role in intestinal epithelial cell proliferation. Paracrine or autocrine EGF receptor signaling in response to damage can also occur via subepithelial sources of amphiregulin (AREG) from stromal cells, mast cells, regulatory T cells (Treg), and innate lymphoid type 2 cells (ILC2). AREG plays a key role in intestinal epithelial cell differentiation as well as proliferation. If damage is so severe that EGF and AREG can no longer be produced, intestinal epithelial restitution may be compromised.
Acknowledgments
This work is supported in part by the Masonic Cancer Center, University of Minnesota.
Footnotes
Financial disclosure: The authors of this manuscript have no relevant conflicts of interest to disclose.
The authors have no conflicts of interest to disclose and are familiar with the journal’s definition of potential conflicts of interest. All authors have read the journal’s authorship agreement.
References
- 1.Calmettes C, et al. Risk Factors for Steroid-Refractory Acute GVHD after Allogeneic SCT from Matched Related or Unrelated Donors. Biol Blood Marrow Transplant. 2015 doi: 10.1016/j.bbmt.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Holtan SG, Pasquini M, Weisdorf DJ. Acute graft-versus-host disease: a bench-to-bedside update. Blood. 2014;124(3):363–73. doi: 10.1182/blood-2014-01-514786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cruz-Correa M, et al. Endoscopic findings predict the histologic diagnosis in gastrointestinal graft-versus-host disease. Endoscopy. 2002;34(10):808–13. doi: 10.1055/s-2002-34257. [DOI] [PubMed] [Google Scholar]
- 4.Melson J, et al. Crypt loss is a marker of clinical severity of acute gastrointestinal graft-versus-host disease. Am J Hematol. 2007;82(10):881–6. doi: 10.1002/ajh.20976. [DOI] [PubMed] [Google Scholar]
- 5.Ertault-Daneshpouy M, et al. Pericapillary hemorrhage as criterion of severe human digestive graft-versus-host disease. Blood. 2004;103(12):4681–4. doi: 10.1182/blood-2003-05-1548. [DOI] [PubMed] [Google Scholar]
- 6.MacMillan ML, et al. Early antithymocyte globulin therapy improves survival in patients with steroid-resistant acute graft-versus-host disease. Biol Blood Marrow Transplant. 2002;8(1):40–6. doi: 10.1053/bbmt.2002.v8.pm11858189. [DOI] [PubMed] [Google Scholar]
- 7.Westin JR, et al. Steroid-Refractory Acute GVHD: Predictors and Outcomes. Adv Hematol. 2011;2011:601953. doi: 10.1155/2011/601953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Fante C, et al. Platelet lysate mucohadesive formulation to treat oral mucositis in graft versus host disease patients: a new therapeutic approach. AAPS PharmSciTech. 2011;12(3):893–9. doi: 10.1208/s12249-011-9649-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sidky YA, Auerbach R. Lymphocyte-Induced Angiogenesis - Quantitative and Sensitive Assay of Graft-Vs-Host Reaction. Journal of Experimental Medicine. 1975;141(5):1084–1100. doi: 10.1084/jem.141.5.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Senger DR, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219(4587):983–5. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 11.Ribatti D, Vacca A, Presta M. The discovery of angiogenic factors: a historical review. Gen Pharmacol. 2000;35(5):227–31. doi: 10.1016/s0306-3623(01)00112-4. [DOI] [PubMed] [Google Scholar]
- 12.Ribatti D, Crivellato E. Immune cells and angiogenesis. J Cell Mol Med. 2009;13(9A):2822–33. doi: 10.1111/j.1582-4934.2009.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voron T, et al. Control of the immune response by pro-angiogenic factors. Front Oncol. 2014;4:70. doi: 10.3389/fonc.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brent L, Medawar P. Quantitative studies on tissue transplantation immunity. VII. The normal lymphocyte transfer reaction. Proc R Soc Lond B Biol Sci. 1966;165(1000):281–307. doi: 10.1098/rspb.1966.0069. [DOI] [PubMed] [Google Scholar]
