Abstract
An effective Zika virus (ZIKV) vaccine will require long-term durable protection. Several ZIKV vaccine candidates have demonstrated protective efficacy in nonhuman primates, but such studies have typically involved ZIKV challenge shortly following vaccination at peak immunity. In this study, we show that a single immunization with an adenovirus vector-based vaccine, as well as two immunizations with a purified inactivated virus vaccine, afforded robust protection against ZIKV challenge in rhesus monkeys at 1 year following vaccination. In contrast, two immunizations with an optimized DNA vaccine, which provided complete protection at peak immunity, resulted in reduced protective efficacy at 1 year that was associated with declining neutralizing antibody titers to sub-protective levels. These data define a microneutralization log titer of 2.0-2.1 as the threshold required for durable protection against ZIKV challenge in this model. Moreover, our findings demonstrate that protection against ZIKV challenge in rhesus monkeys is possible for at least 1 year with a single-shot vaccine.
Introduction
The development of a safe and effective ZIKV vaccine has emerged as a global health priority (1–5). ZIKV infection has been shown to be associated with fetal microcephaly and other congenital malformations (6–9) as well as Guillain-Barre syndrome in healthy adults (10). Protective efficacy of DNA vaccines, RNA vaccines, adenovirus (Ad) vector-based vaccines, purified inactivated virus (PIV) vaccines, and live attenuated virus (LAV) vaccines has been demonstrated against ZIKV challenge in rodents and nonhuman primates (11–19), and several vaccine candidates are currently in clinical trials (3–5).
Nonhuman primate challenge studies reported to date have only assessed protection at peak immunity shortly after vaccination (11, 13, 15). In this study, we report the 1-year protective efficacy of three leading vaccine platforms (PIV, DNA, Ad) in rhesus monkeys as well as the immune correlates of protection.
Results
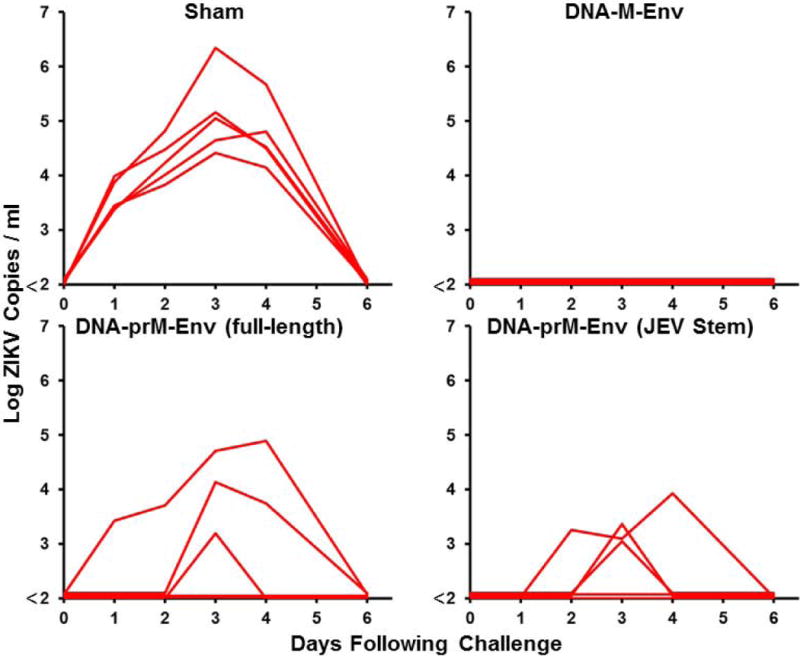
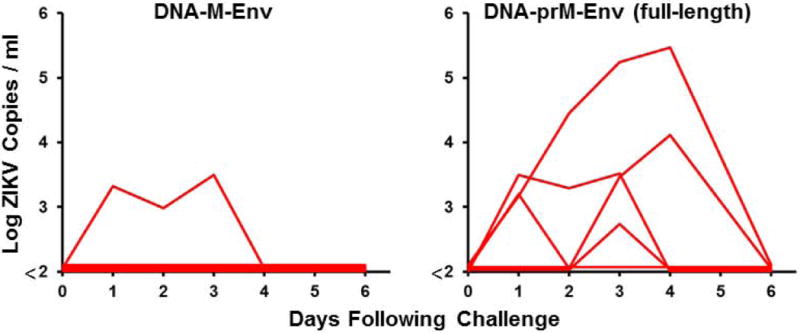
We previously designed a DNA vaccine expressing an engineered form of ZIKV BeH815744 prM-Env containing a deletion of the cleavage peptide (amino acids 216-794; also termed M-Env), and we showed that this vaccine protected against ZIKV challenge in both mice and rhesus monkeys (11, 12). We compared antigen expression and immunogenicity of DNA vaccines expressing this engineered M-Env, the corresponding full-length prM-Env, and full-length prM-Env containing the stem region of Japanese encephalitis virus (JEV), which has been shown to increase secretion of soluble subviral particles (15) (Fig. 1A). The DNA-M-Env vaccine exhibited the highest Env expression by Western blot (Fig. 1B). Groups of Balb/c mice (N=5/group) were then immunized by the intramuscular route with a single 50 μg immunization of DNA vaccines expressing M-Env, prM-Env (full-length), or prM-Env (JEV stem). The DNA-M-Env vaccine induced the highest antibody responses by ELISA at week 4 (P=0.003 and P=0.002 comparing titers induced by DNA-M-Env titers with titers induced by DNA-prM-Env (full-length) and DNA-prM-Env (JEV Stem), respectively; Fig. 1B). Following challenge with 105 viral particles (VP) [102 plaque-forming units (PFU)] of ZIKV-BR by the intravenous route (12), only the DNA-M-Env vaccine afforded complete protection (Fig. 1C). Env-specific log ELISA titers >2.0 were associated with protection (P<0.0001, Fig. S1). We speculate that the improved performance of the deleted M-Env immunogen may reflect the inefficiency of natural cleavage in the full-length prM-Env immunogen and the lack of the cleavage peptide in the deleted M-Env immunogen.
Figure 1. ZIKV prM-Env antigen development.
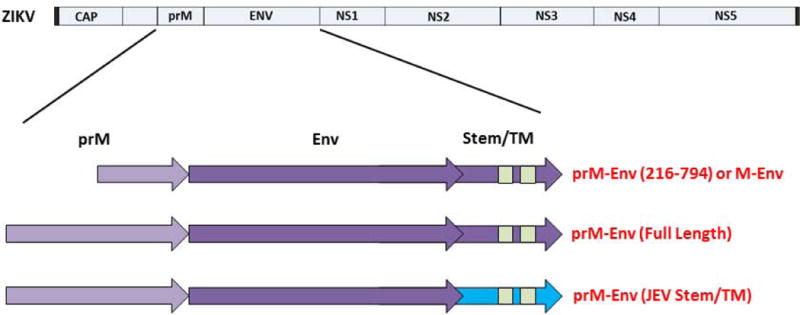
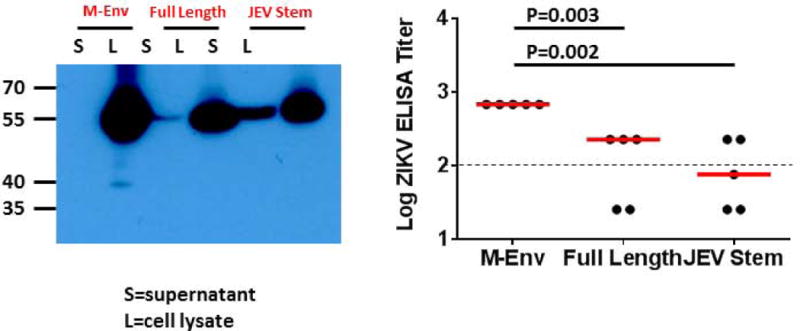
(A) Scheme of the ZIKV prM-Env antigens tested: cleavage peptide-deleted prM-Env (amino acids 216-794; also termed M-Env), full-length prM-Env, and full-length prM-Env with the stem and transmembrane (TM) region of Japanese encephalitis virus (JEV). (B) Expression from DNA vaccines expressing these three antigens by Western blot and immunogenicity in Balb/c mice (N=5/group) by Env-specific ELISA following a single immunization of 50 μg of DNA vaccines expressing M-Env, prM-Env (full-length), or prM-Env (JEV stem). P-values were determined by t-test. The dotted line reflects log ELISA titers of 2.0. Red lines reflect medians. (C) Mice were challenged by the i.v. route with 105 VP (102 PFU) ZIKV-BR. Viral loads were determined in serum on days 0, 1, 2, 3, 4, and 6.
