Abstract
Introduction
Phosphatidylinositol-3-kinase (PI3K) inhibitors comprise a novel class of agents that are effective for the treatment of chronic lymphocytic leukemia (CLL) and indolent non-Hodgkin lymphoma (iNHL). The demonstrated efficacy and subsequent FDA approval of the prototypical PI3Kδ inhibitor idelalisib has led to development of multiple compounds targeting this pathway.
Areas Covered
We review the hypothesized therapeutic mechanisms of PI3K inhibitors, including abrogation of tonic signaling from the B cell receptor, blockade of pro-survival signals from the microenvironment, and enhancement of anti-tumor immunity. We examine toxicities of idelalisib, including bacterial infections and sepsis (possibly secondary to drug-induced neutropenia), opportunistic infections (possibly attributable to on-target inhibition of T cell function), and organ toxicities such as transaminitis and enterocolitis (possibly autoimmune, secondary to on-target inhibition of p110δ in regulatory T cells). We then summarize PI3K inhibitors that have entered clinical trials for the treatment of lymphoma, focusing on agents with significant selectivity for PI3Kα and PI3Kδ.
Expert Opinion
PI3K inhibitors, particularly those that target p110δ, have robust efficacy in the treatment of CLL and iNHL. However, in comparison to other novel agents, idelalisib has infectious and autoimmune toxicities that have limited its use. Outside of clinical trials, the use of PI3K inhibitors should be restricted to CLL patients who have progressed on ibrutinib or iNHL patients who have progressed on two prior therapies. Whether newer PI3K inhibitors will demonstrate differentiated toxicity profiles in comparable patient populations while retaining efficacy remains to be seen.
Keywords: PI3-kinase inhibitors, chronic lymphocytic leukemia, indolent non-Hodgkin lymphoma, idelalisib, duvelisib, umbralisib, copanlisib
1. Introduction
The first effective pharmacotherapy used for the treatment of lymphoma was cytotoxic chemotherapy, initially identified in the 1940s when single-agent nitrogen mustards were shown to induce remissions in patients with Hodgkin and non-Hodgkin lymphomas [1]. Over the subsequent decades additional effective cytotoxic agents were identified; however, the next novel approach to anti-lymphoma pharmacotherapy was not realized until the approval of rituximab in 1997, ushering in the era of monoclonal antibodies. Within the past decade, a third type of agent to treat lymphoma, small molecule targeted therapies, has emerged. These drugs are designed to specifically inhibit signaling pathways critical for the survival and interaction of neoplastic cells with their stromal environment. This review will focus on one successful category of these targeted therapies – those that inhibit the phosphatidylinositol-3-kinase (PI3K) signaling pathway. Specifically, we will review the role of PI3K-alpha (PI3Kα) and PI3K-delta (PI3Kδ) inhibition in lymphoma therapy. Pan-PI3K inhibitors and combination PI3K – mammalian target of rapamycin (mTOR) inhibitors have been reviewed elsewhere [2].
2. Introduction to Phosphatidylinositol-3-Kinase Signaling
Phosphatidylinositol is composed of a membrane-bound lipid with a six carbon inositol head. Hydroxyl groups attached to these carbons can be phosphorylated by phosphatidylinositol kinases. Class I PI3-kinases produce phosphoinositide 3,4,5-triphosphates (PIP3) from phosphoinositide 4,5-diphosphosphates. Upstream agonists, including receptor tyrosine kinases (RTKs) and G-protein coupled receptors, ultimately lead to PI3K activation by freeing the catalytic subunit of the PI3K (referred to as p110α, p110β, p110γ, and p110δ for the four different isoforms) from inhibition by its associated regulatory subunit [3]. The generated PIP3 serves as a lipid second messenger that can be recognized by other proteins within the cell, activating downstream signaling pathways. The effects of PI3K activation (and, conversely, PI3K inhibition by small molecule inhibitors) ultimately depend on cellular context as well as which PI3K isoform is engaged. PI3Kδ and PI3Kγ expression is largely restricted to hematopoietic cells, while PI3Kα and PI3Kβ are ubiquitously expressed [3,4]. While the presence of multiple isoforms and multiple cellular contexts is complex, it also adds a built-in layer of specificity when considering drug targets. For example, the on-target toxicities of a PI3Kδ-specific inhibitor are limited to its effects on leukocytes since the enzyme is primarily expressed in these cells.
3. The Physiologic Role of PI3K Signaling in Hematopoietic Cells
3.1 PI3K Signaling in B Lymphocytes
Murine studies in which the p110δ catalytic subunit was knocked out [4, 5] or replaced with a kinase-dead version [6] have identified a critical role for p110δ throughout the life of the B lymphocyte (Figure 1(a)). Data from these mice demonstrate that generation of PIP3 after B cell receptor (BCR) engagement is largely dependent on p110δ [4, 6]. Additionally, downstream signaling from cytokine/chemokine receptors and RTKs in B cells is also deficient in mice that lack p110δ [6]. The lack of input from these key signal transduction elements leads to a reduction in absolute number of three types of mature B cells: follicular (B2) cells, marginal zone B cells, and peritoneal (B1) B cells [4, 6, 7] . Histologically, this manifests as a lack of germinal centers in the spleen, lymph nodes, and Peyer’s patches [6]. Mice without functional p110δ have reduced levels of immunoglobulins and reduced humoral immune responses to antigens. Deletion of p110α [7], p110β [7], and p110γ [8] has no overt effect on B cell number or function. However, combined deletion of p110α and p110δ leads to an arrest of B cell development at the pre-B cell stage [7]. One reason for this may be the dependence of signaling from the BCR on the p110α and p110δ isoforms. Absence of tonic signaling from the BCR then leads to prolonged Rag expression and excessive VDJH recombination [7].
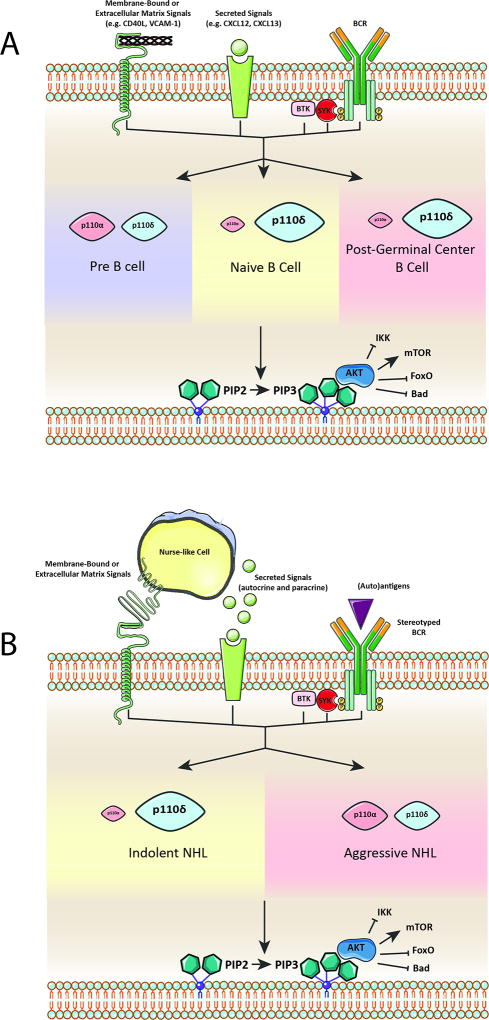
Figure 1.
(a) Upstream signals from the B cell receptor, secreted molecules (e.g. cytokines), and membrane-bound and extracellular matrix proteins stimulate the PI3K pathway. At different stages of B cell development, certain isoforms of the catalytic subunit p110 are responsible for the majority of PI3K pathway output (represented by relative size). p110β and p110γ are not included as knockout mice have no overt B cell phenotype and the role of these isoforms in B cell development is less studied. Downstream effects of PI3K activation include inhibition of IKK, FOXO, and the proapoptotic protein Bad as well as activation of mTOR. (b) A neoplastic B cell co-opts upstream signals from the microenvironment to constitutively activate the PI3K pathway. A more prominent role for p110α in aggressive NHL is hypothesized based on improved efficacy of p110α/δ combinatorial inhibitors for the treatment of these diseases. BCR – B cell receptor, NHL – Nonhodgkin Lymphoma.
3.2 PI3K Signaling in Conventional and Regulatory T Lymphocytes
Murine studies also implicate p110δ and p110γ as the isoforms responsible for the majority of PIP3 signaling in conventional T cells. The mature T cell receptor (TCR) signals primarily through p110δ [6, 9], while chemokine receptors often utilize p110γ [9, 10]. Thus, T cell phenotypes upon ablation of either p110δ or p110γ are different. Sole inhibition of p110δ impairs CD8+ cytotoxic T cell function [11] and also blocks the ability of native CD4+ cells to proliferate, expand, and differentiate into helper T cell subsets [12]. On the other hand, genetic ablation of p110γ reduces chemotaxis of activated CD4+ and CD8+ T cells [13, 14]. While individual deletion of either p110δ or p110γ isoform alone has no effects on T cell development, simultaneous genetic ablation of both isoforms blocks T cell development in the thymus [8].
The role of p110δ in regulatory T cell (Treg) function requires special attention as it may explain some of the toxicities seen with p110δ inhibitors. p110δ is critical for Treg survival and function [15]. For example, p110δ-deficient mice develop an autoimmune colitis thought to be due to an impaired ability of Tregs to restrain inflammation in response to colonic microbiota [6, 16]. In sum, while the inhibition of p110δ does impair the function of conventional T cells, this is often counterbalanced by a decrease in Treg function.
3.3 PI3K Signaling in Innate Immune Cells
PI3-kinases play a critical role in the innate immune systems as well as the adaptive immune system. Toll-like receptor signaling in macrophages is enhanced with genetic p110δ inhibition [16]. Additionally, p110δ is required for dendritic cells to switch from a pro-inflammatory to anti-inflammatory state after exposure to lipopolysaccharide, and inhibition of p110δ leads to prolonged pro-inflammatory responses in dendritic cells [17]. PI3K signaling, specifically from p110γ, is critical for many aspects of neutrophil function, including chemotaxis, phagosome formation, and the oxidative burst [18, 19]. Activated PI3Kγ promotes immune suppression and tolerance of cancer by blocking pro-inflammatory NF-κB signaling in tumor associated macrophages [20]. Conversely, mice that lack p110γ or mice that are treated with p110γ-specific inhibitors mount macrophage-mediated pro-inflammatory responses upon exposure to pathogenic stimuli (including cancer cells) [20]. These data suggest that, in certain contexts, p110δ and p110γ inhibition may facilitate heightened – and perhaps excessive – innate immune responses.
4. The Pathophysiologic Role of PI3K Signaling in Lymphomas
The PI3K pathway that is necessary for normal B cell development and activation is subverted by B cell lymphomas to promote unlimited growth and survival (Figure 1(b)). While activating mutations in PI3K enzymes are more commonly found in solid malignancies, mutations in PIK3CA, the gene encoding p110α, are found in about 1–8% of DLBCLs [21, 22], and inactivating mutations in PTEN, which negatively regulates the PI3K pathway, are found in 3–22% of DLBCLs [23]. Furthermore, p110α gene amplification [24, 25] and PTEN loss of expression [23] are commonly seen in mantle cell lymphoma (MCL) and diffuse large B cell lymphoma (DLBCL). Preclinical and clinical studies demonstrate that inhibition of PI3K is an effective therapeutic strategy for the treatment of lymphomas. At least three mechanisms of action may account for the effectiveness of PI3K inhibitors – inhibition of signaling from the BCR, inhibition of cytokine signaling from the microenvironment, and enhancement of anti-tumor immunity. Hypothesized mechanisms have also been reviewed in more detail elsewhere [26].