- 15.Luft T, et al. Steroid-refractory GVHD: T-cell attack within a vulnerable endothelial system. Blood. 2011;118(6):1685–92. doi: 10.1182/blood-2011-02-334821. [DOI] [PubMed] [Google Scholar]
- 16.Deschaumes C, et al. CD95 ligand-dependant endothelial cell death initiates oral mucosa damage in a murine model of acute graft versus host disease. Lab Invest. 2007;87(5):417–29. doi: 10.1038/labinvest.3700541. [DOI] [PubMed] [Google Scholar]
- 17.Janin A, et al. CD95 engagement induces disseminated endothelial cell apoptosis in vivo: immunopathologic implications. Blood. 2002;99(8):2940–7. doi: 10.1182/blood.v99.8.2940. [DOI] [PubMed] [Google Scholar]
- 18.Holtan SG, et al. Circulating Angiogenic Factors associated with Response and Survival in Patients with Acute Graft-versus-Host Disease: Results from Blood and Marrow Transplant Clinical Trials Network 0302 and 0802. Biol Blood Marrow Transplant. 2015 doi: 10.1016/j.bbmt.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Penack O, et al. Inhibition of neovascularization to simultaneously ameliorate graft-vs-host disease and decrease tumor growth. J Natl Cancer Inst. 2010;102(12):894–908. doi: 10.1093/jnci/djq172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holtan SG, et al. Circulating Angiogenic Factors as Biomarkers of Acute GVHD Onset and Response to Therapy: Repair and Regeneration versus Endothelial Damage and Inflammation. Blood. 2014 2014 ASH Abstract #2489. [Google Scholar]
- 21.He F, et al. Relationship of Epidermal Growth Factor to Cyclosporine-Associated Magnesium Wasting and Clinical Outcomes Post-Allogeneic Hematopoietic Cell Transplantation. Blood. 2014 ASH 2014 abstract. [Google Scholar]
- 22.Oikonomou KA, et al. Downregulation of serum epidermal growth factor in patients with inflammatory bowel disease. Is there a link with mucosal damage? Growth Factors. 2010;28(6):461–6. doi: 10.3109/08977194.2010.527967. [DOI] [PubMed] [Google Scholar]
- 23.Poulsen SS, et al. Immunohistochemical localization of epidermal growth factor in rat and man. Histochemistry. 1986;85(5):389–94. doi: 10.1007/BF00982668. [DOI] [PubMed] [Google Scholar]
- 24.Levine JE, et al. Low Paneth cell numbers at onset of gastrointestinal graft-versus-host disease identify patients at high risk for nonrelapse mortality. Blood. 2013;122(8):1505–9. doi: 10.1182/blood-2013-02-485813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright NA, Pike C, Elia G. Induction of a novel epidermal growth factor-secreting cell lineage by mucosal ulceration in human gastrointestinal stem cells. Nature. 1990;343(6253):82–5. doi: 10.1038/343082a0. [DOI] [PubMed] [Google Scholar]
- 26.McKenna KJ, et al. Epidermal growth factor enhances intestinal mitotic activity and DNA content after acute abdominal radiation. Surgery. 1994;115(5):626–32. [PubMed] [Google Scholar]
- 27.Procaccino F, et al. Protective effect of epidermal growth factor in an experimental model of colitis in rats. Gastroenterology. 1994;107(1):12–7. doi: 10.1016/0016-5085(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 28.McCole DF, et al. Epidermal growth factor partially restores colonic ion transport responses in mouse models of chronic colitis. Gastroenterology. 2005;129(2):591–608. doi: 10.1016/j.gastro.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Coursodon CF, Dvorak B. Epidermal growth factor and necrotizing enterocolitis. Curr Opin Pediatr. 2012;24(2):160–4. doi: 10.1097/MOP.0b013e3283504ddb. [DOI] [PubMed] [Google Scholar]