We next compared the immunogenicity and protective efficacy of multiple ZIKV vaccine candidates in Balb/c mice. Groups of mice (N=5/group) were immunized once by the intramuscular route with 109 VP Ad26-M-Env, 109 VP RhAd52-M-Env, 1 μg PIV with alum, 50 μg DNA-M-Env, 50 μg DNA-prM-Env, or sham vaccine. Env-specific ELISA titers were higher in the Ad26-M-Env, RhAd52-M-Env, and PIV groups as compared with the DNA-M-Env and DNA-prM-Env groups over 20 weeks of follow-up (Fig. 2A). At week 20, all mice were challenged with ZIKV-BR as described above. Complete protection was observed in the groups of mice that received the Ad26-M-Env, RhAd52-M-Env, and PIV vaccines (Fig. 2B). In contrast, protection was observed in only 80% (4 of 5) of mice that received the DNA-M-Env vaccine and in only 20% (1 of 5) of mice that received the DNA-prM-Env vaccine (Fig. 2C), which elicited the lowest Env-specific antibody responses (Fig. 2B), consistent with the previous experiment.
Figure 2. Long-term immunogenicity and protective efficacy of ZIKV vaccines in Balb/c mice.
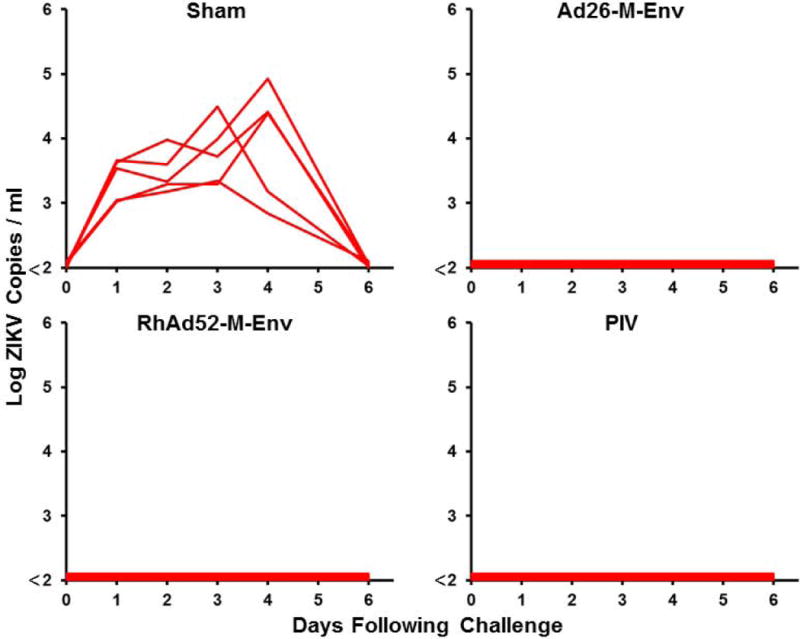
(A) Balb/c mice (N=5/group) were immunized once by the intramuscular route with 109 VP Ad26-M-Env, 109 VP RhAd52-M-Env, 1 μg PIV with alum, 50 μg DNA-M-Env, 50 μg DNA-prM-Env, or sham vaccine. Median Env-specific ELISA titers are shown. Error bars reflect S.E.M. The dotted line reflects log ELISA titers of 2.0. (B, C) Mice were challenged by the i.v. route with 105 VP (102 PFU) ZIKV-BR. Viral loads were determined in serum on days 0, 1, 2, 3, 4, and 6.
To evaluate the durability of ZIKV vaccine efficacy in nonhuman primates, we immunized 16 rhesus monkeys by the subcutaneous route with 5 μg ZIKV PIV vaccine with alum (N=8) or sham vaccine (alum only) (N=8) twice at weeks 0 and 4 (11). We followed ZIKV-specific neutralizing antibodies by microneutralization (MN50) assays (11, 12) over 52 weeks (Fig. 3A). Median log MN50 titers in the PIV vaccinated monkeys were 1.88 at week 4 after the initial immunization and increased to 3.71 at week 8 after the boost immunization. Neutralizing antibody titers then declined by 1.33 logs over the next 10 weeks to median log MN50 titers of 2.38 at week 18, and titers then remained largely stable until week 52. Low Env-specific cellular immune responses were also observed by interferon (IFN)-γ ELISPOT assays (Fig. S2).
Figure 3. Long-term immunogenicity and protective efficacy of the ZIKV PIV vaccine in rhesus monkeys.
