Abstract
IMPORTANCE
Glioblastoma is an incurable tumor, and the therapeutic options for patients are limited.
OBJECTIVE
To determine whether the systemic administration of HER2-specific chimeric antigen receptor (CAR)–modified virus-specific T cells (VSTs) is safe and whether these cells have antiglioblastoma activity.
DESIGN, SETTING, AND PARTICIPANTS
In this open-label phase 1 dose-escalation study conducted at Baylor College of Medicine, Houston Methodist Hospital, and Texas Children’s Hospital, patients with progressive HER2-positive glioblastoma were enrolled between July 25, 2011, and April 21, 2014. The duration of follow-up was 10 weeks to 29 months (median, 8 months).
INTERVENTIONS
Monotherapy with autologous VSTs specific for cytomegalovirus, Epstein-Barr virus, or adenovirus and genetically modified to express HER2-CARs with a CD28.ζ-signaling endodomain (HER2-CAR VSTs).
MAIN OUTCOMES AND MEASURES
Primary end points were feasibility and safety. The key secondary end points were T-cell persistence and their antiglioblastoma activity.
RESULTS
A total of 17 patients (8 females and 9 males; 10 patients ≥ 18 years [median age, 60 years; range, 30–69 years] and 7 patients <18 years [median age, 14 years; range, 10–17 years]) with progressive HER2-positive glioblastoma received 1 or more infusions of autologous HER2-CAR VSTs (1 × 106/m2 to 1 × 108/m2) without prior lymphodepletion. Infusions were well tolerated, with no dose-limiting toxic effects. HER2-CAR VSTs were detected in the peripheral blood for up to 12 months after the infusion by quantitative real-time polymerase chain reaction. Of 16 evaluable patients (9 adults and 7 children), 1 had a partial response for more than 9 months, 7 had stable disease for 8 weeks to 29 months, and 8 progressed after T-cell infusion. Three patients with stable disease are alive without any evidence of progression during 24 to 29 months of follow-up. For the entire study cohort, median overall survival was 11.1 months (95% CI, 4.1–27.2 months) from the first T-cell infusion and 24.5 months (95% CI, 17.2–34.6 months) from diagnosis.
CONCLUSIONS AND RELEVANCE
Infusion of autologous HER2-CAR VSTs is safe and can be associated with clinical benefit for patients with progressive glioblastoma. Further evaluation of HER2-CAR VSTs in a phase 2b study is warranted as a single agent or in combination with other immunomodulatory approaches for glioblastoma.
Glioblastoma is the most aggressive primary brain cancer. Despite multimodal therapy that combines maximal surgical resection with postoperative adjuvant chemoradiotherapy, 5-year overall survival (OS) rates have remained less than 4% for adults and less than 16% for children.1,2 Tumor-targeted immunotherapy has the potential to improve outcomes because it does not rely on the cytotoxic mechanisms of conventional therapies to which glioblastoma cells are resistant. Results from completed early-phase clinical trials with peptide, tumor cell, or dendritic cell vaccines for patients with glioblastoma have been encouraging, demonstrating clinical benefit.3–6
Cellular immunotherapy with adoptively transferred chimeric antigen receptor (CAR)–modified T cells is an attractive option to improve the outcomes for patients with glioblastoma.6,7 Chimeric antigen receptors usually recognize unprocessed antigens expressed on the surface of cancer cells. For glioblastoma-directed CAR T-cell therapy, several cell surface proteins are actively targeted in preclinical models, including interleukin 13Rα2 (IL-13Rα2), EphA2, EGFRvIII, and HER2.8–12 For example, Ahmed et al11 have shown that HER2-CART cells kill both “bulk” glioma cells and glioma-initiating cells and have potent antitumor activity in preclinical xenograft models derived from patients with glioblastoma.
Despite the potential benefit of HER2-CAR T cells, safety concerns were raised by the death of 1 patient, who received 1 × 1010 T cells expressing a third-generation HER2-CAR with a trastuzumab-based antigen recognition exodomain and a CD28.41BB.ζ signaling endodomain, and IL-2 after lymphodepleting chemotherapy.13 We therefore developed a second-generation HER2-CAR with an FRP5-based exodomain and a CD28.ζ endodomain. An initial safety evaluation of HER2-CAR T cells (up to 1 × 108/m2) in patients with sarcoma demonstrated no evident toxic effects and some indicators of antitumor activity; however, T-cell persistence was limited.14
One potential strategy to optimize the persistence of adoptively transferred T cells relies on the expression of CARs in virus-specific T cells (VSTs).15 These cells not only provide antitumor activity through their CAR but may also receive appropriate costimulation following native T-cell receptor (αβTCR) engagement by latent virus antigens presented by professional antigen-presenting cells. Our center has established the safety of adoptively transferred polyclonal VST lines, enriched for cytomegalovirus (CMV), Epstein-Barr virus (EBV), and adenovirus (Adv), in hematopoietic stem cell transplant recipients.16,17 We thus developed a phase 1 dose-escalation study of infusing HER2-CAR–modified autologous VSTs (HER2-CAR VSTs) in patients with progressive glioblastoma and, herein, report on their safety, persistence, and antitumor activity.