4.1 Inhibition of Tonic BCR Signaling by PI3K Inhibitors
While the BCR classically activates downstream signaling pathways after antigen engagement, it is now clear that persistent BCR signaling is critical at some points in the physiologic life of a lymphocyte, as well as in the life of a neoplastic lymphoma cell [27]. This constitutive level of BCR signaling can be achieved through ligand-dependent or ligand-independent means. For example, in chronic lymphocytic leukemia (CLL) cells, the BCR may recognize auto-antigens or common exo-antigens (e.g. β-glucan) to become activated and promote cell survival and clonal expansion [28]. In the activated B-cell-like (ABC) subtype of DLBCL, chronic signaling from the BCR is required to prevent apoptosis. This chronic active signaling can be achieved through mutations in CARD11, CD79A, or CD79B that lead to downstream activation of NF-κB, mimicking the effects of antigen binding [29]. Analysis of the immunoglobulin variable regions from multiple subtypes of lymphoma indicates that while somatic hypermutation is ongoing, cells appear to be selected to maintain the proper reading frame and integrity of the immunoglobulin locus, consistent with a need for constitutive signaling from this receptor [27].
In agreement with the hypothesis that tonic BCR signaling activates p110δ and downstream pro-survival pathways, treatment of DLBCL cell lines, Hodgkin lymphoma cell lines, and follicular lymphoma (FL) cell lines with idelalisib induces apoptosis in all three [30–32]. In CLL cells, the enzymatic activity of p110δ is higher than in normal B cells [33]. However, treatment of primary CLL cells and CLL cell lines with the p110δ-specific inhibitor idelalisib causes only a modest reduction in cell viability and the IC50 exceeds 10uM [33, 34]. This unimpressive level of in vitro cytotoxicity contrasts with the significant efficacy of this agent in the clinic. Thus, a significant portion of the in vivo efficacy of p110δ inhibition may be due to inhibition of cues from the microenvironment (see section 4.2 below).
For complete abrogation of signaling from the BCR, inhibition of both p110α and p110δ may be required, as suggested by mouse models. Copanlisib, a pan-PI3K inhibitor with preferential activity against p110α and p110δ, was more effective at inhibiting BCR-mediated downstream activation of NF-κB than idelalisib in DLBCL cells lines [35]. Direct comparison of copanlisib and idelalisib treatment of primary CLL cells revealed IC50 values of 450nM and >10uM, respectively, while both had minimal effects on B cells from normal controls [36]. Treatment of mantle cell lymphoma (MCL) cell lines and primary samples with the combined p110α/δ inhibitor pictilisib was more effective at blocking downstream BCR pathway activation and inducing apoptosis than use of the p110δ-specific inhibitor idelalisib alone [37]. MCL patient samples at relapse had higher levels of p110α and were more resistant to idelalisib in vitro, raising the possibility that other PI3K isoforms may be able to compensate when p110δ is blocked [37].
4.2 Inhibition of Cytokine Signaling From the Microenvironment by PI3K Inhibitors
In addition to blockade of pro-survival signals mediated by tonic signaling from the BCR, some of the therapeutic efficacy of PI3K inhibitors may be attributable to their inhibition of paracrine and autocrine pro-survival signals. This has been best demonstrated for p110δ inhibitors in CLL. Multiple cell types in the tumor microenvironment -- including mesenchymal stromal cells, monocyte-derived nurse-like cells, follicular dendritic cells, T cells, and endothelial cells -- communicate with and support the CLL cells [38]. This crosstalk is mediated by adhesion molecules, chemokines, and soluble factors. Many of these messengers activate downstream signaling pathways that converge on p110δ. In vitro, p110δ inhibition with idelalisib reduces CLL cell responsiveness to microenvironmental signals such as CD40L, CXCL-12, CXCL-13, and also decreases secretion of multiple cytokines and chemokines [30, 31]. In systems where CLL cells are co-cultured with supportive stromal cells and thus rendered relatively resistant to standard chemotherapeutic agents, p110δ inhibition abrogates that protective effect and causes enhanced cytotoxicity even as monotherapy [31, 33].
Clinically, a redistribution lymphocytosis is often seen after treatment with p110δ inhibitors and other agents that target this pathway (e.g. BTK inhibitors) [39]. This phenomenon is presumably due to drug interference with homing and adhesion signals from stromal cells that keep CLL cells fixed in the peripheral lymphoid tissues [38, 40]. Circulating CLL cells may be more amenable to chemotherapeutic and antibody-mediated therapies, providing rationale for the combination of PI3K inhibitors with other classes of agents.
4.3 Enhancement of Anti-tumor Immunity by PI3K Inhibitors
Mice with genetic inactivation of p110δ are resistant to tumorigenesis, and p110δ inactivation in Tregs is both necessary and sufficient to confer this tumor resistance [11]. While not yet definitely demonstrated in the clinical setting, this pre-clinical data suggests that inhibition of Treg function via p110δ blockade may facilitate recognition and elimination of tumor cells. This benefit extended across many cancer models including lung adenocarcinoma, thymoma, breast adenocarcinoma, and pancreatic adenocarcinoma. Presumably, such a mechanism could contribute to the anti-tumor efficacy of p110δ inhibition in lymphoma as well. Additionally, PI3K inhibition can enhance the innate immune response (see section 3.3), and administration of a pan-PI3K inhibitor augmented the anti-tumor effects of a dendritic cell vaccine in murine xenograft models [41].
5. PI3Kα and PI3Kδ Inhibitors in Clinical Development for the Treatment of Lymphoma
Based upon the rationale described above and strong preclinical data, there has been intensive effort to develop PI3K inhibitors for the treatment of lymphoid malignancies. While development of pan-PI3K inhibitors has been hampered by toxicity, the creation of isoform-selective inhibitors has proven to be a more successful approach. Idelalisib was the first FDA-approved PI3K inhibitor for any malignancy after demonstrating success in the treatment of both CLL and FL. Here, we review recent data regarding the development of idelalisib for the treatment of multiple types of lymphoma, and then move on to review clinical trials of other isoform-selective PI3K inhibitors, with a particular focus on those whose targets include either p110α or p110δ.
5.1 Idelalisib
Idelalisib (CAL-101, GS-1101) is the first FDA-approved PI3K inhibitor. It is a p110δ-selective inhibitor (Table 1) approved for used in relapsed CLL in combination with rituximab, relapsed FL after two lines of prior therapy, and relapsed small lymphocytic lymphoma (SLL) after two lines of prior therapy.
Table 1.
IC50 Values (nM) of Selected PI3K Inhibitors Against Recombinant Enzymes
| Drug Name | p110α | p110β | p110δ | p110γ | Reference |
|---|---|---|---|---|---|
| Idelalisib | 820 | 565 | 2.5 | 89 | [30] |
| Duvelisib | 1602 | 85 | 2.5 | 27 | [42] |
| Umbralisib | >10000 | 1116 | 22 | 1065 | [43] |
| RP-6530 | >7000 | >2000 | 24.5 | 33.2 | [44] |
| INCB050465 | >20000 | >20000 | 1 | >20000 | [45] |
| ACP-319 | 33000 | 270 | 18 | 85 | [46] |
| Pictilisib | 3 | 33 | 3 | 75 | [47] |
| Acalisib | 5441 | 3377 | 12.7 | 1398 | [48] |
| Copanlisib | 0.5 | 3.7 | 0.7 | 6.4 | [49] |
5.1.1 Idelalisib Monotherapy
Early studies of idelalisib examined its efficacy in the treatment of indolent non-Hodgkin lymphomas (iNHL). A phase I dose escalation study in 64 patients with relapsed/refractory iNHL demonstrated responses rates of 45% in FL, 55% in SLL, 33% in marginal zone lymphoma, and 55% in lymphoplasmacytic lymphoma [50]. Formal DLT criteria were not met, but transaminase elevations were seen in 48% of patients (grade ≥3 25%), along with diarrhea (36%, grade ≥3 9.4%), pneumonia (18.8%, grade ≥3 17.2%), neutropenia (44%, grade ≥3 23%), and rash (25%, grade ≥3 3.1%). The high response rates eventually led to a phase II trial of idelalisib monotherapy in iNHL that was refractory to both rituximab and an alkylating agent [51]. In this single-arm, multicenter study of 123 patients, the overall response rate (ORR) was 54% in the FL subgroup (including 8% complete responses) and 58% in the SLL subgroup (0% complete responses), confirming the promising results of the phase I study and ultimately leading to accelerated FDA approval. After a median follow up of 9.7 months, the median progression-free survival (PFS) was 11.0 months (range, 0.03–16.6 months) and median overall survival (OS) was 20.3 months (range, 0.7–22.0 months). The toxicities were manageable, particularly given that the median age of subjects was 64 years and the median number of prior regimens was 4. Transaminitis was seen in 47% of patients, with only 13% grade ≥3, and led to discontinuation in only 4% of subjects. Diarrhea was seen in 43% (grade ≥3 13%).
The response rates above were exceeded in the CLL patients enrolled on the phase I study of idelalisib monotherapy, in which an ORR of 72% (all partial responses) was observed after a median dosing duration of 15 months [39]. Promisingly, patients with high-risk del17p/TP53 mutated disease also had a high ORR of 54%. The median PFS was 15.8 months at all dose levels but 32 months in patients who received ≥150mg twice daily, the selected phase II dose. Neutropenia was particularly frequent in this population (57%, grade ≥3 43%), with lower rates of transaminitis (28%, grade ≥3 2%) and diarrhea/colitis (30%, grade ≥3 6%). These results led to an initial registration program in CLL that included three randomized combination trials, reviewed below. Combination trials were employed because prolonged lymphocytosis was common in the phase I CLL study, and at that time concerns remained about how this would be managed in the response criteria.
Finally, 40 patients with heavily pretreated MCL were enrolled on the phase I study [52]. At the 150 mg twice-daily dose, the ORR was 69%. The median PFS was 3.7 months, relatively short compared to idelalisib in CLL. Common adverse events were (total %/grade ≥3%) diarrhea (40/18), neutropenia (30/10), rash (23/3), pneumonia (13/10), and transaminitis (60/20). No responses were seen in a small number of subjects who received idelalisib monotherapy for the treatment of DLBCL [52].
