Abstract
Beta-amyloid (Aβ) positive individuals hyper-activate brain regions compared to those not at-risk; however, hyperactivation is then thought to diminish as Alzheimer’s disease symptomatology begins, evidencing eventual hypoactivation. It remains unclear when in the disease staging this transition occurs. We hypothesized that differential levels of amyloid burden would be associated with both increased and decreased activation (i.e., a quadratic trajectory) in cognitively-normal adults. Participants (N=62; aged 51–94) underwent an fMRI spatial distance-judgment task and Amyvid-PET scanning. Voxelwise regression modeled age, linear-Aβ, and β quadratic-A as predictors of BOLD activation to difficult spatial distance-judgments. A significant quadratic-Aβ effect on BOLD response explained differential activation in bilateral angular/temporal and medial prefrontal cortices, such that individuals with slightly elevated Aβ burden exhibited hyperactivation whereas even higher Aβ burden was then associated with hypoactivation. Importantly, in high-Aβ individuals, Aβ load moderated the effect of BOLD activation on behavioral task performance, where in lower-elevation, greater deactivation was associated with better accuracy, but in higher-elevation, greater deactivation was associated with poorer accuracy during the task. This study reveals a dose-response, quadratic relationship between increasing Aβ burden and alterations in BOLD activation to cognitive challenge in cognitively-normal individuals that suggests 1) the shift from hyper- to hypo-activation may begin early in disease staging, 2) depends, in part, on degree of Aβ burden, and 3) tracks cognitive performance.
Keywords: Aging, Beta-amyloid, fMRI, preclinical Alzheimer’s Disease, nonlinear BOLD activation, cognition
1. Introduction
Alzheimer’s disease (AD) is a complex neurodegenerative disorder for which a precise diagnosis in living persons remains elusive. Despite this limitation, there has been general agreement on several biomarkers, as well as the staging of these biomarkers, such that individuals at-risk for transitioning to AD can be identified in pre-clinical, asymptomatic states (Albert et al., 2011; Dubois et al., 2016; Jack et al., 2010, 2013; Sperling et al., 2011). Increased beta-amyloid (Aβ) deposition is thought to be the earliest biomarker for AD, followed by tau deposition and brain atrophy (Jack et al., 2010, 2013), with Aβ deposition occurring 15–30 years before the onset of AD symptoms (Dubois et al., 2016; Jansen et al., 2015; Rowe et al., 2010). Importantly, while Aβ is a necessary component of AD pathology, individuals have been identified with clinically significant Aβ burden who exhibit no AD behavioral symptomatology (Delaère, He, Fayet, Duyckaerts, & Hauw, 1993). However, evidence suggests that within cognitively normal aging, elevated Aβ burden may alter patterns of functional brain activation.
In clinically-normal older adults performing cognitive (typically episodic memory) tasks during scanning, those with measurable Aβ burden tend to show increased brain activation (i.e., hyperactivation) in select brain regions such as the hippocampus, parietal cortex, precuneus, posterior cingulate, and temporal cortex, compared to older adults without Aβ burden (e.g., Elman et al., 2014; Huijbers et al., 2014; Leal et al., 2017; Mormino et al., 2012; Oh et al., 2015; Oh, Steffener, Razlighi, Habeck, & Stern, 2016; Sperling et al., 2009). Similarly, older adults diagnosed with mild cognitive impairment (MCI) also exhibit functional hyperactivation (for review see Sperling et al., 2011), although this phenomenon is likely limited to individuals at the earliest identifiable stage of MCI (e.g., Celone et al., 2006; Dickerson et al., 2005; Foster et al., 2016). Furthermore, longitudinal research following early MCI individuals with hyperactivation at baseline suggests that these individuals may experience more rapid cognitive decline than their non-hyperactivating MCI peers (e.g., Dickerson et al., 2004; Miller et al., 2008; O’Brien et al., 2010; Sperling et al., 2010). Thus, it appears that hyperactivation may be a specific marker for individuals in the earlier phases of AD development (i.e., early MCI) and a predictor of poorer cognitive outcomes. While the mechanism driving Aβ-related hyperactivation is still unclear, hyperactivation occurs in regions that activate or deactivate in response to cognitive tasks (e.g., Huijbers et al., 2014; Oh et al., 2015, 2016; Sperling et al., 2009). These results suggest that a similar mechanism, likely reduced inhibition (Sperling, Mormino, & Johnson, 2014), underlies hyperactivation regardless of the region or direction of activation.
Interestingly, hyperactivation appears to eventually transition to decreased activation (i.e., hypoactivation) in those individuals farther along the AD spectrum, such as in late MCI or probable AD (e.g., Bosch et al., 2010; Celone et al., 2006; Dickerson et al., 2005; Sperling, 2011; Sperling et al., 2010), suggesting a quadratic trajectory of functional brain activation changes across the AD continuum: preclinical AD to MCI/prodromal AD (hyperactivation) and prodromal AD/MCI to probable AD (hypoactivation). While the transition to hypo- from hyperactivation has previously been thought to occur after the onset of AD symptomatology (e.g., Celone et al., 2006; Dickerson et al., 2005), there is also evidence that hypoactivation may occur in older, cognitively-normal individuals with significant Aβ burden (Kennedy et al., 2012), suggesting that the effect of Aβ on brain activation is complex, likely quadratic, and that the transition between these states may occur earlier than previously thought.
To assess whether Aβ is associated with a quadratic change in activation within a sample of cognitively healthy middle-aged and older adults, we utilized a spatial distance-judgment task with three levels of difficulty (Rieck, Rodrigue, Boylan, & Kennedy, 2017). This task affords the ability to investigate the dynamic range over which the brain responds (or modulates) to task difficulty; however, in the current study we compare the hardest level of the task to the control condition, optimizing the potential to find Aβ-related changes in functional brain activation in healthy aging. We hypothesized that differential levels of Aβ burden would be associated with both increases and decreases (i.e., nonlinearity) in activation to a cognitively challenging spatial distance-judgment task. Further, we hypothesized that Aβ burden-related activation would be associated with task performance.
2. Methods
2.1. Participants
Participants included 62 healthy adults (mean age = 67.73 ± 10.21; age range 51–94 years) who were drawn from a larger study of 181, of whom 73 had both fMRI and amyloid-PET data. Eighteen participants were deemed to have elevated Aβ burden using a standardized uptake value ratio (SUVR) cutoff of 1.11 (Clark et al., 2011; see Table 1). A sample of 42 younger adults (mean age = 27.45 ± 4.40; age range 20–35) were also included to provide visual estimates of task-related activity as a reference, however these individuals were not included in the Aβ analysis and did not undergo amyloid-PET data collection. All participants were recruited from the Dallas-Fort Worth metroplex and screened to ensure they were right-handed, fluent English speakers, with normal or corrected-to-normal vision. When required, MRI-compatible glasses were used during scanning. Participants were also screened against a history of metabolic, neurological or psychiatric conditions, head trauma, drug or alcohol problems, significant cardiovascular disease, depression (Center for Epidemiological Study - Depression < 16 Radloff, 1977), and to be cognitively intact (Mini Mental State Exam ≥ 26 Folstein, Folstein, & McHugh, 1975). Twenty-two participants in the current sample self-reported a diagnosis of hypertension. PET scanning took place on average within a year of MRI acquisition (M = 12.16, SD = 5.24 months).