- 30.Lu N, et al. Activation of the epidermal growth factor receptor in macrophages regulates cytokine production and experimental colitis. J Immunol. 2014;192(3):1013–23. doi: 10.4049/jimmunol.1300133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reeves JR, Richards RC, Cooke T. The effects of intracolonic EGF on mucosal growth and experimental carcinogenesis. Br J Cancer. 1991;63(2):223–6. doi: 10.1038/bjc.1991.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinha A, et al. Epidermal growth factor enemas with oral mesalamine for mild-to-moderate left-sided ulcerative colitis or proctitis. N Engl J Med. 2003;349(4):350–7. doi: 10.1056/NEJMoa013136. [DOI] [PubMed] [Google Scholar]
- 33.Sullivan PB, et al. Intestinal mucosa remodeling by recombinant human epidermal growth factor(1–48) in neonates with severe necrotizing enterocolitis. J Pediatr Surg. 2007;42(3):462–9. doi: 10.1016/j.jpedsurg.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 34.Yan F, et al. A Lactobacillus rhamnosus GG-derived soluble protein, p40, stimulates ligand release from intestinal epithelial cells to transactivate epidermal growth factor receptor. J Biol Chem. 2013;288(42):30742–51. doi: 10.1074/jbc.M113.492397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zijlstra RT, et al. Effect of orally administered epidermal growth factor on intestinal recovery of neonatal pigs infected with rotavirus. J Pediatr Gastroenterol Nutr. 1994;19(4):382–90. doi: 10.1097/00005176-199411000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Riegler M, et al. Epidermal growth factor attenuates Clostridium difficile toxin A- and B-induced damage of human colonic mucosa. Am J Physiol. 1997;273(5 Pt 1):G1014–22. doi: 10.1152/ajpgi.1997.273.5.G1014. [DOI] [PubMed] [Google Scholar]
- 37.Buret A, et al. Effects of orally administered epidermal growth factor on enteropathogenic Escherichia coli infection in rabbits. Infect Immun. 1998;66(10):4917–23. doi: 10.1128/iai.66.10.4917-4923.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider DS, Ayres JS. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol. 2008;8(11):889–95. doi: 10.1038/nri2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zaiss DM, et al. Emerging Functions of Amphiregulin in Orchestrating Immunity, Inflammation, and Tissue Repair. Immunity. 2015;42(2):216–226. doi: 10.1016/j.immuni.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanash AM, et al. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity. 2012;37(2):339–50. doi: 10.1016/j.immuni.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahluwalia A, Tarnawski AS. Critical role of hypoxia sensor--HIF-1alpha in VEGF gene activation. Implications for angiogenesis and tissue injury healing. Curr Med Chem. 2012;19(1):90–7. doi: 10.2174/092986712803413944. [DOI] [PubMed] [Google Scholar]
- 42.Nevala WK, et al. Evidence of systemic Th2-driven chronic inflammation in patients with metastatic melanoma. Clin Cancer Res. 2009;15(6):1931–9. doi: 10.1158/1078-0432.CCR-08-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohm JE, et al. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. 2003;101(12):4878–86. doi: 10.1182/blood-2002-07-1956. [DOI] [PubMed] [Google Scholar]
- 44.Gabrilovich D, et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92(11):4150–66. [PubMed] [Google Scholar]
- 45.Alfaro C, et al. Influence of bevacizumab, sunitinib and sorafenib as single agents or in combination on the inhibitory effects of VEGF on human dendritic cell differentiation from monocytes. Br J Cancer. 2009;100(7):1111–9. doi: 10.1038/sj.bjc.6604965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Voron T, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015 doi: 10.1084/jem.20140559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Min CK, et al. Vascular endothelial growth factor (VEGF) is associated with reduced severity of acute graft-versus-host disease and nonrelapse mortality after allogeneic stem cell transplantation. Bone Marrow Transplant. 2006;38(2):149–56. doi: 10.1038/sj.bmt.1705410. [DOI] [PubMed] [Google Scholar]