5.1.2 Idelalisib Combination Therapy
The efficacy of idelalisib as monotherapy, as well as the non-overlapping toxicities of PI3K inhibition with standard chemoimmunotherapy, has led to numerous trials combining idelalisib with monoclonal antibodies, chemotherapy, or both. In CLL, multiple phase III trials of idelalisib combination therapy have now been published. Idelalisib plus rituximab had a higher ORR (77 vs. 15%) and higher PFS (not reached vs. 5.5 months) compared to rituximab plus placebo in patients with relapsed/refractory CLL. Neutropenia (60%/grade ≥3 37%), diarrhea/colitis (21%, grade ≥3 5%), and transaminitis (40%, grade ≥3 8%) were seen at higher rates in the group receiving idelalisib [53,54]. Unlike the other phase III trials below, no increased risk of infection was identified, although median exposure to therapy at first report was only 5 months, compared to much longer follow-up times in the other studies. The addition of an anti-CD20 monoclonal antibody blunted the redistribution lymphocytosis seen with idelalisib. An international, double-blind, placebo-controlled trial of bendamustine and rituximab (BR) in addition to either idelalisib or placebo in 416 relapsed/refractory CLL patients was also recently reported [55]. The addition of idelalisib to BR was clearly efficacious, with a significant improvement in PFS (20.8 months vs. 11.1 months) and OS (not reached vs. 31.6 months), although complete response rates after a median follow up of 14 months were essentially equivalent. However, this result was tempered by the finding that the addition of idelalisib to BR increased the risk of infection and other toxicities. The frequency of infections – mostly bacterial – in the idelalisib group was 69% (39% grade ≥3, with six infectious deaths) and in the placebo group was 59% (25% grade ≥3, with three infectious deaths). Reactivation of cytomegalovirus was rare but has been observed, primarily in the context of idelalisib with BR. Rates of toxicities (total %/grade ≥3%) including neutropenia (64/60), transaminitis (61/21), and diarrhea (38/9) were also higher in the idelalisib plus BR arms compared to placebo plus BR arms (55/47, 32/3, and 23/2, respectively). Finally, an open-label, randomized phase III trial compared idelalisib plus ofatumumab to ofatumumab monotherapy in relapsed/refractory CLL [56]. Median PFS was doubled in the idelalisib/ofatumumab group (16.3 months vs. 8.0 months), but serious infections, opportunistic infections, and treatment-related deaths (22 deaths vs. 6 deaths) were more common in the group receiving idelalisib. For example, rates of sepsis (6% vs. 1%) and Pneumocystis jirovecii pneumonia (5% vs. 1%) were higher in subjects receiving idelalisib/ofatumumab versus ofatumumab alone. Because of an increase in serious infections and infectious mortality in idelalisib-treated patients, the manufacturer Gilead Sciences halted six trials of combination therapy in less heavily pretreated CLL and iNHL patients in March 2016.
Based on these developments and on the fact that the drug is already FDA approved, few studies involving idelalisib treatment for lymphomas in earlier lines of therapy are actively enrolling. A dose-optimization study is currently being sponsored by Gilead Sciences (NCT02536300) to investigate whether the lower dose of 100 mg twice-daily affects the overall safety profile or ORR of the drug in relapsed follicular lymphoma. Idelalisib is also being evaluated in a phase II single-arm study (NCT02332980) in combination with pembrolizumab for the treatment of relapsed iNHL and CLL. The toxicities of hepatitis, colitis, and pneumonitis, believed to be autoimmune (discussed below), will be of special interest in this study. Finally, idelalisib use in the posttransplant setting is also being explored, both as a maintenance therapy after reduced intensity conditioning allogeneic stem cell transplant for B cell malignancies (NCT03151057) and as monotherapy for persistent disease after allogeneic stem cell transplant (NCT02662296).
5.1.3 Toxicities of Idelalisib
The major toxicities of idelalisib can be divided into three groups. First, there are increased rates of bacterial infection. This could be secondary to drug-induced neutropenia and may be particularly exacerbated when the drug is given with chemotherapy. Second, there appears to be an increased risk for opportunistic infections. One possible mechanism for this, based on preclinical data, is impairment of T cell function with p110δ blockade. Prophylaxis for Pneumocystis jirovecii and monitoring for cytomegalovirus reactivation is now mandatory during treatment with idelalisib. In addition to the two types of infectious toxicities, inflammatory toxicities of presumed autoimmune origin – including colitis, hepatitis, and pneumonitis – have been seen with idelalisib. Diarrhea/colitis is seen in 20–40% of idelalisib-treated patients. An early self-limited form of diarrhea tends to occur within the first 8 weeks of therapy and is responsive to antidiarrheal agents. A second type of diarrhea occurs later and is responsive to steroids [57]. Hepatitis is prominent when idelalisib is given in the up-front setting or combined with immunomodulatory agents. For example, a study of rituximab, lenalidomide, and idelalisib in iNHL was halted after six out of the seven patients developed transaminitis, in two cases leading to death [58]. Two other ALLIANCE trials of idelalisib, lenalidomide, and rituximab in iNHL also had to be suspended because of cases of transaminitis, rash, fevers, and hypotension seen in 4 out of the first 8 patients enrolled; these toxicities were still seen after an amendment to remove rituximab from the dosing schema [59]. A phase I study of idelalisib combined with ofatumumab in up-front CLL found that 79% of enrolled patients experienced any grade transaminase elevation, with 54% experiencing grade ≥3 transaminase elevation [60]. A steroid-responsive pneumonitis and rash have also been reported in patients on idelalisib [50,56,60]. Multiple lines of clinical evidence support the hypothesis that an autoimmune mechanism underlies these toxicities. They respond to steroids [57,60]. Activated cytotoxic T cell infiltrates are seen in intestinal and liver biopsies from patients experiencing colitis and hepatitis, respectively [60–61]. Combination with the immunomodulatory agent lenalidomide increased the toxicity profile [58,59].
Preclinical evidence suggests that these three classes of toxicities may be due to on target effects. p110δ inhibition decreases the function of neutrophils and adaptive immune cells (perhaps increasing the risk of infection) as well as the function of Tregs (perhaps predisposing to autoimmunity). Toxicities seem to be more severe in less heavily pretreated patients [60], perhaps because patients who have experienced multiple rounds of therapy have a less intact immune system. Management algorithms for these toxicities have been published [62].
5.2 Duvelisib
Duvelisib (IPI-145) is different from idelalisib in that it targets both p110δ and p110γ (Table 1) [42]. Thus, studies with this agent may provide insight into the role of p110γ inhibition in the efficacy and safety of PI3K inhibitors. Preclinical data supported further studies of this agent in various types of lymphoma. In primary CLL cells, duvelisib is cytotoxic at micromolar doses (with no demonstrable effect on normal B cells) and antagonizes activation of downstream signaling pathways after BCR crosslinking at nanomolar doses [63,64]. This occurs even in the presence of the BTK C481S resistance mutation, suggesting that duvelisib may have a role in the treatment of patients who develop acquired resistance to ibrutinib. Duvelisib had moderate efficacy when tested against various DLBCL cell lines, causing growth inhibition in approximately half of tested cell lines [65]. Its efficacy in DLBCL cell lines may be increased by combination with ibrutinib, as together the duo led to prolonged blockade of AKT activation, enhanced caspase activation, and greater tumor growth inhibition in xenograft models [66]. Finally, in MCL, duvelisib blocked cell line growth both in vitro and in murine patient-derived xenograft models [67].
5.2.1 Duvelisib monotherapy
As with idelalisib, trials investigating the role of duvelisib monotherapy have primarily focused on iNHL and CLL. The initial phase I data in iNHL were quite promising, with an ORR of 62% (including five complete responses in FL) with the most common grade ≥3 toxicities including transaminitis (37%), diarrhea (16%), and neutropenia (22%) [68]. However, the subsequent phase II single-arm study of duvelisib monotherapy in relapsed/refractory iNHL (DYNAMO) reported an ORR of 46% (41% in FL, 33% in marginal zone lymphoma, and 68% in SLL) in subjects who had received a median of 3 prior treatments, with a median PFS of 8.4 months [69,70]. Although cross-trial comparison is difficult, the response rate here was less than the 50–70% response rates reported for idelalisib monotherapy in similar patient populations, and the median PFS was also shorter than the 11 months seen in the phase II registration trial of idelalisib. Thus, although the trial met its primary end point, the results were generally considered disappointing. The toxicity profile was similar to that seen with idelalisib and included neutropenia (28%) and diarrhea (15%). One patient had a pneumocystis infection and three subjects had cytomegalovirus reactivation. With regards to CLL, a phase Ib study showed an ORR to duvelisib monotherapy of 58% [71]. The most common grade ≥3 adverse events included neutropenia (39%), febrile neutropenia (15%), and pneumonia (11%). More patients discontinued for adverse events (31%) than for disease progression (24%). A subset analysis restricted to patients who had progressed on ibrutinib showed that one out of six CLL patients had a partial response to duvelisib [72]. Seventeen previously untreated patients with CLL could receive duvelisib through an expansion cohort of this phase I study, and they had a very promising ORR of 82% [70,73]. These results led to the phase III DUO study of duvelisib against the comparator arm of ofatumumab monotherapy in relapsed/refractory CLL [74]. This trial has completed enrollment, with results expected later this year. Verastem has now taken over development of duvelisib from Infinity Pharmaceuticals.
5.2.2 Duvelisib combination therapy
By combining duvelisib with chemoimmunotherapy, one may be able to enhance response rates and durability of response without adding significant toxicity. In previously untreated follicular lymphoma, a two arm study examined the combination of duvelisib with rituximab (DR) or duvelisib with obinutuzumab (DO) [75]. The ORR was comparable between the two arms, at 81% in the DO arm and 92% in the DR arm. The addition of the monoclonal antibodies did not significantly alter the toxicity profile of duvelisib, with the most common grade ≥3 adverse events including transaminitis, neutropenia, and diarrhea/colitis. There were two events of P. jirovecii pneumonia, both in patients not receiving prophylaxis. A three-arm study to investigate duvelisib in combination with either rituximab, bendamustine, or BR in relapsed/refractory iNHL and CLL has completed enrollment [76]. At a median follow up of 16.3 months, the ORR in NHL was 64% and in CLL was 92%. Duvelisib plus fludarabine, cyclophosphamide, and rituximab (FCR) was evaluated in approximately 30 previously untreated patients with CLL under the age of 65 [77]. The rate of minimal residual disease negativity in the bone marrow of subjects was 89%, substantially higher than that seen with FCR alone. Longer follow up may indicate if this finding leads to more durable responses.
5.3 Umbralisib
Umbralisib (TGR-1202) is a p110δ inhibitor that possesses a different chemical structure than the previously described compounds idelalisib and duvelisib, with nitrogen atoms in the heterocyclic quinazolinone backbone replaced by carbon and oxygen atoms [43]. This change in chemical structure may potentially alter the toxicity profile based on the hypothesis that nitrogen-based heterocyclic backbones can cause hepatotoxicity. In addition, this different chemical structure leads to inhibition of casein kinase-1ε (CK-1ε) as well as p110δ, a property not shared by idelalisib or duvelisib [78]. This introduces some confusion regarding umbralisib’s ultimate mechanism of action. Umbralisib is effective at inducing apoptosis as a single agent in primary CLL cells in vitro, independent of TP53 status; this efficacy is comparable to that achieved in vitro with idelalisib [79]. Furthermore, in preclinical studies, the combination of carfilzomib plus umbralisib was synergistic at in vitro killing of primary samples from patients with multiple types of lymphoma as well as multiple lymphoma cell lines (CLL, SLL, MCL, and DLBCL). This synergy was due to the additional ability of umbralisib to inhibit CK-1ε and was not seen when idelalisib was combined with carfilzomib [78].