Table 1.
Participant Demographics and Task Performance
| Low SUVR | High SUVR | Total | |
|---|---|---|---|
|
| |||
| N (% Female) | 44 (66) | 18 (39) | 62 (58.07) |
| Mean Age (SD) | 65.36 (10.21)* | 73.50 (7.14) | 67.73 (10.21) |
| Mean Education (SD) | 15.30 (2.53) | 16.33 (2.50) | 15.60 (2.54) |
| Mean MMSE (SD) | 28.86 (0.82) | 28.89 (0.68) | 28.87 (0.78) |
| Mean CESD (SD) | 3.68 (3.79) | 3.94 (3.83) | 3.76 (3.77) |
| fMRI Task Accuracy | |||
| Control (SD) | 97.47 (5.71) | 95.53 (7.73) | 96.91 (6.36) |
| Easy (SD) | 95.24 (7.72) | 93.57 (9.49) | 94.76 (8.23) |
| Medium (SD) | 87.25 (15.02) | 86.22 (15.95) | 86.95 (15.17) |
| Hard (SD) | 71.69 (20.85) | 76.18 (17.46) | 72.99 (19.89) |
| fMRI Task RT (sec) | |||
| Control (SD) | .71 (.18) | .67 (0.10) | .70 (.16) |
| Easy (SD) | .86 (.16) | .82 (0.12) | .85 (.15) |
| Medium (SD) | .93 (.18) | .87 (0.12) | .92 (.16) |
| Hard (SD) | 1.07 (.24) | .97 (0.17) | 1.04 (.22) |
Note: Low SUVR – less than 1.11 standardized uptake value ratio; High SUVR – greater than or equal to 1.11 standardized uptake value ratio; There were no significant group differences on any measure (p’s > .146) other than age (t(60) = −3.080, p = .003). MMSE - Mini Mental State Exam; CESD – Center for Epidemiologic Study-Depression; Accuracy reported as mean percent accuracy; Response time (RT) reported as a mean of medians in seconds; SD – standard deviation;
p < .05.
Eleven of the initial 73 participants were excluded from analysis due to MRI acquisition issues: excessive in-scanner motion (n = 4); poor functional image acquisition (n = 2); no response on greater than 15% of trials (n = 1); or < 70% accuracy on the control condition (n = 4). The 11 excluded participants did not differ significantly from the included participants, respectively, in age [t(71) = −1.20, p = .24; 71.91 ± 13.74 SD vs 67.73 ±10.21], education [t(71) = .58, p = .56; 15.09 ± 3.27 vs 15.60 ± 2.54], MMSE [t(71) = 1.66, p = .10; 28.45 ± .69 vs 28.87 ± .78], or CESD [t(71) = .03, p = .98; 3.73 ± 3.32 vs 3.76 ± 3.77]. One excluded participant was amyloid positive.
2.2. Imaging Protocol
2.2.1. PET Acquisition
On a separate session, participants were scanned on a single Siemens ECAT HR PET scanner at UT Southwestern Medical School. All participants were injected with 370 MBq (10 mCi) of 18F-Florbetapir (Avid Radiopharmaceuticals/Eli Lilly). Approximately 30 minutes post-injection, participants were placed on the imaging table and foam wedges were used to secure the participant’s head. A 2-minute scout was acquired to ensure the brain was within the field of view. Fifty minutes post-injection, an internal rod source transmission scan was acquired for 7 minutes immediately followed by a 2-frame by 5 minutes each dynamic emission acquisition. The transmission image was reconstructed using back-projection with a 6-mm full-width at half-maximum (FWHM) Gaussian filter. Emission images were processed by iterative reconstruction, 4 iterations and 16 subsets with a 3-mm FWHM ramp filter.
2.2.2. PET Data Processing
Each participant’s PET scan was first registered to their T1-weighted image with a rigid affine registration using Advanced Normalization Tools (ANTs) (Avants, Tustison, Song, & Gee, 2009) scripts and visually inspected for registration quality. Freesurfer (Fischl, 2012) parcellations of interest that correspond to the traditionally used 7 ROIs for amyloid deposition (i.e., anterior cingulate, posterior cingulate, precuneus, lateral temporal, lateral parietal, middle frontal, and inferior frontal) were also registered to each subject’s T1 image. Using methods outlined in Rodrigue et al., 2012, uptake counts were extracted from each ROI and normalized to whole cerebellar counts to yield standardized uptake value ratios (SUVRs) for each ROI. All ROIs were averaged to form mean cortical amyloid index.
2.2.3. MRI Acquisition
Participants were scanned on a single Philips Achieva 3T whole-body scanner equipped with a 32-channel head coil. High-resolution anatomical images were collected with a T1-weighted MP-RAGE sequence with the following parameters: 160 sagittal slices, 1 × 1 × 1 mm3 voxels; FOV = 256 mm × 204 mm × 160 mm, FOV = 256 mm, TE = 3.8 ms, TR = 8.3 ms, FA = 12°. Blood Oxygenation Level Dependent (BOLD) fMRI data were acquired using a T2*-weighted echo planar imaging sequence in 29 interleaved axial slices parallel to AC-PC line: 64 × 64 matrix, 3.4 × 3.4 × 5 mm3 voxels, FOV = 220 mm × 145 mm × 220 mm, TE = 30 ms, TR = 1500 ms.
2.2.4. fMRI Distance Judgment task
For a detailed description of the distance judgment task see Rieck et al., 2017. In brief, participants performed a coordinate judgment task modeled after Baciu et al., 1999 with three levels of difficulty as well as a categorical judgment control condition (see supplemental figure 1). For the categorical condition, a dot was presented to the right or left of a centrally positioned bar. Participants indicated, with button presses, on which side of the bar the dot was located. For the coordinate distance judgment task, participants were first cued with a vertical reference line. Then they were presented with a horizontal bar in the center of the screen. The participant indicated with a button press on each trial whether the dot was “closer to” or “farther away” from the horizontal bar than the length of the vertical reference line, which varied parametrically across three levels of difficulty (i.e., easy, medium, and hard).
2.2.5. fMRI Data Processing
Individual participant time-series data were preprocessed with Statistical Parametric Mapping 8 (SPM8; Wellcome Department of Cognitive Neurology, London, UK) according to a standard pipeline of procedures. In order, images were corrected for differences in slice-time acquisition, individual volumes were corrected for within-run participant movement, and images were normalized to a common MNI space and smoothed with an isotropic 8 mm3 FWHM Gaussian kernel. ArtRepair (Mazaika, Hoeft, Glover, & Reiss, 2009) was used to identify outlier volumes. For each participant, runs that had more than 15% outlier volumes (~30 volumes) with > 3% deviation from the mean in global intensity spikes or > 2 mm of motion displacement were excluded. Participants with more than one run flagged for excessive outlier volumes were excluded from the study entirely (n = 4).