Methods
Study Design and Participants
This open-label phase 1 clinical trial was approved by the protocol review committee of the Dan L. Duncan Comprehensive Cancer Center at Baylor College of Medicine, the institutional biosafety committee and the institutional review board of Baylor College of Medicine, the Recombinant DNA Advisory Committee of the National Institutes of Health, and the US Food and Drug Administration (NCT01109095). All procedures involving human participants were carried out in accordance to the Declaration of Helsinki.18 Written informed consent was obtained from patients or guardians before enrollment in the study. This trial used the modified continual assessment method, with a cohort size of 3 patients per dose level. Patients received 1 or more intravenous infusions of autologous HER2-CAR VST at 5 dose levels (1 × 106/m2, 3 × 106/m2, 1 × 107 /m2,3 × 107/m2, and 1 × 108/m2). Patients who had an objective response at 6 weeks or at subsequent evaluation were eligible to receive up to 6 additional doses of T cells at 6- to 12-week intervals at the same dose level. All patients received infusions between July 25, 2011, and April 21, 2014. The median follow-up period was 8 months (range, 10 weeks to 29 months).
Patients with histologically proven progressive recurrent glioblastoma(World Health Organization grade IV glioma)were enrolled in the study after the diagnosis was confirmed by 2 independent pathologists (J.H. and S.Z.P.) (Figure 1). All patients underwent magnetic resonance imaging (MRI) to assess their disease before T-cell infusion. Eligibility criteria included HER2-positive glioblastoma, CMV seropositivity, normal left ventricular ejection fraction, Karnofsky/Lansky performance score of 50 or more, and life expectancy of 6 weeks or more at the time of T-cell infusion. Patients had to have completed (and recovered from) cytotoxic therapy at least 4 weeks before T-cell infusion. One exception waste mozolomide (TMZ); owing to its extremely short half-life, patients were allowed to receive TMZ up to 2 days prior to T-cell infusion. Exclusion criteria included human immunodeficiency virus seropositivity, inadequate liver function, and renal insufficiency.
Figure 1.
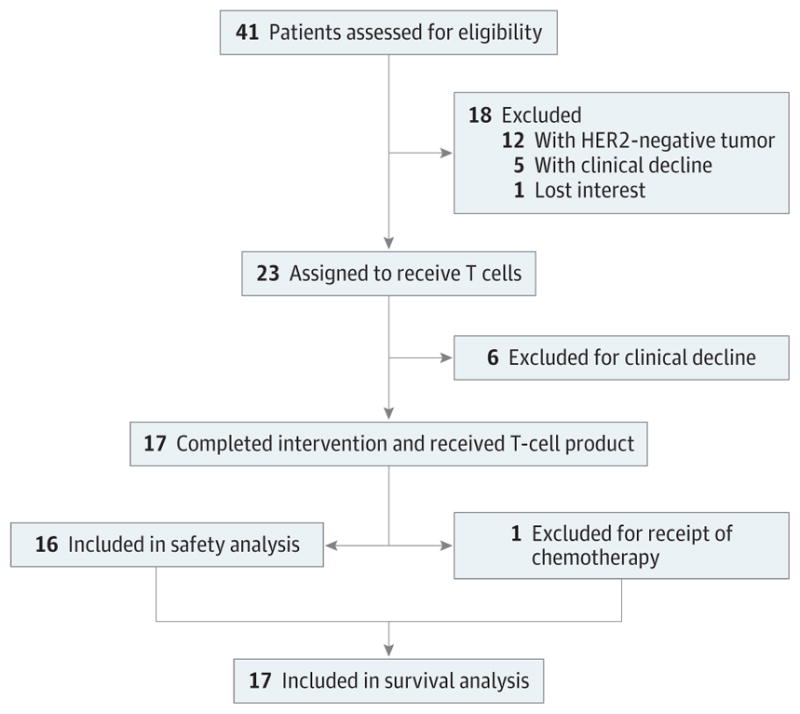
CONSORT Flow Diagram
Procedures
Autologous HER2-CARVST products were manufactured from up to 90 mL of peripheral blood according to current Good Manufacturing Practice guidelines, as previously described,16,17 and are outlined in detail in the eAppendix in the Supplement. The clinical grade retroviral vector used for transduction encoded a second-generation HER2-CAR(FRP5.CD28.ζ).14 HER2-CAR VSTs were tested for sterility, HLA identity, immunophenotype, and specificity for HER2 and virus (Adv, CMV, or EBV) at the time of cryopreservation.
Toxic effects were monitored using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.X.19 Peripheral blood samples were obtained prior to T-cell infusions and then at regular predetermined time points to evaluate for infusion-related toxic effects and to perform correlative studies (quantitative real-time polymerase chain reaction [qPCR] and enzyme-linked immunospot [Elispot] assays [eAppendix in the Supplement]).