- 48.Nachbaur D, et al. Vascular endothelial growth factor and activin-a serum levels following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13(8):942–7. doi: 10.1016/j.bbmt.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 49.Kim DH, et al. Vascular endothelial growth factor gene polymorphisms may predict the risk of acute graft-versus-host disease following allogeneic transplantation: preventive effect of vascular endothelial growth factor gene on acute graft-versus-host disease. Biol Blood Marrow Transplant. 2008;14(12):1408–16. doi: 10.1016/j.bbmt.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 50.Kim AR, et al. Blockade of Vascular Endothelial Growth Factor (VEGF) Aggravates the Severity of Acute Graft-versus-host Disease (GVHD) after Experimental Allogeneic Hematopoietic Stem Cell Transplantation (allo-HSCT) Immune Netw. 2011;11(6):368–75. doi: 10.4110/in.2011.11.6.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lunn RA, et al. Cytokine profiles in stem cell transplantation: possible use as a predictor of graft-versus-host disease. Hematology. 2005;10(2):107–14. doi: 10.1080/10245330400001975. [DOI] [PubMed] [Google Scholar]
- 52.Porkholm M, et al. Higher angiopoietin-2 and VEGF levels predict shorter EFS and increased non-relapse mortality after pediatric hematopoietic SCT. Bone Marrow Transplant. 2013;48(1):50–5. doi: 10.1038/bmt.2012.101. [DOI] [PubMed] [Google Scholar]
- 53.Medinger M, et al. GVHD after allogeneic haematopoietic SCT for AML: angiogenesis, vascular endothelial growth factor and VEGF receptor expression in the BM. Bone Marrow Transplant. 2013;48(5):715–21. doi: 10.1038/bmt.2012.200. [DOI] [PubMed] [Google Scholar]
- 54.Dewerchin M, Carmeliet P. PlGF: a multitasking cytokine with disease-restricted activity. Cold Spring Harb Perspect Med. 2012;2(8) doi: 10.1101/cshperspect.a011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Falco S. The discovery of placenta growth factor and its biological activity. Exp Mol Med. 2012;44(1):1–9. doi: 10.3858/emm.2012.44.1.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oura H, et al. A critical role of placental growth factor in the induction of inflammation and edema formation. Blood. 2003;101(2):560–7. doi: 10.1182/blood-2002-05-1516. [DOI] [PubMed] [Google Scholar]
- 57.Selvaraj SK, et al. Mechanism of monocyte activation and expression of proinflammatory cytochemokines by placenta growth factor. Blood. 2003;102(4):1515–24. doi: 10.1182/blood-2002-11-3423. [DOI] [PubMed] [Google Scholar]
- 58.Lieu CH, et al. The association of alternate VEGF ligands with resistance to anti-VEGF therapy in metastatic colorectal cancer. PLoS One. 2013;8(10):e77117. doi: 10.1371/journal.pone.0077117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim KJ, Cho CS, Kim WU. Role of placenta growth factor in cancer and inflammation. Exp Mol Med. 2012;44(1):10–9. doi: 10.3858/emm.2012.44.1.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holtan SG, et al. Prognostic Impact of Follistatin in Acute Graft-Versus-Host Disease: Results from BMT CTN 0302 and 0802. Biol Blood Marrow Transplant. 2015 ASBMT/CIBMTR Tandem Meetings Abstract #39 2015. [Google Scholar]
- 61.Leonard JT, et al. Tissue Expression of Placental Growth Factor in Acute Graft Versus Host Disease after Hematopoietic Stem Cell Transplantation. Blood. 2014 2014 ASH Abstract #1171. [Google Scholar]
- 62.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A. 1993;90(22):10705–9. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hornig C, et al. Release and complex formation of soluble VEGFR-1 from endothelial cells and biological fluids. Lab Invest. 2000;80(4):443–54. doi: 10.1038/labinvest.3780050. [DOI] [PubMed] [Google Scholar]
- 64.Lorquet S, et al. Soluble forms of VEGF receptor-1 and -2 promote vascular maturation via mural cell recruitment. FASEB J. 2010;24(10):3782–95. doi: 10.1096/fj.09-149070. [DOI] [PubMed] [Google Scholar]