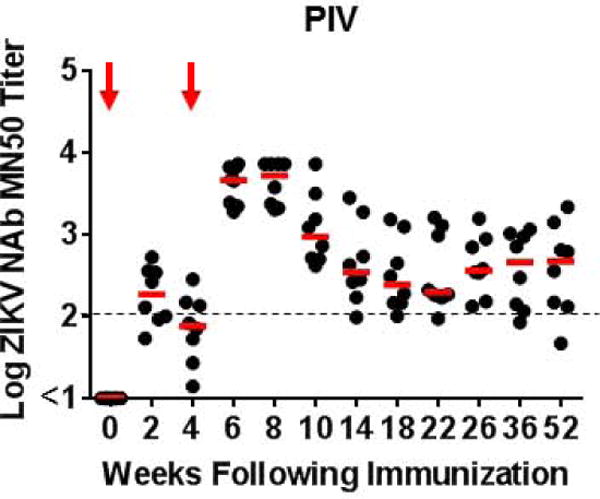
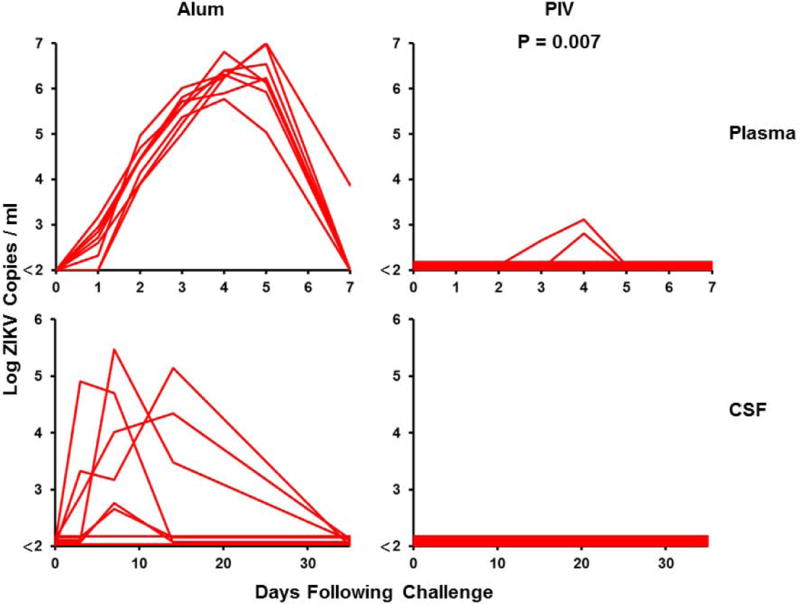
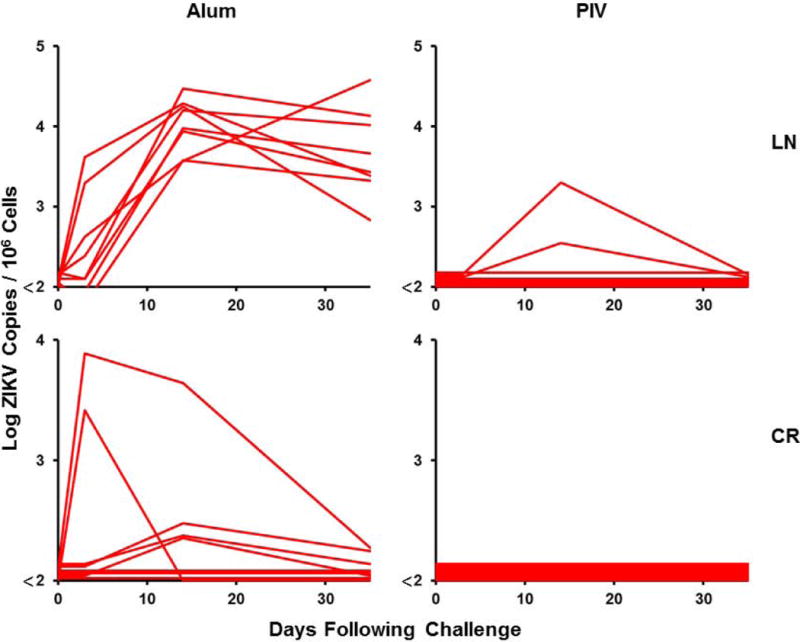
(A) Log ZIKV-specific microneutralization (MN50) titers following immunization of rhesus monkeys by the s.c route with 5 μg ZIKV PIV vaccine (N=8) at weeks 0 and 4 (red arrows). The dotted line reflects log MN50 titers of 2.0. Red bars reflect medians. PIV vaccinated and sham control rhesus monkeys (N=8/group) were challenged by the s.c route with 106 VP (103 PFU) ZIKV-BR. Viral loads are shown in (B) plasma, (C) cerebrospinal fluid (CSF), and (D) lymph nodes (LN). Viral loads were determined on days 0, 1, 2, 3, 4, 5, and 7 for the plasma samples and on days 0, 3, 14, and 35 for the other samples. P-value determined by Fisher’s exact test.
At week 52, all monkeys were challenged with 106 VP (103 PFU) of ZIKV-BR by the subcutaneous route as previously described (11, 20). Viral loads were quantitated by RT-PCR. Sham control monkeys exhibited approximately 7 days of viremia with median peak viral loads of 6.47 on day 4-5 following challenge (Fig. 3B). Virus was detected for a longer period of time in certain tissue compartments of the sham controls, including cerebrospinal fluid (CSF) and lymph nodes (LN) (Fig. 3C–D), consistent with previous findings from our laboratory and others (20–23). In contrast, PIV vaccinated monkeys showed no detectable viremia (<2 log copies/ml) in 75% (6 of 8) of animals (P=0.007 compared with sham controls), and low and transient viral blips in 25% (2 of 8) of animals. These two PIV vaccinated monkeys also showed low levels of virus in LN.
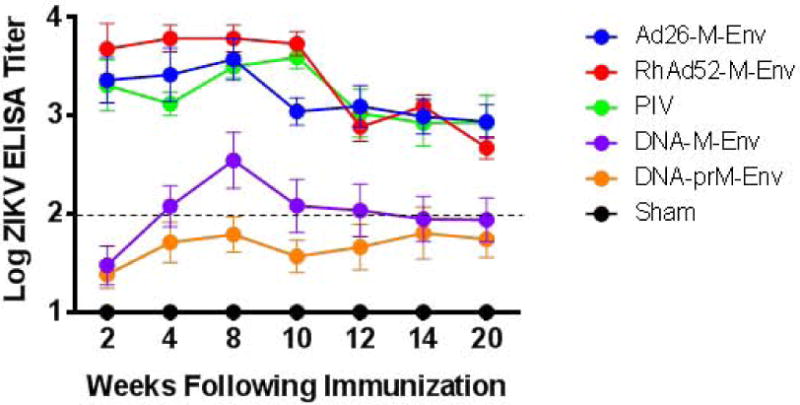
We next evaluated the durability of protection afforded by the DNA-M-Env and the RhAd52-M-Env vaccines in nonhuman primates. We immunized 15 rhesus monkeys by the intramuscular route with two immunizations of 5 mg DNA-M-Env at weeks 0 and 4 (N=7), a single-shot immunization of 1011 VP RhAd52-M-Env at week 0 (N=4), or sham vaccine (N=4). MN50 titers were low after the first DNA-M-Env vaccination but reached peak median log titers of 2.23 at week 8 after the boost immunization (Fig. 4A). Median log MN50 titers in the DNA-M-Env vaccinated animals declined rapidly to 1.43 by week 14 but then remained largely stable until week 52. Of note, only 2 of 7 DNA-M-Env vaccinated animals exhibited log MN50 titers of 2.0 or higher during this follow-up period. In contrast, a single immunization of the RhAd52-M-Env induced median log MN50 titers of 2.38 by week 2 (Fig. 4A). MN50 titers in these animals persisted and proved remarkably stable over a year of follow-up, with median log MN50 titers of 2.42 (range 2.30–2.54) at week 52 (Fig. 4A). Env-specific cellular immune responses were also induced in these animals (Fig. S3).
Figure 4. Long-term immunogenicity and protective efficacy of the ZIKV DNA-M-Env and RhAd52-M-Env vaccines in rhesus monkeys.