Clinical Response Criteria
Clinical response to T-cell infusion was evaluated by performing MRI prior to and 6 weeks after T-cell infusion. Disease response was defined as a complete response (disappearance of all initial markers of disease), a partial response (30% decrease in the longest diameter of the tumor), a progressive disease (20% increase in the measurement of tumor), or a stable disease (small changes that do not meet the criteria for partial response or progressive disease). Patients with evidence of clinical benefit (complete response, stable disease, or partial response) at the 6-week evaluation were eligible to receive additional doses of T cells.
Outcomes
The primary objective of this study was to define the maximum tolerated dose and safety of autologous HER2-CARVSTs in patients with glioblastoma. Secondary objectives were to determine the in vivo fate of infused T cells and their antiglioblastoma activity.
Statistical Analysis
Safety data were described by the number and proportion of patients who had treatment-related toxic effects. Progression-free survival and OS were analyzed using Kaplan-Meier methods. Transgene expression and Elispot assays were summarized over time using descriptive statistics. The significance between groups was determined by use of the 2-tailed t test or the Fisher exact test. P < .05 was considered statistically significant. Univariate Cox proportional hazards regression analysis was used to analyze the associations of potential risk factors with response and survival outcomes.
Results
Patient Characteristics
Between July 25, 2011, and April 21, 2014, 17 patients (8 females and 9 males) with recurrent or progressive glioblastoma were enrolled in the study (eTables 1 and 2 in the Supplement). Ten of 17 patients were 18 years or older (median age,60 years; range, 30–69 years). Seven patients were younger than 18 years of age (median age, 14 years; range, 10–17 years). Sixteen patients were seropositive for CMV, and all 17 patients had a HER2-positive glioblastoma (eFigure 1 in the Supplement). Sixteen patients had surgical resections followed by radiotherapy with concomitant TMZ. Eight of 17 patients (47%) had undergone 2 or more surgical resections. All adult patients and 3 of 7 pediatric patients had received TMZ for 6 months or more. Ten patients (59%) had failed 1 to 5 lines of additional salvage therapies, and 6 patients (35%) had received investigational therapies prior to study enrollment. One patient (patient 5) received TMZ until 2 days prior to T-cell infusion, while 16 patients had not received chemotherapeutic or investigational agents for at least 4 weeks. The median time to T-cell infusion from diagnosis was 12.9 months (range, 5.9–27.2 months). All enrolled patients had normal absolute lymphocyte counts (mean, 1130/μL; range, 421–2318/μL [to convert to ×109 per liter, multiply by 0.001]) at the time of T-cell infusion.
Characteristics of Clinical Grade HER2-CAR VSTs
The mean HER2-CAR transduction efficiency of HER2-CAR VSTs was 39% (range, 18%–67%; eFigure 2A in the Supplement). Cell products contained CD3+/CD8+ (mean, 71%; range, 16%–97%) and CD3+/CD4+ (mean, 24%; range, 0.3%–88%) T cells. Most T cells had a memory phenotype consisting of effector and central memory T-cell subsets (eFigure 2B in the Supplement). In standard assays for cytotoxic effects, HER2-CAR VSTs had significant cytotoxic effects against the HER2-positive glioma cell line U373, in contrast to unmodified VSTs. Only background killing was observed against HER2-negative K562 (eFigure 2C in the Supplement). HER2-CARVSTs of all patients with a glioblastoma contained Adv- and EBV-specific T cells; in addition, all HER2-CAR VSTs from CMV-seropositive patients contained CMVpp65-specific T cells as judged by interferongamma (IFN-γ) Elispot assays (eFigure 2D in the Supplement).
Safety and In Vivo Detection of HER2-CAR VSTs
Seventeen patients received a total of 30 infusions, with 6 patients receiving multiple infusions (eTable 3 in the Supplement). No dose-limiting toxicity was observed; however, patients 3 and 16 had grade 2 seizures and/or headaches, which were probably related to the T-cell infusion (Table). At 6 weeks after the infusion, the results of cardiac function studies showed that the left ventricular ejection fractions were unchanged from their preinfusion values. HER2-CAR VSTs were detected by qPCR in all patients after the infusion. Fifteen of 17 patients had their highest frequency of HER2-CAR VSTs 3 hours after the infusion (mean, 7.8 copies/μg DNA; range, 1.4–27.8 copies/μg DNA), 1 patient had the highest frequency of HER2-CAR VSTs at 1 week after the infusion (2.0 copies/μg DNA), and 1 patient had the highest frequency of HER2-CAR VSTs at 2 weeks after the infusion (7.2 copies/μg DNA) (eFigure 3A and 3B in the Supplement). At 6 weeks after the infusion, HER2-CAR VSTs were present in 7 of 15 patients (mean, 2.0 copies/μg DNA; range, 0.7–3.8). Six weeks after infusion, the frequency of qPCR-positive blood samples declined, with 1 of 6 samples positive at 3 months, 2 of 7 samples positive at 6 months, 2 of 3 samples positive at 9 months, 2 of 6 samples positive at 12 months, and no samples positive at 18 or 24 months (eFigure 3B in the Supplement). These results indicate that HER2-CAR VSTs did not expand after the infusion but could persist for 1 year at a low frequency. Infusing multiple doses of HER2-CAR VSTs did not change their in vivo fate (eFigure 3C in the Supplement).