- 65.Esch FS, et al. Structural characterization of follistatin: a novel follicle-stimulating hormone release-inhibiting polypeptide from the gonad. Mol Endocrinol. 1987;1(11):849–55. doi: 10.1210/mend-1-11-849. [DOI] [PubMed] [Google Scholar]
- 66.Nakamura T, et al. Activin-binding protein from rat ovary is follistatin. Science. 1990;247(4944):836–8. doi: 10.1126/science.2106159. [DOI] [PubMed] [Google Scholar]
- 67.de Kretser DM, et al. The roles of activin A and its binding protein, follistatin, in inflammation and tissue repair. Mol Cell Endocrinol. 2012;359(1–2):101–6. doi: 10.1016/j.mce.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 68.Phan C, et al. Activated lymphocytes promote endothelial cell detachment from matrix: a role for modulation of endothelial cell beta 1 integrin affinity. J Immunol. 1999;163(8):4557–63. [PubMed] [Google Scholar]
- 69.Almici C, et al. Changes in circulating endothelial cells count could become a valuable tool in the diagnostic definition of acute graft-versus-host disease. Transplantation. 2014;98(7):706–12. doi: 10.1097/TP.0000000000000385. [DOI] [PubMed] [Google Scholar]
- 70.Enninga EA, et al. Fetal Sex-Based Differences in Maternal Hormones, Angiogenic Factors, and Immune Mediators During Pregnancy and the Postpartum Period. Am J Reprod Immunol. 2014 doi: 10.1111/aji.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Samlowski WE, et al. Marrow ablative doses of gamma-irradiation and protracted changes in peripheral lymph node microvasculature of murine and human bone marrow transplant recipients. Lab Invest. 1987;56(1):85–95. [PubMed] [Google Scholar]
- 72.Zeng L, et al. Endothelial injury, an intriguing effect of methotrexate and cyclophosphamide during hematopoietic stem cell transplantation in mice. Transplant Proc. 2008;40(8):2670–3. doi: 10.1016/j.transproceed.2008.06.038. [DOI] [PubMed] [Google Scholar]
- 73.Kochi S, et al. Induction of apoptosis in mouse brain capillary endothelial cells by cyclosporin A and tacrolimus. Life Sci. 2000;66(23):2255–60. doi: 10.1016/s0024-3205(00)00554-3. [DOI] [PubMed] [Google Scholar]
- 74.Carmona A, et al. Distinct deleterious effects of cyclosporine and tacrolimus and combined tacrolimus-sirolimus on endothelial cells: protective effect of defibrotide. Biol Blood Marrow Transplant. 2013;19(10):1439–45. doi: 10.1016/j.bbmt.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 75.Liu HT, et al. Rapamycin inhibits re-endothelialization after percutaneous coronary intervention by impeding the proliferation and migration of endothelial cells and inducing apoptosis of endothelial progenitor cells. Tex Heart Inst J. 2010;37(2):194–201. [PMC free article] [PubMed] [Google Scholar]
- 76.Cheng SL, et al. Increased expression of placenta growth factor in COPD. Thorax. 2008;63(6):500–6. doi: 10.1136/thx.2007.087155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iwasaki H, et al. PlGF repairs myocardial ischemia through mechanisms of angiogenesis, cardioprotection and recruitment of myo-angiogenic competent marrow progenitors. PLoS One. 2011;6(9):e24872. doi: 10.1371/journal.pone.0024872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mueller RJ, et al. Persistence of recipient-type endothelium after allogeneic hematopoietic stem cell transplantation. Haematologica. 2011;96(1):119–27. doi: 10.3324/haematol.2010.030288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park JE, et al. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J Biol Chem. 1994;269(41):25646–54. [PubMed] [Google Scholar]
- 80.Biedermann BC, et al. Endothelial injury mediated by cytotoxic T lymphocytes and loss of microvessels in chronic graft versus host disease. Lancet. 2002;359(9323):2078–83. doi: 10.1016/S0140-6736(02)08907-9. [DOI] [PubMed] [Google Scholar]