At the individual subject level, BOLD response to each condition (categorical, easy, medium, hard) was modeled in SPM as a block convolved with a canonical hemodynamic response function; six directions of motion-estimates for each volume generated from ArtRepair were also included as nuisance covariates. To maximize differences in dynamic range of activation/deactivation, our contrast of interest was [Hard Coordinate vs. Categorical], as a measure of activation (hard > categorical) or deactivation (categorical > hard) to cognitive challenge.
2.2.6. fMRI Data Analysis
A voxel-wise linear regression was conducted with an intercept, mean centered age at the time of fMRI, mean centered cortical amyloid SUVR (Aβ), and the squared term (i.e., quadratic) of mean cortical amyloid SUVR (Aβ2) as independent variables in a model with task activation [Hard vs. Categorical] as the dependent variable. All results were cluster corrected to FWE p < .05 using SnPM with a height threshold of p < .005 and 5000 permutations (SnPM13; http://warwick.ac.uk/snpm). As the primary test of our hypothesis, that Aβ burden would relate to both increases and decreases in BOLD activation, we tested the quadratic effect of amyloid on BOLD activation. To test for regions that showed a linear effect, but no significant quadratic effect, a mask was created from the quadratic effect using a height threshold of p < .05 with a zero-voxel cluster extent. This mask was used as an exclusion mask when investigating the linear effect, tested within the full model.
To provide the pattern of regions that increased and decreased activation to hard vs. categorical contrast, we conducted a positive and negative test of the intercept in the older adults. In this model, because each variable was mean centered, tests of this effect represented a conditional estimate of activation and deactivation at the mean of all other variables in the model. Similarly, to provide a visual reference for the pattern of deactivations in young adults (a population without significant amyloid deposition), a one-sample t-test of [Categorical > Hard] was conducted in the sample of 42 younger adults.
To gauge the potential effects of relevant covariates (APOE status, brain size), three separate follow-up analyses were also run controlling for APOE status, average gray matter thickness (estimated using Freesurfer and averaged across all gray matter parcels), and total gray matter volume (estimated using Freesurfer and summing over all gray matter parcels) after adjusting for manually traced intra-cranial volume (Raz et al., 2005). We re-estimated the same model (i.e., intercept, mean centered age, mean centered Aβ, and Aβ2) with gray matter volume or thickness entered as continuous covariates. While gray matter thickness and volume measures were available for all participants, only 59 of the 62 participants had APOE genotype information (see Kennedy et al., 2015 for genotyping details). Therefore, to maximize sample size and power, including APOE as a covariate was conducted as a follow-up to the primary model. Again, re-estimating the same model (i.e., intercept, mean centered age, mean centered Aβ, and Aβ2) but with the reduced number of individuals, APOE status (ε4- and ε4+) was entered as a dichotomous covariate where ε4- individuals were the reference group.
3. Results
3.1. Behavioral Data
To evaluate effects of difficulty manipulation, age, and Aβ burden on the in-scanner task, we ran repeated-measures ANOVAs with the four difficulty conditions as a within-subject variable, age and SUVR as between-subjects variables, and all interactions as predictors of accuracy or response time (RT). There was a significant effect of task difficulty on accuracy, (F(3,174) = 32.398, p < .001), and response time, (F(3,174) = 69.724, p < .001), as well as an effect of age on accuracy, (F(1,58) = 4.128, p = .047). There were no other significant effects on either accuracy or response time (ps > .142). Overall the results revealed that age had a minimal effect on task performance, and that increasing task difficulty produced poorer accuracy and slower response times (see Table 1). Amyloid burden was not related to accuracy or reaction time, therefore, amyloid-related effects on BOLD activation are not confounded with task performance.
3.2. Effects of Amyloid Burden on BOLD Activation in the Hard Compared to Categorical Condition
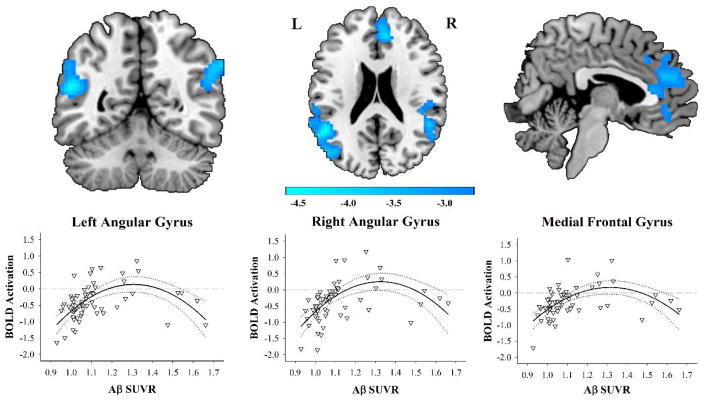
The results of the voxelwise linear regression on BOLD activation revealed a significant effect of Aβ2 on activation. Effect of age was not significant. There were significant negative quadratic effects of Aβ on BOLD deactivation in three large clusters: in the left angular gyrus/middle temporal gyrus, the right angular gyrus/superior temporal gyrus, and the bilateral medial frontal gyrus and anterior cingulate (see Figure 1 and Table 2). The quadratic relationship indicated that within these regions, compared to individuals with lower SUVR, individuals with slightly increased SUVR exhibited hyperactivation. In individuals with even greater SUVR, however, the BOLD response began to pseudonormalize (to borrow a phrase from Sperling et al., 2010), or transition to hypoactivation. A significant positive linear effect of Aβ burden was found in the same regions as the quadratic effect, further supporting the results that SUVR is associated with increased and then decreased activation. After masking out voxels with significant quadratic effects, there were no regions exhibiting only a significant linear effect of Aβ. Further, there were no significant non-linear effects in the positive direction and no significant negative linear effects.
Figure 1. Brain regions exhibiting a quadratic effect of Aβ on Activation to the Hard Compared to Categorical Conditions.
Aβ SUVR shows a nonlinear association to BOLD activation in bilateral angular/temporal gyri and medial frontal gyrus. Compared to individuals with lower SUVR, slightly elevated SUVR is associated with hyperactivation. Individuals with greater SUVR show hypoactivation. The nonlinear BOLD activation effect remains the same after controlling for gray matter volume and thickness. Blue scale represents t-values from the negative quadratic effect. Quadratic lines shown with 95% confidence intervals.
Table 2.