Table.
Adverse Events Within the First 6 Weeks After HER2-CAR VST Infusion
| Adverse Event | No. (%) of Patients (N = 17) | ||
|---|---|---|---|
| Grade 2 | Grade 3 | Grade 4 | |
| Probably related | |||
| Central nervous system | 0 | 0 | 0 |
| Headache | 1 (6) | 0 | 0 |
| Seizure | 2 (12) | 0 | 0 |
| Unrelated | |||
| Hematologic toxic effects | |||
| Anemia | 1 (6) | 0 | 0 |
| Lymphopenia | 7 (41) | 2 (12) | 0 |
| Neutropenia | 2 (12) | 0 | 1 (6) |
| Thrombocytopenia | 1 (6) | 0 | 0 |
| Nonhematologic toxic effects | |||
| General | |||
| Anorexia | 1 (6) | 0 | 0 |
| Fatigue | 0 | 1 (6) | 0 |
| Somnolence | 1 (6) | 0 | 0 |
| Weakness | 2 (12) | 1 (6) | 0 |
| HEENT | |||
| Eye paralysis, lateral | 1 (6) | 0 | 0 |
| Gastrointestinal | |||
| Nausea | 2 (12) | 0 | 0 |
| Diarrhea | 1 (6) | 0 | 0 |
| Constipation | 1 (6) | 0 | 0 |
| Vomiting | 2 (12) | 0 | 0 |
| Cardiac | |||
| Bradycardia | 1 (6) | 0 | 0 |
| Respiratory | |||
| Atelectasis | 1 (6) | 0 | 0 |
| Pain | |||
| Extremity | 1 (6) | 0 | 0 |
| Bone | 1 (6) | 0 | 0 |
| Myalgia | 1 (6) | 0 | 0 |
| Musculoskeletal | |||
| Edema, localized | 1 (6) | 0 | 0 |
| Fracture | 1 (6) | 0 | 0 |
| Central nervous system | |||
| Headache | 0 | 2 (2) | 0 |
| Seizure | 1 (6) | 0 | 0 |
| Gait disturbance | 2 (12) | 0 | 0 |
| Memory impairment | 1 (6) | 0 | 0 |
| Tremors | 1 (6) | 0 | 0 |
| Cerebral edema | 0 | 0 | 1 (6) |
| Hydrocephalus | 0 | 1 (6) | 0 |
| Infectious | |||
| Urinary tract infection | 1 (6) | 0 | 0 |
| Laboratory test results | |||
| Elevated ALT | 1 (6) | 0 | 0 |
| Elevated AST | 1 (6) | 0 | 0 |
| Hyperbilirubinemia | 1 (6) | 0 | 0 |
| Hyperkalemia | 1 (6) | 0 | 0 |
| Hypernatremia | 1 (6) | 0 | 0 |
| Hyponatremia | 1 (6) | 1 (6) | 0 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; HEENT, head, ears, eyes, nose, and throat; HER2-CAR VST, HER2–chimeric antigen receptor virus-specific T cell.
HER2-CAR VST products contained T cells that were specific for CMV (pp65), Adv, and EBV (eFigure 2D in the Supplement). The IFN-γ Elispot assays were used to measure the frequency of these VSTs in the peripheral blood of patients who received infusions. The precursor frequency of CMV (pp65)-, Adv-, and EBV-specific T cells did not change in comparison with an endogenous control (CMV IE1-specific T cells), confirming our qPCR result that infused HER2-CAR VSTs did not expand (eFigure 4 in the Supplement).