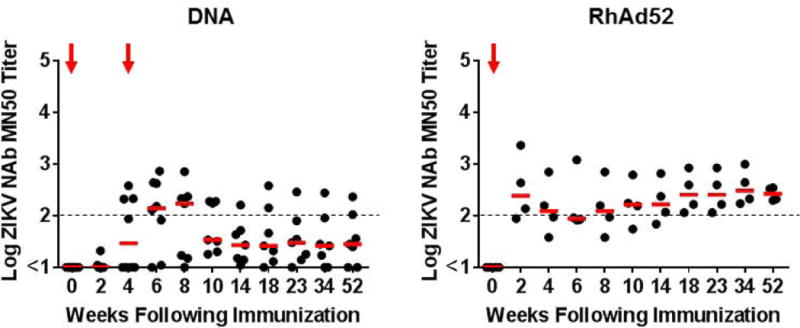
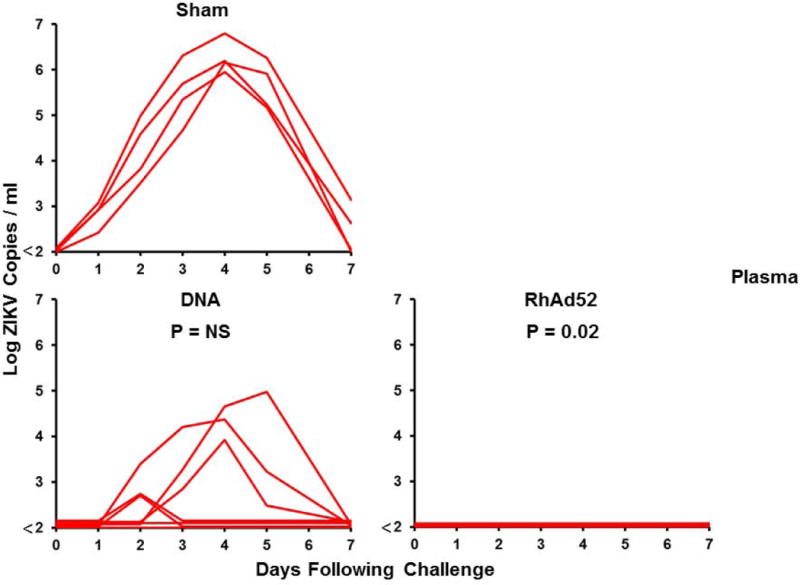
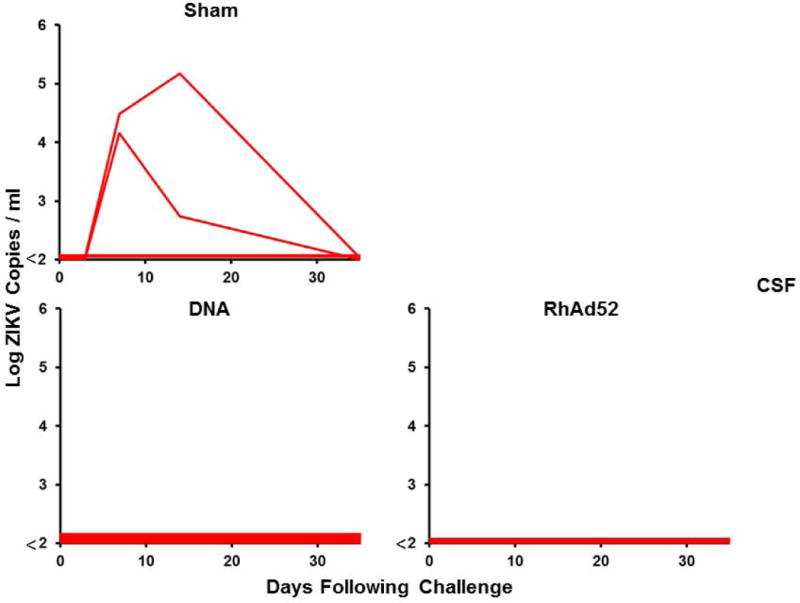
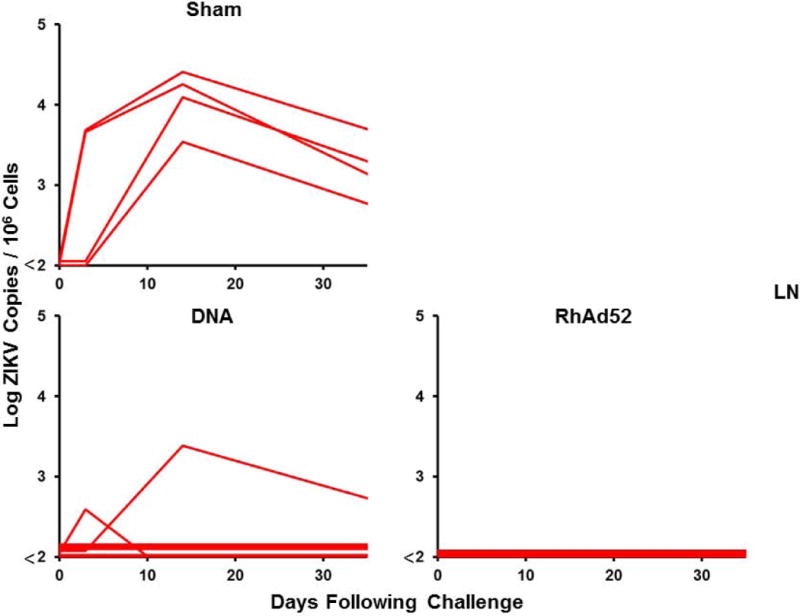
(A) Log ZIKV-specific microneutralization (MN50) titers following immunization of rhesus monkeys by the i.m. with two immunizations of 5 mg DNA-M-Env (N=7) at weeks 0 and 4 (red arrows) or a single-shot immunization of 1011 VP RhAd52-M-Env (N=4) at week 0 (red arrow). The dotted lines reflect log MN50 titers of 2.0. Red bars reflect medians. Vaccinated and sham control rhesus monkeys were challenged by the s.c route with 106 VP (103 PFU) ZIKV-BR. Viral loads are shown in (B) plasma, (C) cerebrospinal fluid (CSF), and (D) lymph nodes (LN). Viral loads were determined on days 0, 1, 2, 3, 4, 5, and 7 for the plasma samples and on days 0, 3, 14, and 35 for the other samples. P-values determined by Fisher’s exact tests.
Following challenge with 106 VP (103 PFU) of ZIKV-BR at week 52, only 29% (2 of 7) of DNA-M-Env vaccinated animals were protected, and 71% (5 of 7) of animals in this group exhibited viremia (Fig. 4B). Of note, the 2 DNA-M-Env vaccinated monkeys that were protected were the animals with the highest log MN50 titers. Since the DNA-M-Env vaccine afforded complete protection when challenged at peak immunity (11), we speculate that the abrogation of protection reflects the decline of neutralizing antibody titers over the year prior to challenge to sub-protective levels. In contrast, a single immunization with RhAd52-M-Env provided protection in 100% (4 of 4) of monkeys at 1 year (P=0.02 compared with sham controls, Fig. 4B–D), likely reflecting the persistent MN50 titers in these animals.
We next assessed the capacity of week 52 pre-challenge serum from the PIV, DNA-M-Env, and RhAd52-M-Env vaccinated monkeys to neutralize a panel of ZIKV strains, and we observed cross-neutralization of viral strains from Brazil (BR), Uganda (UG), Thailand (TH), Philippines (PH), and Puerto Rico (PR) (Fig. S4). We also evaluated the capacity of serum antibodies to enhance ZIKV infection in vitro in K562 cells. As expected, all animals with detectable neutralizing antibodies resulted in enhanced infection in K562 cells at relatively high dilutions of sera (Figs. S5, S6), suggesting that this in vitro assay does not readily distinguish between protective versus enhancing antibodies. No animals demonstrated enhanced ZIKV viremia in this study, including monkeys with sub-protective neutralizing antibodies and enhanced infection in K562 cells. We also observed that MN50 titers increased in all the vaccinated animals following challenge (Figs. S7, S8), which may reflect either a lack of complete sterilizing immunity or alternatively an immunologic boost by the 106 VP dose of the challenge virus. Supporting the latter possibility is the lack of observed increased cellular immune responses in the RhAd52-M-Env vaccinated animals following challenge (Fig. S9).