Cluster peaks for Quadratic Effect of Amyloid on BOLD Activation
| Cluster Label | BA | k | x | y | z | Cluster FWEp |
|---|---|---|---|---|---|---|
| L middle temporal gyrus, L angular gyrus, L middle occipital gyrus | 39, 40 | 491 | −51 | −51 | 21 | .044 |
| −48 | −75 | 24 | ||||
| −33 | −87 | 27 | ||||
| R angular gyrus, R middle temporal gyrus, R superior temporal gyrus | 39, 40 | 617 | 63 | −51 | 36 | .032 |
| 54 | −48 | 21 | ||||
| 45 | −18 | −6 | ||||
| R anterior cingulate, R medial frontal gyrus, L anterior cingulate, L medial frontal gyrus | 24, 32, 10 | 572 | 9 | 42 | 21 | .035 |
| 12 | 39 | 36 | ||||
| 6 | 33 | 12 |
Note: BA – Brodmann Area; k – cluster extent; L – Left; R – Right. Cluster-wise p-values obtained from SnPM using family-wise error correction.
3.3. Effects of Aβ burden and BOLD Activation on Task Performance
To explore relationships between BOLD activation and Aβ burden with behavior, a repeated-measures ANOVA was conducted that included a within-subject variable of task difficulty (easy, medium, and hard) and between-subjects variables of age, BOLD activation (averaged across all significant voxels exhibiting the quadratic effect), Aβ, and BOLD activation x Aβ interaction. In the full sample, there was a significant effect of difficulty (F(2,114) = 28.77, p < .001), such that accuracy decreased with increasing difficulty, but no other effects reached significance (p’s > .247). To further explore these relationships, we limited the analysis to individuals with high Aβ burden using an SUVR cutoff of 1.11 (n = 18; see Table 1). The results again indicated a significant effect of task difficulty (F(2,26) = 5.147, p = .013) such that accuracy decreased as the difficulty of the task increased. No other within-subject effects of age by difficulty, amyloid by difficulty, activation by difficulty, or the amyloid by activation by difficulty interaction were significant ps > .447. There was however, a significant between-subjects effect of age on average task accuracy (F(1,13) = 6.351, p = .026), such that accuracy decreased with age, as well as a BOLD activation x Aβ interaction (F(1,13) = 6.506, p =.024) on average task accuracy. No other effects were significant (ps > .555). To decompose the nature of this interaction we used simple slopes analysis (Preacher, Curran, & Bauer, 2006).
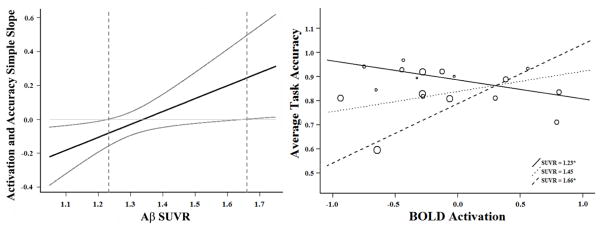
A simple slopes analysis tests the estimated slope (i.e., the relationship between the predictor variable and dependent variable) continuously along the moderator variable, providing a precise breakdown of how the moderator (in this case Aβ burden) alters the association between activation and average task accuracy. The results of the analysis indicate that in the high-Aβ (SUVR > 1.11) group, at SUVR less than 1.23, deactivation was significantly associated with higher task accuracy (Figure 2 left panel, first dotted vertical line; right panel, solid line). However, at SUVR greater than 1.66, deactivation was significantly associated with lower task accuracy (Figure 2 left panel, second vertical dotted line; right panel, dashed line). Figure 2, right panel illustrates the estimated slope at each of these inflections of significance and at the midway between these points (SUVR = 1.45) of the eighteen individuals with elevated amyloid. There was no relationship between average task accuracy and activation averaged across all significant voxels when the analysis was limited to the 44 individuals without significant Aβ (r(41) = −.021, p = .894).
Figure 2. In high-Aβ individuals, Aβ load moderates the effect of BOLD activation on task accuracy.
Within the high-Aβ group, there was a significant interaction between SUVR and BOLD activation on task accuracy. Simple slopes analysis revealed that in the high-Aβ (SUVR > 1.11) group, at Aβ SUVR less than 1.23 a significant relationship between activation and task accuracy exists, such that greater deactivation was associated with higher task accuracy (left panel, first dotted vertical line; right panel, solid line). However, at SUVR greater than 1.66, increased deactivation was significantly associated with lower task accuracy (left panel, second vertical dotted line; right panel, dashed line). The right panel illustrates the estimated slope at each inflection of significance and midway between these points (SUVR M = 1.45) for the eighteen individuals with elevated amyloid. Each dot in the right panel corresponds to an individual in the analysis and the size of the dot represents the amount of amyloid burden.
In sum, individuals at or slightly above the cutoff for clinically relevant Aβ burden exhibit a beneficial relationship between deactivation and task performance. Importantly, this effect flips in individuals with more severely elevated Aβ burden, such that deactivation is now associated with poorer task performance.
3.4 Overall Effects of Task on BOLD Response
To place the quadratic amyloid effects in context, we tested the conditional effect of Hard vs. Categorical from the full model in the 62 older adults (i.e., the intercept). Several regions activated and deactivated in response to the difficult distance judgment task (see Kennedy, Rieck, Boylan, & Rodrigue, 2017 for similar results in the entire sample). Regions that activated included the bilateral parietal lobes, bilateral middle frontal gyrus, and medial superior frontal gyrus (see Supplemental Figure 2). Regions that deactivated included a set of regions typically associated with the default network: the bilateral posterior cingulate, bilateral medial frontal, and bilateral middle temporal gyrus (see Supplemental Figure 2 and Figure 3, left panel). To further place the quadratic effects in context we separately contrasted the Categorical > Hard conditions within a sample of 42 younger adults. Notably, younger adults (Figure 3, right panel) significantly deactivate a similar set of regions to those observed in the conditional and quadratic effects in older adults. These results provide evidence that these default regions deactivate similarly in younger, healthy individuals in response to the challenging distance judgment task (Figure 3; and see Kennedy, Rieck, Boylan, & Rodrigue, 2017 for parent sample data).
Figure 3. Deactivation in Response to the Hard Compared to Categorical Conditions in Older and Younger Adults.