Tumor Responses and Survival After HER2-CAR VST Infusion
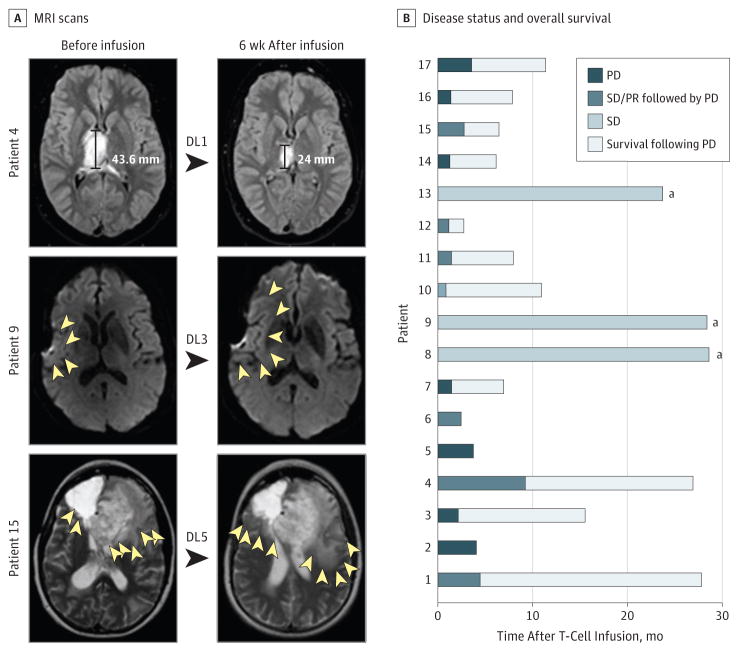
To evaluate the antiglioblastoma activity of HER2-CAR VSTs, MRI of the brain was performed 6 weeks after T-cell infusion (Figure 2A). Patient 14 received chemotherapy within the first 6 weeks of T-cell infusion and was excluded from the response analysis. Of 16 evaluable patients, 1 (6%; patient 4) had a partial response, and 7 (44%) had a stable disease for 8 weeks to 29 months after the first T-cell infusion (eTable 3 in the Supplement). Patient 4, a 17-year-old male with an unresectable right thalamic glioblastoma (maximum dimension, 4.6 cm), received HER2-CAR VSTs (1 × 106/m2) and had a partial response that lasted for 9.2 months (Figure 2A). He then had a stable disease after a second infusion at the same dose level and survived for 26.9 months from the first infusion (eTable 3 in the Supplement). Three patients (18%; patients 8, 9, and 13) are alive with a stable disease for 29.0, 28.8, and 24.0 months of follow-up. Eight patients had a progressive disease based on Response Evaluation Criteria In Solid Tumors criteria.20 Despite a progressive disease, 6 patients survived for more than 6 months (range, 6.1–15.5 months; Figure 2B). For the entire study cohort, the median time to progression was 3.5 months; median OS was 11.1 months (95% CI, 4.1–27.2 months) after the first T-cell infusion and 24.5 months (95% CI, 17.2–34.6 months) after diagnosis. We performed an extensive univariate Cox proportional hazards regression analysis to evaluate if age at diagnosis, sex, grade or intensity of HER2 positivity, number of T-cell infusions, or phenotype of infused T cells were associated with OS (eTable 4 in the Supplement). We found no correlation except that patients who did not receive salvage therapy prior to infusion had a significantly longer median OS (27.2 months) than did patients who received infusion after salvage therapy (6.7 months; P = .02; eTable 4 in the Supplement).
Figure 2. Clinical Outcome After HER2–Chimeric Antigen Receptor Virus-Specific T-Cell (HER2-CAR VST) Infusions.
A, Magnetic resonance imaging (MRI) scan of the brain before and 6 weeks after HER2-CAR VST infusion. The MRI scan of patient 4 shows a partial response (PR); MRI scans of patients 9 and 15 show increased edema after HER2-CAR VST infusions (yellow arrowheads outline the margin of edema). B, Swimmer plot showing disease status and overall survival in 17 patients treated with HER2-CAR VSTs. DL indicates dose level; PD, progressive disease; PR, partial response; and SD, stable disease.
aAlive.
Discussion
In this phase 1 dose-escalation study, we established the safety of autologous HER2-CAR VSTs in 17 patients with progressive glioblastoma. Although HER2-CARVSTs did not expand, they were detectable in the peripheral blood for up to 12 months. Eight patients had clinical benefit as defined by a partial response (n = 1) and a stable disease (n = 7). The median OS was 11.1 months after T-cell infusion and 24.5 months after diagnosis. Three patients with a stable disease were alive at the time of last follow-up with no disease progression.
Chimeric antigen receptor T-cell therapies are an attractive strategy to improve the outcomes for patients with glioblastoma. To our knowledge, only 1 study in which 3 patients with glioblastoma received an intratumoral injection of T cells that were genetically modified with a first-generation IL-13Rα2-specific CAR has been published.8 Local injections were well tolerated, and 2 of the 3 patients had a transient clinical response. In our study, we infused HER2-CAR VSTs intravenously because T cells can travel to the brain after intravenous injections, as evidenced by clinical responses after the infusion of tumor-infiltrating lymphocytes for melanoma brain metastasis21 and by detection of CD19-CAR T cells in the cerebrospinal fluid of patients with B-precursor leukemia.22
Infusion of HER2-CAR VSTs of up to 1 × 108/m2 was well tolerated without dose-limiting toxic effects. HER2-CARVSTs did not expand but were present in the peripheral blood for up to 1 year after infusion. The observation of no T-cell expansion is in agreement with previous studies in which patients with glioblastoma received unmodified CMV-specific T cells,23 patients with EBV-positive nasopharyngeal carcinoma received EBV-specific T cells,24,25 or patients with neuroblastoma received EBV-specific T cells genetically modified to express GD2-CARs(GD2-CAR/EBV T cells).15 Lack of in vivo expansion of CMV cells, EBV T cells, and GD2-CAR/EBV T cells in these studies contrasts with the significant expansion of VSTs in hematopoietic stem cell transplant recipients, who are severely lymphodepleted and have experienced reactivation of the corresponding virus.16,26 Since patients with glioblastoma in our study had normal absolute lymphocyte counts at the time of T-cell infusion, lymphodepleting chemotherapy and/or the provision of viral antigens in the form of vaccines are potential strategies to increase their invivo expansion. Lymphodepleting chemotherapy has been shown to be critical for the robust expansion of CD19-CAR T cells in patients with hematologic malignant neoplasms,22,27 and vaccines have been used successfully to boost the expansion of CAR VSTs in preclinical models.28