5.3.1 Umbralisib monotherapy and combination therapy
Safety data from phase I studies of umbralisib either as monotherapy or in combination with ublituximab (an anti-CD20 monoclonal antibody) in both CLL and NHL indicate that this agent has a different safety profile than idelalisib or duvelisib. Across all patients (40 with CLL/SLL and 112 with NHL), toxicity rates included transaminitis at 6% (grade≥3 3%), pneumonitis at 1% (grade ≥3 less than 1%), neutropenia at 19% (grade ≥3 16%) and diarrhea at 42% (grade ≥3 2%) [80]. These rates are less than those reported for other p110δ inhibitors in similar patient populations. With regards to CLL, the combined ORR (including 27 patients who received both umbralisib monotherapy and umbralisib plus ublituximab) was 89% with a median PFS of 24 months. A phase III trial comparing ublituximab plus umbralisib to obinutuzumab plus chlorambucil in both previously untreated and relapsed patients is currently enrolling.
Given the published preclinical data, umbralisib is being studied more extensively in aggressive NHL as well as iNHL. Among patients with FL and marginal zone lymphoma, the ORR to combination therapy (ublituximab plus umbralisib) was 71% (with 24% complete responses); in patients with aggressive NHL (DLBCL, MCL, and Richter’s transformation), the ORR was 32% (with 16% complete responses) [81]. A separate study has explored umbralisib, ublituximab, and bendamustine in patients with advanced DLBCL and relapsed/refractory FL [82]. The response rate in DLBCL was encouraging at 63% (with 44% complete responses), and 88% in FL. No cases of transaminitis were reported. A phase IIb trial comparing ublituximab plus umbralisib to umbralisib alone is currently enrolling patients with DLBCL. Finally, based on the preclinical data showing synergy between umbralisib and carfilzomib [78], a trial of this duo in relapsed/refractory Hodgkin and non-Hodgkin lymphoma is actively recruiting subjects (NCT02867618).
As constitutive BCR signaling is critical for survival of many types of lymphoma cells, and BTK and PI3Kδ both lie within this pathway, dual inhibition of both PI3Kδ and BTK results in more complete BCR pathway blockade in vitro [83]. In the clinic, this combination may achieve synergistic cell kill – attaining more durable remissions – and may also delay the development of resistance. Therefore, multiple trials are centered on the therapeutic backbone of ibrutinib combined with umbralisib. A phase I/Ib study has identified a dosing strategy of umbralisib 800 mg daily and ibrutinib (560 mg daily for MCL, 420 mg daily for CLL) as the recommended phase II dose, and expansion cohorts are now accruing [84]. The ORR in relapsed/refractory MCL was 85% (after median time on study of 10.9 months) and in CLL was 89% (after median time on study of 11 months). A phase I trial investigating the chemotherapy-free triplet of umbralisib, ibrutinib, and ublituximab in multiple types of lymphoma did not reach a maximum tolerated dose, with common adverse events including diarrhea (39%), nausea (32%), and neutropenia (29%, grade ≥3 16%) [85]. Response rates were encouraging in CLL/SLL (100%), FL (80%), marginal zone lymphoma (100%) and MCL (100%), but less encouraging in DLBCL (17%).
5.4 Copanlisib
Copanlisib (BAY 80–6946) is an intravenous PI3K inhibitor that was recently approved for the treatment of relapsed follicular lymphoma. The drug is a pan-PI3K inhibitor, and although it has a slight (10-fold) preference for p110α and p110δ isoforms when tested against purified recombinant enzymes, it does have significant activity against all p110 isoforms, and is actually a more potent p110γ inhibitor than any other compound mentioned in this review (Table 1) [49]. As mentioned in section 4.1, the drug is cytotoxic to primary CLL cells as well as DLBCL, marginal zone lymphoma, and MCL cell lines at nanomolar doses [35,36,86]. Its unique pharmacokinetics allow it to be dosed on a weekly basis.
5.4.1 Copanlisib monotherapy
The first-in-human study showed promising results in the NHL cohort of nine patients, with a 77% ORR and manageable toxicities of hyperglycemia and hypertension [87], leading to multiple phase II studies. An open-label phase II trial of copanlisib (CHRONOS-1, NCT01660451) examined efficacy and safety in patients who failed at least two prior lines of therapy for indolent or aggressive NHL (part A) with a second portion of the study specifically devoted to patients with relapsed/refractory indolent lymphoma (part B). Results from part A demonstrated an ORR of 44% in the indolent lymphoma cohort (n = 33) and 27% in the aggressive lymphoma cohort (n = 51) [89]. Toxicities (all grades/grade ≥3) included hyperglycemia (60%/25%), hypertension (55%/41%), neutropenia (35%/30%), diarrhea (41%/4.8%), transaminitis (26%/3%), and pneumonitis (3.6%/2.4%). Hyperglycemia is a known side effect of p110α inhibition. The hypertension is unexplained and is not a side effect commonly seen with other pan-PI3K inhibitors [87]. Both the hyperglycemia and hypertension are transient, resolving within 24 h of the infusion. The lower incidence of diarrhea/colitis and transaminitis could be attributed to an intermittent dosing schedule, a broader spectrum of kinase inhibition, or bypass of hepatic first-pass metabolism, since the drug is administered intravenously. Results from part B of CHRONOS-1 confirmed efficacy in iNHL, with ORRs of 59% (12% CR) in FL (n = 104) and 70% (9% CR) in marginal zone lymphoma (n = 23) [90]. The most common adverse events were again transient hyperglycemia (49%/40%) and hypertension (29%/23%). It was on the basis of this trial that the drug received accelerated approval for the treatment of relapsed follicular lymphoma in patients who have received at least two prior therapies. A phase II study of copanlisib monotherapy in relapsed/refractory DLBCL (NCT02391116) established modest efficacy of the drug in the germinal center B-cell-like (GCB) subtype of DLBCL (ORR 14%, CR 4.5%) but greater efficacy in the activated B-cell-like (ABC) subtype (ORR 38%, CR 25%) [91]. A phase III study of copanlisib versus placebo in rituximab-refractory iNHL (NCT02369016, CHRONOS-2) has completed enrollment [88].
5.4.2 Copanlisib Combination Therapy
Two phase III trials of copanlisib-based therapy regimens are currently enrolling patients with iNHL. In CHRONOS-3 (NCT02367040), patients with indolent lymphoma who have progressed after a rituximab containing regimen will be randomized to either rituximab plus copanlisib or rituximab alone, with a primary outcome measure of PFS. In CHRONOS-4 (NCT02626455), patients are stratified based on prior therapy. Those who have progressed after receiving R-CHOP or R-CVP for iNHL will be randomized to BR-copanlisib or BR. Those who have progressed after BR will be randomized to R-CHOP-copanlisib or R-CHOP. In this trial, copanlisib is administered for a fixed duration of 12 months in patients without progressive disease.
5.5 RP-6530
RP-6530 is a small-molecule p110δ/γ inhibitor developed by Rhizen Pharmaceuticals. In preclinical assays, it inhibited activationof PI3K and subsequent cellular proliferation in DLBCLcell lines. Patient-derived primary cells from DLBCL, CLL, MCL, and marginal zone lymphoma were also sensitive to the drug at a concentration of 4µM [44]. This prompted a phase I study in relapsed/refractory lymphomas, with an ORR of 20% [92,93]. The relatively low ORR may be due to predominant enrollment of patients with aggressive NHL (e.g. DLBCL, MCL, and peripheral T cell lymphoma) and Hodgkin lymphoma, as opposed to other trials of PI3K inhibitors which have focused on CLL and iNHL. No dose-limiting toxicities were observed. A phase II study is planned in NHL patients.
5.6 INCB050465
This compound developed by Incyte Pharmaceuticals is a potent, specific inhibitor of p110δ (Table 1) [45]. As a single agent, it induces apoptosis in DLBCL and MCL cell lines when used in the nanomolar range [45]. The pharmacokinetic and pharmacodynamics data are favorable. The drug can be taken once daily, and at the 30-mg dose, the steady-state average blood concentration is 15 times the IC90, with maximal p110δ inhibition at all doses tested [94]. Side effects were similar to those seen with idelalisib and duvelisib, and included colitis/diarrhea and pneumonitis, although none of the 52 patients had grade ≥2 elevated transaminases. The ORR was 61%, and varied by lymphoma subtype: DLBCL (36%), FL (78%), marginal zone lymphoma (100%), MCL (75%), CLL (33%), and Hodgkin lymphoma (13%). Disease specific trials are planned, including an open-label study of single agent INCB050465 in marginal zone lymphoma (NCT03144674) and relapsed/refractory FL [95]. The dosing schedule in this latter trial is unusual; after receiving daily dosing of INCB050465 20 mg for 2 months, subjects will then receive 20 mg on a weekly basis. This may represent an attempt to mitigate toxicities. Intriguingly, a head-to-head phase II trial comparing INCB050465 to idelalisib in relapsed/refractory FL is also planned (NCT03126019). In addition to these studies of monotherapy, a study evaluating INCB050465 in combination with bendamustine and obinutuzumab for the treatment of relapsed/refractory FL is actively accruing [96].
5.7 ACP-319
ACP-319 (known as AMG-319 before acquisition by Acerta) inhibits p110δ with submicromolar affinity in cell-free assays (Table 1) [46]. The phase 1 first-in-human study was completed in subjects with CLL and iNHL with results reported in abstract form [97]. The dose limiting toxicity was a case of hemolytic anemia; grade ≥3 treatment-related adverse events included colitis (10%), infection (7%), and transaminitis (4%). Intriguingly, declines in the Treg population in CLL patients treated with the drug were observed; this may be reflective of primary actions of the drug or may be secondary to improvement in disease burden. This decline in Treg abundance mirrors later findings in patients treated with idelalisib who experienced autoimmune toxicities while on therapy [60]. A phase I pilot study of the next-generation BTK inhibitor acalabrutinib in combination with ACP-319 in 12 subjects with relapsed/refractory CLL has finished enrollment (NCT02157324).
5.8 Acalisib
Acalisib (GS-9820) is a second generation PI3Kδ inhibitor developed by the maker of idelalisib, Gilead Sciences [98]. A phase Ib, open-label, dose-escalation and expansion study has completed accrual [99]. The ORR for the NHL subset (including MCL and DLBCL) was 33.3% and for the CLL subset was 85%. Transaminitis, diarrhea, and rash were seen. Based on the drug’s similar efficacy and toxicity profile to idelalisib, no further clinical development was planned.
5.9 Pictilisib
Pictilisib (GDC-0941) is equipotent against p110α and p110δ, with modest selectivity over p110β and p110γ (Table 1) [47]. It demonstrated moderate effectiveness in murine models of spontaneous lymphomas driven by hyperactive PI3K signaling [99]. A phase I study has been completed in a combined population of patients with solid malignancies and lymphomas, but only results from the solid tumor arm have been reported [100]. The dose-limiting toxicity was rash; a case of grade 3 pneumonitis and a case of grade 4 hyperglycemia were seen. Hyperglycemia is a known side effect of PI3Kα inhibition which has not been seen with p110δ or p110γ inhibitors.
5.10 KA-2237
Karus Therapeutics is developing KA-2237, a small molecule inhibitor of p110β and p110δ (although IC50 data in cell-free assays for each of the isoforms has not been publicly released). Preclinical studies show strong activity of the agent in MCL cell lines in PDX models [101]. It was more effective at inducing apoptosis than duvelisib or idelalisib, and was also effective at treating ibrutinib-resistant patient-derived xenografts. A phase I study of KA-2237 monotherapy is now enrolling patients with relapsed /refractory B cell lymphomas (NCT02679196).