Given the heterogeneous outcome of the challenge studies with the PIV, DNA-M-Env, and RhAd52-M-Env vaccines, we performed an immune correlates analysis to define the threshold MN50 titer required for protection. In the vaccinated animals, the log MN50 titer at the time of challenge (week 52) was inversely correlated with the peak log ZIKV viral load following challenge (R=−0.81, P<0.0001; Fig. 5). Moreover, MN50 titers were higher in protected animals than in infected animals (P<0.0001). Specifically, 92% (12 of 13) of animals with MN50 titers >2.0 and 100% (12 of 12) of animals with MN50 titers >2.1 at week 52 were protected. In contrast, 100% (6 of 6) of animals with MN50 titers <2.0 were infected. Similar results were obtained by an immune correlates analysis that included all animals including the sham controls (Fig. S10). Moreover, adoptive transfer studies using purified IgG from week 52 plasma samples confirmed that the vaccine-induced rhesus monkey antibodies afforded passive protection in Balb/c mice (Fig. S11).
Figure 5. Immune correlates analysis in vaccinated rhesus monkeys.
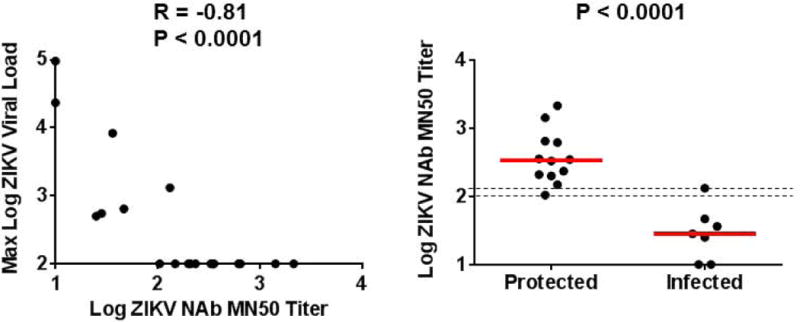
Correlation of maximum log viral loads following ZIKV-BR challenge with log MN50 titers at week 52 prior to challenge (left). P-value determined by Spearman rank-correlation test. Comparison of log MN50 titers at week 52 in protected versus infected animals (right). P-value determined by Wilcoxon rank-sum test. The dotted lines reflect log MN50 titers of 2.0 and 2.1. Red lines reflect medians.
Discussion
In this study, we demonstrate that a single-shot immunization with RhAd52-M-Env provided complete protection against ZIKV-BR challenge in 100% (4 of 4) rhesus monkeys after 1 year. Two immunizations with the ZIKV PIV vaccine also provided robust protection in 75% (6 of 8) animals after 1 year. In contrast, DNA vaccines expressing the same optimized M-Env insert elicited neutralizing antibody titers that declined to sub-protective levels during this time period. Protective efficacy strongly correlated with MN50 titers at the time of challenge, which defined the threshold of protection in this model to be log MN50 titers of 2.0-2.1 (MN50 titers of 100–125).
Previous ZIKV vaccine studies in nonhuman primates from our laboratory and others have challenged animals shortly following vaccination at peak immunity (11, 13, 15). While these data provide an important assessment of the theoretical short-term protective efficacy of vaccine candidates, it is critical for a ZIKV vaccine to provide long-term durable protection. Vaccine-elicited antibody responses typically decline with different kinetics depending on the vaccine modality and are likely impacted by multiple immunologic and other factors. The PIV vaccine induced high MN50 titers following vaccination that declined over 3 months but importantly still remained above the protective threshold in the majority of animals. In contrast, the DNA-M-Env vaccine induced moderate MN50 titers that were sufficient for protection at peak immunity (11), but these responses declined to sub-protective levels within 2-3 months. The RhAd52-M-Env vaccine induced moderate MN50 titers after a single-shot immunization, but these responses remained stable with minimal decline over 52 weeks. The immunologic basis of the persistent neutralizing antibody responses elicited by RhAd52-M-Env remains to be determined.
The strong correlation between ZIKV-specific antibody responses and protective efficacy in both mice and rhesus monkeys, as well as the robustness of this immune correlate across different antigens and different vaccine platforms, suggest the potential generalizability of these observations. Taken together with previous adoptive transfer studies using polyclonal antibodies from vaccinated animals (11, 12) as well as monoclonal antibodies (24), we suggest that ZIKV-specific neutralizing antibodies represent the primary mechanistic correlate of protection for ZIKV vaccines. These insights should prove useful in the clinical development of ZIKV vaccines, although the quantitative titer threshold required for ZIKV protection may differ between rhesus monkeys and humans. For other flavivirus vaccines in humans, neutralizing antibody titers of >10 have been reported as correlates of protection (25–27). Whether or not higher titers will be required for protection against ZIKV in humans remains to be determined. Future studies should also define the Env regions and epitopes that are the target of protective neutralizing antibodies.
The potential for cross-reactive DENV-specific antibodies to interfere with the immunogenicity and/or protective efficacy of ZIKV vaccines is an important research question. Previous studies have suggested that DENV-specific antibodies can increase ZIKV replication in vitro and in mice (28–30), but studies in primates have to date not replicated these findings (31, 32). Dedicated studies of ZIKV vaccines in DENV-exposed animals and humans are therefore warranted. It also remains uncertain whether vaccine protection against virus replication in peripheral blood and tissues will translate into prevention of congenital Zika syndrome.
Taken together, our data demonstrate durable 1-year protection against ZIKV challenge by a recombinant adenovirus vector-based vaccine and a purified inactivated virus vaccine in rhesus monkeys. ZIKV Ad, PIV, DNA, and RNA vaccines are currently being evaluated in clinical trials (33). Our study also defines the threshold MN50 titers that correlate with long-term protection in this model, although the relationship between the rhesus monkey model and humans remains to be determined. Nevertheless, these findings provide insights that support clinical development of ZIKV vaccines for humans.
Materials and Methods
Study Design
The objective of these studies was to evaluate the immunogenicity and protective efficacy of ZIKV vaccines in mice and rhesus monkeys. Studies were powered (N=4-8/group) to detect large differences in protective efficacy. Animals were randomly allocated to groups. Immunologic and virologic assays were performed blinded. All animal studies were approved by the appropriate Institutional Animal Care and Use Committee (IACUC).
Animals, vaccines, and challenges
Female Balb/c mice were purchased from commercial vendors and housed at Beth Israel Deaconess Medical Center. 31 outbred, Indian-origin male and female rhesus monkeys (Macaca mulatta) were housed at Bioqual, Rockville, MD. Vaccine constructs have been described previously (11, 12). In the first monkey vaccine study, animals were immunized by the s.c. route with 5 μg ZIKV purified inactivated virus (PIV) vaccine derived from the PRVABC59 isolate with alum (Alhydrogel; Brenntag Biosector) or alum alone at weeks 0 and 4 (N=8/group). In the second monkey vaccine study, animals were immunized by the i.m. route with 5 mg DNA vaccines expressing M-Env (prM-Env amino acids 216–794 derived from the BeH815744 isolate with the cleavage peptide deleted) at weeks 0 and 4 (N=7), a single immunization of 1011 VP RhAd52 expressing M-Env at week 0 (N=4), or sham controls (N=4). Rhesus monkeys were challenged at week 52 by the s.c route with 106 viral particles (VP) [103 plaque-forming units (PFU)] ZIKV-BR (Brazil ZKV2015). Studies in Balb/c mice used 1 μg ZIKV PIV, 50 μg DNA vaccines, or 109 VP RhAd52 vaccines and were challenged with 105 viral particles (VP) [102 plaque-forming units (PFU)] ZIKV-BR.