Although we did not observe an expansion of HER2-CAR VSTs in the peripheral blood, T cells could have expanded at glioblastoma sites. At 6 weeks after T-cell infusion, the MRI scans of patients 3, 7, 10, 16, and 17 showed an increase in peritumoral edema. Although these patients were classified as having a progressive disease, it is likely that the imaging changes for some of these patients were due to inflammatory responses, indicative of local T-cell expansion, especially since these patients survived for more than 6 months. Local inflammatory responses, so-called pseudoprogression, have been observed in several immunotherapy studies, including those for glioblastoma.3 In this regard, the Response Assessment for Neuro-Oncology working group recently published their recommendation for immunotherapy studies.29
We infused T cells that could potentially recognize HER2 and pp65 expressed in glioblastoma. Five patients had a glioblastoma that was positive for pp65; 2 of these patients had a progressive disease, and 3 had a stable disease. A larger cohort of patients with pp65-positive glioblastoma is needed to determine if pp65 expression is associated with the antiglioblastoma activity of HER2-CARVSTs. Outcomes data on post-progression survival of patients with glioblastoma are limited.30 One recent Italian study performed a retrospective outcomes analysis of 232 patients with glioblastoma who received second-line chemotherapy at disease progression after radiotherapy with TMZ. 30 The median progression-free survival was 2.5 months, and the median postprogression survival was 8.6 months. A randomized controlled clinical phase 2 trial compared the combination of bevacizumab plus lomustine with single-agent bevacizumab or lomustine in patients with glioblastoma whose front-line therapy had failed.31 Although bevacizumab and lomustine were well tolerated, the lomustine dose needed to be reduced in the bevacizumab plus lomustine arm. Fifty-two patients who received bevacizumab plus lomustine had the best outcome, with a median OS of 12 months and an 18-month OS of 20%. In our cohort, in which second-line therapy failed for 10 of the 17 patients with a glioblastoma, we achieved similar outcomes (median OS, 11.1 months; 18-month OS, 29.4%) with a median of 1 HER2-CAR VST infusion (range, 1–6 infusions) without evident toxic effects. Limiting the analysis to the 7 patients who received infusions without having received salvage therapy revealed a median OS of 27.2 months and an 18-month OS of 43%.
Limitations
No definitive conclusions regarding survival benefit based on historical controls can be made from the results of phase 1 clinical studies like ours. In addition to the small sample size, we also had attrition from screening patients for HER2 positivity to T-cell infusion (Figure 1). However, of 23 clinical-grade HER2-CAR VST products generated, 17 were infused, which is within the 25% to 30% expected rate of attrition for cell therapy studies at our center, and all patients who received infusions were included in the survival analysis. Inclusion of children(<18 years), who have a better prognosis than adults, might have also affected the outcome.2 However, there was no significant difference between the survival probability for children and that for adults in this clinical study. Although the small sample size most likely contributed to our finding, 4 of 8 pediatric patients were also heavily pretreated, and 1 patient had no resection of the primary tumor owing to its location.
There is a need to improve the antiglioblastoma activity of HER2-CAR VSTs. Besides lymphodepletion and/or immunization with a CMVpp65 vaccine to enhance the in vivo expansion and persistence of adoptively transferred T cells, other manipulations of the immunesystem might be necessary, such as blocking inhibitory molecules that are expressed on the cell surface (eg, programmed cell death ligand 1 [PD-L1]) or secreted (eg, transforming growth factor β [TGF-β]) by glioma cells.32,33 In this regard, it would have been informative to have performed additional correlative studies to monitor the phenotype of lymphocytes and myeloid cells before and after T-cell infusion. However, owing to sample availability, we restricted our analysis to the discussed transgene qPCRs and Elispot assays. In addition, performing studies (eg, CD107a degranulation or cytotoxicity assay) to determine the functionality of HER2-CARVSTs after infusion would have been informative, but these studies were not possible owing to the low frequency of HER2-CAR VSTs. Finally, antigen expression in glioblastoma is heterogeneous, and targeting multiple antigens may also have the potential to improve response rates and outcomes.34,35
Conclusions
Treatment of progressive glioblastoma with HER2-CAR VSTs is feasible and safe and resulted in clinical benefit for 8 of 17 patients. Although these data support larger studies, they also highlight the need to improve the antiglioblastoma activity of HER2-CARVSTs by augmenting their expansion, function, and persistence.
Supplementary Material
Key Points.
Question
What is the safety and antitumor efficacy of autologous HER2-specific chimeric antigen receptor (CAR)–modified virus-specific T cells (VSTs) in patients with progressive glioblastoma?