6. Conclusion
PI3K pathway inhibitors represent a novel class of targeted therapies for the treatment of lymphomas. Multiple mechanisms of action likely contribute to their effectiveness, and include inhibition of tonic BCR signaling required by lymphoma cells, interference with pro-survival signals provided by the microenvironment, and possibly enhancement of anti-tumor immunity. Agents with activity against the p110δ isoform have had the most clinical success so far, primarily in the treatment of CLL (a disease that has a particular dependence on p110δ) and iNHLs (Table 2). Newer agents have activity against other PI3K isoforms, but exactly how activity against p110α, p110β, and p110γ will affect efficacy or toxicity is currently unknown. Enthusiasm for p110δ inhibitors has been tempered by the recognition of three types of toxicity in the prototypical agent of this class, idelalisib: increased bacterial infections, increased opportunistic infections, and presumed autoimmune toxicities (including enteritis/colitis, transaminitis, and pneumonitis). However, the incidence of these toxicities varies widely among other agents within this class, and it remains to be seen if such adverse events are due to on-target or off-target effects. Until clarity is achieved, trials of PI3K inhibitors should mandate opportunistic infection prophylaxis and careful surveillance for neutropenia as well as autoimmune toxicities, and should clearly report all infectious and autoimmune complications. Future research directions include the continued development of novel agents within this class, the evaluation of PI3K inhibitors as components of novel-novel combination therapy, elucidation of the mechanisms of resistance to these agents, and further mechanistic studies to try to understand, prevent, and treat the set of toxicities seen with these drugs.
Table 2.
Selected Trials of PI3K Inhibitors
| Drug | Additional Agents |
Disease | Phase | Patient no. |
Median age (range) |
Mean time on therapy (mos)1 |
Median Follow-up (mos) |
ORR (CR) | Median PFS (mos) |
Neutropenia (All / Grade 3) |
Diarrhea (All / Grade 3) |
Transaminitis (All / Grade 3) |
NCT | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Idelalisib | ||||||||||||||
| - | R/R iNHL | 1 | 64 | 64 (32–91) | 9.3 | NR | 47% (1.6%) | 7.6 | 44 / 23 | 36/9 | 48/25 | NCT00710528 | [50] | |
| - | R/R CLL | 1 | 54 | 63 (37–82) | 15 | NR | 72% (0%) | 15.8 | 57/43 | 30/6 | 28/2 | NCT01090414 | [39] | |
| - | R/R MCL | 1 | 40 | 69 (52–83) | 6.3 | NR | 40% (5%) | 3.7 | 30/10 | 40/18 | 60/20 | NCT00710528 | [52] | |
| 101-08, 2 | - | 1L CLL | 2 | 37 | 70 | 4.8 | NR | 81% (0%) | NR | NR/20 | 8/3 | NR/8 | NCT01203930 | [102] |
| 101-08, 1 | rituximab | 1L CLL | 2 | 64 | 71 (65–90) | 22.41 | NR | 97% (19%) | Not reached | 53/28 | 64/42 | 67/23 | [103] | |
| ofatumumab | 1L CLL | 2 | 24 | 67 (58–85) | 7.71 | 14.7 | NR | NR | 46/29 | 46/17 | 79/54 | NCT02135133 | [60] | |
| 101-09 (DELTA) | - | R/R iNHL | 2 | 125 | 64 (33–87) | 6.61 | 9.7 | 57% (6%) | 11.0 | 56/27 | 43/13 | 47/13 | NCT01282424 | [51] |
| Gilead 116 | rituximab | R/R CLL | 3 | 110 | 71 | 5 | 13 | 77 | Not reached | 60/37 | 21/5 | 40/8 | NCT01539512 | [54] |
| Gilead 115 | BR | R/R CLL | 3 | 207 | 62 (656-69) | 14.8 | 14 | 70% (1%) | 20.8 | 64/60 | 38/9 | 61/21 | NCT01569295 | [55] |
| Gilead 119 | ofatumumab | R/R CLL | 3 | 174 | 68 (61–74) | 13.91 | 16.1 | 75% (<1%) | 16.3 | 71/47 | 54/23 | 53/11 | NCT01659021 | [56] |
| Duvelisib | ||||||||||||||
| - | R/R iNHL | 1 | 36 | 64 (37–78) | 11.81 | NR | 62% (21%) | Not reached | 22/22 | 32/16 | 47/37 | NCT01476657 | [68] | |
| - | R/R CLL | 1 | 54 | 66 (42–82) | NR | NR | 58% (2%) | 15.7 | 53/42 | 44/9 | 29/9 | [71] | ||
| - | 1L CLL | 1 | 18 | NR | 13.2 | NR | 82% (0%) | Not reached | NR/39 | NR/22 | NR/17 | [73] | ||
| DYNAMO | - | R/R iNHL | 2 | 129 | 65 (30–90) | 61 | 12 | 46% (0%) | 8.4 | 32/23 | 44/15 | 14/6 | NCT01882803 | [69] |
| CONTEMPO | rituximab | 1L FL | 1/2 | 28 | 58 | 3.9 | NR | 87% (22%) | NR | 14/11 | 46/14 | 32/25 | NCT02391545 | [75] |
| obinutuzumab | 1L FL | 1/2 | 27 | 58 | 4.5 | NR | 91% (18%) | NR | 22/22 | 26/11 | 44/26 | |||
| FCR | 1L CLL | 1/2 | 12 | 57 (46–65) | NR | NR | 100% (33%) | NR | 67/50 | 42/8 | 25/17 | NCT02158091 | [77] | |
| Umbralisib | ||||||||||||||
| - / ublituximab2 | R/R CLL | 1 | 43 | 65 (22–86) | NR | NR | 88%3 | 24,4 Not Reached5 | 21/18 | 47/3 | 8/3 | NCT01767766 | [80] | |
| - / ublituximab2 | R/R NHL | 1 | 122 | 53% | 274 | |||||||||
| ibrutinib | R/R MCL | 1 | 14 | 67 (50–83) | NR | 14 | 79% (9%) | 8.4 | 36/7 | 36/0 | 22/0 | NCT02268851 | [84] | |
| R/R CLL | 1 | 18 | 67 (48–86) | NR | 94% (6%) | Not reached | 38/17 | 28/0 | ||||||
| ublituximab, ibrutinib | CLL6 | 1 | 20 | 65 (32–85) | 11.1 | NR | 100% (32%) | NR | 32/18 | 47/3 | None reported | NCT02006485 | [85] | |
| ublituximab, ibrutinib | NHL | 1 | 18 | 91% (36%) | ||||||||||
| ublituximab and bendamustine | R/R DLBCL and FL | 1 | 68 (31–81) | NR | DLBCL: 63% (44%) FL: 88% (50%) | NR | 24/24 | 36/9 | None reported | NCT02006485 | [82] | |||
| Copanlisib | ||||||||||||||
| -7 | R/R NHL | 1 | 9 | 65 (33–86) | 101 | NR | 78% (11%) | NR | NR | 16/2 | NR | NCT00962611 | [87] | |
| CHRONOS-1 Part A | - | R/R iNHL | 2 | 33 | 68 (46–89) | 5.61 | NR | 44% (9%) | 9.8 | 39/33 | 39/3 | 34/6 | NCT01660451 | [89] |
| R/R aNHL8 | 2 | 34 | 63 (22–90) | 2.01 | NR | 27% (8%) | 2.3 | 31/28 | 41/6 | 24/2 | ||||
| CHRONOS-1 Part B | - | R/R iNHL | 2 | 142 | NR | NR | NR | 59% (12%) | 11.3 | 25/19 | 18/4 | 28/NR | [90] | |
| - | R/R DLBCL | 2 | 67 | 69 (25–93) | NR | NR | 25% (12.5%)9 | NR | NR | 36/2 | NR | NCT02391116 | [90,91] | |
| RP-6530 | ||||||||||||||
| - | R/R NHL10 | 1 | 26 | 59 (20–83) | 2.5 | NR | 20% (10%) | NR | None reported | None reported | None reported | NCT02017613 | [92] | |
| INCB050465 | ||||||||||||||
| - | R/R NHL | 1 | 52 | 65 (30–88) | 3.31 | NR | 61% (32%) | NR | NR/21 | 31/6 | NR/0 | NCT02018861 | [94] | |
| ACP-319 | ||||||||||||||
| - | R/R NHL | 1 | 28 | 68 | NR | NR | NR | NR | NR | NR/10% | NR/4% | NCT01300026 | [97] | |
1L = first line, BO = bendamustine plus obinutuzumab, BR = bendamustine plus rituximab, FL = follicular lymphoma, FCR = fludarabine, cyclophosphamide, and rituximab, iNHL = indolent non-Hodgkin lymphoma, MCL = mantle cell lymphoma, MZL = marginal zone lymphoma, NR = not reported, Ofa = ofatumumab, R/R = relapsed/refractory, R-CHOP = rituximab, cyclophosphamide, vincristine, doxorubicin, and prednisone
In certain trials, only median time on therapy was provided. Time on therapy refers to time on PI3K inhibitor if multiple agents were involved.
Results of monotherapy and combination therapy with ublituximab were reported together.
Response rate restricted to 16 patients who received the phase 3 dose.
ORR in patients treated with umbralisib monotherapy.
ORR in patients treated with umbralisib plus ublituximab.
Three CLL patients were treatment naïve.
This trial enrolled both patients with solid and liquid malignancies. Median time on therapy and response rates are for NHL only. Age and toxicities are for all enrolled subjects.
The aggressive non Hodgkin lymphoma cohort included patients with DLBCL, FL grade 3b, MCL, mediastinal large B-cell lymphoma, peripheral T cell lymphoma, and transformed FL.
ORR and CR rate are for the 40 patients included in the per-protocol analysis (i.e. those who received at least three doses of drug and had pre- and post-treatment CT scans for comparison)
Diseases included Hodgkin lymphoma (9), T-cell lymphoma (4), DLBCL (4), MCL (3), CLL/SLL (2), FL (1), MZL (1), lymphoplasmacytic lymphoma (1), and multiple myeloma (1).
7. Expert Opinion
The range of therapeutic options available for the treatment of lymphomas has expanded greatly in recent years. Novel monoclonal antibodies, BTK inhibitors, thalidomide analogs, and PI3K inhibitors have all been shown to be efficacious in particular lymphoma subtypes and clinical contexts. The exact timing of PI3K inhibitor use in the treatment course of NHL is still being understood, because despite their clear efficacy (even in patients with high risk [e.g. del17p/TP53 mutated] disease) they also have serious toxicities. In CLL, because of higher rates of toxicity with idelalisib when compared to ibrutinib, PI3K inhibition is best reserved for patients who have contraindications to the use of BTK inhibitors, are intolerant of BTK inhibitors, or have progressed on BTK inhibitors. We have relatively little data regarding the effectiveness of PI3K inhibitors specifically in such patients, but retrospective data do suggest activity particularly in the context of those who discontinue ibrutinib for adverse events [104]. In relapsed/refractory FL, idelalisib should be reserved until subjects have failed at least two prior lines of therapy.