RT-PCR
RT-PCR assays were utilized to monitor viral loads, essentially as previously described (11, 12). RNA was extracted from plasma or other samples with a QIAcube HT (Qiagen). The wildtype ZIKV BeH815744 Cap gene was utilized as a standard. RNA was purified (Zymo Research), and RNA quality and concentration was assessed by the BIDMC Molecular Core Facility. Log dilutions of the RNA standard were reverse transcribed and included with each RT-PCR assay. Viral loads were calculated as virus particles (VP) per ml or per 1×106 cells and were confirmed by PFU assays. Assay sensitivity was 100 copies/ml or 1×106 cells.
Adoptive transfer studies
Polyclonal immunoglobulin G (IgG) was purified with protein G purification kits (Thermo Fisher Scientific) from week 52 plasma samples from rhesus monkeys vaccinated with the PIV, RhAd52-M-Env, DNA-M-Env, and sham vaccines. Total IgG was buffer-exchanged into 1× PBS and pooled for each group. Purified IgG was infused intravenously into groups of naïve recipient Balb/c mice (N=5/group) prior to ZIKV-BR challenge 2 h after infusion. Mice received 400 µl (high dose) or 25 µl (low dose) of a 10 mg/ml solution of purified IgG.
ELISA
Mouse and monkey ZIKV Env ELISA kits (Alpha Diagnostic International) were used to determine endpoint binding antibody titers using a modified protocol. 96-well plates coated with ZIKV Env protein were first equilibrated at room temperature with 300 µl of kit working wash buffer for 5 min. 6 µl of serum was added to the top row, and 3-fold serial dilutions were tested in the remaining rows. Samples were incubated at room temperature for 1 h, and plates washed 4 times. 100 µl of anti-mouse or anti-human IgG HRP-conjugate working solution was then added to each well and incubated for 30 min at room temperature. Plates were washed 5 times, developed for 15 min at room temperature with 100 µl of TMB substrate, and stopped by the addition of 100 µl of stop solution. Plates were analyzed at 450nm/550nm on a VersaMax microplate reader using Softmax Pro 6.0 software (Molecular Devices). ELISA endpoint titers were defined as the highest reciprocal serum dilution that yielded an absorbance >2-fold over background values. Log10 endpoint titers are reported.
Neutralization assay
A high-throughput ZIKV microneutralization (MN) assay was utilized for measuring ZIKV-specific neutralizing antibodies, essentially as previously described (11, 12). Briefly, serum samples were serially diluted three-fold in 96-well micro-plates, and 100 µl of ZIKV-PR (PRVABC59) containing 100 PFU were added to 100 µl of each serum dilution and incubated at 35ºC for 2 h. Supernatants were then transferred to microtiter plates containing confluent Vero cell monolayers (World Health Organization, NICSC-011038011038). After incubation for 4 d, cells were fixed with absolute ethanol: methanol for 1 h at –20°C and washed three times with PBS. The pan-flavivirus monoclonal antibody 6B6-C1 conjugated to HRP (6B6-C1 was a gift from JT Roehrig, CDC) was then added to each well, incubated at 35°C for 2 h, and washed with PBS. Plates were washed, developed with 3,3′,5,5′–tetramethylbenzidine (TMB) for 50 min at room temperature, stopped with 1:25 phosphoric acid, and absorbance was read at 450 nm. For a valid assay, the average absorbance at 450 nm of three non-infected control wells had to be ≤ 0.5, and virus-only control wells had to be ≥ 0.9. Normalized absorbance values were calculated, the MN50 titer was determined by a log mid-point linear regression model. The MN50 titer was calculated as the reciprocal of the serum dilution that neutralized ≥ 50% of ZIKV, and seropositivity was defined as a titer ≥ 10, with the maximum measurable titer 7,290. Log10 MN50 titers are reported. For the cross-strain virus neutralization assays, the following ZIKV strains were utilized: Brazil (BR; Fortaleza/2015, renamed Paraiba/2015), Uganda (UG; Uganda/1947; MR766), Thailand (TH; SV0127/14), Philippines (PH; CPCC074000Y01U00B001), and Puerto Rico (PR; PRVABC59).
Antibody-dependent enhancement (ADE) assay
Two-fold serial dilutions of heat-inactivated sera were mixed with an equal volume of ZIKV (sufficient to achieve approximately 15% infection of 5×104 K562-DC-SIGN cells) and incubated for 1 hr at 37°C. This mixture was added to a 96-well plate containing medium (RPMI-1640 supplemented with 10% FBS, 1% penicillin/streptomycin, 1% L-glutamine (200mM), and 1% non-essential amino acids (10mM)) with 5×104 K562 cells per well in duplicate and incubated 18-20 h overnight in a 37°C, 5% CO2, humidified incubator. Following overnight incubation, the cells are fixed, permeabilized and immunostained with flavivirus group-reactive mouse monoclonal antibody 4G2, and secondary polyclonal goat anti-mouse IgG PE-conjugated antibody (catalog no. 550589, BD Biosciences). The percent infected cells are quantified on a BD Accuri C6 Plus flow cytometer (BD Biosciences).
Statistical analyses
Analysis of virologic and immunologic data was performed using GraphPad Prism v6.03 (GraphPad Software). Comparisons of groups were performed using t-tests and Wilcoxon rank-sum tests. Correlations were assessed by Spearman rank-correlation tests.
Supplementary Material
Figure S1. Immune correlates analysis in mice.
Figure S2. Cellular immune responses in the ZIKV PIV vaccine study.
Figure S3. Cellular immune responses in the ZIKV DNA-M-Env and RhAd52-M-Env vaccine study.
Figure S4. Cross-strain neutralization of a panel of ZIKV strains.
Figure S5. Antibody-dependent enhancement assays in the ZIKV PIV vaccine study.
Figure S6. Antibody-dependent enhancement assays in the ZIKV DNA-M-Env and RhAd52-M-Env vaccine study following challenge.
Figure S7. MN50 titers in the ZIKV PIV vaccine study following challenge.
Figure S8. MN50 titers in the ZIKV DNA-M-Env and RhAd52-M-Env vaccine study following challenge.
Figure S9. Cellular immune responses in the ZIKV DNA-M-Env and RhAd52-M-Env vaccine study following challenge.
Figure S10. Immune correlates analysis in vaccinated and sham control rhesus monkeys.
Figure S11. Adoptive transfer studies of rhesus monkey IgG in mice.
One sentence summary.
A single immunization with an adenovirus vector-based vaccine, as well as two immunizations with a purified inactivated virus vaccine, afforded robust protection against ZIKV challenge in rhesus monkeys at 1 year following vaccination.
Acknowledgments
We thank R. Olson, K. Kabra, N. Botero, G. Ballarini, C. Springer, Y. Bedh, A. Dean, M. Iampietro, A. Brinkman, M. Kamath, E. Mendes, C. Kannadka, J. Misamore, and B. Finneyfrock for generous advice, assistance, and reagents. The data presented in this paper are tabulated in the main paper and in the supplementary materials. P.A., R.A.L., D.H.B., R.A.D., R.G.J., K.H.E., and S.J.T. are co-inventors on pending patent applications related to ZIKV vaccines, antigens, and vectors, and licensure discussions with industry partners are currently ongoing. P.A. and D.H.B. are co-founders and equity holders in AVVI Biotech. Correspondence and requests for materials should be addressed to D.H.B. (dbarouch@bidmc.harvard.edu). ZIKV challenge stocks and vaccine constructs are available with appropriate MTAs. We acknowledge support from the U.S. Military Research and Materiel Command and the U.S. Military HIV Research Program through its cooperative agreement with the Henry M. Jackson Foundation (W81XWH-11-2-0174); the National Institutes of Health (AI095985, AI096040, AI124377); and the Ragon Institute of MGH, MIT, and Harvard. The views expressed in this manuscript are those of the authors and do not represent the official views of the Department of the Army or the Department of Defense.