Findings
This phase 1 dose-escalation study established the safety of autologous HER2-CAR VSTs in 17 patients with progressive glioblastoma with no serious adverse events. Eight patients had a clinical benefit, with a median overall survival of 11.1 months after T-cell infusion and 24.5 months after diagnosis, and 3 patients were alive with no disease progression at the last follow-up.
Meaning
Infusion of autologous HER2-CAR VSTs is safe, with some indication of clinical benefit, and evaluation in a phase 2b study is warranted.
Acknowledgments
Funding/Support: This study was funded by the Alliance for Cancer Gene Therapy, Cancer Prevention and Research Institute of Texas grant RP110553, Alex’s Lemonade Stand Pediatric Cancer Foundation, Stand Up To Cancer/St Baldrick’s Pediatric Dream Team Translational Research grant SU2C-AACR-DT1113, the Clinical Research Center at Texas Children’s Hospital, the Dan L. Duncan Institute for Clinical and Translational Research at Baylor College of Medicine, and by shared resources through grant P30CA125123 from the National Institutes of Health. Stand Up To Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research.
Footnotes
Conflict of Interest Disclosures: Drs Ahmed, Hegde, Bielamowicz, Dotti, Brenner, Heslop, Wels, and Gottschalk and Ms Brawley have patents and/or patent applications pertaining to adoptive cell therapy. No other disclosures were reported.
Additional Contributions: We thank the referring physicians, the staff of the clinical research unit for assisting with patient follow-up, and the staff of the Good Manufacturing Practice facility for assisting in T-cell line production and analysis. We also thank the patients who participated in this study and the parents who entrusted the care of their children to us.
Author Contributions: Drs Ahmed and Gottschalk had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Ahmed, Rooney, Dotti, Grilley, Hicks, Brenner, Heslop, Wels, Gottschalk.
Acquisition, analysis, or interpretation of data: Ahmed, Brawley, Hegde, Bielamowicz, Kalra, Landi, Robertson, Gray, Diouf, Wakefield, Ghazi, Gerken, Yi, Ashoori, Wu, Liu, Gee, Su, Kew, Baskin, Zhang, New, Stojakovic, Hicks, Powell, Grossman, Gottschalk.
Drafting of the manuscript: Ahmed, Brawley, Hegde, Bielamowicz, Kalra, Landi, Robertson, Gray, Wakefield, Ghazi, Gerken, Yi, New, Stojakovic, Hicks, Gottschalk.
Critical revision of the manuscript for important intellectual content: Ahmed, Hegde, Bielamowicz, Landi, Diouf, Ashoori, Wu, Liu, Rooney, Dotti, Gee, Su, Kew, Baskin, Zhang, Grilley, Hicks, Powell, Brenner, Heslop, Grossman, Wels, Gottschalk.
Statistical analysis: Ahmed, Bielamowicz, Landi, Wakefield, Wu, Liu, Hicks.
Obtained funding: Ahmed, Gottschalk.
Administrative, technical, or material support: Ahmed, Brawley, Kalra, Landi, Robertson, Diouf, Wakefield, Ghazi, Gerken, Yi, Ashoori, Rooney, Gee, Kew, Baskin, Zhang, New, Grilley, Stojakovic, Hicks, Powell, Brenner, Heslop, Grossman, Wels, Gottschalk.
Study supervision: Ahmed, Hegde, Landi, Rooney, Stojakovic, Powell, Brenner, Heslop, Gottschalk.