The toxicities seen with p110δ inhibitors include increased bacterial infections, opportunistic infections, and inflammation of presumed autoimmune origin including colitis, hepatitis, and pneumonitis. Rational arguments based on pre-clinical data can be made to suggest that these toxicities are due to on-target p110δ inhibition. Nevertheless, reported rates of these toxicities vary widely among p110δ inhibitors, raising the possibility that these may be off-target effects. However, caution must be taken when making cross-trial comparisons of the toxicities of various inhibitors. Toxicities are dependent on the pharmacodynamic effects of the drug, the patient population in which the drug is administered, and the length of time that subjects spend on the drug. Toxicity rates are difficult to interpret if pharmacodynamic data are not provided (particularly since the IC50 for p110δ varies widely among compounds). Furthermore, inhibition of additional kinases (such as other PI3K isoforms or – in the case of umbralisib – CK-1ε) may alter the frequency of treatment-related complications. The patient population in which the drug is administered can also cause adverse event rates to vary widely. For example, rates of grade ≥3 transaminitis seen with idelalisib ranged from 2% in the phase I trial of heavily pretreated CLL patients to 52% in the phase II trial of untreated CLL patients. Rates of toxicities with idelalisib have been higher in less heavily pre-treated patients and younger patients, perhaps because their immune system is more intact. Finally, length of drug exposure affects toxicity rates. For example, idelalisib-related colitis increases in incidence after about 7 months on the drug, and trials with shorter median follow-up may underestimate colitis rates. Possible ways to mitigate toxicity that should be explored in the future include combination therapy, alternative dosing schedules, and identification of factors that can predict which patients may be at highest risk for these events.
Moving forward, combination therapy involving PI3K inhibitors holds promise because it may be able to achieve durable responses while lessening toxicity. The combination of PI3K inhibitors with BTK inhibitors is particularly attractive because of the complementary mechanisms of action of these agents, blocking the BCR signaling pathway at multiple levels. Furthermore, their toxicities are non-overlapping. Combination therapy with either ibrutinib or chemoimmunotherapy may ameliorate toxicities if the effects of these added agents on the immune system can counteract the effects of p110δ inhibition. Additionally, while idelalisib has only demonstrated efficacy in iNHL, other PI3K inhibitors in earlier development (particularly those targeting p110α and p110δ) do have activity against aggressive NHLs, and phase 3 studies in these diseases are awaited.
Multiple unanswered issues remain within this field. However, the efficacy of these agents is unquestionable and therefore necessitates that we identify the safest way to use these agents for the treatment of our patients.
References
- 1.Goodman LS, Wintrobe MM, et al. Nitrogen mustard therapy; use of methyl-bis (beta-chloroethyl) amine hydrochloride and tris (beta-chloroethyl) amine hydrochloride for Hodgkin’s disease, lymphosarcoma, leukemia and certain allied and miscellaneous disorders. J Am Med Assoc. 1946 Sep 21;132:126–32. doi: 10.1001/jama.1946.02870380008004. [DOI] [PubMed] [Google Scholar]
- 2.Westin JR. Status of PI3K/Akt/mTOR pathway inhibitors in lymphoma. Clin Lymphoma Myeloma Leuk. 2014 Oct;14(5):335–42. doi: 10.1016/j.clml.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, et al. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010 May;11(5):329–41. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 4.Clayton E, Bardi G, Bell SE, et al. A crucial role for the p110delta subunit of phosphatidylinositol 3-kinase in B cell development and activation. J Exp Med. 2002 Sep 16;196(6):753–63. doi: 10.1084/jem.20020805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jou ST, Carpino N, Takahashi Y, et al. Essential, nonredundant role for the phosphoinositide 3-kinase p110delta in signaling by the B-cell receptor complex. Mol Cell Biol. 2002 Dec;22(24):8580–91. doi: 10.1128/MCB.22.24.8580-8591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okkenhaug K, Bilancio A, Farjot G, et al. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002 Aug 09;297(5583):1031–4. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 7.Ramadani F, Bolland DJ, Garcon F, et al. The PI3K isoforms p110alpha and p110delta are essential for pre-B cell receptor signaling and B cell development. Sci Signal. 2010 Aug 10;3(134):ra60. doi: 10.1126/scisignal.2001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Webb LM, Vigorito E, Wymann MP, et al. Cutting edge: T cell development requires the combined activities of the p110gamma and p110delta catalytic isoforms of phosphatidylinositol 3-kinase. J Immunol. 2005 Sep 01;175(5):2783–7. doi: 10.4049/jimmunol.175.5.2783. [DOI] [PubMed] [Google Scholar]
- 9.Garcon F, Patton DT, Emery JL, et al. CD28 provides T-cell costimulation and enhances PI3K activity at the immune synapse independently of its capacity to interact with the p85/p110 heterodimer. Blood. 2008 Feb 01;111(3):1464–71. doi: 10.1182/blood-2007-08-108050. [DOI] [PubMed] [Google Scholar]
- 10.Janas ML, Varano G, Gudmundsson K, et al. Thymic development beyond beta-selection requires phosphatidylinositol 3-kinase activation by CXCR4. J Exp Med. 2010 Jan 18;207(1):247–61. doi: 10.1084/jem.20091430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali K, Soond DR, Pineiro R, et al. Inactivation of PI(3)K p110delta breaks regulatory T-cell-mediated immune tolerance to cancer. Nature. 2014 Jun 19;510(7505):407–11. doi: 10.1038/nature13444. •• A basic research paper that uses genetically engineered mice to demonstrate that p110δ signaling within regulatory T cells is necessary and sufficient for the tumor-resistant phenotype of p110δ-deficient mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okkenhaug K, Patton DT, Bilancio A, et al. The p110delta isoform of phosphoinositide 3-kinase controls clonal expansion and differentiation of Th cells. J Immunol. 2006 Oct 15;177(8):5122–8. doi: 10.4049/jimmunol.177.8.5122. [DOI] [PubMed] [Google Scholar]
- 13.Martin AL, Schwartz MD, Jameson SC, et al. Selective regulation of CD8 effector T cell migration by the p110 gamma isoform of phosphatidylinositol 3-kinase. J Immunol. 2008 Feb 15;180(4):2081–8. doi: 10.4049/jimmunol.180.4.2081. [DOI] [PubMed] [Google Scholar]
- 14.Thomas MS, Mitchell JS, DeNucci CC, et al. The p110gamma isoform of phosphatidylinositol 3-kinase regulates migration of effector CD4 T lymphocytes into peripheral inflammatory sites. J Leukoc Biol. 2008 Sep;84(3):814–23. doi: 10.1189/jlb.0807561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patton DT, Garden OA, Pearce WP, et al. Cutting edge: the phosphoinositide 3-kinase p110 delta is critical for the function of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2006 Nov 15;177(10):6598–602. doi: 10.4049/jimmunol.177.10.6598. [DOI] [PubMed] [Google Scholar]
- 16.Steinbach EC, Kobayashi T, Russo SM, et al. Innate PI3K p110delta regulates Th1/Th17 development and microbiota-dependent colitis. J Immunol. 2014 Apr 15;192(8):3958–68. doi: 10.4049/jimmunol.1301533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aksoy E, Taboubi S, Torres D, et al. The p110delta isoform of the kinase PI(3)K controls the subcellular compartmentalization of TLR4 signaling and protects from endotoxic shock. Nat Immunol. 2012 Nov;13(11):1045–1054. doi: 10.1038/ni.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koyasu S. The role of PI3K in immune cells. Nat Immunol. 2003 Apr;4(4):313–9. doi: 10.1038/ni0403-313. [DOI] [PubMed] [Google Scholar]
- 19.Sasaki T, Irie-Sasaki J, Jones RG, et al. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000 Feb 11;287(5455):1040–6. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- 20.Kaneda MM, Messer KS, Ralainirina N, et al. PI3Kgamma is a molecular switch that controls immune suppression. Nature. 2016 Nov 17;539(7629):437–442. doi: 10.1038/nature19834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abubaker J, Bavi PP, Al-Harbi S, et al. PIK3CA mutations are mutually exclusive with PTEN loss in diffuse large B-cell lymphoma. Leukemia. 2007 Nov;21(11):2368–2370. doi: 10.1038/sj.leu.2404873. [DOI] [PubMed] [Google Scholar]
- 22.Baohua Y, Xiaoyan Z, Tiecheng Z, et al. Mutations of the PIK3CA gene in diffuse large B cell lymphoma. Diagn Mol Pathol. 2008 Sep;17(3):159–65. doi: 10.1097/PDM.0b013e31815d0588. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Huang H, Young KH. The PTEN tumor suppressor gene and its role in lymphoma pathogenesis. Aging (Albany NY) 2015 Dec;7(12):1032–49. doi: 10.18632/aging.100855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Psyrri A, Papageorgiou S, Liakata E, et al. Phosphatidylinositol 3’-kinase catalytic subunit alpha gene amplification contributes to the pathogenesis of mantle cell lymphoma. Clin Cancer Res. 2009 Sep 15;15(18):5724–32. doi: 10.1158/1078-0432.CCR-08-3215. [DOI] [PubMed] [Google Scholar]
- 25.Cui W, Cai Y, Wang W, et al. Frequent copy number variations of PI3K/AKT pathway and aberrant protein expressions of PI3K subunits are associated with inferior survival in diffuse large B cell lymphoma. J Transl Med. 2014 Jan 13;12:10. doi: 10.1186/1479-5876-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okkenhaug K, Graupera M, Vanhaesebroeck B. Targeting PI3K in Cancer: Impact on Tumor Cells, Their Protective Stroma, Angiogenesis, and Immunotherapy. Cancer Discov. 2016 Oct;6(10):1090–1105. doi: 10.1158/2159-8290.CD-16-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaffer AL, 3rd, Young RM, Staudt LM. Pathogenesis of human B cell lymphomas. Annu Rev Immunol. 2012;30:565–610. doi: 10.1146/annurev-immunol-020711-075027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burger JA, Chiorazzi N. B cell receptor signaling in chronic lymphocytic leukemia. Trends Immunol. 2013 Dec;34(12):592–601. doi: 10.1016/j.it.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis RE, Ngo VN, Lenz G, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010 Jan 07;463(7277):88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lannutti BJ, Meadows SA, Herman SE, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011 Jan 13;117(2):591–4. doi: 10.1182/blood-2010-03-275305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoellenriegel J, Meadows SA, Sivina M, et al. The phosphoinositide 3’-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011 Sep 29;118(13):3603–12. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meadows SA, Vega F, Kashishian A, et al. PI3Kdelta inhibitor, GS-1101 (CAL-101), attenuates pathway signaling, induces apoptosis, and overcomes signals from the microenvironment in cellular models of Hodgkin lymphoma. Blood. 2012 Feb 23;119(8):1897–900. doi: 10.1182/blood-2011-10-386763. [DOI] [PubMed] [Google Scholar]