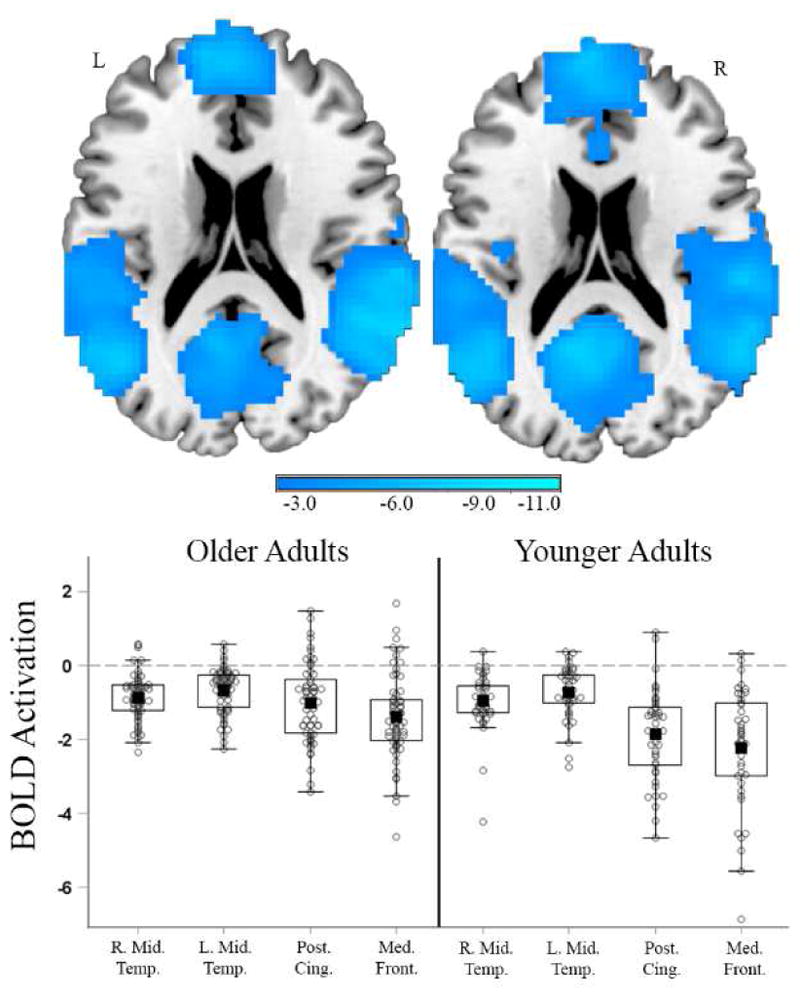
The left panel shows the conditional effect of deactivation for older adults at mean age and mean amyloid derived from the full model with age, amyloid, and the quadratic effect of amyloid. The right panel shows a sample of 42 young adults (age range 20 – 35) and a one-sample t-test of Hard vs. Categorical. Young adult results are presented as a visual reference of expected activity in individuals who are devoid of Aβ. Individual parameter estimates for both groups were extracted from 4mm spheres surrounding peak coordinates. Cluster FWE corrections were calculated in SnPM using a height threshold of p < .005. Filled black square = mean; Box = 25th and 75th percentile; Whiskers = min and max values; beyond whiskers are individuals greater than 1.5 times the interquartile range. R. Mid Temp. = Right Middle Temporal Gyrus, MNI xyz = 63, −48, 9; L. Mid. Temp. = Left Middle Temporal Gyrus, MNI xyz = −57, −63, 9; Post. Cing = Posterior Cingulate, MNI xyz = −6, −45, 33; Med. Front. = Medial Frontal Gyrus, MNI xyz = −3, 57, −6.
3.5 Effect of Covariates on Model Results
Prior research has established that APOEε4 is associated with greater Aβ burden in healthy individuals (for review see Liu, Kanekiyo, Xu, & Bu, 2013). To assess the potential impact of APOE status on the model results between Aβ and BOLD activation we tested the same model including APOE status as a dichotomous variable in the smaller sample of individuals with APOE genotyping, Amyloid PET imaging, and fMRI data (n = 59). The results remained largely the same, however, the quadratic effect in the left and right angular gyrus/temporal clusters expanded to include the left and right hippocampus respectively (see Supplemental Figure 3). As in the primary analysis, there was no significant effect of APOE group on positive BOLD activation. Further, in models controlling for gray matter thickness or volume, the quadratic cluster results remained unchanged.
4. Discussion
The current study tested the hypothesis that varying levels of Aβ burden would be associated with quadratic change in BOLD activation in response to a difficult spatial-judgment task. Prior research has indicated that early AD-related symptomatology, as well as Aβ burden in healthy older adults, is associated with increased activation throughout the brain. This hyperactivation occurs in both regions that increase activation in response to a task (e.g., hippocampus; Dickerson et al., 2005; Huijbers et al., 2014; Mormino et al., 2012; Oh et al., 2015) as well as in regions that decrease activation in response to a task (e.g., default mode regions; Celone et al., 2006; Mormino et al., 2012; Sperling et al., 2009). Importantly, regardless of whether the region typically activates or deactivates, increases in activation are the most typically reported change. Diminution of hyperactivation, or increased deactivation, after the onset of significant AD symptomatology has been reported (e.g., Bosch et al., 2010; Celone et al., 2006; Dickerson et al., 2005); however, there is some indication that decreases in BOLD activation may begin in asymptomatic older adults at-risk for AD (Kennedy et al., 2012).
The results from the current study provide further evidence that Aβ burden is associated with significant alteration in BOLD activation in response to cognitive tasks, and that this association is complex and dependent on amyloid load. Middle-aged and older adults without Aβ burden robustly deactivate in response to a difficult spatial judgment task. Slightly greater amounts of Aβ, however, are associated with hyperactivation in regions typically associated with the default mode network (i.e., bilateral angular/temporal gyrus and medial frontal/anterior cingulate cortex). Even greater amounts of Aβ burden, however, were associated with pseudonormalization of the activation, or the beginning of the transition to hypoactivation. This quadratic finding helps explain why absolute level of deactivation in Aβ-positive individuals mirrors that of Aβ-negative individuals, as it suggests that this is capturing different physiological processes (healthy deactivation vs transition from hyper- to hypo-activation) at potentially different stages of AD-related pathology (cognitively-normal aging vs preclinical AD).
Critically, we found that both hyper- and hypo-activation tracked performance on the fMRI task. In individuals with elevated Aβ burden, Aβ load significantly moderated the effect of BOLD activation on task accuracy. Specifically, greater deactivation was associated with better performance in individuals with slightly elevated amyloid, whereas in those individuals carrying the highest Aβ burden, greater deactivation was revealed to be associated with poorer task performance. This relationship between activation, amyloid, and behavior helps to clarify the nature of the pseudonormalization of activation (Sperling et al., 2010). Healthy older individuals with significant Aβ burden are not somehow maintaining apparently normal levels of deactivation despite Aβ burden, but rather they are most likely beginning the transition into hypoactivation that is thought to occur after more significant pathology takes place. Therefore, the present results suggest that the shift from hyper- to hypoactivation, along with information about amyloid status, may prove to be a sensitive, early marker for cognitive decline. For example, previous research has shown that those exhibiting hyperactivation tend to exhibit steeper rates of cognitive decline over time than their non-hyperactivating peers (Dickerson et al., 2004), and that the relationship between hippocampal hyperactivation and longitudinal decline in cognition is mediated by longitudinal change in amyloid accumulation (Leal et al., 2017). However, with knowledge of an individual’s amyloid status, it may be possible to further delineate cognitively normal individuals who do not exhibit hyperactivation into those who are deactivating normally and those who are deactivating pseudonormally. Further research is needed to investigate if this association between functional brain activation and behavior is indeed reflective of preclinical AD processes or is the reflection of non-pathological aging processes.