Role of the Funder/Sponsor: The Alliance for Cancer Gene Therapy, the Cancer Prevention and Research Institute of Texas, Alex’s Lemonade Stand Pediatric Cancer Foundation, the Clinical Research Center at Texas Children’s Hospital, the Dan L. Duncan Institute for Clinical and Translational Research at Baylor College of Medicine, and National Institutes of Health contributed to the conduct of the study. The funding sources had no role in the design of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.Stupp R, Taillibert S, Kanner AA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314(23):2535–2543. doi: 10.1001/jama.2015.16669. [DOI] [PubMed] [Google Scholar]
- 2.Cohen KJ, Pollack IF, Zhou T, et al. Temozolomide in the treatment of high-grade gliomas in children: a report from the Children’s Oncology Group. Neuro Oncol. 2011;13(3):317–323. doi: 10.1093/neuonc/noq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pollack IF, Jakacki RI, Butterfield LH, et al. Antigen-specific immune responses and clinical outcome after vaccination with glioma-associated antigen peptides and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in children with newly diagnosed malignant brainstem and nonbrainstem gliomas. J Clin Oncol. 2014;32(19):2050–2058. doi: 10.1200/JCO.2013.54.0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sampson JH, Heimberger AB, Archer GE, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28(31):4722–4729. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell DA, Batich KA, Gunn MD, et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature. 2015;519(7543):366–369. doi: 10.1038/nature14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suryadevara CM, Verla T, Sanchez-Perez L, et al. Immunotherapy for malignant glioma. Surg Neurol Int. 2015;6(suppl 1):S68–S77. doi: 10.4103/2152-7806.151341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown CE, Alizadeh D, Starr R, et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med. 2016;375(26):2561–2569. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown CE, Badie B, Barish ME, et al. Bioactivity and safety of IL13Rα2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin Cancer Res. 2015;21(18):4062–4072. doi: 10.1158/1078-0432.CCR-15-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson LA, Scholler J, Ohkuri T, et al. Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci Transl Med. 2015;7(275):275ra22. doi: 10.1126/scitranslmed.aaa4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow KK, Naik S, Kakarla S, et al. T cells redirected to EphA2 for the immunotherapy of glioblastoma. Mol Ther. 2013;21(3):629–637. doi: 10.1038/mt.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed N, Salsman VS, Kew Y, et al. HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clin Cancer Res. 2010;16(2):474–485. doi: 10.1158/1078-0432.CCR-09-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sampson JH, Choi BD, Sanchez-Perez L, et al. EGFRvIII mCAR-modified T-cell therapy cures mice with established intracerebral glioma and generates host immunity against tumor-antigen loss. Clin Cancer Res. 2014;20(4):972–984. doi: 10.1158/1078-0432.CCR-13-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18(4):843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed N, Brawley VS, Hegde M, et al. Human epidermal growth factor receptor 2 (HER2)–specific chimeric antigen receptor–modified T cells for the immunotherapy of HER2-positive sarcoma. J Clin Oncol. 2015;33(15):1688–1696. doi: 10.1200/JCO.2014.58.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14(11):1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leen AM, Myers GD, Sili U, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12(10):1160–1166. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- 17.Leen AM, Bollard CM, Mendizabal AM, et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood. 2013;121(26):5113–5123. doi: 10.1182/blood-2013-02-486324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 19.National Cancer Institute, National Institutes of Health. [Accessed March 19, 2017];CTCAE files. https://evs.nci.nih.gov/ftp1/CTCAE/About.html. Updated May 17, 2010.
- 20.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 21.Hong JJ, Rosenberg SA, Dudley ME, et al. Successful treatment of melanoma brain metastases with adoptive cell therapy. Clin Cancer Res. 2010;16(19):4892–4898. doi: 10.1158/1078-0432.CCR-10-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuessler A, Smith C, Beagley L, et al. Autologous T-cell therapy for cytomegalovirus as a consolidative treatment for recurrent glioblastoma. Cancer Res. 2014;74(13):3466–3476. doi: 10.1158/0008-5472.CAN-14-0296. [DOI] [PubMed] [Google Scholar]
- 24.Louis CU, Straathof K, Bollard CM, et al. Adoptive transfer of EBV-specific T cells results in sustained clinical responses in patients with locoregional nasopharyngeal carcinoma. J Immunother. 2010;33(9):983–990. doi: 10.1097/CJI.0b013e3181f3cbf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Louis CU, Straathof K, Bollard CM, et al. Enhancing the in vivo expansion of adoptively transferred EBV-specific CTL with lymphodepleting CD45 monoclonal antibodies in NPC patients. Blood. 2009;113(11):2442–2450. doi: 10.1182/blood-2008-05-157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heslop HE, Slobod KS, Pule MA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115(5):925–935. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooper LJ, Al-Kadhimi Z, Serrano LM, et al. Enhanced antilymphoma efficacy of CD19-redirected influenza MP1-specific CTLs by cotransfer of T cells modified to present influenza MP1. Blood. 2005;105(4):1622–1631. doi: 10.1182/blood-2004-03-1208. [DOI] [PubMed] [Google Scholar]
- 29.Okada H, Weller M, Huang R, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 2015;16(15):e534–e542. doi: 10.1016/S1470-2045(15)00088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franceschi E, Ermani M, Bartolini S, et al. Post progression survival in glioblastoma: where are we? J Neurooncol. 2015;121(2):399–404. doi: 10.1007/s11060-014-1651-7. [DOI] [PubMed] [Google Scholar]
- 31.Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–953. doi: 10.1016/S1470-2045(14)70314-6. [DOI] [PubMed] [Google Scholar]
- 32.Preusser M, Lim M, Hafler DA, Reardon DA, Sampson JH. Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nat Rev Neurol. 2015;11(9):504–514. doi: 10.1038/nrneurol.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roy LO, Poirier MB, Fortin D. Transforming growth factor-beta and its implication in the malignancy of gliomas. Target Oncol. 2015;10(1):1–14. doi: 10.1007/s11523-014-0308-y. [DOI] [PubMed] [Google Scholar]
- 34.Hegde M, Mukherjee M, Grada Z, et al. Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. J Clin Invest. 2016;126(8):3036–3052. doi: 10.1172/JCI83416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hegde M, Corder A, Chow KK, et al. Combinational targeting offsets antigen escape and enhances effector functions of adoptively transferred T cells in glioblastoma. Mol Ther. 2013;21(11):2087–2101. doi: 10.1038/mt.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.