- 33.Herman SE, Gordon AL, Wagner AJ, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010 Sep 23;116(12):2078–88. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shull AY, Noonepalle SK, Awan FT, et al. RPPA-based protein profiling reveals eIF4G overexpression and 4E–BP1 serine 65 phosphorylation as molecular events that correspond with a pro-survival phenotype in chronic lymphocytic leukemia. Oncotarget. 2015 Jun 10;6(16):14632–45. doi: 10.18632/oncotarget.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paul J, Soujon M, Wengner AM, et al. Simultaneous Inhibition of PI3Kdelta and PI3Kalpha Induces ABC-DLBCL Regression by Blocking BCR-Dependent and -Independent Activation of NF-kappaB and AKT. Cancer Cell. 2017 Jan 09;31(1):64–78. doi: 10.1016/j.ccell.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Gockeritz E, Kerwien S, Baumann M, et al. Efficacy of phosphatidylinositol-3 kinase inhibitors with diverse isoform selectivity profiles for inhibiting the survival of chronic lymphocytic leukemia cells. Int J Cancer. 2015 Nov 01;137(9):2234–42. doi: 10.1002/ijc.29579. [DOI] [PubMed] [Google Scholar]
- 37.Iyengar S, Clear A, Bodor C, et al. P110alpha-mediated constitutive PI3K signaling limits the efficacy of p110delta-selective inhibition in mantle cell lymphoma, particularly with multiple relapse. Blood. 2013 Mar 21;121(12):2274–84. doi: 10.1182/blood-2012-10-460832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ten Hacken E, Burger JA. Microenvironment interactions and B-cell receptor signaling in Chronic Lymphocytic Leukemia: Implications for disease pathogenesis and treatment. Biochim Biophys Acta. 2016 Mar;1863(3):401–13. doi: 10.1016/j.bbamcr.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown JR, Byrd JC, Coutre SE, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110delta, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014 May 29;123(22):3390–7. doi: 10.1182/blood-2013-11-535047. •• This phase I study with a relatively long median duration of follow up highlights the tolerability and effectiveness of idelalisib in a heavily pretreated patient population. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fiorcari S, Brown WS, McIntyre BW, et al. The PI3-kinase delta inhibitor idelalisib (GS-1101) targets integrin-mediated adhesion of chronic lymphocytic leukemia (CLL) cell to endothelial and marrow stromal cells. PLoS One. 2013;8(12):e83830. doi: 10.1371/journal.pone.0083830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marshall NA, Galvin KC, Corcoran AM, et al. Immunotherapy with PI3K inhibitor and Toll-like receptor agonist induces IFN-gamma+IL-17+ polyfunctional T cells that mediate rejection of murine tumors. Cancer Res. 2012 Feb 01;72(3):581–91. doi: 10.1158/0008-5472.CAN-11-0307. [DOI] [PubMed] [Google Scholar]
- 42.Winkler DG, Faia KL, DiNitto JP, et al. PI3K-delta and PI3K-gamma inhibition by IPI-145 abrogates immune responses and suppresses activity in autoimmune and inflammatory disease models. Chem Biol. 2013 Nov 21;20(11):1364–1374. doi: 10.1016/j.chembiol.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 43.Burris HA, Patel MR, Lanasa MC, et al. Activity of TGR-1202, a novel once-daily PI3Kδ inhibitor, in patients with relapsed or refractory hematologic malignancies [Meeting Abstract] J Clin Oncol. 2014;32(15_suppl):2513–2513. [Google Scholar]
- 44.Viswanadha S, Gaudio E, Zucca E, et al. Dual PI3Kδ/γ Inhibition By RP6530 Induces Apoptosis and Cytotoxicity In B-Lymphoma Cells [Meeting Abstract] Blood. 2013;122(21):4411–4411. [Google Scholar]
- 45.Shin N, Koblish H, Covington M, et al. INCB050465, a novel PI3K delta inhibitor, synergizes with PIM protein kinase inhibition to cause tumor regression in a model of DLBCL [Meeting Abstract] [Meeting Abstract] Cancer Research. 2015 Aug;75:2. [Google Scholar]
- 46.Cushing TD, Hao X, Shin Y, et al. Discovery and in vivo evaluation of (S)-N-(1-(7-fluoro-2-(pyridin-42-yl)quinolin-3-yl)ethyl)-9H–purin-6-amine (AMG319) and related PI3Kdelta inhibitors for inflammation and autoimmune disease. J Med Chem. 2015 Jan 08;58(1):480–511. doi: 10.1021/jm501624r. [DOI] [PubMed] [Google Scholar]
- 47.Folkes AJ, Ahmadi K, Alderton WK, et al. The identification of 2-(1H–indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-t hieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J Med Chem. 2008 Sep 25;51(18):5522–32. doi: 10.1021/jm800295d. [DOI] [PubMed] [Google Scholar]
- 48.Shugg RP, Thomson A, Tanabe N, et al. Effects of isoform-selective phosphatidylinositol 3-kinase inhibitors on osteoclasts: actions on cytoskeletal organization, survival, and resorption. J Biol Chem. 2013 Dec 06;288(49):35346–57. doi: 10.1074/jbc.M113.507525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu N, Rowley BR, Bull CO, et al. BAY 80-6946 is a highly selective intravenous PI3K inhibitor with potent p110alpha and p110delta activities in tumor cell lines and xenograft models. Mol Cancer Ther. 2013 Nov;12(11):2319–30. doi: 10.1158/1535-7163.MCT-12-0993-T. [DOI] [PubMed] [Google Scholar]
- 50.Flinn IW, Kahl BS, Leonard JP, et al. Idelalisib, a selective inhibitor of phosphatidylinositol 3-kinase-delta, as therapy for previously treated indolent non-Hodgkin lymphoma. Blood. 2014 May 29;123(22):3406–3413. doi: 10.1182/blood-2013-11-538546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gopal AK, Kahl BS, De Vos S, et al. PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014 Mar 13;370(11):1008–1018. doi: 10.1056/NEJMoa1314583. • This landmark phase II study demonstrated idelalisib’s effectiveness in the treatment of patients with relapsed/refractory lymphoma and was subsequently used as the basis for its regulatory approval in this setting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kahl B, Byrd JC, Flinn IW, et al. Clinical Safety and Activity In a Phase 1 Study of CAL-101, An Isoform-Selective Inhibitor of Phosphatidylinositol 3-Kinase P110δ, In Patients with Relapsed or Refractory Non-Hodgkin Lymphoma [Meeting Abstract] Blood. 2010;116(21):1777–1777. [Google Scholar]
- 53.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014 Mar 13;370(11):997–1007. doi: 10.1056/NEJMoa1315226. • This phase III study established the effectiveness of the idelalisib/rituximab combination in a relapsed CLL population including those with high-risk disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharman JP, Coutre SE, Furman RR, et al. Second Interim Analysis of a Phase 3 Study of Idelalisib Plus Rituximab for Relapsed Chronic Lymphocytic Leukemia: Efficacy Analysis in Patient Subpopulations with Del(17p) and Other Adverse Prognostic Factors [Meeting Abstract] Blood. 2014;124(21):330–330. [Google Scholar]
- 55.Zelenetz AD, Barrientos JC, Brown JR, et al. Idelalisib or placebo in combination with bendamustine and rituximab in patients with relapsed or refractory chronic lymphocytic leukaemia: interim results from a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2017 Mar;18(3):297–311. doi: 10.1016/S1470-2045(16)30671-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones JA, Robak T, Brown JR, et al. Efficacy and safety of idelalisib in combination with ofatumumab for previously treated chronic lymphocytic leukaemia: an open-label, randomised phase 3 trial. Lancet Haematol. 2017 Mar;4(3):e114–e126. doi: 10.1016/S2352-3026(17)30019-4. [DOI] [PubMed] [Google Scholar]
- 57.Coutre SE, Barrientos JC, Brown JR, et al. Management of adverse events associated with idelalisib treatment: expert panel opinion. Leuk Lymphoma. 2015;56(10):2779–86. doi: 10.3109/10428194.2015.1022770. • This expert consensus statement highlights the common toxicities experienced by patients on idelalisib and provides recommendations on their management. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheah CY, Nastoupil LJ, Neelapu SS, et al. Lenalidomide, idelalisib, and rituximab are unacceptably toxic in patients with relapsed/refractory indolent lymphoma. Blood. 2015;125(21):3357–3359. doi: 10.1182/blood-2015-03-633156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith SM, Pitcher BN, Jung SH, et al. Safety and tolerability of idelalisib, lenalidomide, and rituximab in relapsed and refractory lymphoma: the Alliance for Clinical Trials in Oncology A051201 and A051202 phase 1 trials. Lancet Haematol. 2017 Apr;4(4):e176–e182. doi: 10.1016/S2352-3026(17)30028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lampson BL, Kasar SN, Matos TR, et al. Idelalisib given front-line for treatment of chronic lymphocytic leukemia causes frequent immune-mediated hepatotoxicity. Blood. 2016 Jul 14;128(2):195–203. doi: 10.1182/blood-2016-03-707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weidner AS, Panarelli NC, Geyer JT, et al. Idelalisib-associated Colitis: Histologic Findings in 14 Patients. Am J Surg Pathol. 2015 Dec;39(12):1661–7. doi: 10.1097/PAS.0000000000000522. [DOI] [PubMed] [Google Scholar]
- 62.Cheah CY, Fowler NH. Idelalisib in the management of lymphoma. Blood. 2016 Jul 21;128(3):331–6. doi: 10.1182/blood-2016-02-702761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dong S, Guinn D, Dubovsky JA, et al. IPI-145 antagonizes intrinsic and extrinsic survival signals in chronic lymphocytic leukemia cells. Blood. 2014 Dec 04;124(24):3583–6. doi: 10.1182/blood-2014-07-587279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Balakrishnan K, Peluso M, Fu M, et al. The phosphoinositide-3-kinase (PI3K)-delta and gamma inhibitor, IPI-145 (Duvelisib), overcomes signals from the PI3K/AKT/S6 pathway and promotes apoptosis in CLL. Leukemia. 2015 Sep;29(9):1811–22. doi: 10.1038/leu.2015.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thompson R, Villegas V, Proctor J, et al. The Potent PI3K-δ γ Inhibitor IPI-145 Exhibits Differential Activity In Diffuse Large B-Cell Lymphoma (DLBCL) Cell Lines. Blood. 2013;122(21):1832–1832. [Google Scholar]
- 66.White K, Murphy E, Faia K, et al. Abstract 376: Combination of duvelisib with either ibrutinib or dexamethasone prevents mTOR-dependent feedback in aggressive B-cell lymphoma cell lines. Cancer Research. 2016;76(14 Supplement):376–376. doi: 10.1158/1538-7445.am2016-376. [DOI] [Google Scholar]