While the quadratic effect of Aβ on BOLD activation occurred in many key regions of the default mode network, Aβ load was not found to have a quadratic or linear effect on BOLD activation in the posterior cingulate/precuneus. The lack of a relationship occurred despite this region exhibiting strong deactivation to the task overall, and the fact that prior studies have implicated a relationship between Aβ (Sperling et al., 2009) and/or Alzheimer’s disease diagnosis (e.g., Celone et al., 2006; Lustig et al., 2003; Pihlajamaki et al., 2010) and activation in the posterior cingulate/precuneus, although this is not always seen (Oh et al., 2015, 2016). It is possible that these discrepant previous findings are influenced by different cognitive demands. For example, intentional encoding tasks were used in studies implicating the posterior cingulate/precuneus (Celone et al., 2006; Lustig et al., 2003; Pihlajamaki et al., 2010; Sperling et al., 2009), while incidental encoding and a working memory task were used in the studies that did not implicate this region (Oh et al., 2015, 2016). The current study used a task most similar to a working memory task, and therefore, this may account for the lack of a relationship between the posterior cingulate/precuneus and Aβ burden.
It is also possible that the posterior cingulate/precuneus does not follow a nonlinear course of progression in preclinical AD individuals. Previous work within our own lab indicated that across the lifespan, cognitively normal APOEε4+ individuals show a linear decrease in BOLD modulation to difficulty, whereas APOEε4- individuals showed no age-related differences in modulation (Foster, Kennedy, & Rodrigue, 2017). These age-related modulation differences in ε4 carriers could be related to Aβ burden, as ε4+ individuals are at increased risk for both amyloid accumulation and for conversion to AD. Relatedly, there is some evidence that increased activation in the precuneus does not decline, but increases further across MCI and probable AD (Lustig et al., 2003; Pihlajamaki et al., 2010; Sperling et al., 2009). Thus, while a large number of brain regions have been shown to exhibit nonlinear change in BOLD activation across the AD continuum, it is possible that the precuneus may not follow this trajectory. It will be important for future research to investigate whether the precuneus simply exhibits a delay in the quadratic effect, or whether it exhibits a fundamentally different change in response to AD pathology and specifically, Aβ burden.
Importantly, the current study provides evidence that nonlinear change in deactivation, specifically the transition to hypoactivation, may begin in asymptomatic (preclinical) individuals at-risk for AD. Previously, this transition was thought to begin in individuals at more severe stages, such as late MCI or early AD (Bosch et al., 2010; Celone et al., 2006; Dickerson et al., 2005), and thus, hypoactivation was thought to manifest in relation to disease processes associated with more severe pathology. To our knowledge, this is the first evidence to suggest that within the same region, amyloid load-related increases and decreases in task activation occur in individuals without AD-related cognitive symptoms.
There are multiple potential mechanisms that may contribute to the observed quadratic change in deactivation. First, it is plausible that Aβ deposition may drive both hyper- and hypoactivation changes. For example, relatively small increases in Aβ are thought to facilitate excitatory activity while high concentrations of Aβ are thought to lead to reduced synaptic activity (for review see Palop & Mucke, 2010). Indeed, an inhibition/excitation imbalance theory is gaining support in aging research (Legon et al., 2016). Therefore, the initial hyperactivation in default mode regions in the current study may represent the effect of increased excitatory activity. As Aβ accumulates further, it may act to reduce excitatory activity, and thus, the hypoactivation observed in individuals with greater Aβ burden may represent the simple effect of excessive Aβ deposition (Palop & Mucke, 2010). Alternately, the initial hyperactivation could be driven by Aβ while hypoactivation could be driven by a separate mechanism, for example recent research has suggested that Aβ and tau may act in concert such that Aβ is associated with hyperconnectivity and tau is associated with hypoconnectivity (Schultz et al., 2017). It will be important for future research to establish whether the quadratic effect of activation is also driven by tau accumulation. Finally, the Aβ-dependent nonlinear effects on activation were not related to either average gray matter volume or thickness. This finding is not surprising given that brain atrophy is thought to occur much later in the AD process (Jack et al., 2010). Taken together, the results suggest that hyper- and hypoactivation are related to Aβ-related altered neural function, but not frank tissue loss.
The results of the present study should be interpreted in the context of its constraints. First, we recognize that the nonlinear effect is reported from a somewhat small sample size and with a limited number of individuals with clinically relevant Aβ burden (n = 18). However, only 20 – 30% of the cognitively normal population show significant Aβ burden, suggesting our proportion of high SUVR individuals falls within the expected range (Rodrigue et al., 2012). Because of this, obtaining large sample sizes of cognitively normal individuals with significant Aβ burden will always be problematic, limiting the strength of the conclusions that can be drawn from any one study. Despite this, we find strong nonlinear effects localized to three clusters typically associated with the default mode network, and these shifts in activation direction track performance differences. Interestingly, several prior studies investigating amyloid, activation, and behavior have found somewhat similar relationships that are limited to high amyloid individuals such that activation (Kennedy et al., 2012; Mormino et al., 2012) or amyloid (Rodrigue et al., 2012) relate to cognition. Second, we note that the current study is cross-sectional and thus cannot speak to changes in amyloid over time and their association with BOLD response. In future studies it will be critically important to both replicate this effect in larger cross-sectional samples spanning additional AD stages, and to track change in deactivation within-persons over time in relation to one’s amyloid progression.
To conclude, we report a quadratic association between amyloid level and degree of activation to a difficult spatial distance-judgment task, with low-Aβ individuals exhibiting increased activation relative to Aβ-negative individuals, and the highest amyloid individuals exhibiting hypoactivation relative to lower Aβ individuals. Critically, amyloid load significantly moderates the effect of activation on task performance in high-Aβ individuals, such that deactivation is beneficial to performance for lower-Aβ elevated individuals, however deactivation (i.e., hypoactivation) relates to poorer performance in higher-Aβ individuals. Greater understanding of the factors that drive both hyper- and hypo-activation will greatly improve our current understanding of functional brain changes in adults at-risk for AD and the traits that allow some individuals to retain significant pathology with no clinical symptomatology.
Supplementary Material
Supplemental Figure 1. Schematic of fMRI task. While in scanner, participants made coordinate near/far spatial distance judgments at varying levels of difficulty (easy, medium, and hard). They also made categorical left/right spatial judgments as a control task.
Supplemental Figure 2. Effect of Hard vs. Categorical on BOLD Activation. The results of a conditional effect of Hard vs. Categorical from the full model including mean centered age, Aβ, and Aβ2 revealed several clusters that activated and deactivated in response to a difficult distance judgment task. A set of regions including the bilateral parietal lobes, bilateral middle frontal gyrus, and medial superior frontal gyrus activated while a set of regions typically associated with the default network including the bilateral posterior cingulate, bilateral medial frontal, and bilateral middle temporal gyrus deactivated to hard compared to categorical conditions. N = 62, mean age = 69 years.