Footnotes
Author Contributions
P.A., R.A.L., N.L.M., S.J.T., and D.H.B. designed the studies. P.A., M.B., M.K., R.P., Z.L., O.N., R.N., and N.B.M. produced the DNA and Ad vaccines and conducted the virologic assays. R.A.D., G.D.G., K.M., R.G.J., K.H.E., S.J.T., and N.L.M. produced the PIV vaccines and conducted the virus neutralization and antibody enhancement assays. M.G.L. led the clinical care of the rhesus monkeys. R.A.L., K.V., E.N.B., A.C., D.J., J.J., B.C.L., S.M., P.G., J.L., and S.K conducted the monkey and mouse studies and performed the immunologic assays. D.H.B. wrote the paper with all co-authors.
References and Notes
- 1.Fauci AS, Morens DM. Zika Virus in the Americas–Yet Another Arbovirus Threat. N Engl J Med. 2016;374:601–604. doi: 10.1056/NEJMp1600297. [DOI] [PubMed] [Google Scholar]
- 2.Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika Virus. N Engl J Med. 2016;374:1552–1563. doi: 10.1056/NEJMra1602113. [DOI] [PubMed] [Google Scholar]
- 3.Barouch DH, Thomas SJ, Michael NL. Prospects for a Zika Virus Vaccine. Immunity. 2017;46:176–182. doi: 10.1016/j.immuni.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durbin A, Wilder-Smith A. An update on Zika vaccine developments. Expert Rev Vaccines. 2017 doi: 10.1080/14760584.2017.1345309. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez E, Diamond MS. Vaccination strategies against Zika virus. Current opinion in virology. 2017;23:59–67. doi: 10.1016/j.coviro.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, Kolenc M, Resman Rus K, Vesnaver Vipotnik T, Fabjan Vodusek V, Vizjak A, Pizem J, Petrovec M, Avsic Zupanc T. Zika Virus Associated with Microcephaly. N Engl J Med. 2016;374:951–958. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- 7.Brasil P, Pereira JP, Jr, Raja Gabaglia C, Damasceno L, Wakimoto M, Ribeiro Nogueira RM, Carvalho de Sequeira P, Machado Siqueira A, Abreu de Carvalho LM, Cotrim da Cunha D, Calvet GA, Neves ES, Moreira ME, Rodrigues Baiao AE, Nassar de Carvalho PR, Janzen C, Valderramos SG, Cherry JD, Bispo de Filippis AM, Nielsen-Saines K. Zika Virus Infection in Pregnant Women in Rio de Janeiro - Preliminary Report. N Engl J Med. 2016 doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika Virus and Birth Defects–Reviewing the Evidence for Causality. N Engl J Med. 2016;374:1981–1987. doi: 10.1056/NEJMsr1604338. [DOI] [PubMed] [Google Scholar]
- 9.Johansson MA, Mier YTRL, Reefhuis J, Gilboa SM, Hills SL. Zika and the Risk of Microcephaly. N Engl J Med. 2016 doi: 10.1056/NEJMp1605367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brasil P, Sequeira PC, Freitas AD, Zogbi HE, Calvet GA, de Souza RV, Siqueira AM, de Mendonca MC, Nogueira RM, de Filippis AM, Solomon T. Guillain-Barre syndrome associated with Zika virus infection. Lancet. 2016;387:1482. doi: 10.1016/S0140-6736(16)30058-7. [DOI] [PubMed] [Google Scholar]
- 11.Abbink P, Larocca RA, De La Barrera RA, Bricault CA, Moseley ET, Boyd M, Kirilova M, Li Z, Ng’ang’a D, Nanayakkara O, Nityanandam R, Mercado NB, Borducchi EN, Agarwal A, Brinkman AL, Cabral C, Chandrashekar A, Giglio PB, Jetton D, Jimenez J, Lee BC, Mojta S, Molloy K, Shetty M, Neubauer GH, Stephenson KE, Peron JP, Zanotto PM, Misamore J, Finneyfrock B, Lewis MG, Alter G, Modjarrad K, Jarman RG, Eckels KH, Michael NL, Thomas SJ, Barouch DH. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science. 2016;353:1129–1132. doi: 10.1126/science.aah6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larocca RA, Abbink P, Peron JP, Zanotto PM, Iampietro MJ, Badamchi-Zadeh A, Boyd M, Ng’ang’a D, Kirilova M, Nityanandam R, Mercado NB, Li Z, Moseley ET, Bricault CA, Borducchi EN, Giglio PB, Jetton D, Neubauer G, Nkolola JP, Maxfield LF, De La Barrera RA, Jarman RG, Eckels KH, Michael NL, Thomas SJ, Barouch DH. Vaccine protection against Zika virus from Brazil. Nature. 2016;536:474–478. doi: 10.1038/nature18952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pardi N, Hogan MJ, Pelc RS, Muramatsu H, Andersen H, DeMaso CR, Dowd KA, Sutherland LL, Scearce RM, Parks R, Wagner W, Granados A, Greenhouse J, Walker M, Willis E, Yu JS, McGee CE, Sempowski GD, Mui BL, Tam YK, Huang YJ, Vanlandingham D, Holmes VM, Balachandran H, Sahu S, Lifton M, Higgs S, Hensley SE, Madden TD, Hope MJ, Kariko K, Santra S, Graham BS, Lewis MG, Pierson TC, Haynes BF, Weissman D. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature. 2017 doi: 10.1038/nature21428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richner JM, Himansu S, Dowd KA, Butler SL, Salazar V, Fox JM, Julander JG, Tang WW, Shresta S, Pierson TC, Ciaramella G, Diamond MS. Modified mRNA Vaccines Protect against Zika Virus Infection. Cell. 2017;168:1114–1125 e1110. doi: 10.1016/j.cell.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dowd KA, Ko SY, Morabito KM, Yang ES, Pelc RS, DeMaso CR, Castilho LR, Abbink P, Boyd M, Nityanandam R, Gordon DN, Gallagher JR, Chen X, Todd JP, Tsybovsky Y, Harris A, Huang YS, Higgs S, Vanlandingham DL, Andersen H, Lewis MG, De La Barrera R, Eckels KH, Jarman RG, Nason MC, Barouch DH, Roederer M, Kong WP, Mascola JR, Pierson TC, Graham BS. Rapid development of a DNA vaccine for Zika virus. Science. 2016;354:237–240. doi: 10.1126/science.aai9137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffin BD, Muthumani K, Warner BM, Majer A, Hagan M, Audet J, Stein DR, Ranadheera C, Racine T, De La Vega MA, Piret J, Kucas S, Tran KN, Frost KL, De Graff C, Soule G, Scharikow L, Scott J, McTavish G, Smid V, Park YK, Maslow JN, Sardesai NY, Kim JJ, Yao XJ, Bello A, Lindsay R, Boivin G, Booth SA, Kobasa D, Embury-Hyatt C, Safronetz D, Weiner DB, Kobinger GP. DNA vaccination protects mice against Zika virus-induced damage to the testes. Nature communications. 2017;8:15743. doi: 10.1038/ncomms15743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sumathy K, Kulkarni B, Gondu RK, Ponnuru SK, Bonguram N, Eligeti R, Gadiyaram S, Praturi U, Chougule B, Karunakaran L, Ella KM. Protective efficacy of Zika vaccine in AG129 mouse model. Scientific reports. 2017;7:46375. doi: 10.1038/srep46375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim E, Erdos G, Huang S, Kenniston T, Falo LD, Jr, Gambotto A. Preventative Vaccines for Zika Virus Outbreak: Preliminary Evaluation. EBioMedicine. 