- 67.Wang J, Zhang V, Bell T, et al. The Effects of PI3K-δ/γ Inhibitor, Duvelisib, in Mantle Cell Lymphoma in Vitro and in Patient-Derived Xenograft Studies. Blood. 2016;128(22):3016–3016. [Google Scholar]
- 68.Flinn I, Oki Y, Patel M, et al. a Phase 1 Evaluation of Duvelisib (IPI-145), a PI3K-δ,γ Inhibitor, in Patients with Relapsed/Refractory iNHL. Blood. 2014;124(21):802–802. [Google Scholar]
- 69.Zinzani P, Wagner-Johnston N, Miller C, et al. DYNAMO: A Phase 2 Study Demonstrating the Clinical Activity of Duvelisib in Patients with Double-Refractory Indolent Non-Hodgkin Lymphoma [Meeting Abstract] Hematological Oncology. 2017;35:69–70. doi: 10.1002/hon.2437_57. [DOI] [Google Scholar]
- 70.Patel MR, O’Brien SM, Faia K, et al. Early clinical activity and pharmacodynamic effects of duvelisib, a PI3K-δ,γ inhibitor, in patients with treatment-naïve CLL. Journal of Clinical Oncology. 2015;33(15_suppl):7074–7074. [Google Scholar]
- 71.O’Brien S, Patel M, Kahl BS, et al. Duvelisib (IPI-145), a PI3K-δ,γ Inhibitor, Is Clinically Active in Patients with Relapsed/Refractory Chronic Lymphocytic Leukemia. Blood. 2014;124(21):3334–3334. [Google Scholar]
- 72.Porcu P, Flinn I, Kahl BS, et al. Clinical Activity of Duvelisib (IPI-145), a Phosphoinositide-3-Kinase-δ,γ Inhibitor, in Patients Previously Treated with Ibrutinib. Blood. 2014;124(21):3335–3335. [Google Scholar]
- 73.O’Brien S, Faia K, White K, et al. Early Clinical Activity and Pharmacodynamic Effects of Duvelisib, a PI3K-delta/gamma Inhibitor, in Patients with Treatment-Naive CLL [Meeting Abstract] Haematologica. 2015 Jun;100:154–155. [Google Scholar]
- 74.Flinn I, Jäger U, Offner F, et al. DUO: A phase 3 trial of the PI3K-δ,γ inhibitor IPI-145 versus ofatumumab in patients with relapsed or refractory chronic lymphocytic leukemia or small lymphocytic lymphoma. Journal of Clinical Oncology. 2014;32(15_suppl):TPS7122–TPS7122. doi: 10.1200/jco.2014.32.15_suppl.tps7122. [DOI] [Google Scholar]
- 75.Casulo C, Sancho J-M, Van Eygen K, et al. Contempo: Preliminary Results in First-Line Treatment of Follicular Lymphoma with the Oral Dual PI3K-δ,γ Inhibitor, Duvelisib, in Combination with Rituximab or Obinutuzumab. Blood. 2016;128(22):2979–2979. [Google Scholar]
- 76.Flinn IW, Cherry M, Maris M, et al. Combination Trial of Duvelisib (IPI-145) with Bendamustine, Rituximab, or Bendamustine/Rituximab in Patients with Lymphoma or Chronic Lymphocytic Leukemia. Blood. 2015;126(23):3928–3928. [Google Scholar]
- 77.Davids MS, Kim HT, Gilbert E, et al. Preliminary Results of a Phase Ib Study of Duvelisib in Combination with FCR (dFCR) in Previously Untreated, Younger Patients with CLL. Blood. 2015;126(23):4158–4158. [Google Scholar]
- 78.Deng C, Lipstein MR, Scotto L, et al. Silencing c-Myc translation as a therapeutic strategy through targeting PI3Kdelta and CK1epsilon in hematological malignancies. Blood. 2017 Jan 05;129(1):88–99. doi: 10.1182/blood-2016-08-731240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Friedman DR, Lanasa MC, Brander DM, et al. Comparison of the PI3K-δ Inhibitors TGR1202 and GS-1101 in Inducing Cytotoxicity and Inhibiting Phosphorylation of Akt in CLL Cells in Vitro [Meeting Abstract] Blood. 2012;120(21):3914–3914. [Google Scholar]
- 80.Mato A, Burris HA, Flinn I, et al. Long-term Follow-up of the Next Generation PI3K-delta Inhibitor TGR-1202 Demonstrates Safety and High Response Rates in CLL: Integrated Analysis of TGR-1202 Monotherapy and Combined with Ublituximab [Meeting Abstract] [Meeting Abstract] Haematologica. 2016 Jun;101:50–51. [Google Scholar]
- 81.O’Connor OA, Flinn I, Lunning M, et al. Long-term Follow-up of the Next Generation PI3K-delta Inhibitor TGR-1202 Demonstrates Safety and High Response Rates in NHL: Integrated Analysis of TGR-1202 Monotherapy and Combined with Ublituximab [Meeting Abstract] [Meeting Abstract] Haematologica. 2016 Jun;101:102–103. [Google Scholar]
- 82.Lunning MA, Vose JM, Bierman PJ, et al. Combination of TGR-1202, Ublituximab, and Bendamustine Is Safe and Highly Active in Patients with Advanced DLBCL and Follicular Lymphoma [Meeting Abstract] Hematological Oncology. 2017;35:266–267. [Google Scholar]
- 83.de Rooij MF, Kuil A, Kater AP, et al. Ibrutinib and idelalisib synergistically target BCR-controlled adhesion in MCL and CLL: a rationale for combination therapy. Blood. 2015 Apr 02;125(14):2306–9. doi: 10.1182/blood-2014-12-619163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Davids MS, Kim HT, Nicotra A, et al. Updated Results of a Multicenter Phase I/Ib Study of TGR-1202 in Combination with Ibrutinib in Patients with Relapsed or Refractory MCL or CLL [Meeting Abstract] Hematological Oncology. 2017;35:54–55. [Google Scholar]
- 85.Nastoupil L, Lunning MA, Vose JM, et al. Chemo-free Triplet Combination of TGR-1202, Ublituximab, and Ibrutinib Is Well Tolerated and Highly Active in Patients with Advanced CLL and NHL [Meeting Abstract] Hematological Oncology. 2017;35:112–113. [Google Scholar]
- 86.Gaudio E, Kwee I, Spriano F, et al. The phosphatidylinositol-3-kinase (PI3K) inhibitor (i) copanlisib is active in preclinical models of B-cell lymphomas as single agent and in combination with conventional and targeted agents including venetoclax and palbociclib [Meeting Abstract] Cancer Research. 2017;77(13 Supplement):154–154. [Google Scholar]
- 87.Patnaik A, Appleman LJ, Tolcher AW, et al. First-in-human phase I study of copanlisib (BAY 80-6946), an intravenous pan-class I phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors and non-Hodgkin’s lymphomas. Ann Oncol. 2016 Oct;27(10):1928–40. doi: 10.1093/annonc/mdw282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nowakowski GS, Gorbatchevsky I, Hiemeyer F, et al. CHRONOS-2: A randomized, double-blind phase III study of phosphatidylinositol-3 kinase alpha/delta inhibitor copanlisib versus placebo in patients with rituximab-refractory indolent non-Hodgkin’s lymphoma (iNHL) [Meeting Abstract] Cancer Research. 2015;75(15 Supplement):CT212–CT212. [Google Scholar]
- 89.Dreyling M, Morschhauser F, Bouabdallah K, et al. Phase II study of copanlisib, a PI3K inhibitor, in relapsed or refractory, indolent or aggressive lymphoma. Ann Oncol. 2017 Jun 14; doi: 10.1093/annonc/mdx289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dreyling M, Santoro A, Mollica L, et al. Copanlisib in Patients with Relapsed or Refractory Indolent B-Cell Lymphoma (CHRONOS-1) [Meeting Abstract] Hematological Oncology. 2017;35:119–120. doi: 10.1002/hon.2437_107. [DOI] [Google Scholar]
- 91.Lenz G, Hawkes E, Verhoef G, et al. Clinical Outcomes and Molecular Characterization from a Phase II Study of Copanlisib in Patients with Relapsed or Refractory Diffuse Large B Cell Lymphoma [Meeting Abstract] Hematological Oncology. 2017;35:68–69. doi: 10.1002/hon.2437_56. [DOI] [Google Scholar]
- 92.Carlo-Stella C, Barde P, Delarue R, et al. Safety and Clinical Activity of RP6530, a Dual PI3Kδ/γ Inhibitor, In Patients with Advanced Hematologic Malignancies: Final Analysis of a Phase I Multi-Center Study [Meeting Abstract] Hematological Oncology. 2017;35:263–263. doi: 10.1002/hon.2438_133. [DOI] [Google Scholar]
- 93.Carlo-Stella C, Delarue R, Barde PJ, et al. Clinical Activity and Safety of RP6530, a Dual PI3Kδ/γ Inhibitor, in Patients with Advanced Hematologic Malignancies: Final Analysis of a Phase 1 Multicenter Study [Meeting Abstract] Blood. 2016;128(22):3011–3011. [Google Scholar]
- 94.Caimi P, Ramchandren R, Phillips TJ, et al. Ongoing Phase 1/2 Study of INCB050465, a Selective PI3Kδ Inibitor, for the Treatment of Patients with Relapsed/Refractory B-Cell Malignancies (CITADEL-101) [Meeting Abstract] Hematological Oncology. 2017;35:268–268. doi: 10.1002/hon.2438_139. [DOI] [Google Scholar]
- 95.Coleman M, Forero-Torres A, Ribrag V, et al. Phase 2 study of the safety and efficacy of INCB050465 in patients with relapsed or refractory (R/R) diffuse large b-cell lymphoma (DLBCL) (CITADEL-202) [Meeting Abstract] Journal of Clinical Oncology. 2017;35(15_suppl):TPS7579–TPS7579. doi: 10.1200/JCO.2017.35.15_suppl.TPS7579. [DOI] [Google Scholar]
- 96.Coleman M, Salar A, Munoz J, et al. Phase 1 study of the safety and efficacy of INCB050465 combined with obinutuzumab and bendamustine for relapsed or refractory (R/R) follicular lymphoma (FL) (CITADEL-102) [Meeting Abstract] Journal of Clinical Oncology. 2017;35(15_suppl):TPS7578–TPS7578. doi: 10.1200/JCO.2017.35.15_suppl.TPS7578. [DOI] [Google Scholar]
- 97.Glenn M, Mato AR, Allgood SD, et al. First-In-Human Study Of AMG 319, a Highly Selective, Small Molecule Inhibitor Of PI3Kδ, In Adult Patients With Relapsed Or Refractory Lymphoid Malignancies [Meeting Abstract] Blood. 2013;122(21):678–678. [Google Scholar]
- 98.Kater A, Tonino S, Kersten M, et al. Interim Analysis of a Phase 1B Study Evaluating the Safety of GS-9820, a Second Generation PI3K-delta Inhibitor, in Relapsed/Refractory Lymphoid Malignancies [Meeting Abstract] Haematologica. 2015 Jun;100:458–458. [Google Scholar]
- 99.Garcia-Martinez JM, Wullschleger S, Preston G, et al. Effect of PI3K- and mTOR-specific inhibitors on spontaneous B-cell follicular lymphomas in PTEN/LKB1-deficient mice. Br J Cancer. 2011 Mar 29;104(7):1116–25. doi: 10.1038/bjc.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sarker D, Ang JE, Baird R, et al. First-in-human phase I study of pictilisib (GDC-0941), a potent pan-class I phosphatidylinositol-3-kinase (PI3K) inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2015 Jan 01;21(1):77–86. doi: 10.1158/1078-0432.CCR-14-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang S, Nastoupil LJ, Guo H, et al. Pre-Clinical Evaluation of the PI3K-p110β/δ Inhibitor KA2237 in Mantle Cell Lymphoma [Meeting Abstract] Blood. 2016;128(22):1837–1837. [Google Scholar]
- 102.Zelenetz AD, Lamanna N, Kipps TJ, et al. A Phase 2 Study of Idelalisib Monotherapy in Previously Untreated Patients ≥65 Years with Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma (SLL) [Meeting Abstract] Blood. 2014;124(21):1986–1986. [Google Scholar]
- 103.O’Brien SM, Lamanna N, Kipps TJ, et al. A phase 2 study of idelalisib plus rituximab in treatment-naive older patients with chronic lymphocytic leukemia. Blood. 2015 Dec 17;126(25):2686–94. doi: 10.1182/blood-2015-03-630947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mato AR, Hill BT, Lamanna N, et al. Optimal sequencing of ibrutinib, idelalisib, and venetoclax in chronic lymphocytic leukemia: results from a multicenter study of 683 patients. Ann Oncol. 2017 May 01;28(5):1050–1056. doi: 10.1093/annonc/mdx031. [DOI] [PubMed] [Google Scholar]