Supplemental Figure 3. The Quadratic Model of Age and Amyloid on BOLD Activation including APOE Status. A subsample of 59 older adults had APOE genotype information. Fourteen of these individuals were ε4+ and seven were in the high-SUVR group. The same model was conducted with APOE status as an independent variable and the results remained largely the same. The left angular/temporal and right angular/temporal clusters, however, expanded to include the left and right hippocampus respectively. Blue scale represents t-values. Parameter estimates were extracted from the same 4mm spheres from the model without APOE (see Table 2).
Acknowledgments
This work was supported in part by the National Institutes of Health [grant numbers AG-036848 to KR and AG-036818 to KK]; and by an Investigator Initiated Trial grant from Eli Lilly and Company for the Amyvid ligand.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, … Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s and Dementia. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. https://doi.org/10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Gee JC. ANTS: Advanced Open-Source Normalization Tools for Neuroanatomy. Penn Image Computing and Science Laboratory; 2009. [Google Scholar]
- Baciu M, Koenig O, Vernier MP, Bedoin N, Rubin C, Segebarth C. Categorical and coordinate spatial relations: fMRI evidence for hemispheric specialization. Neuroreport. 1999;10(6):1373–1378. doi: 10.1097/00001756-199904260-00040. https://doi.org/10.1097/00001756-199904260-00040. [DOI] [PubMed] [Google Scholar]
- Bosch B, Bartres-Faz D, Rami L, Arenaza-urquijo EM, Fernandez-Espejo D, Junque C, … Molinuevo JL. Cognitive reserve modulates task-induced activations and deactivations in healthy elders, amnestic mild cognitive impairment and mild Alzheimer’s disease. Cortex. 2010;46:451–461. doi: 10.1016/j.cortex.2009.05.006. https://doi.org/10.1016/j.cortex.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Celone Ka, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, … Sperling Ra. Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: an independent component analysis. The Journal of Neuroscience. 2006;26(40):10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. https://doi.org/10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CM, Schneider JA, Bedell BJ, Beach TG, Bilker WB, Mintun MA, … Skovronsky DM. Use of Florbetapir-PET for Imaging β-Amyloid Pathology. JAMA. 2011;305(3):275–283. doi: 10.1001/jama.2010.2008. https://doi.org/10.1001/jama.2010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaère P, He Y, Fayet G, Duyckaerts C, Hauw JJ. βA4 deposits are constant in the brain of the oldest old: An immunocytochemical study of 20 french centenarians. Neurobiology of Aging. 1993;14(2):191–194. doi: 10.1016/0197-4580(93)90096-t. https://doi.org/10.1016/0197-4580(93)90096-T. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, … Sperling RA. Medial temporal lobe function and structure in mild cognitive impairment. Annals of Neurology. 2004;56(1):27–35. doi: 10.1002/ana.20163. https://doi.org/10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, … Sperling RA. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65(3):404–411. doi: 10.1212/01.wnl.0000171450.97464.49. https://doi.org/10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, … Jack CR. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimer’s and Dementia. 2016;12(3):292–323. doi: 10.1016/j.jalz.2016.02.002. https://doi.org/10.1016/j.jalz.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman Ja, Oh H, Madison CM, Baker SL, Vogel JW, Marks SM, … Jagust WJ. Neural compensation in older people with brain amyloid-β deposition. Nature Neuroscience. 2014;17(10):1316–8. doi: 10.1038/nn.3806. https://doi.org/10.1038/nn.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. NeuroImage. 2012;62(2):774–781. doi: 10.1016/j.neuroimage.2012.01.021. https://doi.org/10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. https://doi.org/10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Foster CM, Addis DR, Ford JH, Kaufer DI, Burke JR, Browndyke JN, … Giovanello KS. Prefrontal contributions to relational encoding in amnestic mild cognitive impairment. NeuroImage: Clinical. 2016;11:158–166. doi: 10.1016/j.nicl.2016.01.008. https://doi.org/10.1016/j.nicl.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster CM, Kennedy KM, Rodrigue KM. Differential aging trajectories of modulation of activation to cognitive challenge in APOE ε4 groups: Reduced modulation predicts poorer cognitive performance APOE ε4 and brain modulation. Journal of Neuroscience. 2017;37(29):6894–6901. doi: 10.1523/JNEUROSCI.3900-16.2017. https://doi.org/10.1523/JNEUROSCI.3900-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbers W, Mormino EC, Wigman SE, Ward AM, Vannini P, McLaren DG, … Sperling RA. Amyloid Deposition Is Linked to Aberrant Entorhinal Activity among Cognitively Normal Older Adults. Journal of Neuroscience. 2014;34(15):5200–5210. doi: 10.1523/JNEUROSCI.3579-13.2014. https://doi.org/10.1523/JNEUROSCI.3579-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, … Trojanowski JQ. Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. The Lancet Neurology. 2013;12(2):207–216. doi: 10.1016/S1474-4422(12)70291-0. https://doi.org/10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, … Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. The Lancet Neurology. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. https://doi.org/10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FRJ, … Zetterberg H. Prevalence of Cerebral Amyloid Pathology in Persons Without Dementia: A Meta-analysis. JAMA. 2015;313(19):1924–1938. doi: 10.1001/jama.2015.4668. https://doi.org/10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Reese ED, Horn MM, Sizemore AN, Unni AK, Meerbrey ME, … Rodrigue KM. BDNF val66met polymorphism affects aging of multiple types of memory. Brain Research. 2015;1612:104–117. doi: 10.1016/j.brainres.2014.09.044. https://doi.org/10.1016/j.brainres.2014.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Rieck JR, Boylan MA, Rodrigue KM. Functional magnetic resonance imaging data of incremental increases in visuo-spatial difficulty in an adult lifespan sample. Data in Brief. 2017;11:54–60. doi: 10.1016/j.dib.2017.01.004. https://doi.org/10.1016/j.dib.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Rodrigue KM, Devous MD, Hebrank AC, Bischof GN, Park DC. Effects of beta-amyloid accumulation on neural function during encoding across the adult lifespan. NeuroImage. 2012;62(1):1–8. doi: 10.1016/j.neuroimage.2012.03.077. https://doi.org/10.1016/j.neuroimage.2012.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal SL, Landau SM, Bell RK, Jagust WJ, Wills H, States U. Hippocampal activation is associated with longitudinal amyloid accumulation and cognitive decline. eLife, (Mci) 2017:1–15. doi: 10.7554/eLife.22978. https://doi.org/10.7554/eLife.22978. [DOI] [PMC free article] [PubMed]
- Legon W, Punzell S, Dowlati E, Adams SE, Stiles AB, Moran RJ. Altered Prefrontal Excitation/Inhibition Balance and Prefrontal Output: Markers of Aging in Human Memory Networks. Cerebral Cortex. 2016;26(11):4315–4326. doi: 10.1093/cercor/bhv200. https://doi.org/10.1093/cercor/bhv200. [DOI] [PubMed] [Google Scholar]
- Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms, and therapy. Nature Reviews Neurology. 2013;9(2):106–118. doi: 10.1038/nrneurol.2012.263. https://doi.org/10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, Brien KCO, Mcavoy M, Raichle ME, … Buckner RL. Functional deactivations: Change with age and dementia of the Alzheimer type. Proceedings of the National Academy of Sciences. 2003;100(24):14504–14509. doi: 10.1073/pnas.2235925100. https://doi.org/10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaika PK, Hoeft F, Glover GH, Reiss AL. Human Brain Mapping. 2009. Methods and Software for fMRI Analysis for Clinical Subjects. [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, … Sperling Ra. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2181–2186. doi: 10.1073/pnas.0706818105. https://doi.org/10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormino EC, Brandel MG, Madison CM, Marks S, Baker SL, Jagust WJ. Aβ Deposition in aging is associated with increases in brain activation during successful memory encoding. Cerebral Cortex. 2012;22(8):1813–1823. doi: 10.1093/cercor/bhr255. https://doi.org/10.1093/cercor/bhr255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien JL, O’Keefe KM, Laviolette PS, Deluca AN, Blacker D, Dickerson BC, Sperling RA. Longitudinal fMRI in elderly reveals loss of hippocampal activation with clinical decline. Neurology. 2010;74(24):1969–1976. doi: 10.1212/WNL.0b013e3181e3966e. https://doi.org/10.1212/WNL.0b013e3181e3966e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Steffener J, Razlighi QR, Habeck C, Stern Y. β-Amyloid Deposition Is Associated with Decreased Right Prefrontal Activation during Task Switching among Cognitively Normal Elderly. The Journal of Neuroscience. 2016;36(6):1962–70. doi: 10.1523/JNEUROSCI.3266-15.2016. https://doi.org/10.1523/JNEUROSCI.3266-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Steffener J, Razlighi R, Habeck C, Liu D, Gazes Y, … Stern Y. Aβ-related hyperactivation in fronto-parietal control regions in cognitively normal elderly. Neurobiology of Aging. 2015;36(12):3247–3254. doi: 10.1016/j.neurobiolaging.2015.08.016. https://doi.org/10.1016/j.neurobiolaging.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nature Neuroscience. 2010;13(7):812–818. doi: 10.1038/nn.2583. https://doi.org/10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajamaki M, O’Keefe K, Bertram L, Tanzi RE, Dickerson BC, Blacker D, … Sperling RA. Evidence of altered posteromedial cortical fMRI activity in subjects at risk for Alzheimer disease. Alzheimer Disease and Associated Disorders. 2010;24(1):28–36. doi: 10.1097/WAD.0b013e3181a785c9. https://doi.org/10.1097/WAD.0b013e3181a785c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;31(4):437–448. https://doi.org/10.3102/10769986031004437. [Google Scholar]
- Radloff LS. A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas. 1977;1(3):385–401. https://doi.org/10.1177/014662167700100306. [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, … Acker JD. Regional Brain Changes in Aging Healthy Adults: General Trends, Individual Differences and Modifiers. Cerebral Cortex. 2005;15(11):1676–1689. doi: 10.1093/cercor/bhi044. https://doi.org/10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Rieck JR, Rodrigue KM, Boylan MA, Kennedy KM. Age-related Reduction of BOLD Modulation to Cognitive Difficulty Predicts Poorer Task Accuracy and Poorer Fluid Reasoning Ability. NeuroImage. 2017 doi: 10.1016/j.neuroimage.2016.12.022. In Press https://doi.org/10.1016/j.neuroimage.2016.12.022. [DOI] [PMC free article] [PubMed]
- Rodrigue KM, Kennedy KM, Devous MD, Reick JR, Hebrank AC, Diaz-Arrastia R, … Park DC. β-Amyloid burden in healthy aging: Regional distribution and cognitive consequences. Neurology. 2012;78:387–395. doi: 10.1212/WNL.0b013e318245d295. https://doi.org/10.1212/WNL.0b013e318245d295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, … Villemagne VL. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiology of Aging. 2010;31(8):1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. https://doi.org/10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Schultz AP, Chhatwal JP, Hedden T, Mormino EC, Hanseeuw BJ, Sepulcre J, … Sperling RA. Phases of hyper and hypo connectivity in the Default Mode and Salience networks track with amyloid and Tau in clinically normal individuals. The Journal of Neuroscience. 2017;(617):3263–16. doi: 10.1523/JNEUROSCI.3263-16.2017. https://doi.org/10.1523/JNEUROSCI.3263-16.2017. [DOI] [PMC free article] [PubMed]
- Sperling RA. The potential of functional MRI as a biomarker in early Alzheimer’s disease. Neurobiology of Aging. 2011;32(Suppl 1):1–11. doi: 10.1016/j.neurobiolaging.2011.09.009. https://doi.org/10.1016/j.neurobiolaging.2011.09.009.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, … Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s and Dementia. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. https://doi.org/10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Dickerson BC, Pihlajamaki M, Vannini P, LaViolette PS, Vitolo OV, … Johnson KA. Functional alterations in memory networks in early alzheimer’s disease. NeuroMolecular Medicine. 2010;12(1):27–43. doi: 10.1007/s12017-009-8109-7. https://doi.org/10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, LaViolette PS, O’Keefe K, O’Brien J, Rentz DM, Pihlajamaki M, … Johnson KA. Amyloid Deposition Is Associated with Impaired Default Network Function in Older Persons without Dementia. Neuron. 2009;63(2):178–188. doi: 10.1016/j.neuron.2009.07.003. https://doi.org/10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Mormino EC, Johnson K. The evolution of preclinical Alzheimer’s disease: Implications for prevention trials. Neuron. 2014;84(3):608–622. doi: 10.1016/j.neuron.2014.10.038. https://doi.org/10.1016/j.neuron.2014.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Schematic of fMRI task. While in scanner, participants made coordinate near/far spatial distance judgments at varying levels of difficulty (easy, medium, and hard). They also made categorical left/right spatial judgments as a control task.
Supplemental Figure 2. Effect of Hard vs. Categorical on BOLD Activation. The results of a conditional effect of Hard vs. Categorical from the full model including mean centered age, Aβ, and Aβ2 revealed several clusters that activated and deactivated in response to a difficult distance judgment task. A set of regions including the bilateral parietal lobes, bilateral middle frontal gyrus, and medial superior frontal gyrus activated while a set of regions typically associated with the default network including the bilateral posterior cingulate, bilateral medial frontal, and bilateral middle temporal gyrus deactivated to hard compared to categorical conditions. N = 62, mean age = 69 years.
Supplemental Figure 3. The Quadratic Model of Age and Amyloid on BOLD Activation including APOE Status. A subsample of 59 older adults had APOE genotype information. Fourteen of these individuals were ε4+ and seven were in the high-SUVR group. The same model was conducted with APOE status as an independent variable and the results remained largely the same. The left angular/temporal and right angular/temporal clusters, however, expanded to include the left and right hippocampus respectively. Blue scale represents t-values. Parameter estimates were extracted from the same 4mm spheres from the model without APOE (see Table 2).