2016;13:315–320. doi: 10.1016/j.ebiom.2016.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shan C, Muruato AE, Nunes BTD, Luo H, Xie X, Medeiros DBA, Wakamiya M, Tesh RB, Barrett AD, Wang T, Weaver SC, Vasconcelos PFC, Rossi SL, Shi PY. A live-attenuated Zika virus vaccine candidate induces sterilizing immunity in mouse models. Nat Med. 2017;23:763–767. doi: 10.1038/nm.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aid M, Abbink P, Larocca RA, Boyd M, Nityanandam R, Nanayakkara O, Martinot AJ, Moseley ET, Blass E, Borducchi EN, Chandrashekar A, Brinkman AL, Molloy K, Jetton D, Tartaglia LJ, Liu J, Best K, Perelson AS, De La Barrera RA, Lewis MG, Barouch DH. Zika Virus Persistence in the Central Nervous System and Lymph Nodes of Rhesus Monkeys. Cell. 2017;169:610–620 e614. doi: 10.1016/j.cell.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osuna CE, Lim SY, Deleage C, Griffin BD, Stein D, Schroeder LT, Omange R, Best K, Luo M, Hraber PT, Andersen-Elyard H, Ojeda EF, Huang S, Vanlandingham DL, Higgs S, Perelson AS, Estes JD, Safronetz D, Lewis MG, Whitney JB. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat Med. 2016 doi: 10.1038/nm.4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dudley DM, Aliota MT, Mohr EL, Weiler AM, Lehrer-Brey G, Weisgrau KL, Mohns MS, Breitbach ME, Rasheed MN, Newman CM, Gellerup DD, Moncla LH, Post J, Schultz-Darken N, Schotzko ML, Hayes JM, Eudailey JA, Moody MA, Permar SR, O’Connor SL, Rakasz EG, Simmons HA, Capuano S, Golos TG, Osorio JE, Friedrich TC, O’Connor DH. A rhesus macaque model of Asian-lineage Zika virus infection. Nature communications. 2016;7:12204. doi: 10.1038/ncomms12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirsch AJ, Smith JL, Haese NN, Broeckel RM, Parkins CJ, Kreklywich C, DeFilippis VR, Denton M, Smith PP, Messer WB, Colgin LM, Ducore RM, Grigsby PL, Hennebold JD, Swanson T, Legasse AW, Axthelm MK, MacAllister R, Wiley CA, Nelson JA, Streblow DN. Zika Virus infection of rhesus macaques leads to viral persistence in multiple tissues. PLoS Pathog. 2017;13:e1006219. doi: 10.1371/journal.ppat.1006219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sapparapu G, Fernandez E, Kose N, Cao B, Fox JM, Bombardi RG, Zhao H, Nelson CA, Bryan AL, Barnes T, Davidson E, Mysorekar IU, Fremont DH, Doranz BJ, Diamond MS, Crowe JE. Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature. 2016 doi: 10.1038/nature20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hombach J, Solomon T, Kurane I, Jacobson J, Wood D. Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva, 2–3 September, 2004. Vaccine. 2005;23:5205–5211. doi: 10.1016/j.vaccine.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Kreil TR, Burger I, Bachmann M, Fraiss S, Eibl MM. Antibodies protect mice against challenge with tick-borne encephalitis virus (TBEV)-infected macrophages. Clin Exp Immunol. 1997;110:358–361. doi: 10.1046/j.1365-2249.1997.4311446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mason RA, Tauraso NM, Spertzel RO, Ginn RK. Yellow fever vaccine: direct challenge of monkeys given graded doses of 17D vaccine. Applied microbiology. 1973;25:539–544. doi: 10.1128/am.25.4.539-544.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dejnirattisai W, Supasa P, Wongwiwat W, Rouvinski A, Barba-Spaeth G, Duangchinda T, Sakuntabhai A, Cao-Lormeau VM, Malasit P, Rey FA, Mongkolsapaya J, Screaton GR. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat Immunol. 2016 doi: 10.1038/ni.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, Vanzetta F, Minola A, Jaconi S, Mele F, Foglierini M, Pedotti M, Simonelli L, Dowall S, Atkinson B, Percivalle E, Simmons CP, Varani L, Blum J, Baldanti F, Cameroni E, Hewson R, Harris E, Lanzavecchia A, Sallusto F, Corti D. Specificity, cross-reactivity and function of antibodies elicited by Zika virus infection. Science. 2016 doi: 10.1126/science.aaf8505. [DOI] [PubMed] [Google Scholar]
- 30.Bardina SV, Bunduc P, Tripathi S, Duehr J, Frere JJ, Brown JA, Nachbagauer R, Foster GA, Krysztof D, Tortorella D, Stramer SL, Garcia-Sastre A, Krammer F, Lim JK. Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science. 2017;356:175–180. doi: 10.1126/science.aal4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pantoja P, Perez-Guzman EX, Rodriguez IV, White LJ, Gonzalez O, Serrano C, Giavedoni L, Hodara V, Cruz L, Arana T, Martinez MI, Hassert MA, Brien JD, Pinto AK, de Silva A, Sariol CA. Zika virus pathogenesis in rhesus macaques is unaffected by pre-existing immunity to dengue virus. Nature communications. 2017;8:15674. doi: 10.1038/ncomms15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCracken MK, Gromowski GD, Friberg HL, Lin X, Abbink P, De La Barrera R, Eckles KH, Garver LS, Boyd M, Jetton D, Barouch DH, Wise MC, Lewis BS, Currier JR, Modjarrad K, Milazzo M, Liu M, Mullins AB, Putnak JR, Michael NL, Jarman RG, Thomas SJ. Impact of prior flavivirus immunity on Zika virus infection in rhesus macaques. PLoS Pathog. 2017;13:e1006487. doi: 10.1371/journal.ppat.1006487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.NCT02840487, NCT02809443, NCT02887482, NCT02963909, NCT02952833, NCT02937233.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Immune correlates analysis in mice.
Figure S2. Cellular immune responses in the ZIKV PIV vaccine study.
Figure S3. Cellular immune responses in the ZIKV DNA-M-Env and RhAd52-M-Env vaccine study.
Figure S4. Cross-strain neutralization of a panel of ZIKV strains.
Figure S5. Antibody-dependent enhancement assays in the ZIKV PIV vaccine study.
Figure S6. Antibody-dependent enhancement assays in the ZIKV DNA-M-Env and RhAd52-M-Env vaccine study following challenge.
Figure S7. MN50 titers in the ZIKV PIV vaccine study following challenge.
Figure S8. MN50 titers in the ZIKV DNA-M-Env and RhAd52-M-Env vaccine study following challenge.
Figure S9. Cellular immune responses in the ZIKV DNA-M-Env and RhAd52-M-Env vaccine study following challenge.
Figure S10. Immune correlates analysis in vaccinated and sham control rhesus monkeys.
Figure S11. Adoptive transfer studies of rhesus monkey IgG in mice.


